Abstract
Background.
There is limited research on the interaction of both positive and negative daily-life environments with stress-related genetic variants on psychotic experiences (PEs) and negative affect (NA) across the extended psychosis phenotype. This study examined whether the FK506 binding protein 51 (FKBP5) variability moderates the association of positive and negative experiences in the moment with PEs and NA in participants with incipient psychosis and their nonclinical counterparts.
Methods.
A total of 233 nonclinical and 86 incipient psychosis participants were prompted for a 1-week period to assess their day-to-day experiences. Participants were genotyped for four FKBP5 single nucleotide polymorphisms (rs3800373, rs9296158, rs1360780, and rs9470080).
Results.
Multilevel analyses indicated that, unlike the risk haplotype, the protective FKBP5 haplotype moderated all the associations of positive experiences with diminished PEs and NA in incipient psychosis compared with nonclinical group.
Conclusions.
Participants with incipient psychosis showed symptomatic improvement when reporting positive appraisals in the interpersonal domain, which suggests that these act as a powerful coping mechanism. The fact that this occurred in daily-life underscores the clinical significance of this finding and pinpoints the importance of identifying protective mechanisms. In addition, results seem to concur with the vantage sensitivity model of gene–environment interaction, which poses that certain genetic variants may enhance the likelihood of benefiting from positive exposures.
Key words: Gene–environment interaction, ecological validity, experience sampling methodology, incipient psychosis, vantage sensitivity
Introduction
Extensive evidence indicates that the psychosis phenotype is expressed across a dynamic continuum that ranges from nonclinical to clinical manifestations [1, 2]. Individuals with nonclinical, subclinical, and early-stage manifestations offer important assets for the study of both risk and protective mechanisms, as they are less affected by the wide array of confounding effects associated with overt psychopathology and chronicity. Thus, investigating continuities as well as discontinuities between clinical and nonclinical expressions may help to elucidate the heterogeneity that characterizes pathways to psychosis and the identification of protective factors [3]. In this regard, the study of patterns of gene–environment interactions (G × E) in clinical and nonclinical populations may contribute to our understanding of common and differential mechanisms operating across the psychosis continuum [4].
Recent G × E studies indicate that the interaction of genetic variants on the FK506 binding protein 5 (FKBP5) gene with psychosocial stressors is associated with psychotic experiences (PEs) in clinical and nonclinical samples [5-8]. Compelling evidence has suggested that individual variation in the FKBP5 gene is linked to the dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis, which has been identified as a critical neurobiological mechanism underlying the emergence of psychotic symptoms [9]. In particular, the minor risk alleles (C, A, T, T) of at least 4 FKBP5 single nucleotide polymorphisms (SNPs; rs3800373, rs9296158, rs1360780, and rs9470080, respectively), as compared with the nonrisk or protective alleles (A, G, C, C), have been associated with a decreased sensitivity of the glucocorticoid receptor to circulating cortisol, entailing a diminished negative feedback regulation of the HPA axis that results in an abnormal prolongation of the stress response [10]. Importantly, the rs1360780 SNPs included in a functional haplotype confers differential effects in FKBP5 mRNA and protein levels mediated by a differential chromatin conformation, resulting in different transcriptional effects between risk and protective alleles [11]. So far, research has predominantly focused on analyzing the effects of the interplay of FKBP5 variation with adverse environmental exposures on psychotic phenomena guided by diathesis-stress model for schizophrenia [12], greatly understudying the interaction of genetic variation with positive, and thus putative protective, environmental factors. However, individuals may differ in their susceptibility to the environment across a range of exposures (not just negative ones) and, therefore, moderation effects by genetic variation should also be expected in relation to the benefit that individuals may obtain from positive experiences [13]. In light of this, new frameworks under which to consider G × E interactions have been developed. For example, the differential susceptibility model [14] highlights that individuals traditionally considered to carry greater vulnerability may be better conceptualized as being more plastic, sensitive, or malleable to the environment (“for worse and for better”). That is, it suggests that the same genetic variants involved in increasing the negative effects of adverse experiences could also be involved in enhancing the likelihood of benefiting from positive ones. Another relevant model of G × E interactions is vantage sensitivity [15], which poses that certain genetic variants may enhance the likelihood of benefiting from positive exposures (without also implying an increase in the susceptibility to negative exposures)—that is, vantage sensitivity is more than the “bright side” of environmental sensitivity as covered in differential susceptibility models. Although these approaches have been scarcely considered within the psychosis field, the pertinence of incorporating the assessment of positive environmental experiences and examining potential beneficial effects is underscored by G × E investigations in other stress-related phenotypes. For instance, in depression research, it has been shown that the impact of BDNF Val66Met genotype on stress sensitivity may be dependent on the experience of daily-life positive emotions [16].
