Abstract
In its severe manifestation, coronavirus disease 2019 (COVID-19) compromises oxygenation in a manner that is refractory to maximal conventional support and requires escalation to extracorporeal membrane oxygenation (ECMO). Maintaining ECMO support for extended durations requires a delicately balanced anticoagulation strategy to maintain circuit viability by preventing thrombus deposition while avoiding excessive anticoagulation yielding hemorrhage—a task that is complicated in COVID-19 secondary to an inherent hypercoagulable state.
Bivalirudin, a member of the direct thrombin inhibitor drug class, offers potential advantages during ECMO, including to its ability to exert its effect by directly attaching to and inhibiting freely circulating and fibrin-bound thrombin. Herein, the successful use of an anticoagulation strategy using the off-label use of a continuous infusion of bivalirudin in a case of severe hypoxemic and hypercarbic respiratory failure caused by COVID-19 requiring venovenous ECMO is reported. Importantly, therapeutic anticoagulation intensity was achieved rapidly with stable pharmacokinetics, and there was no need for any circuit interventions throughout the patient's 27-day ECMO course. In COVID-19, bivalirudin offers a potential option for maintaining systemic anticoagulation during ECMO in a manner that may mitigate the prothrombotic nature of the underlying pathophysiologic state.
Key Words: Bivalirudin, Direct thrombin inhibitors, Extracorporeal membrane oxygenation, ECMO, Coronavirus disease, COVID, Extracorporeal
IN 2019 a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of coronavirus disease 2019 (COVID-19).1 Severe cases of COVID-19 result in profound hypoxemia refractory to maximal conventional ventilator therapy that requires escalation to extracorporeal membrane oxygenation (ECMO) in carefully selected individuals. Given the extended duration of COVID-19 symptoms, maintaining circuit integrity for that period requires a finely tuned anticoagulation strategy that balances the need for anticoagulation to prevent thrombus deposition with avoiding hemorrhage. Achieving this harmony is complicated in COVID-19 secondary to an inherent hypercoagulable state, with elevated risk for both venous and arterial thromboembolic complications.2, 3, 4
Most typically, unfractionated heparin is used during ECMO due to its rapid onset of action, widespread availability, and the ability to reverse its action with protamine. However, heparin carries inherent limitations that predispose to persistent fluctuations in dose sensitivity and heparin-induced thrombocytopenia (HIT).5 Direct thrombin inhibitors, of which bivalirudin is a member, produce transient inhibition of thrombin to mitigate the cleavage of fibrinogen to its active form. Furthermore, they offer potential advantages during ECMO due to their ability to exert this effect by directly attaching to and inhibiting both freely circulating and fibrin-bound thrombin. In addition, bivalirudin offers reliable pharmacokinetics because of its largely organ-independent clearance.6 A growing body of evidence has emerged demonstrating the safety of bivalirudin during ECMO, with some studies reporting a superior balance between thrombosis and hemorrhage.7, 8, 9
Herein, the successful use of an anticoagulation strategy using a continuous infusion of bivalirudin in a 65-year-old female with severe hypoxemic and hypercarbic respiratory failure caused by COVID-19 requiring venovenous ECMO (VV-ECMO) is reported.
Case Report
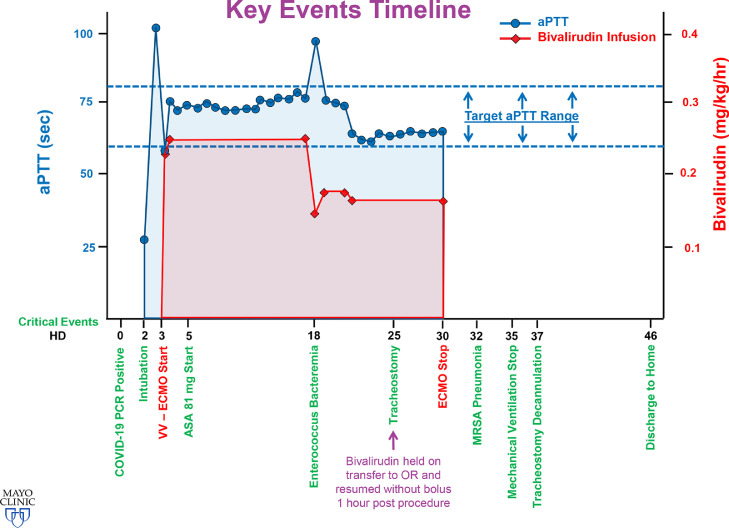
A 65-year-old female, with a medical history significant only for hypertension and hyperlipidemia, was admitted to the hospital in hypoxemic and hypercarbic respiratory failure due to confirmed COVID-19 on a polymerase chain reaction assay. After admission to an outside institution she was treated initially with noninvasive ventilation and self-proning. Due to progressive hypoxemia, she was intubated on hospital day (HD) 2, which was complicated by severe subcutaneous emphysema and pneumomediastinum, concerning for tracheal injury. She was transferred to the authors’ institution for consideration of ECMO therapy. Important clinical events are summarized in Fig 1 .
