Abstract
The international outbreak of respiratory illness termed coronavirus disease 2019 (COVID-19) began in December 2019 that has affected >0.8 million individuals. Self-limiting respiratory tract involvement, severe pneumonia, multiorgan failure and death are the spectrum of COVID-19. To date, there are no especial therapeutic agents for COVID-19 infections. One such medication includes the antimalarial hydroxychloroquine (HCQ), which recently reported as a possible therapy for shortening the duration of COVID-19 symptoms, reducing inflammatory reactions to infection, impairing the exacerbation of pneumonia and boosting lung imaging findings. Like all medications, HCQ has side effects and may occur in COVID-19 patients. Here, we report on the case of a 42-year-old woman, presented with fever and dry cough, who had COVID-19 and 2 days later presented with a pruritic erythematous maculopapular rash, which started from the distal of upper extremities and rapidly, involved the entire body.
INTRODUCTION
The international outbreak of respiratory illness termed coronavirus disease 2019 (COVID-19) began in December 2019, has affected >0.8 million individuals and has been leaded to 40 598 deaths until 1 April 2020 [1]. The disease is highly contagious, with the pandemic reported in the 51st World Health Organization Status Report on 11 March 2020 [2]. Self-limiting respiratory tract involvement, severe pneumonia, multiorgan failure and death are the spectrum of COVID-19. To date, there are no specific and definitive therapeutic agents for COVID-19 infection. One of the drugs prescribed to improve the condition of COVID-19 patients is the antimalarial hydroxychloroquine (HCQ), which recently reported as a supportive drug for shortening the duration of COVID-19 symptoms, reducing inflammatory reactions to infection, impairing the exacerbation of pneumonia and boosting lung imaging findings [3]. Like all medications, HCQ has side effects and may occur in COVID-19 patients. Despite its positive effect, its side effects should be taken into consideration and replaced with other effective medications if possible. We decided to report a case of the HCQ side effects and the appropriate management to it.
CASE REPORT
A 42-year-old woman, presented with fever and dry cough in the past 2 days to her family physician. She had a history of contacting someone with similar symptoms and no underlying problems. No abnormality was found in the physical examination, her temperature was 38°C and other vital signs were normal. Oxygen saturation was 98%. Imaging and laboratory tests were requested. Lab tests revealed elevated lactate dehydrogenase (648 units/liter (U/L), normal: 140–280 U/L), C-reactive protein level (52 milligrams/Liter (mg/L), normal: < 10 mg/L), aspartate aminotransferase (59 U/L, normal: 10–40 U/L), thrombocytopenia, and leukopenia (white blood cells = 2600/microliter, 31% lymphocyte cells). Serology for rheumatoid factor, antinuclear factor, antideoxyribonucleic acid, antismooth muscle and antimitochondrial antibodies were negative as well as hepatitis B surface antigen and anti-hepatitis C virus antibodies. Mild bilateral patchy ground-glass opacification/opacity (GGO) was seen in lung computed tomography (CT) scan (Fig. 1). Due to COVID-19 pandemic and clinical findings, the nasopharyngeal swab test was done, and severe acute respiratory syndrome coronavirus 2 nucleic acid was detected by reverse transcription-polymerase chain reaction. HCQ 200 mg twice daily was started, and 500 mg of acetaminophen every 6 h. After two days, the patient presented with a pruritic erythematous maculopapular rash and flat atypical targets that started from the distal of upper extremities (Fig. 2), rapidly involved the entire body and torn blisters that were only be seen as ulcers on orolabial area (Fig. 3). She had genital mucosal involvement but did not allow us to take pictures. The Nikolsky sign was positive (Fig. 4). Finally, a diagnosis of Stevens–Johnson syndrome (SJS) was made.
Figure 1.
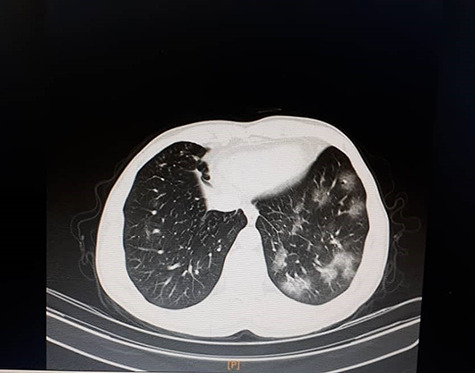
Chest CT showed mild bilateral patchy GGO.
Figure 2.

Pruritic erythematous maculopapular rash on the distal extremities due to HCQ consumption.
Figure 3.
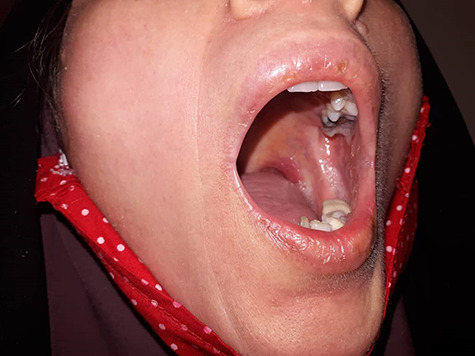
Small blisters on orolabial area.
Figure 4.

Nonpruritic scalded skin.