Another relevant issue that has received increasing attention in the context of G × E research has been the importance of refining environmental measures [17]. In this regard, the enhancement of precision, reliability, and ecological validity offered by the use of ambulatory assessment strategies (such as experience sampling methodology [ESM] or ecological momentary assessment) should be helpful for examining genetic moderation of the effects of both positive and negative microlevel experiences (i.e., those occurring in the moment in real-life contexts; [18]). To the best of our knowledge, there are no ambulatory assessment studies investigating the potential moderation of individual genetic variation involved in the regulation of the stress system, such as FKBP5 variants, in the association of positive and negative momentary experiences on the psychosis phenotype. Importantly, there is also a lack of studies with ecological validity that examine plausible genetic-environmental interplay differences between nonclinical and clinical individuals, which should allow to identify risk and resilience mechanisms and targets for prophylactic interventions [19].
Therefore, the present study used ESM to elucidate the extent to which the interplay of FKBP5 variability with both positive and negative exposures impacts the expression of PEs, as well as negative affect (NA), in daily-life across nonclinical and clinical levels of expression of the extended psychosis phenotype. Specifically, we examined whether the interaction effects of positive and negative experiences with the FKBP5 haplotype on PEs and NA differed between subjects with and without need for care, that is, in incipient psychosis and nonclinical groups. We predicted that the association of both positive and negative momentary experiences with symptoms and NA would be greater in an incipient psychosis group than in a nonclinical group, and that these associations would be moderated by FKBP5 variability.
Materials and Methods
Participants
Data were collected as part of an ongoing longitudinal investigation examining psychosis risk and resilience [20]. The present study included a total of 319 participants reflecting different levels of expression of the psychosis phenotype for which ESM and genetic data were available. The nonclinical group was comprised of 233 students recruited from university and technical schools who scored across a range on questionnaire assessments of schizotypy and psychosis-proneness questionnaires (mean age = 20.0 years, SD = 2.9 years; 25.3% males). We invited participants from our screening samples who had standard scores based upon sample norms of at least 1.0 on the positive or negative schizotypy dimensions of the Wisconsin Schizotypy Scales [21], the suspiciousness scale of the Schizotypal Personality Questionnaire [22], or the positive symptom subscale of the Community Assessment of Psychic Experiences [23], and randomly selected participants who had standard scores below 1.0 on each of these. The goal of this enrichment procedure was to ensure adequate variability of schizotypy traits and avoid having a “super healthy” control sample. The final nonclinical sample contained 198 university and 35 technical school students.
The incipient psychosis group included 86 patients recruited at the Sant Pere Claver Health Foundation (mean age = 22.3 years, SD = 4.7 years; 69.8% males). Specifically, the sample for this study consisted of 55 diagnosed with at-risk mental states for psychosis (ARMS) and 31 first episode psychosis (FEP) patients (6 of them met criteria for affective psychoses including 4 with bipolar I disorder and 2 with unipolar depression with psychosis). Patients’ inclusion criteria were age between 14 and 40 years and IQ ≥ 75. ARMS-criteria were established by the Comprehensive Assessment of At-Risk Mental States [24] and/or the Schizophrenia Proneness Instrument-Adult version [25]. FEP-patients met DSM-IV-TR criteria for any psychotic disorder or affective disorder with psychotic symptoms as established by the Structured Clinical Interview for DSM-IV [26]. Ethical approval was obtained from the University Ethics committee and participants provided written informed consent.
Measures
Participants received personal digital assistants that signaled them randomly eight times daily for 1 week to complete brief assessments of affect, cognition, activities, and PEs on 7-point scales ranging from 1 (not at all) to 7 (very much). A detailed description of the ESM assessment and validation data can be found in previous studies [20]. The positive and negative experience items used in the present study focused on two main domains of daily-life: situational and interpersonal. We focused on two content areas that have been shown as highly relevant in psychosis and tested out in ESM research: situational stress and appraisals of social rejection [20]. Thus, the negatively valenced items were: “My current situation is stressful” and (when alone) “I am alone because people do not want to be with me.” The positively valenced items were: “My current situation is positive” and “Right now I feel that others care about me.” Two ESM indices were created and used as outcome measures: (i) PEs was computed by averaging the scores for 10 items: unusual senses, unusual thoughts, feeling weird, losing control, difficulty controlling thoughts, familiar things seeming strange, feeling suspicious, feeling mistreated, hearing/seeing things others could not, and passivity (coefficient α = 0.95); (ii) NA was the mean of four items: feeling anxious, sad, angry, and guilty (coefficient α = 0.89).