Fig 1.
Key events timeline. Important events by hospital day are shown on the x-axis; the y-axis shows an overlay of activated partial thromboplastin time values (scale on left margin in seconds) and bivalirudin dose (scale on the right margin in mg/kg/h). The target range activated partial thromboplastin time is portrayed by the dotted line and is defined as 60 to 80 seconds. aPTT, activated partial thromboplastin time; ASA, aspirin; COVID-19, coronavirus disease 2019; HD, hospital day; MRSA, methicillin-resistant Staphylococcus aureus; OR, operating room; PCR, polymerase chain reaction; VV-EMCO, venovenous extracorporeal membrane oxygenation.
Initial arterial blood gas revealed a normal partial pressure of carbon dioxide but a markedly depressed ratio of arterial oxygen partial pressure- to- fractional inspired oxygen of 76. Maintenance of ventilation was positional and dependent on endotracheal tube positioning <1 cm above the carina. An attempt at bronchoscopy to delineate a suspected tracheal tear was unsuccessful given the degree of hypoxemia (arterial oxygen partial pressure 48-76); poor respiratory compliance, with elevated airway pressures (continuous mandatory ventilation with tidal volume of 260 mL, peak airway pressure 40, positive end-expiratory pressure 14); and concurrent hemodynamic instability, with the patient receiving norepinephrine, 0.05 µg/kg/min. Given this constellation of findings and a point-of-care transthoracic echocardiograph confirming normal biventricular function, VV-ECMO was initiated on HD 3 via a 25- Fr drainage cannula placed in the right atrium via the right common femoral vein and a 17- Fr return cannula placed in the superior vena cava via the right internal jugular vein. Circulatory and oxygenation support were achieved with a Cardiohelp device (Getinge, Gothenburg, Sweden). ECMO blood flows were maintained between 4 and 4.25 L/min during her ECMO course. Subsequent bronchoscopy revealed absence of tracheal injury. With the reductions in airway pressures (peak, plateau, and driving pressures) facilitated by extracorporeal support, her subcutaneous emphysema improved markedly over the subsequent 72 hours.
Per the authors’ institutional standard ECMO initiation protocol, an initial bolus of 100 U/kg of unfractionated heparin was administered immediately preceding cannula insertion. Subsequent anticoagulation during her ECMO course consisted of a continuous bivalirudin infusion to maintain a targeted activated partial thromboplastin time (aPTT) of 60- to- 80 seconds. For the majority of her ECMO course, aPTT was maintained between 73 and 75 seconds (see Fig 1). Relevant hematologic laboratory parameters at the time of ECMO initiation are provided in Table 1 . At therapeutic aPTT levels, her dose range of bivalirudin was between 0.15 and 0.25 mg/kg/h. She had 2 plateaus in her dosing, with the first portion of her ECMO course requiring a dose of 0.25 mg/kg/h and the second portion, after the diagnosis of Enterococcus bacteremia, requiring a dose of 0.16 mg/kg/h to maintain the same aPTT levels. On HD 5, aspirin at a dose of 81 mg once daily was added to her anticoagulation.
Table 1.
