Abstract
Background and objective:
The main purpose of treatment in patients with malignant pleural effusion (MPE) is symptom palliation. Currently, patients undergo repeat thoracenteses prior to receiving a definitive procedure as clinicians are not aware of the risk factors associated with fluid recurrence. The primary objective of this study was to identify risk factors associated with recurrent symptomatic MPE.
Methods:
Retrospective multicentre cohort study of patients who underwent first thoracentesis was performed. The primary outcome was time to fluid recurrence requiring intervention in patients with evidence of metastatic disease. We used a cause-specific hazard model to identify risk factors associated with fluid recurrence. We also developed a predictive model, utilizing Fine–Gray subdistribution hazard model, and externally validated the model.
Results:
A total of 988 patients with diagnosed metastatic disease were included. Cumulative incidence of recurrence was high with 30% of patients recurring by day 15. On multivariate analysis, size of the effusion on chest X-ray (up to the top of the cardiac silhouette (hazard ratio (HR): 1.84, 95% CI: 1.21–2.80, P = 0.004) and above the cardiac silhouette (HR: 2.22, 95% CI: 1.43–3.46, P = 0.0004)), larger amount of pleural fluid drained (HR: 1.06, 95% CI: 1.04–1.07, P < 0.0001) and higher pleural fluid LDH (HR: 1.008, 95% CI: 1.004–1.011, P < 0.0001) were associated with increased hazard of recurrence. Negative cytology (HR: 0.52, 95% CI: 0.43–0.64, P < 0.0001) was associated with decreased hazard of recurrence. The model had low prediction accuracy.
Conclusion:
Pleural effusion size, amount of pleural fluid drained, LDH and pleural fluid cytology were found to be risk factors for recurrence.
Keywords: effusion recurrence, pleural effusion, thoracentesis, malignant pleural effusion
SUMMARY AT A GLANCE
Factors such as larger pleural effusion size, amount of pleural fluid drained, LDH and pleural fluid cytology were found to be risk factors for pleural fluid recurrence. Knowing what risk factors are associated with recurrence of pleural effusion would allow physicians to identify patients who are more likely to recur.
INTRODUCTION
Malignant pleural effusion (MPE) is associated with a median survival of 3–6 months and can cause significant dyspnoea resulting in poor quality of life.1,2 The main purpose of treatment in patients with MPE is symptom palliation. There are a number of treatment alternatives available. Some, such as thoracentesis, achieve only temporary relief while others such as placement of an indwelling pleural catheter (IPC), chest tube with chemical pleurodesis and pleuroscopy with chemical pleurodesis are more definitive solutions.
Current recommendations on the management of pleural effusion in patients with malignancy propose that a therapeutic thoracentesis be performed first in order to determine the effect of drainage on breathlessness and rate and degree of recurrence of the pleural effusion.3 However, in patients with advanced-stage tumours with high suspicion of metastatic pleural disease that do not have any other alternative diagnoses (i.e. empyema, pneumonia and heart failure), a thoracentesis, primarily for palliation of dyspnoea, may be less effective and may increase costs as compared to a definitive treatment up front.4
Many patients with previously diagnosed metastatic disease elsewhere present with new pleural effusions and therefore do not require thoracentesis for staging but rather only need thoracentesis for therapeutic palliation of dyspnoea. In these patients, it would be useful to know what the risk factors for recurrence are and which effusions are likely to rapidly recur after initial thoracentesis. If a patient has risk factors for recurrence, performing a definitive intervention rather than thoracentesis as the first procedure would be warranted. Conversely, if a patient does not have risk factors for recurrence such that the probability of requiring a second intervention was low, then an initial thoracentesis with follow-up would be a good strategy. Clearly, this decision depends on the probability of recurrence and the predicted survival time of the patient.
