Abstract
The evolving coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a rapid expansion of knowledge on the disease's clinical manifestations, laboratory and radiographic abnormalities, and patient trajectories. One area of particular focus is the effect that this illness may have on pregnancy and maternal-fetal disease. As of April 24, 2020, we identified 55 English language reports in the scientific literature summarizing data for 339 women and 258 fetuses and neonates. The majority of these data have focused on maternal-fetal transmission and neonatal outcomes. One systematic review and meta-analysis including the spectrum of coronaviruses [Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and COVID-19] in pregnancy noted increased rates of adverse outcomes associated with this group of infections. Here, we report the case of a COVID-19 positive woman presenting to our emergency department (ED) at 34 weeks gestation with preeclampsia. This case highlights the unique diagnostic and therapeutic challenges associated with treating patients with these concomitant diseases.
Keywords: COVID-19, SARS-CoV-2, Preeclampsia, Emergency medicine, Emergency department
1. Introduction
Changes that occur during pregnancy make pregnant women and their fetuses more susceptible to adverse outcomes and disease complications [1]. Research indicates that, compared to the general pregnant population, those infected with influenza are at increased risk for pontaneous abortion, preterm delivery, low birth weight, birth of a small-for-gestational-age infant, and fetal demise [2]. Systematic reviews and meta-analyses conclude that pregnant women experiencing coronavirus infection are at increased risk of miscarriage, preeclampsia, cesarean birth and perinatal death [3]. Given these risks, there has been notable concern about the health and well being of pregnant patients, their fetuses, and neonates during the current COVID-19 pandemic. Data specific to the effects of COVID-19 on the pregnant patient and fetus are lacking, with the majority of research focusing on maternal-fetal transmission and neonatal outcomes. Reports on small numbers of patients suggest pregnant women infected with COVID-19 may be at risk for preterm delivery, but fetal effects seem to be limited [4]. To our knowledge, there is only one report of pre-eclampsia in a COVID-19 patient; however, details of the case are not available [3]. Here, we share the case of a patient who presented to a community emergency department (ED) at 34 weeks gestation for evaluation of respiratory symptoms in the setting of COVID-19.
2. Case report
A 31-year-old female (Gravida 2, Para 0) with a history of hypertension presented to the ED at 34 weeks gestation with 3 days of cough, shortness of breath, and new onset right upper quadrant pain. She denied visual changes, headache, contractions, vaginal discharge, or bleeding. She reported no known sick contacts or COVID-19 exposure and had been sheltering in place for several weeks.
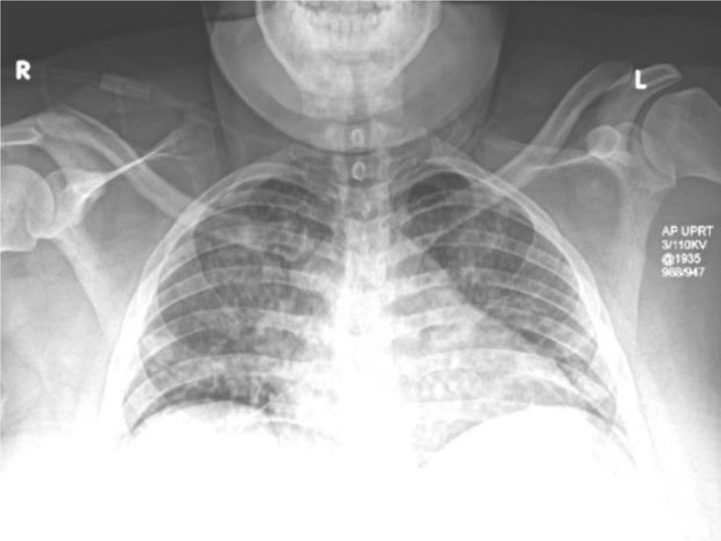
In the ED, her temperature was 38.9 °C, heart rate 129 beats per minute (bpm), blood pressure 162/86 mm Hg, respiratory rate 36 breaths per minute and oxygen saturation 98% on room air; physical exam was notable for tachypnea with clear lung sounds and suprapubic tenderness. Laboratory abnormalities included: platelets 138 thou/μL and lymphocytes 0.88 thou/μL; AST 66 U/L and ALT 46 IU/L; D-dimer 877 μg/mL FEU; CRP 97 mg/L; and LDH 311 U/L. Chest radiography revealed prominent interstitial markings (Fig. 1 ). Bedside transabdominal ultrasound demonstrated normal fetal movement and heart rate 167 bpm. Nasopharyngeal swab for COVID-19 resulted positive on hospital day 2. While in the ED, elevated blood pressures prompted treatment with 10 mg of labetalol intravenously (IV) as well as 4 g of magnesium sulfate IV for presumed preeclampsia with severe features. She was transferred to our tertiary hospital for definitive care.
Fig. 1.
Chest X-ray on day of presentation showing prominent interstitial markings.