Hematologic and Inflammatory Parameters at ECMO Initiation
| Laboratory Test | Result | Unit |
|---|---|---|
| Hemoglobin | 8.6 | g/dL |
| Platelet count | 219,000 | /L |
| Prothrombin time | 19.2 | s |
| Activated partial thromboplastin time | 104 | s |
| Antithrombin activity | 76 | % |
| Fibrinogen | 517 | mg/dL |
| D-dimer | 1,584 | ng/mL |
| Ferritin (serum) | 691 | µ/L |
| Procalcitonin (serum) | 0.69 | ng/mL |
| Interleukin 6 (plasma) | 50.5 | pg/mL |
Abbreviation: ECMO, extracorporeal membrane oxygenation.
Her ECMO course was complicated by Enterococcus bacteremia on HD 18. She underwent a tracheostomy on HD 25 and subsequently was weaned and decannulated from ECMO on HD 30. Bivalirudin infusion was discontinued, and heparin 5,000 U subcutaneous 3 times per day was initiated for deep vein thrombosis prophylaxis. Her post-ECMO course was complicated by methicillin-resistant Staphylococcus aureus pneumonia on HD 32. She required mechanical ventilation until HD 35. Her tracheostomy was decannulated on HD 37 with no further complications. She participated in physical therapy and was discharged home on room air on HD 46.
Importantly, no evidence for thrombotic or embolic complications was identified, including a negative Doppler ultrasound of all 4 extremities post-decannulation, normal renal and hepatic function, and absence of neurologic deficits. In addition, her oxygenator pressure delta remained stable, and plasma-free hemoglobin ranged from 10.9 to 13.4 mg/dL (normal range 0.0-15.2 mg/dL). Furthermore, ECMO circuit interventions were not required during her course, including failure of her oxygenator or pump head, nor was there evidence for reduction of oxygenator performance.
Discussion
COVID-19 perturbs coagulation performance, with an inherent hypercoagulable state; derangements in laboratory parameters (elevations of D-dimer, fibrin degradation products, prothrombin time, and aPTT); and both venous and arterial thromboembolic disease.2 , 4 Particularly concerning are reports of microthrombi that may precipitate or exacerbate hypoxemic respiratory failure. Superimposed upon the effect of COVID-19 is the inherently prothrombotic nature of extracorporeal circuits, triggered by contact phase activation and enhanced during periods of inflammation, which necessitates administration of systemic anticoagulation.10 Recent reports have identified an increase in the incidence of prolonged aPTT, with the majority of these cases being positive for lupus anticoagulant.11 However, this has not been associated with a bleeding tendency and the lupus anticoagulant may, in fact, predispose to a thrombotic tendency resulting in suggestions that a prolonged aPTT not be cited as a barrier for the use of anticoagulation in patients with COVID-19. With these considerations in mind, recommendations have been published suggesting targeting higher-than-typical anticoagulation intensity, with concurrent potential benefit from antiplatelet agents in ECMO patients with COVID-19.3
To the authors’ knowledge, this is the first reported case of the use of direct thrombin inhibitors for maintenance anticoagulation during ECMO used in the setting of COVID-19. There remains a relative dearth of published evidence for ECMO in the setting of COVID-19, with a complete absence of reports of anticoagulation management in this population. The authors’ center transitioned to a bivalirudin infusion as a first- line anticoagulant for adult and pediatric ECMO in 2017. As such, using bivalirudin in the patient described herein was not a novel strategy chosen, due to the prothrombotic nature of COVID-19. It is worth stating that heparin was not contraindicated in this patient, and its use for the rapid obtaining of therapeutic anticoagulation at the time of ECMO initiation is standard practice at the authors’ institution, with subsequent transition to bivalirudin for maintenance in all patients barring contraindications to its use (absolute or relative stasis due to rapid proteolysis of local stores of bivalirudin, antiphosholipid syndrome because the baseline aPTT may be falsely elevated, and allergy/intolerance).