The main aim of this study was to identify risk factors associated with recurrent symptomatic MPE requiring repeat intervention following first thoracentesis in patients with biopsy proof or strong clinical evidence of metastatic disease. Knowing what risk factors are associated with recurrence of pleural effusion and having this information available prior to thoracentesis would allow physicians to identify patients who are more likely to recur.
METHODS
We performed a retrospective multicentre cohort study of consecutive patients with a pleural effusion who underwent first thoracentesis from 1 January 2010 to 31 December 2013. Approval was obtained from Institutional Review Board Committee 4, PA14–0387 at the University of Texas MD Anderson Cancer Center.
Consecutive patients aged 18 years or older with either proven metastatic cancer or strong clinical evidence of metastatic disease undergoing their first thoracentesis for pleural effusion were included. Strong clinical evidence was defined as imaging demonstrating multiple metastases in a typical clinical pattern, such as positron emission tomography (PET) or bone scan showing multiple bone metastases or computed tomography (CT) or magnetic resonance imaging (MRI) of the brain showing metastatic disease or CT of the chest/abdomen or pelvis with findings sufficiently definitive that the patient was deemed to have metastatic disease.
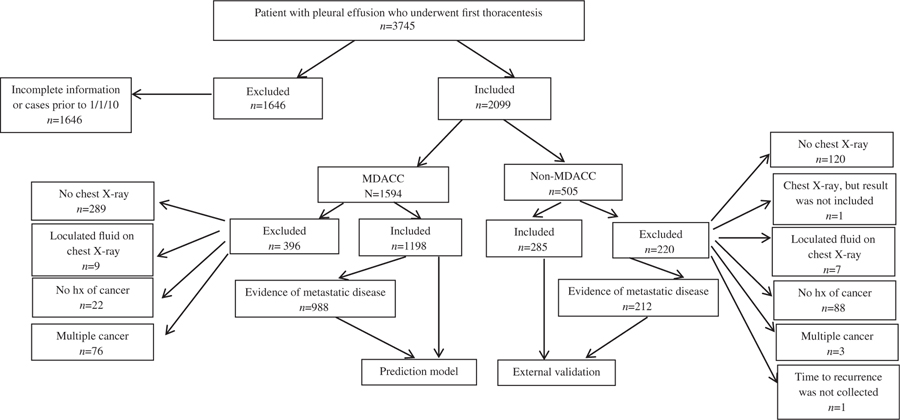
We excluded patients aged <18 years, patients lost to follow-up immediately after the procedure, patients who did not have a chest X-ray, CT or PET within 14 days from first thoracentesis, patients with no history of cancer, suspicion of cancer or active cancer, patients who had loculated pleural effusions on chest X-ray and patients with multiple types of cancer. We also excluded patients who had previous fluid drainage at another institution or history of chest tube placement. In addition to patient demographics, clinical data, pleural fluid characteristics and cytology results, a review of chest X-rays, CT and PET scans was performed (Fig. 1).
Figure 1.
Flow chart of patients selected in the study. hx, history; MDACC-MD Anderson Cancer Center.
Size of the effusion was based on the most recent chest X-ray done within 2 weeks prior to the first thoracentesis. Physicians assessing the chest X-rays were blinded to the fluid recurrence status of the patients. Discordant chest X-ray findings were resolved by two attending physicians (D.E.O. and H.B.G.) who independently reviewed the X-rays. The following categories were used (Fig. 2):
Chest X-ray 1: blunting of the costophrenic angle, but at least part of diaphragm is still visible.
Chest X-ray 2: effusion greater than 1, and up to the inferior border of the vascular pedicle.
Chest X-ray 3: effusion greater than 2, and up to the top of the cardiac silhouette.
Chest X-ray 4: effusion greater than 3, above the cardiac silhouette.
Figure 2.