Genetic data
Genomic DNA was extracted from saliva in the nonclinical sample and blood in the incipient psychosis samples. Four single-nucleotide polymorphisms (SNPs) included in a previously described functional FKBP5 haplotype [27] were genotyped using TaqMan 5′ exonuclease assay (Applied Biosystems): rs3800373, rs9296158, rs1360780, and rs9470080. Compliance with Hardy–Weinberg equilibrium was verified for all polymorphisms in both samples (all p > 0.05). Haplotypes were estimated using PHASE, after confirming that the four SNPs were in high linkage-disequilibrium (D′ > 0.9; r 2 > 0.7). Participants were classified into three groups for analyses based on previous studies [19,28]: (i) carriers of at least one protective haplotype and no risk haplotypes (AGCC/−, n = 157), (ii) carriers of one risk haplotype and one protective haplotype (AGCC/CATT, n = 118), and (iii) carriers of at least one risk haplotype and no protective haplotypes (CATT/−, n = 44). Haplotypic frequencies are presented in Table S1).
Statistical analyses
ESM data have a hierarchical structure in which ESM ratings (level 1 data) are nested within participants (level 2 data). Linear mixed models were used to control for within-subject clustering of multiple observations using the “xtmixed” command in Stata 12 [29]. Graphs were generated with the R program (www.r-project.org).
Two types of multilevel analyses were conducted in the present study. First, in order to examine whether the effect of the FKBP5 haplotype on PEs and NA differed between nonclinical and incipient psychosis groups, we assessed the main effects of level 2 predictors on level 1 outcome variables (PEs and NA). The level 2 predictors were the FKBP5 haplotype (0 = AGCC/−; 1 = AGCC/CATT; 2 = CATT/−), the group status (incipient psychosis vs nonclinical groups), and the interaction term (FKBP5 haplotype × group status).
Second, to examine whether the potential moderating role of the FKBP5 haplotype in the association of momentary appraisals with daily-life outcomes differed between nonclinical and incipient psychosis groups, cross-level interactions were conducted. Cross-level interactions tested whether level 1 slopes (i.e., the association of positive and negative experiences with symptoms and NA) varied as a function of level 2 variables FKBP5 haplotype, group status, and FKBP5 haplotype × group status). Finally, when a level 2 interaction was significant, the effect of the interaction was examined in each FKBP5 haplotype group using simple slopes analyses. Please note that multiple comparisons in multilevel modeling do not present the risk of alpha inflation that is seen in traditional unilevel analyses, so following Gelman et al. [30] multiple testing corrections were not conducted.
Results
Main effects of level 2 predictors (FKBP5 and group)
Results indicated that the FKBP5 haplotype was not associated with momentary PEs or NA ( Table 1). However, as expected, participants in the incipient psychosis group experienced more PEs and NA than individuals in the nonclinical group (p < 0.001). No interaction effects were found between the FKBP5 haplotype and group on PEs or NA in daily-life. Thus, haplotype risk and protective groups were unassociated with the overall reports of symptoms and affect in daily life.
Table 1.
Main effects of the FKBP5 haplotype, group status, and their interaction on psychotic experiences and negative affect (n = 319).
| Level 1 criterion | Level 2 predictors | ||
|---|---|---|---|
| FKBP5 haplotype | Group: early-psychosis vs nonclinical | FKBP5 haplotype × group | |
| γ 01 (df = 316) | γ 02 (df = 316) | γ 03 (df = 315) | |
| ESM psychosis | |||
| Psychotic experiences index | 0.026 (SE = 0.036) | 0.447 (SE = 0.058)*** | 0.138 (SE = 0.082) |
| ESM affect | |||
| Negative affect index | −0.031 (SE = 0.051) | 0.425 (SE = 0.081)*** | 0.122 (SE = 0.114) |
*p < 0.050.
**p < 0.010.
p < 0.001.
The effect of the FKBP5 haplotype × group interaction term was examined over and above the main effects.
Cross-level interactions
Cross-level interaction analyses examined whether the FKBP5 haplotype, group status, and their interaction moderated the associations of positive and negative experiences with PEs and NA in daily-life ( Table 2). All negative appraisals in the moment (situational stress and feeling unwanted by others) were associated with increased PEs and NA in the moment, whereas all positive experiences (current situation being positive and feeling cared for by others) were associated with decreased PEs and NA.
Table 2.
