Abstract
In paediatrics, the overall clinical picture of thiamine deficiency (TD) is not easy to recognize, because it mimics or can be confused with other diseases even in cases of classic beriberi. Unsurprisingly, the likelihood of misdiagnosis of TD is even greater where beriberi has not been described.
Critically ill patients have increased thiamine body consumption and dextrose-based IV fluid increases thiamine cellular demand even further. Consequently, severe acute conditions may result in TD, or trigger TD signs in patients with borderline thiamine status, with life-threatening consequences.
Here, we describe the case of a young patient admitted to a West African hospital where TD is not well documented and diagnosed with severe pneumonia who responded dramatically to thiamine injection.
The lack of rapid diagnostic capacity and the severe outcome of TD justify the use of a therapeutic thiamine challenge in cases with high clinical suspicion. Increased awareness about TD and low threshold for thiamine use should guide clinicians in their practice.
INTRODUCTION
Thiamine (Vitamin B1) is an essential micronutrient with dual coenzymatic and non-coenzymatic functions. It is involved in carbohydrate and branched-chain amino acid metabolism, as well as (though not exclusively) in the production of neurotransmitters, myelin and nucleic acids [1–3]. Thiamine deficiency leads to an acquired mitochondrial disorder.
In humans, there is no endogenous synthesis and the body’s requirements depend exclusively on dietary supply. The combination of limited body storage and a high turnover rate (half-life < 10 days) results in potential depletion of thiamine stores within 2 weeks if it is not continuously replaced. Exclusively breastfed infants are the most vulnerable.
In paediatrics, the overall clinical picture of TD is not easy to recognize, even in cases of classic beriberi, since it mimics or can be confused with other diseases such as sepsis, severe pneumonia, malaria or typhoid fever. Unsurprisingly, the likelihood of misdiagnosis of TD is even greater in some resource-limited settings where TD has been poorly documented due to lack of research and/or access to advanced laboratories [4–6] and therefore low awareness on the matter amongst doctors, especially in Africa.
Critically ill patients have increased thiamine body consumption (associated with hypermetabolism) and dextrose-based IV fluid increases thiamine cellular demand even further. Parenteral nutrition with vitamins is not common practice in many resource-limited settings. Consequently, severe acute conditions may result in TD, or trigger TD signs in patients with borderline thiamine status, with life-threatening consequences [7]. TD classically results from poor intake secondary to monotonous diet with milled rice (but not exclusively). Classic wet beriberi affects young infants but can be seen at all ages.
Here we describe the case of a young patient admitted to a West African hospital where thiamine deficiency is not well documented [8] and diagnosed with severe pneumonia who responded dramatically to thiamine injection.
CLINICAL FINDINGS AND THERAPEUTIC INTERVENTION
A critically ill 3-month-old infant was admitted for acute respiratory distress with a working diagnosis of complicated pneumonia (H0). He presented with fever, tachypnea with bilateral crackles, tachycardia, but no oedemas, poor feeding and hypotonia. Considering the high prevalence of malaria in the area, a malaria rapid test was performed and the result was negative. He received IV ceftriaxone according to Médecins Sans Frontières guidelines for severe pneumonia, IV fluids maintenance with solution containing Ringer Lactate and 5% dextrose and oxygen (O2) via nasal cannulas in order to maintain SpO2 > 92%.
Eight hours later (H8) his condition worsened with hypoxia, lethargy and toxic appearance. An anti-Staphylococcal antibiotic was added (cloxacillin) and oxygen increased.
At H48 in the absence of improvement, azithromycin, trial of steroids and bronchodilators and gastric tube feeding were commenced.
At H52 he was exhausted with intense respiratory distress, head bobbing, increased O2 needs to 5 l/mn, and had become unconscious. In this setting, higher levels of intensive care were unavailable, and in the presence of a life-threatening condition, we decided on a trial of IV thiamine (slow infusion over 30 min, repeated 12 h later). A rapid clinical improvement was observed within 12 h of the thiamine administration with significant reduction of O2 needs and respiratory distress (Fig. 1). After 24 h, the infant was alert with a normal tone and a stable cardiorespiratory status and without signs of respiratory distress. Finally after 48 h, the patient was breastfeeding and off O2 and discharged on Day 5 (Table 1).
Figure 1.
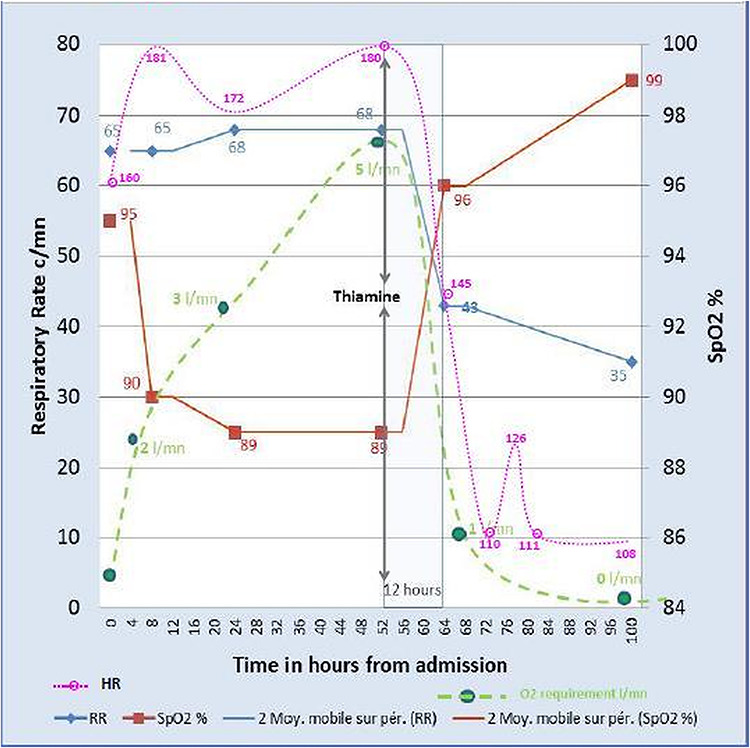
Respiratory rate (RR); heart rate (HR), oxygen saturation (SpO2) and oxygen requirements overtime from admission; admission = H0.
