Abstract
Objective
Little is known about coronavirus disease 2019 (COVID-19)-associated hypercoagulability. We sought to characterize patients with deep venous thrombosis (DVT) identified after admission for COVID-19.
Methods
All adult patients admitted to Montefiore Medical Center from March 1, 2020, to April 10, 2020, and undergoing lower extremity venous duplex for DVT evaluation were included. Patients admitted with suspicion of COVID-19 were divided into severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive and SARS-CoV-2 negative groups based on in-hospital test results. Patients without clinical suspicion for COVID-19 were not tested. A retrospective case-control study design was used to identify potential risk factors for DVT in patients with COVID-19. Demographic, radiographic, and laboratory values were abstracted and analyzed.
Results
During the study period, 3404 patients with confirmed COVID-19 were admitted to the hospital. Of the 135 SARS-CoV-2 patients who underwent duplex scanning, there were 18 (13.3%) noted to have DVT compared with 72 of the 711 patients (10.1%) who were either SARS-CoV-2 negative or untested. The odds ratio for DVT in COVID-19 was 1.35 (95% confidence interval, 0.78-2.34; P = .289). Baseline characteristics for COVID-19 patients with and without DVT were overall similar. COVID-19 patients with DVT had an elevated median first d-dimer (18.88 μg/mL [interquartile range (IQR), 7.79-20.00] vs 2.55 μg/mL [IQR, 1.45-6.28]; P = .002; reference value, <0.5 μg/mL), average in-hospital d-dimer (median, 11.93 μg/mL [IQR, 8.25-16.97] vs 3.54 μg/mL [IQR, 2.05-8.53]; P < .001) and median fibrinogen level (501.0 [IQR, 440.0-629.0] vs 654.5 [IQR, 535.8-780.0]; P = .002; reference range, 187-502 mg/dL). There was a trend to significance for COVID-19 patients with DVT compared with without DVT in median d-dimer levels at the time of the duplex (13.61 μg/mL [IQR, 4.04-19.97] vs 3.58 μg/mL [IQR, 2.51-9.62]; P = .055) and median ferritin levels (1679.0 ng/mL [IQR, 1168.0-2577.0] vs 1103.0 ng/mL [IQR, 703.5-2076.5]; P = .055; reference range, 25-270 ng/mL). Twelve of the 18 patients with COVID who developed DVT did so despite chemical thromboprophylaxis, and 2 developed DVT despite therapeutic anticoagulation
Conclusions
We found only a modestly increased risk of DVT in patients with COVID-19, likely underestimated owing to limitations in duplex testing early in the epidemic. Elevated d-dimer and a less elevated fibrinogen are associated with DVT in patients with COVID-19 who seem to form thrombus despite conventional chemical thromboprophylaxis. Additionally, an increasing d-dimer over time may be a reflection of the development of DVT in patients with COVID-19.
Keywords: COVID, DVT, SARS-CoV-2, D-dimer, Thrombosis
Article Highlights.
-
•
Type of Research: Single-center, retrospective case-control study
-
•
Key Findings: Elevated d-dimer and a less elevated fibrinogen were associated with deep venous thrombosis (DVT) in 18 of 135 patients with coronavirus disease 2019 (COVID-19) who underwent duplex scanning, who seem to form thrombus despite conventional chemical thromboprophylaxis. An increasing d-dimer over time may be a reflection of the development of DVT in patients with COVID-19.
-
•
Take Home Message: The d-dimer level and trend over time may be important in triggering DVT evaluation and therapy in patients with COVID-19.