Moderation by FKBP5 haplotype, group, and the FKBP5 × group interaction of the association between both positive and negative appraisals with psychotic experiences and negative affect (n = 319).
| Level 1 criterion | Level 1 predictora | Level 2 predictors | |||
|---|---|---|---|---|---|
| ESM momentary appraisals | FKBP5 haplotype | Group: early-psychosis vs nonclinical | FKBP5 haplotype × groupb | ||
| γ10 (df = 316) | γ11 (df = 316) | γ12 (df = 316) | γ13 (df = 315) | ||
| Appraisals | Coeff. (SE) | Coeff. (SE) | Coeff. (SE) | ||
| ESM psychosis | Negative appraisals | ||||
| Psychotic experiences index | Situation stressful | 0.042 (0.007)*** | 0.007 (0.007) | 0.080 (0.011)*** | 0.013 (0.015) |
| Alone b/c not wanted | 0.104 (0.032)** | 0.016 (0.029) | 0.041 (0.040) | −0.005 (0.059) | |
| Positive appraisals | |||||
| Situation positive | −0.059 (0.008)*** | 0.005 (0.008) | −0.059 (0.013)*** | 0.035 (0.018)* | |
| Others care about me | −0.034 (0.008)*** | 0.002 (0.008) | −0.045 (0.013)*** | 0.040 (0.018)* | |
| ESM affect | Negative appraisals | ||||
| Negative affect index | Situation stressful | 0.216 (0.015)*** | 0.004 (0.014) | 0.065 (0.023)** | 0.013 (0.033) |
| Alone b/c not wanted | 0.156 (0.050)** | 0.086 (0.046) | 0.001 (0.060) | −0.025 (0.092) | |
| Positive appraisals | |||||
| Situation positive | −0.277 (0.018)*** | 0.021 (0.017) | −0.001 (0.026) | 0.075 (0.037)* | |
| Others care about me | −0.133 (0.015)*** | 0.009 (0.014) | −0.009 (0.023) | 0.078 (0.032)* | |
p < 0.050.
p < 0.010.
p < 0.001.
The table reports the coefficient of the association of the level 1 predictor and criterion for the analyses of FKBP5 and group variables entered simultaneously.
The effect of FKBP5 × group interaction term was examined over and above the main effects.
Regarding moderation effects, the FKBP5 haplotype did not moderate the associations of positive or negative appraisals with PEs or NA. Group status moderated the associations of situational stress with PEs and NA ( Table 2). Analysis of simple slopes showed that the associations were greater in the incipient psychosis group (PEs: 0.126, SE = 0.013, p < 0.001; NA: 0.285, SE = 0.023, p < 0.001) compared with the nonclinical group (PEs: 0.046, SE = 0.04, p < 0.001; NA: 0.218, SE = 0.011, p < 0.001). The group status also moderated the associations of the two positive appraisals with PEs, but not NA, in the moment. That is, as positive experiences increased, incipient psychosis participants experienced greater decreases in symptoms (positive situation: −0.114, SE = 0.014, p < 0.001; others care about me: −0.078, SE = 0.017, p < 0.001) than their nonclinical counterparts (positive situation: −0.055, SE = 0.005, p < 0.001; others care about me: −0.032, SE = 0.004, p < 0.001).
The FKBP5 haplotype by group interaction moderated all the associations of positive, but not negative, experiences with decreased PEs and NA in daily-life ( Table 2). As shown in Figure 1, simple slope analyses indicated that, among protective haplotype carriers (i.e., AGCC/−), the positive appraisal of the situation was associated with decreased PEs in the incipient psychosis group compared with the nonclinical group (PEs: −0.087, SE = 0.018, p < 0.001; NA: −0.043, SE = 0.038, ns). In addition, among protective haplotype carriers, the positive experience of feeling cared for by others was associated with decreased PEs, as well as NA, in the incipient psychosis group compared with the nonclinical group (PEs: −0.096, SE = 0.017, p < 0.001; NA: −0.066, SE = 0.033, p < 0.050; see Figure 2). Furthermore, among the mixed haplotype (i.e., one protective and one risk haplotype carriers; AGCC/CATT), the experience of a positive situation was associated with diminished PEs in incipient psychosis in comparison to nonclinical group (situation positive—PEs: −0.040, SE = 0.020, p < 0.050; NA: 0.002, SE = 0.040, ns; others care about me—PEs: −0.004, SE = 0.022, ns; NA: 0.014, SE = 0.036, ns). Finally, among risk haplotype carriers (i.e., CATT/−), no group differences were found in the association of both positive appraisals with symptoms (situation positive—PEs: −0.017, SE = 0.035, ns; NA: 0.135, SE = 0.079, ns; others care about me—PEs: −0.035, SE = 0.027, ns; NA: 0.088, SE = 0.055, ns).