One potential hindrance to the use of bivalirudin is anticoagulation monitoring, with various laboratory assays having been described including activating clotting time, ecarin clotting time, chromogenic antifactor IIa assays, and aPTT (the most robust experience uses aPTT).12, 13, 14 Although uncommon, bivalirudin resistance, believed to be secondary to elevated factor VIII or fibrinogen, has been reported with recommendations to use alternative assays (ecarin clotting time or chromogenic antifactor IIa) if concern for inaccurate aPTT becomes present.15 At the authors’ center, dosing adjustments rely on an ECMOpatient -derived algorithm that is adjusted for estimated creatinine clearance and guided by aPTT monitoring. In the patient described herein, an elevated aPTT target of 60- to- 80 seconds was used, which differed significantly from non–COVID-19 VV-ECMO patients (typical targets at the authors’ center are 40-60 s for VV-ECMO and 60-80 s for venoarterial ECMO). The decision to select an elevated aPTT target was reinforced by the grossly elevated D-dimer of 1,584 ng/mL, which has been associated with thrombotic complications in COVID-19.4 In addition, low-dose aspirin was added empirically once appropriate hemostasis and anticoagulant tolerance was demonstrated in an effort to mitigate platelet activation. The clinical impact of its addition is unclear.
ECMO circuits are complex systems of tubing built from foreign material, which often are coated with anticoagulant (heparin- bonded) and include a membrane oxygenator with a large surface area. Unfractionated heparin remains the mainstay of continuous anticoagulation therapy in patients on ECMO support due to its rapid onset of action, widespread availability, and the ability to reverse its action with protamine.5 However, inherent limitations exist, including the requirement of the cofactor antithrombin III through which its action is mediated, unpredictable kinetics requiring frequent dose adjustments, and the highly antigenic nature of heparin that may trigger HIT.7 COVID-19 may exacerbate variations in heparin sensitivity secondary to reduced antithrombin III levels.16
Direct thrombin inhibitors have numerous pharmacokinetic and pharmacodynamic advantages versus heparin. Bivalirudin is a member of the direct thrombin inhibitor drug class and has been approved by the US Food and Drug Administration for short-term use (up to 4 h post-procedure) in adult patients undergoing percutaneous coronary intervention.17 It is a semisynthetic bivalent inhibitor of thrombin that produces transient inhibition of thrombin. This action at the terminal step in the cleavage of fibrinogen to its active form establishes the foundation for its potential role in interrupting an important humoral regulatory process that is deranged in acute inflammatory states. Because of its largely organ-independent metabolism, bivalirudin has a short half-life of 25 minutes in patients with normal renal function.6 , 18 Other reported benefits of this drug class, when compared with heparin, include the ability to inhibit circulating and clot-bound thrombin and the absence of association with HIT.18
Sepsis and acute respiratory distress syndrome, including COVID-19, are characterized by a procoagulant state that results in a massive production of thrombin.19 In addition to direct effects on the endothelial barrier by thrombin, the subsequent cleavage of fibrinogen to fibrin results in diffuse alveolar and interstitial fibrin deposition yielding the formation of microthrombi.19 An important interplay between the innate immune system and platelets occurs at the site of endothelial lesions in a mechanism that is upregulated by the effect of thrombin. This mechanistic description underscores an additional potential clinical advantage of direct thrombin inhibitors in ECMO patients with COVID-19.
Over the last decade, an expanding body of evidence has established the safety of bivalirudin in mechanical circulatory support, with emerging data demonstrating superior balance between thrombosis and hemorrhage.7, 8, 9 Ranucci et al. identified reductions of aPTT variations, blood loss, and transfusion requirements with bivalirudin-based anticoagulation in a population of post-cardiotomy VA-ECMO patients.9 Pieri et al. again found a reduction in dose adjustment requirements and a reduced incidence of supratherapeutic aPTT with bivalirudin in 5 VV-ECMO and 5 VA-ECMO patients.8 In a retrospective study of 32 pediatric ECMO patients, Hamzah et al. reported a reduction in bleeding events in patients anticoagulated with bivalirudin.7 Importantly, in this study bivalirudin was found to be more cost-effective despite higher drug acquisition costs. In a cohort of 44 shock patients (92% VA-ECMO), Berei et al. found no differences in thrombotic events or mortality but suggested bivalirudin as a viable alternative to heparin-based protocols.20
The patient described in this report, who was positive for COVID-19 and on ECMO, was anticoagulated with bivalirudin and demonstrated excellent stability in aPTT, with minimal requirements for dose adjustments. Importantly, anticoagulant intensity was achieved rapidly and sustained with only a single supratherapeutic aPTT assay occurring in the setting of Enterococcus bacteremia. Hemostasis was appropriate, with no blood product transfusion requirement, and the patient was separated from ECMO successfully without hematologic complications. Furthermore, the absence of thrombotic, embolic, and circuit complications was encouraging. This is reinforced when considering the entrainment of additional thrombotic risk associated with COVID-19. With the growing evidence of its safety and its superiority described in several recent publications, bivalirudin should be considered for maintenance of anticoagulation for COVID-19 patients requiring ECMO.