Due to the likelihood of a drug reaction, HCQ was discontinued, and COVID-19 treatment was changed to lopinavir/ritonavir 400 mg twice daily in the hospital. Loratadine 10 mg twice daily and diphenhydramine 50 mg three times daily were given. She was discharged after five days with nonpruritic scalded skin on the distal of upper extremities (Fig. 4).
DISCUSSION
COVID-19 is an emerging disease that currently has no specific treatment, and the treatments are purely supportive. Chloroquine and its derivatives, such as HCQ, have a long history of being used as prophylactic drugs in malaria areas. In some studies, HCQ has been used to improve the symptoms of patients with COVID-19. This drug is used to support patients with COVID-19 and is not known as a definitive treatment for the disease [3, 4]. The mechanism of action of HCQ is to affect intracellular components such as endosomes, lysosomes and Golgi vesicles and to increase their pH, which interferes with the steps of virus replication, including fusion and uncoating. The side effects of taking this drug occur when it is not sufficiently dispersed through the relatively small central portion. Therefore, the amount of drug entering the central part is an important factor in causing skin reactions [5]. The most common HCQ usage complications are headache, dizziness and gastrointestinal complications. Some studies of COVID-19 patients have reported complications such as gastrointestinal distress, headache, blurred vision, insomnia and prolonged QT interval [3, 6]. In our case, erythema multiforme with maculopapular rash, and flat atypical targets were seen in the distal part of the limb. The skin complications caused by HCQ use are infrequent. These include acute generalized exanthematous pustulosis, SJS, toxic epidermal necrolysis and rashes. HCQ is a very rare cause of drug-induced SJS. This condition begins as itchy papular erythematous eruptions in the trunk and then affects the face, organs and mucous membranes of the mouth. It also had purulent rashes on the trunk, limbs and face [7]. In a study by Volpe et al. [8], a patient with rheumatoid arthritis after taking HCQ developed diffuse, erythematous exfoliative rash involving trunk and limbs, which diagnosed as a drug rash with eosinophilia and systemic symptoms syndrome. In another study, after taking HCQ, the patient developed skin symptoms of SJS such as a pruritic rash over her abdomen, which described as ‘targets’ with a persisting exfoliating rash and eczematous patches [9].
Numerous studies have shown that the use of HCQ in some people with COVID-19 causes gastrointestinal symptoms and heart problems [3, 6], but so far there have been no reports that taking HCQ leads to the symptoms of SJS, which develops skin rashes in different parts of the body, especially in the oropharynx. Skin manifestations of COVID-19 included erythematous rash, urticaria and chickenpox-like vesicles [10]. In our case, while the rash was most likely caused by HCQ, the increasing skin manifestations seen with COVID-19 raise the intriguing hypothesis that the rash in our patient might have also been a COVID-19 manifestation.
CONCLUSIONS
It is worth noting that although HCQ appears to be safe, it does have known side effects. The boundary between therapeutic and toxic doses is narrow while any severe disorders of their use can be life-threatening. One of the side effects of HCQ is SJS caused by the drug, and given the worldwide pandemic of COVID-19 and the increasing need for this drug, we need to be careful about its use in order to control and manage the side effects of this drug.
ACKNOWLEDGMENTS
Special thanks to the Student Research Committee of Mazandaran University of Medical Sciences for supporting us in this project.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
FUNDING
None.
ETHICAL CONSIDERATION
Written informed consent was obtained from the patient for the publication of this case report as well as accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
GUARANTOR
Alireza Razavi.
REFERENCES
- 1. World Health Organization Coronavirus disease 2019 (COVID-19): situation report, Vol. 72 World Health Organization, 2020. [Google Scholar]
- 2. World Health Organization Coronavirus disease 2019 (COVID-19): situation report, Vol. 51 World Health Organization, 2020. [Google Scholar]
- 3. Zhou D, Dai S-M, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 2020;75:491–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergin C, Philbin M, Gilvarry P, O'Connor M, King F, Adams R, et al. Interim guidance for the use of antiviral therapy in the clinical management of acute respiratory infection with SARS-CoV-2 (COVID-19), [v2. 0]. Health Service Executive, 2020. [Google Scholar]
- 5. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:1–12. [DOI] [PubMed] [Google Scholar]
- 6. Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ 2020;369:m1432. [DOI] [PubMed] [Google Scholar]
- 7. Callaly E, FitzGerald O, Rogers S. Hydroxychloroquine-associated, photo-induced toxic epidermal necrolysis. Clin Exp Dermatol 2008;33:572–4. [DOI] [PubMed] [Google Scholar]
- 8. Volpe A, Marchetta A, Caramaschi P, Biasi D, Bambara LM, Arcaro G. Hydroxychloroquine-induced DRESS syndrome. Clin Rheumatol 2008;27:537. [DOI] [PubMed] [Google Scholar]
- 9. Leckie M, Rees R. Stevens–Johnson syndrome in association with hydroxychloroquine treatment for rheumatoid arthritis. Rheumatology 2002;41:473–4. [DOI] [PubMed] [Google Scholar]
- 10. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020;36:e212–3. [DOI] [PubMed] [Google Scholar]