In late 2019, the first reports of human infection with a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed a highly transmissible, significantly morbid, and potentially fatal disease.1, 2, 3 In addition to the pulmonary impact of this infection, cardiac, renal, and hematologic manifestations have been identified. There have been multiple reports of an increased risk of deep venous thrombosis (DVT) in patients hospitalized with SARS-CoV-2 infection or coronavirus disease 2019 (COVID-19).4 , 5 Interestingly, the rapid and sudden demise, particularly of younger patients, is concerning for undiagnosed massive pulmonary embolism (PE). A recent study found the incidence of a thrombotic complication (arterial or venous) at 31% in intensive care unit (ICU) patients with COVID-19.4 This may also explain the finding that mortality may be improved with anticoagulation for COVID-19.6
Although evidence is accumulating and clinical trials are in development, little is known about the suspected hypercoagulability observed in COVID-19. Whether the purported increase in thrombotic events is the direct result of COVID-19 or simply related to the critical nature of the illness remains unclear. We therefore sought to quantify the proportion of hospitalized patients undergoing lower extremity venous duplex examination who were SARS-CoV-2 positive and to identify risk factors for DVT in this subset of patients.
Methods
Adult patients (≥18 years of age) admitted to Montefiore Medical Center between March 1, 2020, and April 10, 2020, undergoing lower extremity venous duplex examination for suspected DVT were included. SARS-CoV-2 status was obtained from the medical record. Patients were tested for SARS-CoV-2 based on clinical suspicion obtained by history and physical examination. SARS-CoV-2 testing was performed on nasopharyngeal swab specimens by in-house polymerase chain reaction (PCR). Patients with a high clinical suspicion for COVID-19 and negative initial testing received subsequent confirmatory repeat testing. Patients awaiting SARS-CoV-2 test results at the time of data abstraction were excluded. Patients who presented with respiratory symptoms or other influenza-like illness symptoms with negative PCR result were categorized as SARS-CoV-2 negative. Patients admitted for other reasons and without suspicion for COVID-19 were categorized as not tested. Additionally, patients with findings of DVT that predated the COVID-19 admission were excluded from the analysis.
A retrospective case-control study design was used to identify potential risk factors for acute DVT in patients with COVID-19. Demographic variables were abstracted including age, race, body mass index (BMI), sex, ethnicity, medical comorbidities, and treatment medications. Additional variables assessed included laboratory values, particularly on the day of the duplex examination, including hemoglobin, blood cell counts, coagulation parameters, cardiac biomarkers, and creatinine, as well as radiologic studies and any operative variables if patients underwent surgery related to the DVT. d-Dimers were recorded throughout the hospitalization and the average d-dimer for the admission was calculated. d-Dimers obtained the day of the ultrasound examination were analyzed independently, as were the first d-dimer levels obtained during the hospitalization. Acute kidney injury (AKI) was defined using the Acute Kidney Injury Network criteria: increase in serum creatinine of 0.3 mg/dL in 48 hours, increase in serum creatinine of 50% over baseline, or oliguria of less than 0.5 mL/kg/h for more than 6 hours. The fraction of inspired oxygen for nonintubated patients receiving supplemental oxygen by nasal cannula was estimated by assuming 4% of inspired oxygen per every liter as described previously.7 The Sequential Organ Failure Assessment (SOFA) score was calculated for each patient from data from the day of the duplex test.
Duplex testing for acute DVT was obtained at the provider's discretion for patients with significant clinical concern for DVT or in those in whom the results were deemed to impact management (eg, patients who were mechanically ventilated and placed prone for persistently poor oxygenation were deemed too unstable, and those already on anticoagulation for other reasons such as cardiac arrhythmias or a prior history of thrombotic episodes requiring lifelong anticoagulation were unlikely to undergo venous duplex testing). The majority of studies were conducted as portable studies at the bedside. These targeted vascular laboratory and ultrasound testing protocols were implemented hospital-wide to address the increased clinical need, decrease hospital staff exposure, and decrease inadvertent viral transmission of SARS-CoV-2 to noninfected patients. Findings of isolated chronic DVT identified on duplex examination were classified as negative to reflect the absence of an acute thrombosis.