Summary
Bivalirudin, a member of the direct thrombin inhibitor drug class, offers potential advantages during ECMO due to its ability to exert its effect by directly attaching to and inhibiting both freely circulating and fibrin-bound thrombin and its reliable pharmacokinetics as a result of its largely organ-independent clearance. In the COVID-19–positive patient presented herein, a continuous infusion of bivalirudin was used successfully to manage her case of severe hypoxemic and hypercarbic respiratory failure requiring VV-ECMO. A rapid achievement of therapeutic anticoagulation, stable pharmacokinetics, and relative ease of dose titration were demonstrated. Importantly, there was no need for any circuit interventions, including oxygenator exchange or thrombus excision, throughout her 27-day ECMO course. In the COVID-19 era, bivalirudin offers a potential option for maintaining systemic anticoagulation during ECMO in a manner that may mitigate the prothrombotic nature of the underlying pathophysiologic state.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shekar K., Badulak J., Peek G., et al. Extracorporeal Life Support Organization COVID-19 interim guidelines. ASAIO J. 2020 doi: 10.1097/MAT.0000000000001193. Apr 29 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radulescu V.C. Anticoagulation therapy in children. Semin Thromb Hemost. 2017;43:877–885. doi: 10.1055/s-0036-1598004. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin T.E. Bivalent direct thrombin inhibitors: Hirudin and bivalirudin. Best Pract Res Clin Haematol. 2004;17:105–125. doi: 10.1016/j.beha.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Hamzah M., Jarden A.M., Ezetendu C., et al. Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020 doi: 10.1097/PCC.0000000000002384. May 13 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Pieri M., Agracheva N., Bonaveglio E., et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vasc Anesth. 2013;27:30–34. doi: 10.1053/j.jvca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M., Ballotta A., Kandil H., et al. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011;15:R275. doi: 10.1186/cc10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edmunds L.H., Jr, Colman R.W. Thrombin during cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2315–2322. doi: 10.1016/j.athoracsur.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 11.Bowles L., Platton S., Yartey N., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2013656. May 5 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster A., Huebler S., Potapov E., et al. Impact of heparin-induced thrombocytopenia on outcome in patients with ventricular assist device support: Single-institution experience in 358 consecutive patients. Ann Thorac Surg. 2007;83:72–76. doi: 10.1016/j.athoracsur.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 13.Salemi A., Agrawal Y.P., Fontes M.A. An assay to monitor bivalirudin levels on cardiopulmonary bypass. Ann Thorac Surg. 2011;92:332–334. doi: 10.1016/j.athoracsur.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Sanfilippo F., Asmussen S., Maybauer D.M., et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: A systematic review. J Intensive Care Med. 2017;32:312–319. doi: 10.1177/0885066616656333. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale M., Ha M., Liu M.H., et al. Direct thrombin inhibitor resistance and possible mechanisms. Hosp Pharm. 2016;51:922–927. doi: 10.1310/hpj5111-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. Apr 17 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Angiomax (bivalirudin) for injection, for intravenous use [package insert]. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020873s036lbl.pdf. Accessed June 15, 2020.
- 18.Burstein B., Wieruszewski P.M., Zhao Y.J., et al. Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med. 2019;8:87–98. doi: 10.5492/wjccm.v8.i6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: Cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 20.Berei T.J., Lillyblad M.P., Wilson K.J., et al. Evaluation of systemic heparin versus bivalirudin in adult patients supported by extracorporeal membrane oxygenation. ASAIO J. 2018;64:623–629. doi: 10.1097/MAT.0000000000000691. [DOI] [PubMed] [Google Scholar]