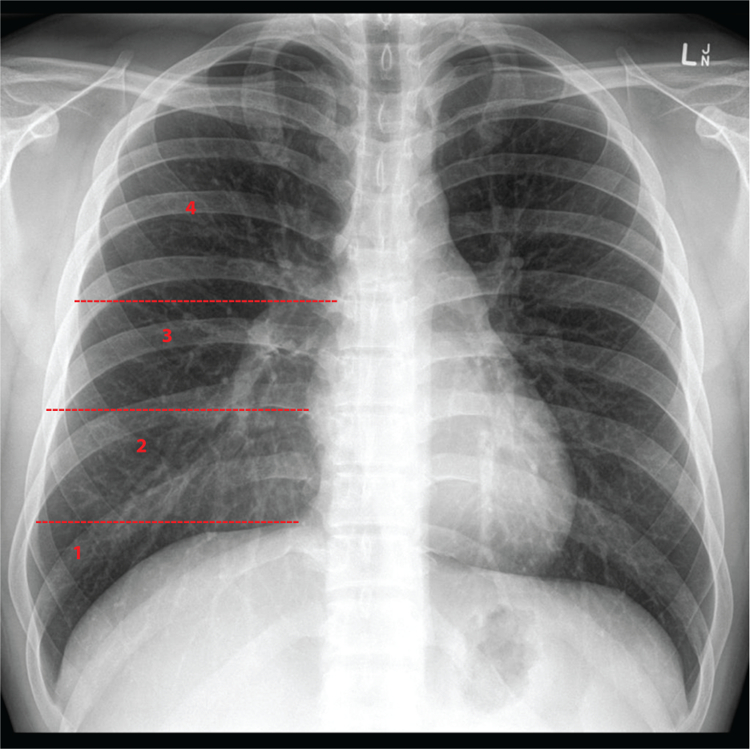
Size of the effusion based on the chest X-ray.
The primary outcome of interest was time to pleural fluid recurrence requiring intervention within the first 100 days of thoracentesis.
Statistical analysis
Descriptive statistics were used to summarize all variables according to 100-day recurrence status. For these characteristics, normally distributed continuous variables are expressed as the mean ± SD. Medians and interquartile ranges were used for non-normally distributed data. Frequencies were used for categorical data, which were compared using the chi-square test or Fisher’s exact test. The Wilcoxon rank-sum (Mann–Whitney) test was used for analysing non-parametrically distributed data. Variables with a P-value of less than 0.20 on univariate analysis were considered candidate variables for multivariate regression models.
We performed the primary analysis using a cause-specific hazard regression model to identify risk factors associated with recurrence within 100 days. This was an explanatory model; therefore, information available after thoracentesis, such as pleural fluid chemistries, could be used as independent variables. All patients alive at the end of 100 days were censored. Backward selection with a P-value of <0.05 to stay in the model was then used to arrive at a parsimonious multivariable model. We checked the proportional hazard assumption by standard methods including complementary log–log plot and Schoenfeld residuals. For variables which violated this assumption, interaction of the variables and a function of time were included in the model.
We performed a secondary analysis, a predictive model, where data from MD Anderson Cancer Center was used to develop a Fine–Gray subdistribution hazard model to identify variables associated with time to pleural fluid recurrence.5 Death was considered a competing risk. This was a predictive model, rather than an explanatory model, so independent variables included only information available to physicians prior to thoracentesis. We used a Fine–Gray subdistribution hazard model with time to pleural fluid recurrence requiring intervention within the first 100 days of thoracentesis as the outcome.
External validation and model assessment were performed using data from five other centres (Johns Hopkins, University of Utah, Henry Ford Health Center, Mayo Clinic and Baylor College of Medicine) (for more details, see Appendices S1 and S2, Supplementary Information).
All statistical analyses were performed in SAS Version 9.4 (SAS Institute, Cary, NC, USA) and R (www.r-project.org). Regression and pec R-packages were used. Brier score and the area under the ROC were calculated using R-functions, calc.myBS and ROC.curve.6
RESULTS
A total of 1231 patients from MD Anderson Cancer Center underwent thoracentesis. Of the 1231 patients in the development cohort, 988 had biopsy proof or strong clinical evidence of metastatic disease prior to thoracentesis and were included in the analysis (see Table 1 for demographics).