The odds ratio for acute DVT in COVID-19 was evaluated with a two-tailed Fisher's exact test. Univariate analysis of risk factors was conducted with t-tests for continuous and χ 2 tests for categorical variables with nonparametric testing as appropriate. Multivariable analysis was not performed owing to small sample sizes. Values for d-dimer, aspartate aminotransferase, alanine aminotransferase, ferritin, activated partial thromboplastin time, creatine phosphokinase, C-reactive protein (CRP), and serum creatinine beyond the limit of detection for the assay were imputed at the threshold value. All analysis was conducted in RStudio (version 1.2.1335) and Microsoft Excel (version 16.0) with alpha set at 0.05. Missing data was assumed to be missing at random and excluded from the analysis for that variable. This study was approved by the Institutional Review Board of Montefiore Medical Center with a waiver of informed consent for this observational review (#2020-11,411).
Results
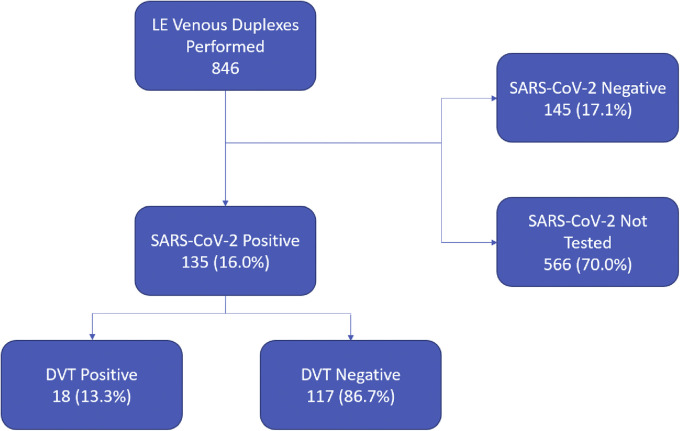
During the study period, a total of 3404 patients were admitted with confirmed COVID-19. After excluding one patient with known arterial thrombosis and two patients with SARS-CoV-2 status pending, there were 846 inpatient duplex studies performed during the study period. Of these, 90 patients (10.6%) were positive for acute DVT. Patients admitted with a high suspicion for COVID-19 accounted for 282 (33.3%) of all lower extremity venous duplex tests performed. Of these, 135 had positive SARS-CoV-2 testing with 18 (13.3%) found to have an acute DVT. During the same time period, 711 patients underwent lower extremity venous duplex who were SARS-CoV-2 negative or not tested. DVT was found in 72 of these patients (10.1%) (Fig 1 ). The odds ratio for DVT in COVID-19 was 1.35 (95% confidence interval, 0.78-2.34; P = .289).
Fig 1.
Flowchart demonstrating patient selection based on duplexes performed. DVT, Deep venous thrombosis; LE, lower extremity; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The demographics of patients testing positive for SARS-CoV-2 and who underwent venous duplex examinations are shown in Table I with laboratory and treatment characteristics shown in Table II . Of these, 18 (13.3%) were ultimately found to have acute DVT by duplex examination. Overall baseline demographics and characteristics were similar to patients with COVID-19 without ultrasound evidence of acute DVT, notably including BMI and congestive heart failure. There was a trend in patients ultimately diagnosed with DVT to have presented with fever (81 [69.2%] vs 17 [94.4%]; P = .051). Importantly, there was no difference noted in the CRP, absolute neutrophil count, neutrophil-to-lymphocyte ratio, SOFA score, need for mechanical ventilation, ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen, or anticoagulant use at the time of the duplex examination between the groups. There were also no significant differences in the rates of AKI, PE, death, or length of stay, although many were still actively hospitalized and undergoing medical care at the time of data analysis.
Table I.