Figure 1.

Group differences between nonclinical and early psychosis groups in the interaction of situation positive with FKBP5 haplotype on psychotic experiences and negative affect.
Figure 2.
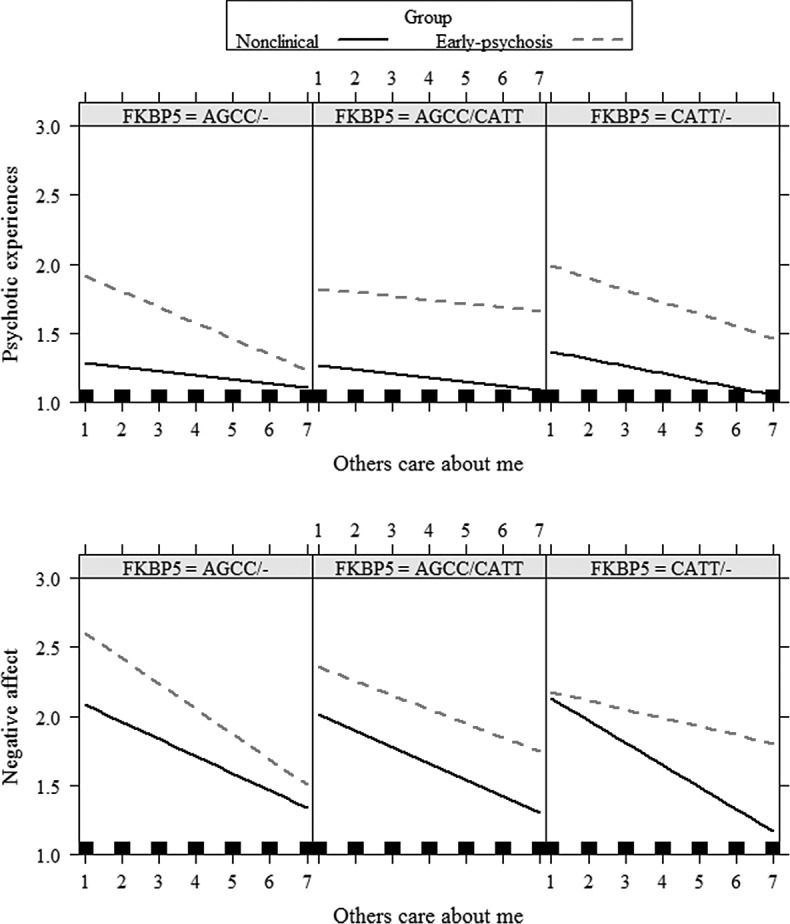
Group differences between nonclinical and early psychosis groups in the interaction of feeling cared for by others with FKBP5 haplotype on psychotic experiences and negative affect.
Discussion
Main findings
The present work investigated whether the interplay between FKBP5 variation and contextual factors is not limited to adverse experiences, but expands into the full spectrum of positive and negative experiences in real-life contexts. The study raised a novel finding indicating that the interaction of FKBP5 variation with positive experiences is associated with diminished PEs and NA, in incipient psychosis compared with nonclinical group. Another relevant finding from the current study was that individuals with incipient psychosis, in comparison to their nonclinical counterparts, reported greater psychotic reactivity to negative, but also to positive, experiences in daily-life. Notably, the incipient psychosis group differed from the nonclinical group in terms of symptomatic reactivity to positive, but not negative, appraisals in the interpersonal domain, suggesting that positive interpersonal appraisals may act as a relevant coping mechanism for help-seeking individuals, ameliorating the intensity of symptom expression in the realm of daily-life.
The interpretation of these findings appears relevant at both basic and applied levels. At a basic science level, they seem to concur with numerous studies showing that environmental enrichment induces dramatic changes on brain and behavior in animal models [31]. It has been shown that environmental enrichment improves behavioral impairment in animal models of schizophrenia. Importantly, environmental enrichment diminishes the long-term negative effects of stress on altered HPA function [32]. A specific effect of environmental enrichment on this reversal role of HPA dysregulation is seen in animal models of transgenerational and multigenerational prenatal stress [33]. In addition, it has been shown that the exposure to environmental enrichment remarkably reduced the stress-sensitive phenotype induced by ancestral stress and also improved several levels of HPA axis function.