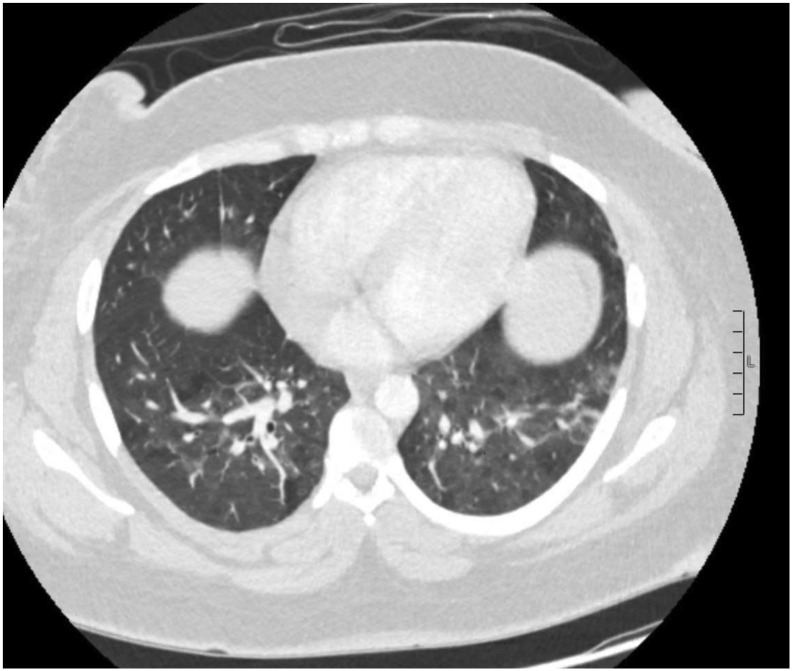
During hospitalization, the patient completed a 4-day course of hydroxychloroquine per infectious disease recommendations. Due to persistent hypertension and climbing liver enzymes (peak AST 225 U/L, ALT 288 IU/L), she underwent an uncomplicated cesarean delivery for management of superimposed preeclampsia with severe features. Her postoperative course was complicated by endometritis treated with 7 days of antibiotics. Her renal function continued to decline, resulting in significant acute kidney injury with associated volume overload. Serum creatinine peaked at 3.87 mg/dL, though she never required dialysis and had gradual improvement in serum creatinine. Intensive care unit (ICU) admission was required on hospital day 10 for worsening hypoxia, which quickly improved after aggressive diuresis. Transesophageal echocardiogram demonstrated normal systolic function, and chest computed tomography was negative for pulmonary embolism but did demonstrate bilateral ground glass opacities typical of COVID-19 infection (Fig. 2 ).
Fig. 2.
Computed tomography scan demonstrating patchy areas of ground glass opacities bilaterally.
Due to her COVID-19+ status and complicated course, contact between mother and baby was restricted to video conferencing and provision of breast milk for her neonate. Mother and infant were discharged on hospital day 17 after two consecutive negative COVID nasopharyngeal swabs (days 13 and 14). Prior to discharge, she was started on hydralazine and amlodipine for chronic hypertension. Discharge plans included biweekly visiting nurses, blood pressure management, and lab draws. Presently, the patient has follow-up appointments with both obstetrics and nephrology and is doing well.
3. Discussion
We describe a patient concomitantly diagnosed with COVID-19 and preeclampsia with severe features, ultimately delivering prematurely at 35 weeks via cesarean section. Data on the effects of COVID-19 on pregnancy are limited - this is the second case of reported preeclampsia in the setting of COVID-19 infection [4]. Due to overlapping laboratory abnormalities (transaminitis, thrombocytopenia), coexisting COVID-19 and preeclampsia can present a diagnostic dilemma. Clinicians must focus on the unique features of these disease entities (significant hypertension in preeclampsia, fever in COVID-19) to identify concurrent pathologies.
As recognized in this case, COVID-19 and preeclampsia have opposing treatment considerations. Given the potential need for emergent delivery, corticosteroids supporting fetal maturation must be considered in preeclampsia, consistent with American College of Obstetrics and Gynecology recommendations [5]. This must be weighed against the possible harmful effects of corticosteroids in patients with COVID-19 [6,7]. The degree of prematurity and anticipated post-delivery pulmonary complications must be evaluated against potential deleterious effects of corticosteroids on the maternal infectious pathology.
Author contributions
All authors (JNH, JH, TDS) conceived the idea for this report, developed the initial draft of the manuscript and revised it for important intellectual content. All authors take full responsibility for the final version of the manuscript.
Declaration of competing interest
The authors report no actual or potential conflicts of interest.
References
- 1.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. NEJM. 2014;370(23):2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosby L.G.I., Rasmussen S.A., Jamieson D.J. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205(1):10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 3.Di Mascio D., Khalil A., Saccone G., et al. Outcomes of Coronavirus spectrum infections (SARS, MERS, COVID 1-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 Mar;25:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Obstetric Practice Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2):e102–e109. doi: 10.1097/AOG.0000000000002237. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. 2020 Mar 13. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available from:
- 7.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 infection. https://www.idsociety.org/COVID19guidelines Available from: [DOI] [PMC free article] [PubMed]