Table 1.
Clinical characteristics of patients with biopsy proof or strong clinical evidence of metastatic disease by 100-day recurrence/death status
| Covariate | Entire group (n = 988) | Alive (n = 286) | Recurrence (n = 465) | Death (n = 237) | P-value† |
|---|---|---|---|---|---|
| Male | |||||
| Yes | 454 (46%) | 140 (49%) | 205 (44.1%) | 109 (46%) | 0.4300 |
| ECOG at baseline | |||||
| 0–1 | 261 (33.2%) | 97 (43.5%) | 129 (34.8%) | 35 (18.1%) | <0.0001 |
| 2–4 | 526 (66.8%) | 126 (56.5%) | 242 (65.2%) | 158 (81.9%) | |
| Cancer type | |||||
| Lung | 199 (20.1%) | 58 (20.3%) | 93 (20%) | 48 (20.3%) | 0.0313 |
| Solid non-lung | 578 (58.5%) | 151 (52.8%) | 291 (62.6%) | 136 (57.4%) | |
| Liquid | 211 (21.4%) | 77 (26.9%) | 81 (17.4%) | 53 (22.4%) | |
| CHF present | |||||
| Yes | 85 (8.6%) | 28 (9.8%) | 41 (8.8%) | 16 (6.8%) | 0.4553 |
| Ascites present | |||||
| Yes | 115 (11.6%) | 29 (10.1%) | 42 (9%) | 44 (18.6%) | 0.0006 |
| High clinical suspicion of pneumonia | |||||
| Yes | 327 (33.1%) | 104 (36.4%) | 134 (28.8%) | 89 (37.6%) | 0.0254 |
| Chemotherapy within 30 days prior to tap | |||||
| Yes | 616 (62.3%) | 173 (60.5%) | 294 (63.2%) | 149 (62.9%) | 0.7405 |
| Radiation within 30 days prior to tap | |||||
| Yes | 121 (12.2%) | 30 (10.5%) | 56 (12%) | 35 (14.8%) | 0.3260 |
| Chemotherapy within 30 days after tap | |||||
| Yes | 582 (58.9%) | 188 (65.7%) | 306 (65.8%) | 88 (37.1%) | <0.0001 |
| Radiation within 30 days after tap | |||||
| Yes | 102 (10.3%) | 24 (8.4%) | 58 (12.5%) | 20 (8.4%) | 0.1117 |
| Surgery within 30 days prior to tap | |||||
| Yes | 44 (4.5%) | 17 (5.9%) | 18 (3.9%) | 9 (3.8%) | 0.3493 |
| Right-sided effusion present | |||||
| Yes | 564 (57.1%) | 165 (57.7%) | 265 (57%) | 134 (56.5%) | 0.9639 |
| Contralateral effusion present | |||||
| Yes | 677 (68.5%) | 204 (71.3%) | 296 (63.7%) | 177 (74.7%) | 0.0057 |
| Size of the effusion on chest X-ray | |||||
| (1) Blunting of costophrenic angle | 103 (10.4%) | 43 (15%) | 25 (5.4%) | 35 (14.8%) | <0.0001 |
| (2) Vascular pedicle | 334 (33.8%) | 126 (44.1%) | 123 (26.5%) | 85 (35.9%) | |
| (3) Cardiac silhouette | 352 (35.6%) | 76 (26.6%) | 191 (41.1%) | 85 (35.9%) | |
| (4) Aortic arch or higher | 199 (20.1%) | 41 (14.3%) | 126 (27.1%) | 32 (13.5%) | |
| Cytology negative | |||||
| Yes | 447 (45.2%) | 186 (65%) | 158 (34%) | 103 (43.5%) | <0.0001 |
Chi-square test was used unless specified.