From a clinical stance, the present findings support increasing evidence indicating that environmental factors in general [34], and daily-life experiences in particular [35], are potent factors in terms of influencing psychotic reactivity and expression. Research has been predominantly guided by diathesis-stress model, and there is a growing criticism regarding the disproportionate focus on stressors and negative life events and the negligence of positive environments. New conceptual frameworks, such as the diathesis-stress-support model [36], suggest that social support can boost skills and competences, prevent risk accumulation, and are essential for an individual’s progress toward recovery. In this sense, the recent growing impact of Positive Psychology [37,38] and Positive Psychiatry [39] has demonstrated that persons with psychosis can experience positive psychological features [40]. Regarding ESM research, one study revealed that individuals with persistent PEs showed more social reward sensitivity than individuals without persistent PEs [41]. This realization has recently encouraged interest in developing momentary clinical interventions with ecologically valid tools [42], providing new opportunities for treatment. The development of recent positive psychological interventions [43] and the new upsurge of real world interventions in mental health [44] may be particularly important in psychosis field.
Clinical relevance of individual genetic differences in the response to positive experiences
Although to the best of our knowledge there are no previous studies investigating whether the FKBP5 haplotype moderates the association of positive experiences with symptom expression in daily-life, findings seem to resonate with recent work in the context of responsiveness to psychotherapy in Posttraumatic stress disorder (PTSD) [45]. The study examined whether improvement in PTSD symptoms after exposure-based therapy differed as a function of FKBP5 (rs1360780) variability. They found that, although no genetic differences on symptoms emerged between baseline and a 4-month follow-up, individuals homozygous for the protective rs1360780 C-allele continued to show a reduction in symptoms in the 10-month follow-up, whereas this was not found for carriers of the risk T-allele [45]. According to Pluess [15], these results suggest that the C-allele may enhance vantage sensitivity in the context of exposure-based therapy in this population. Given that in the present study, the protective haplotype was associated with diminished symptoms in the context of positive experiences for incipient psychosis participants (but not increased symptoms in the context of negative ones), our findings would seem to be in line with a vantage sensitivity interpretation for incipient psychosis as compared with nonclinical group. It must be noted, although, that we did not employ specific statistical tests [46] to formally investigate such pattern of G × E interactions.
Recent etiological evidence that advocates for positive and negative effects of genetic and environment factors on the developmental trajectories of psychosis has not yet been embodied into preventive interventions. In this regard, it is imperative to devise new real-world targeted strategies based on knowledge about the individual capacity of people at-risk or with psychotic symptoms in showing differential reactivity to negative and positive factors. This is a critical issue, as many clinicians, even those with knowledge and sensitivity toward psychological factors in incipient psychosis services with a preventive framework, continue to treat persons with any degree of psychosis expression in a very different manner to that employed with persons presenting with risk for or overt non-psychotic disorders. Most likely, decades of a narrow conceptualization of psychosis as the straightforward product of a genetically determined brain disease, the historically engraved “broken brain” view of schizophrenia [47] and the youth of a developing agreed upon conceptual framework in which to integrate the dynamic, malleable, and reactive nature of psychosis experiences, partly account for this situation.
Strengths and limitations
The present study has a number of strengths, including the estimation of a haplotype that increases the power to detect genetic associations [48] and the use of valid ecological measures that are considered to increase the power and reliability of G × E research [49]. In this sense, the design of the study offers a useful model for researchers wanting to examine complex G × E effects in the real world. As regards to limitations, these include gender differences in the composition of nonclinical and clinical samples, which may limit the generalizability of findings; and the fact that causal inferences of the effects of momentary positive and negative experiences cannot be definitively drawn given that ESM predictor and criterion variables were measured concurrently. Future G × E longitudinal studies should also examine the association of PE and NA interactions over time in large samples allowing examination of potential differences in affective versus nonaffective psychosis diagnoses. This would shed light on the complex relationship between affect and psychosis and potential similarities and differences in phenotypes with a predominance of each component.
Conclusions
Overall, the study provides evidence of individual differences in the interaction of genetic variation with proximal environmental factors in nonclinical and incipient psychosis groups. Future studies should employ ambulatory assessments to investigate potential mechanisms involved in the interplay of positive momentary exposures with protective individual variation in relevant genes for the homeostasis of the HPA axis, which may result in an adaptive response to stress in the realm of daily-life. Moreover, empirical evidence from research examining patterns of reactivity to the environment could encourage real-world targeted strategies based on the knowledge of individual differences in the reactivity from negative to positive factors in the field of psychosis. This is a critical issue given that, despite intense efforts in the early intervention paradigm [50], there continues to be a great amount of therapeutic helplessness associated with psychosis risk outcomes.
Acknowledgments.