Table 1.
Clinical condition over time from admission (colour codes: green, normal; yellow, moderate; orange, moderate ‘advanced’; red, severe)
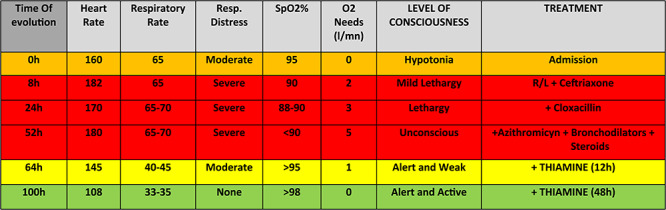
|
DISCUSSION
Acute thiamine deficiency in infancy is well described in Asia but poorly documented in Africa, although outbreaks have been identified [8].
The improvement of our patient’s clinical condition occurred 2.5 days after admission and one could argue that this is the time necessary for the initial treatment—such as antibiotics—to be effective. Nevertheless, the clinical condition was clearly continuing to worsen until thiamine injection was decided as a last treatment resort. The very fast and spectacular clinical improvement within 12 h of thiamine injection as shown in the table and graph is strongly in favour of thiamine deficiency or thiamine responsive disorder, as per the literature [5, 6, 9, 10].
It appears that some West African populations are at risk of thiamine deficiency [10]. Polished rice has become a major food component of the adult population in this area. This is important as the patient was breastfeeding and his mother could have had borderline thiamine body store secondary to her diet and increasing the risk of TD in our patient. It is not known though if she had a monotonous diet and or tingling in the fingers, which can indicate TD. Typically acute cardiac beriberi (or wet beriberi) peaks at 2–4 months of age and our patient was 3 months old. An additional factor, which could have precipitated into clinical thiamine deficiency, was the fact that this critically ill patient was nil by mouth and received only dextrose-based IV fluid for the first 48 h of admission without thiamine supplementation [7].
Thiamine deficiency has a large clinical spectrum not restricted to wet and dry beriberi, and as such it is frequently misdiagnosed, sometimes with fatal consequences or permanent neurological sequelae. Even classic beriberi, presenting as an acute heart failure in very young infants, is still often unrecognized, especially on the African continent where it has not been formally described; thus, increased clinical awareness is essential [8]. Early treatment with thiamine has the potential to rapidly reverse clinical signs and minimize sequelae, before the onset of permanent lesions. The lack of rapid diagnostic tests and absence of higher levels of care in many resource-limited settings justify the use of a therapeutic thiamine challenge in cases with high clinical suspicion. It is effective, inexpensive and easy to administer. Medical awareness on this topic is important.
ACKNOWLEDGEMENTS
We thank Marta A. Balinska for her editorial assistance.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The open-access fees are entirely funded by Médecins Sans Frontières.
INFORMED CONSENT
Oral consent was granted by the child’s mother for reporting the case for pedagogical purposes. We have purposefully removed the name of the town and of the hospital so as to remove any potential identifiers from this case report.
GUARANTOR
Laurent Hiffler.
REFERENCES
- 1. Manzetti S, Zhang J, Spoel D. Thiamine function, metabolism, uptake, and transport. Biochemistry (2014) 53(5):821–835. doi: 10.1021/bi401618y [DOI] [PubMed] [Google Scholar]
- 2. Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med 2001; 1:197–207. doi: 10.2174/1566524013363870 [DOI] [PubMed] [Google Scholar]
- 3. Bettendorff L, Wins P.. Biological functions of thiamine derivatives: focus on non-coenzyme roles. OA Biochem 2013; 1:10. doi: 10.13172/2052-9651 [DOI] [Google Scholar]
- 4. Khounnorath S, Chamberlain K, Taylor AM, Soukaloun D, Mayxay M, Lee SJ, et al. Clinically unapparent infantile thiamine deficiency in Vientiane, Laos PLoS Negl Trop Dis 2011; 5:e969. doi: 10.1371/journal.pntd.0000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rao SN, Chandak GR. Cardiac beriberi: often a missed diagnosis. J Trop Pediatr 2010; 56:284–285. doi: 10.1093/tropej/fmp108 [DOI] [PubMed] [Google Scholar]
- 6. Crook MA, Sriram K.. Thiamine deficiency: the importance of recognition and prompt management. Nutrition 2014; 30(7–8):953–954. doi: 10.1016/j.nut.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 7. Lima LF, Leite HP, Taddei JA. Low blood thiamine concentrations in children upon admission to the intensive care unit: risk factors and prognostic significance. Am J Clin Nutr 2011;93:57–61 [DOI] [PubMed] [Google Scholar]
- 8. Adamolekun B, Hiffler L. A diagnosis and treatment gap for thiamine deficiency disorders in sub-Saharan Africa. Ann N Y Acad Sci 2017. doi: 10.1111/nyas.13509. [DOI] [PubMed] [Google Scholar]
- 9. Sangtawesin C, Leartveravat S.. Pediatric cardiac beriberi: 3 different presentations. J Med Assoc Thai 2008;91Suppl 3:S165–S168 [PubMed] [Google Scholar]
- 10. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci 2018;1430:3–43. doi: 10.1111/nyas.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]