CHF, congestive heart failure; ECOG, Eastern Cooperative Oncology Group.
Risk factors
On univariate analysis compared with liquid tumours, having a solid non-lung malignancy, increasing pleural effusion size on chest X-ray, higher amount of pleural fluid drained, higher pleural fluid LDH, higher pleural protein level and higher pleural fluid cholesterol were associated with increased hazard of recurrence (Table 2). High clinical suspicion of pneumonia, contralateral effusion and negative cytology were associated with a decreased hazard for recurrence (Table 2). On multivariate analysis, increasing pleural effusion size on chest X-ray, larger amount of pleural fluid drained, higher pleural fluid LDH and positive cytology were associated with increased hazard of recurrence (Fig. 3, Table 2).
Table 2.
Explanatory model with risk factors for pleural effusion recurrence requiring intervention in patients with biopsy proof or strong clinical evidence of metastatic disease with censoring at 100 days
| Covariate | HR | Univariate model | P-value | Multivariate model (n = 967) |
||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | HR | 95% CI | P-value | |||||
| Age | 1.001 | 0.995 | 1.008 | 0.6630 | ||||
| Male | 0.912 | 0.760 | 1.095 | 0.3248 | ||||
| ECOG at baseline | ||||||||
| 0–1 | 1.000 | |||||||
| 2–4 | 1.132 | 0.914 | 1.402 | 0.2562‡ | ||||
| Borg at baseline | 1.041 | 0.951 | 1.139 | 0.3834 | ||||
| Cancer type | ||||||||
| Lung cancer | 1.330 | 0.988 | 1.792 | 0.0603 | ||||
| Solid non-lung | 1.486 | 1.161 | 1.901 | 0.0016 | ||||
| Liquid tumour | 1.000 | |||||||
| CHF present | 1.054 | 0.765 | 1.452 | 0.7479 | ||||
| Ascites present | 0.805 | 0.586 | 1.106 | 0.1803† | ||||
| High clinical suspicion of pneumonia | 0.799 | 0.654 | 0.977 | 0.0284† | ||||
| Chemotherapy within 30 days prior to tap | 0.985 | 0.816 | 1.189 | 0.8742 | ||||
| Radiation within 30 days prior to tap | 1.076 | 0.814 | 1.423 | 0.6073 | ||||
| Chemotherapy within 30 day after tap | 1.054 | 0.870 | 1.277 | 0.5932 | ||||
| Radiation within 30 days after tap | 1.306 | 0.992 | 1.719 | 0.0574† | ||||
| Surgery within 30 days prior to tap | 0.761 | 0.475 | 1.219 | 0.2552 | ||||
| Right-sided effusion present | 1.002 | 0.834 | 1.204 | 0.9859 | ||||
| Contralateral effusion present | 0.737 | 0.610 | 0.890 | 0.0015† | ||||
| Duration of effusion prior to thoracentesis (per 10 days increments) | 0.995 | 0.985 | 1.005 | 0.3407 | ||||
| Size of the effusion on chest X-ray | ||||||||
| (1) Blunting of costophrenic angle | 1.000 | 1.000 | ||||||
| (2) Above 1 and up to the vascular pedicle | 1.405 | 0.914 | 2.159 | 0.1215 | 1.254 | 0.814 | 1.933 | 0.3040 |
| (3) Above 2 and up to the top of the cardiac silhouette | 2.463 | 1.623 | 3.737 | <0.0001 | 1.843 | 1.211 | 2.803 | 0.0043 |
| (4) Above the cardiac silhouette | 3.711 | 2.415 | 5.702 | <0.0001 | 2.224 | 1.429 | 3.460 | 0.0004 |
| Amount of fluid drained (100 mL increments) | 1.082 | 1.067 | 1.098 | <0.0001† | 1.061 | 1.044 | 1.078 | <0.0001 |
| Fluid LDH (100 unit/L increments) | 1.008 | 1.006 | 1.011 | <0.0001† | 1.008 | 1.004 | 1.011 | <0.0001 |
| Fluid protein (1 g/dL increments) | 1.068 | 1.031 | 1.106 | 0.0003† | ||||
| Fluid cholesterol(1 mg/dL) | 1.009 | 1.005 | 1.012 | <0.0001† | ||||
| Fluid triglycerides (10 mg/dL increments) | 1.002 | 0.991 | 1.014 | 0.6652 | ||||
| Cytology negative | 0.442 | 0.364 | 0.536 | <0.0001† | 0.526 | 0.431 | 0.640 | <0.0001 |
P-value < 0.2 based on univariate analysis.