This work was supported by the Spanish Ministerio de Economía y Competitividad (Plan Nacional de PSI2017-87512-C2-1-R and PSI2017-87512-C2-2-R; Red de Excelencia PSI2014-56303-REDT, PROMOSAM: Investigación en Procesos, Mecanismos y Tratamientos Psicológicos para la Promoción de la Salud Mental), Fundació La Marató de TV3 (091110) and Comissionat per a Universitats i Recerca of the Generalitat de Catalunya (2017SGR1612). Neus Barrantes-Vidal is supported by the Institució Catalana de Recerca i Estudis Avançats (ICREA) Academia Award.
Conflict of Interest.
The authors declare no conflict of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1192/j.eurpsy.2019.4.
click here to view supplementary material
References
- [1].Kwapil TR, Barrantes-Vidal N. Schizotypy: looking back and moving forward. Schizophr Bull. 2015;41:S366–S373. 10.1093/schbul/sbu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- [3].Barrantes-Vidal N, Grant P, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015;41:S408–S416. 10.1093/schbul/sbu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].European Network of National Networks Studying Gene Environment Interactions in Schizophrenia (EU-GEI). Identifying gene–environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40:729–736. 10.1093/schbul/sbu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ajnakina O, Borges S, Di Forti M, Patel Y, Xu X, Green P, et al. Role of environmental confounding in the association between FKBP5 and first-episode psychosis. Front Psychiatry. 2014;5:84 10.3389/fpsyt.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alemany S, Moya J, Ibáñez MI, Villa H, Mezquita L, Ortet G, et al. Childhood trauma and the rs1360780 SNP of FKBP5 gene in psychosis: a replication in two general population samples. Psychol Med. 2016;46:221–223. 10.1017/S0033291715001695. [DOI] [PubMed] [Google Scholar]
- [7].Collip D, Myin-Germeys I, Wichers M, Jacobs N, Derom C, Thiery E, et al. FKBP5 as a possible moderator of the psychosis-inducing effects of childhood trauma. Br J Psychiatry. 2013;202:261–268. 10.1192/bjp.bp.112.115972. [DOI] [PubMed] [Google Scholar]
- [8].Cristóbal-Narváez P, Sheinbaum T, Rosa A, Ballespí, S, de Castro-Catala M, Peña E, et al. The interaction between childhood bullying and the FKBP5 gene on psychotic-like experiences and stress reactivity in real life. PLoS ONE. 2016;11:e0158809 10.1371/journal.pone.0158809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–S195. 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- [11].Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 2017;104:667–685. 10.1037/0033-295X.104.4.667. [DOI] [PubMed] [Google Scholar]
- [13].Pluess M, Belsky J. Vantage sensitivity: genetic susceptibility to effects of positive experiences In: Pluess M, editor. Genetics of psychological well-being. Oxford, UK: Oxford University Press; 2015, p. 193–210. [Google Scholar]
- [14].Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- [15].Pluess M. Vantage sensitivity: environmental sensitivity to positive experiences as a function of genetic differences. J Pers. 2017;85:38–50. 10.1111/jopy.12218. [DOI] [PubMed] [Google Scholar]
- [16].Wichers M, Kenis G, Jacobs N, Myin-Germeys I, Schruers K, Mengelers R, et al. The psychology of psychiatric genetics: evidence that positive emotions in females moderate genetic sensitivity to social stress associated with the BDNF Val66Met polymorphism. J Abnorm Psychol. 2008;117:699–704. 10.1037/a0012909. [DOI] [PubMed] [Google Scholar]
- [17].Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch. Gen. Psychiatry. 2005;62:473–481. 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- [18].Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. 10.1016/j.neuroscience.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cristóbal-Narváez P, Sheinbaum T, Myin-Germeys I, Kwapil TR, Castro-Catala M, Domínguez-Martínez T, et al. The role of stress-regulation genes in moderating the association of stress and daily-life psychotic experiences. Acta Psychiatr Scand. 2017;136:389–399. 10.1111/acps.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barrantes-Vidal N, Chun C, Myin-Germeys I, Kwapil TR. Psychometric schizotypy predicts psychotic-like, paranoid, and negative symptoms in daily-life. J Abnorm Psychol. 2013;122:1077–1087. 10.1037/a0034793. [DOI] [PubMed] [Google Scholar]
- [21].Kwapil TR, Barrantes-Vidal N, Silva PJ. The dimensional structure of the Wisconsin Schizotypy Scales: factor identification and construct validity. Schizophr Bull. 2008;34:444–457. 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Res. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- [23].Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. 10.1017/S0033291701005141. [DOI] [PubMed] [Google Scholar]
- [24].Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis—the Comprehensive Assessment of At-Risk Mental States (CAARMS). Aust NZJ Psychiatry. 2005;39:964–971. 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- [25].Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. Schizophrenia proneness instrument-adult version (SPI-A). Rome, Italy: Giovanni Fioriti; 2007. [Google Scholar]
- [26].First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders-patient ed. (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department; 1995. [Google Scholar]
- [27].Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- [28].Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13:25–37. 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- [29].StataCorp. Stata statistical software: release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- [30].Gelman A, Hill J, Yahima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189–211. [Google Scholar]
- [31].Burrows EL, Hannan, AJ. Cognitive endophenotypes, gene-environment interactions and experience-dependent plasticity in animal models of schizophrenia. Biol Psychol. 2016;116:82–90. 10.1016/j.biopsycho.2015.11.015. [DOI] [PubMed] [Google Scholar]
- [32].Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci. 2003;18:3367–3374. 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- [33].McCreary JK, Erickson ZT, Hao Y, Ilnytskyy Y, Kovalchuk I, Metz GA. Environmental intervention as a therapy for adverse programming by ancestral stress. Sci Rep. 2016;6:37814 10.1038/srep37814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van Os J, Reininghaus U, Meyer-Lindenberg A. The search for environmental mechanisms underlying the expression of psychosis: introduction. Schiz Bull. 2017;43:283–286. 10.1093/schbul/sbw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reininghaus U, Kempton MJ, Valmaggia L, Craig TK, Garety P, Onyejiaka A, et al. Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: an experience sampling study. Schiz Bull. 2016;42:712–722. 10.1093/schbul/sbv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheng SC, Walsh E, Schepp KG. Vulnerability, stress, and support in the disease trajectory from prodrome to diagnosed schizophrenia: diathesis-stress-support model. Arch Psychiatr Nurs. 2016;30:810–817. 10.1016/j.apnu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- [37].Seligman ME, Csikszentmihalyi M. Positive psychology. An introduction. Am Psychol. 2010;55:5–14. 10.1037/0003-066X.55.1.5. [DOI] [PubMed] [Google Scholar]
- [38].Seligman, ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60:410–421. 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- [39].Jeste DV, Palmer BW, Rettew DC, Boardman S. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76:675–683. 10.4088/JCP.14nr09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Edmonds EC, Martin AS, Palmer BW, Eyler LT, Rana BK, Jeste DV. Positive mental health in schizophrenia and healthy comparison groups: relationships with overall health and biomarkers. Aging Ment Health. 2016;11:1–9. 10.1080/13607863.2016.1251572. [DOI] [PubMed] [Google Scholar]
- [41].Collip D, Wigman JT, van Os J, Oorschot M, Jacobs N, Derom C, et al. Positive emotions from social company in women with persisting subclinical psychosis: lessons from daily life. Acta Psychiatr Scand. 2014;129:202–210. 10.1111/acps.12151. [DOI] [PubMed] [Google Scholar]
- [42].Myin-Germeys I, Klippel A, Steinhart H, Reininghaus U. Ecological momentary interventions in psychiatry. Curr Opin Psychiatry. 2016;29:258–263. 10.1097/YCO.0000000000000255. [DOI] [PubMed] [Google Scholar]
- [43].Jeste DV, Palmer BW, Saks ER. Why we need positive psychiatry for schizophrenia and other psychotic disorders. Schizophr Bull. 2017;43:227–229. 10.1093/schbul/sbw184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Versluis A, Verkuil B, Spinhoven P, van der Ploeg MM, Brosschot JF. Changing mental health and positive psychological well-being using ecological momentary interventions: a systematic review and meta-analysis. J Med Internet Res. 2016;18: e152 10.2196/jmir.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wilker S, Pfeiffer A, Kolassa S, Elbert T, Lingenfelder B, Ovuga E, et al. The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. Transl Psychiatry. 2014;4:e403 10.1038/tp.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: recommendations for evaluating interaction effects. Dev Psychopathol. 2012;24:389–409. 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- [47].Claridge G, Barrantes-Vidal N. The classification of psychosis. Br J Psychiatry. 2011;198:323–324. 10.1192/bjp.198.4.323b. [DOI] [PubMed] [Google Scholar]
- [48].Crawford DC, Nickerson DA. Definition and clinical importance of haplotypes. Annu Rev Med. 2005;56:303–320. 10.1146/annurev.med.56.082103.104540. [DOI] [PubMed] [Google Scholar]
- [49].Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, van Os J. Experience sampling research in psychopathology: opening the black box of daily-life. Psychol Med. 2009;39:1533–1547. 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- [50].McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55:486–497. 10.1177/070674371005500803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1192/j.eurpsy.2019.4.
click here to view supplementary material