P-value < 0.2 based on univariate analysis, but more than 20% values were missing. This variable was not included in the multivariable model due to too many missing values.
CHF, congestive heart failure; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Figure 3.
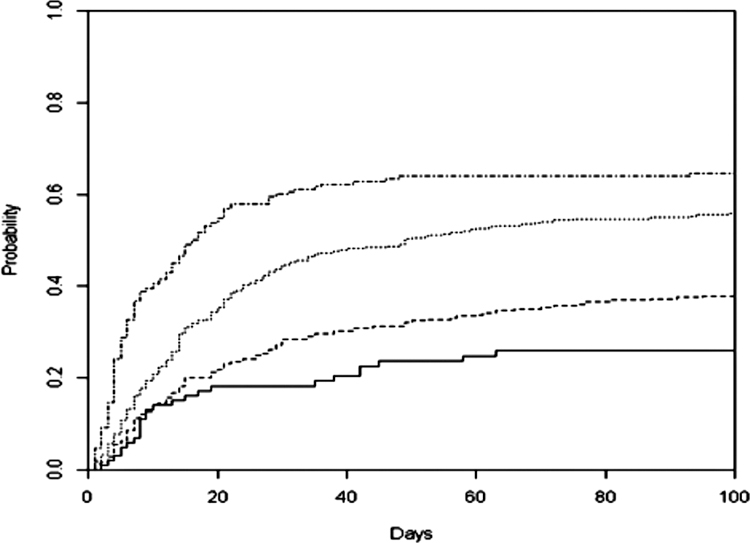
Pleural effusion size on chest X-ray and hazard of recurrence in the development cohort. —, Blunting; ------, vascped; ------, card; ------, aorta, highest.
Predictive model and external validation
In the Fine–Gray model, 30% of patients had pleural fluid recurrence at day 15, 40% of patients had pleural fluid recurrence at day 30, 45% of patients had pleural fluid recurrence at day 60 and 48% of patients had pleural fluid recurrence at day 90.
On multivariate analysis, larger pleural effusion on chest X-ray was associated with increased hazard of recurrence. In addition, having ascites was associated with a higher risk of recurrence in the first 6 days after thoracentesis, but if the effusion did not recur within the first 6 days then the hazard of recurrence was much lower. Contralateral effusion was associated with decreased hazard of recurrence (details of the prediction model analysis are shown in Appendix S1, Table S1 and Fig. S1, Supplementary Information).
A total of 290 patients from five other centres underwent thoracentesis and were used for external validation. Of the 290 patients in the external validation cohort, only 212 had biopsy proof or strong clinical evidence of metastatic disease prior to thoracentesis and were included in the analysis (see Table S2 for demographics; see Appendix S2, Table S2 and Figure S2 for details of the prediction model results and external validation, Supplementary Information). Discrimination as assessed by the time-dependent ROC ranged from 0.55 at day 10 to 0.54 at day 99 (Table S3, Supplementary Information). Assessment of model fit by observed versus predicted plots at 15, 30, 60 and 90 days (P < 0.005) was poor as well (Fig. S3, Supplementary Information). The Brier score was 0.181 at day 10 and deteriorated over time to 0.266 by day 99 (Table S3, Supplementary Information).
DISCUSSION
This is the first study to develop a parsimonious model for time to pleural fluid recurrence requiring intervention in patients with biopsy proof or strong clinical evidence of metastatic disease. The explanatory model showed that larger pleural effusion on chest X-ray, larger amounts of pleural fluid drainage during first thoracentesis, higher pleural fluid LDH and positive cytology were associated with a higher hazard of pleural fluid recurrence. The predictive model shows that early recurrence is fairly common, occurring in 30% of patients by day 15. In addition, the predictive model suggests that larger effusions and absence of contralateral effusion on chest X-ray are risk factors associated with a higher hazard of recurrence. Having ascites at baseline increases the hazard of recurrence initially but then this hazard decreases, and if the effusion has not required intervention by day 6 the hazard of recurrence starts to decrease. While we were able to identify several risk factors that were strongly associated with recurrence risk, the model lacked predictive power.
Our data is consistent with and builds on prior studies showing that a larger amount of fluid drained is associated with an increased hazard of pleural fluid recurrence. This is similar to the study by Boshuizen et al. where higher quantity of fluid drained was associated with a higher probability of recurrence and need for re-intervention.7 This is not to say that draining larger amounts of fluid will cause recurrence, but rather that draining larger amounts of fluid is a marker of increased risk for recurrence. In addition, we observed that higher fluid LDH and positive cytology also increase the hazard of pleural fluid recurrence.
This is the first study to use a Fine–Gray subdistribution hazard prediction model for pleural fluid recurrence in patients with malignancy. Competing risks are particularly good for clinical prediction, as compared with conventional Kaplan–Meier and Cox models. This is because when competing risks are present and occur with high frequency, the Kaplan–Meier survival function will consistently overestimate the crude incidence of the outcome of interest.6
Although we did identify several risk factors that are strongly associated with recurrence, there is still a large amount of inter-patient variability. In the multivariate model, the variables identified only account for a modest portion of the overall variability, such that the model’s discrimination was poor. Also, the model lacked external validity.
The lack of external validity in our study may be due to differences in practice patterns from one institution to another. The patients from the development cohort and validation cohort are very similar with one exception, time to intervention. Compared with the development cohort, the validation cohort had a higher recurrence rate and more interventions after day 20. However, the ultimate cumulative incidence of intervention for recurrent MPE was similar (100-day cumulative incidence of recurrence: 0.48 for the development cohort vs 0.55 for the validation cohort). The development cohort was drawn from a single institution where the care pathway is very algorithmic with mid-level providers following up all patients using the same schedule. In contrast, the validation cohort consisted of five different hospitals, each with their own care pathway, and in these hospitals follow-up was based more on patient and physician discretion. In addition to variations in timing of follow-up, which in turn drives timing of potential intervention, there may also be differences in practice patterns in terms of criteria that determine whether an intervention is done. In the development cohort, the decision is driven by symptoms of dyspnoea as measured by Borg score, improvement of dyspnoea after the initial thoracentesis and radiographic evidence of fluid recurrence to guide whether a second procedure is required. Since the timing of follow-up and the criteria for re-intervention are algorithmic and driven by mid-level providers, there is less between-patient variability within the centre. For example, in the management algorithm, a large effusion that is minimally symptomatic is followed clinically rather than being managed with a definitive intervention. This is done with the knowledge that the patient has ready access to the clinic and will be seen in for scheduled follow-up in short time. These variations in practice conceivably make the development and validation cohort inherently heterogeneous. A prospective study using similar standardized algorithms for timing of follow-up and pre-specified criteria for re-intervention would presumably address this bias.
These results may still prove to be useful in decision-making in patients with large pleural effusion and known metastatic disease in whom staging is not needed. While the model cannot predict the exact time to recurrence, the ultimate outcome of repeat intervention was high and similar between groups. As numerous alternatives for management of MPE are now available, informing physicians and patients appropriately about the actual risk of eventual pleural fluid recurrence (i.e. cumulative incidence proportion) should facilitate a more informed decision process and hopefully provide earlier definitive intervention in selected cases.
We do recognize that our study has limitations such as the retrospective nature of data collection and the potential for bias due to difference in practice patterns and heterogeneity of patient population.
In conclusion, patients with advanced metastatic disease who have large unilateral effusions have a high probability of pleural fluid recurrence in the next 100 days. Risk factors for recurrence included size of the effusion, amount of pleural fluid drained, LDH and pleural fluid cytology. However, the multivariate model demonstrated limited predictive power. Future studies will be needed and should be designed with special attention to standardization of the follow-up schedule and criteria used to justify repeat interventions.
Supplementary Material
Appendix S1 Prediction model development.
Appendix S2 Prediction model results and external validation.
Figure S1 Ascites present and hazard of recurrence with lower hazard of recurrence after day 6.
Figure S2 Developmental cohort.
Figure S3 Validation cohort.
Table S1 Pleural effusion recurrence requiring intervention in patients with biopsy proof or strong clinical evidence of metastatic disease with censoring at 100 days.
Table S2 Clinical characteristics of patients with biopsy proof or strong clinical evidence of metastatic disease for validation cohort by 100-day recurrence/death status.
Table S3 External validation for patients with biopsy proof or strong clinical evidence of metastatic disease based on 100-day recurrence status.
Acknowledgements
The authors acknowledge Cynthia Ray, MD, for her contributions to the writing of this manuscript. This study was funded with contributions by the NIH/NCI under award number P30CA016672, which supports the Biostatistics core facility at The University of Texas MD Anderson Cancer Center.
Abbreviations:
- CHF
congestive heart failure
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- MPE
malignant pleural effusion
- PET
positron emission tomography
- ROC
receiver operating characteristics
Footnotes
Supplementary Information
Additional supplementary information can be accessed via the html version of this article at the publisher’s website.
REFERENCES
- 1.Musani AI, Haas AR, Seijo L, Wilby M, Sterman DH. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration 2004; 71: 559–66. [DOI] [PubMed] [Google Scholar]
- 2.Putnam JB Jr, Light RW, Rodriguez RM, Ponn R, Olak J, Pollak JS, Lee RB, Payne DK, Graeber G, Kovitz KL. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999; 86: 1992–9. [PubMed] [Google Scholar]
- 3.Official Statement of the American Thoracic Society adopted by the ATS Board of Directors, March 2000. Management of malignant pleural effusions. Am. J. Respir. Crit. Care Med 2000; 162: 1987–2001.11069845 [Google Scholar]
- 4.Shafiq M, Frick KD, Lee H, Yarmus L, Feller-Kopman DJ. Management of malignant pleural effusion: a cost-utility analysis. J. Bronchology Interv. Pulmonol 2015; 22: 215–25. [DOI] [PubMed] [Google Scholar]
- 5.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am. J. Epidemiol 2009; 170: 244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshuizen RC, Vincent AD, Heuvel MM. Comparison of modified Borg scale and visual analog scale dyspnea scores in predicting re-intervention after drainage of malignant pleural effusion. Support. Care Cancer 2013; 21: 3109–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Prediction model development.
Appendix S2 Prediction model results and external validation.
Figure S1 Ascites present and hazard of recurrence with lower hazard of recurrence after day 6.
Figure S2 Developmental cohort.
Figure S3 Validation cohort.
Table S1 Pleural effusion recurrence requiring intervention in patients with biopsy proof or strong clinical evidence of metastatic disease with censoring at 100 days.
Table S2 Clinical characteristics of patients with biopsy proof or strong clinical evidence of metastatic disease for validation cohort by 100-day recurrence/death status.
Table S3 External validation for patients with biopsy proof or strong clinical evidence of metastatic disease based on 100-day recurrence status.