Demographic factors and clinical characteristics for patients with coronavirus disease 2019 (COVID-19) who underwent venous duplex testing
| DVT negative | DVT positive | P value | |
|---|---|---|---|
| Total number | 117 | 18 | |
| Age, years | 64.0 (53.0-73.0) | 59.00 (49.0-64.0) | .06 |
| Male sex | 61 (52.1) | 11 (61.1) | .65 |
| Race | .46 | ||
| Asian | 2 (1.7) | 0 (0.0) | |
| Black | 26 (22.2) | 3 (16.7) | |
| White | 3 (2.6) | 2 (11.1) | |
| Other | 71 (60.7) | 11 (61.1) | |
| Unknown/declined | 15 (12.8) | 2 (11.1) | |
| Ethnicity | .12 | ||
| Not Hispanic | 68 (58.1) | 6 (33.3) | |
| Hispanic | 42 (35.9) | 11 (61.1) | |
| Unknown/declined | 7 (6.0) | 1 (5.6) | |
| BMI | 28.7 (24.6-32.7) | 30.3 (28.4-37.0) | .07 |
| History of HTN | 81 (69.2) | 13 (72.2) | 1 |
| History of DM | 45 (38.5) | 6 (33.3) | .88 |
| History of HLD | 45 (38.5) | 5 (27.8) | .54 |
| History of smoking | .33 | ||
| None | 77 (66.4) | 15 (83.3) | |
| Former | 20 (17.2) | 2 (11.1) | |
| Current | 19 (16.4) | 1 (5.6) | |
| History of CAD | 15 (12.8) | 1 (5.6) | .62 |
| History of COPD | 13 (11.1) | 0 (0.0) | .29 |
| History of CHF | 8 (6.8) | 0 (0.0) | .54 |
| History of CKD | 25 (21.4) | 3 (16.7) | .88 |
| History of prior DVT/PE | 9 (7.9) | 1 (5.6) | 1 |
| History of recent surgery | 4 (3.5) | 0 (0.0) | .95 |
| History of malignancy | 17 (14.5) | 3 (16.7) | 1 |
| Presenting complaint | |||
| Fever | 81 (69.2) | 17 (94.4) | .051 |
| Cough | 74 (63.2) | 13 (72.2) | .63 |
| Myalgia | 33 (28.2) | 5 (27.8) | 1 |
| Fatigue | 38 (32.5) | 7 (38.9) | .79 |
| Diarrhea | 23 (19.7) | 5 (27.8) | .63 |
| Nausea/vomiting | 16 (13.7) | 1 (5.6) | .56 |
| Known SARS-CoV-2 exposure | 24 (20.7) | 1 (5.6) | .23 |
BMI, Body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; DVT, deep venous thrombosis; HLD, hyperlipidemia; HTN, hypertension; PE, pulmonary embolism; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Values are median (interquartile range or number %). Demographic variables were relatively comparable between the acute DVT positive and negative groups.
Table II.
Laboratory values and treatment characteristics for patients with coronavirus disease 2019 (COVID-19) who underwent venous duplex testing
| DVT negative | DVT positive | P value | |
|---|---|---|---|
| Total number | 117 | 18 | |
| WBC, × 109/L | 9.9 (6.8-13.6) | 10.6 (8.12-16.6) | .20 |
| Neutrophil count, × 109/L | 8.0 (4.9-11.4) | 8.7 (6.6-14.3) | .12 |
| Lymphocyte count, × 109/L | 0.9 (0.6-1.3) | 1.0 (0.7-1.1) | .91 |
| Monocyte count, × 109/L | 0.60 (0.40-1.00) | 0.50 (0.40-0.78) | .33 |
| Eosinophil count, × 109/L | 0.0 (0.0-0.2) | 0.0 (0.0-0.1) | .25 |
| Neutrophil to lymphocyte ratio | 8.2 (4.1-15.2) | 10.0 (6.2-18.4) | .16 |
| Hemoglobin, g/dL | 10.59 ± 2.81 | 10.92 ± 2.52 | .64 |
| Platelet count, × 109/L | 280.0 (178.5-403.0) | 243.5 (214.5-273.0) | .23 |
| Prothrombin time, seconds | 15.5 (14.7-16.5) | 16.0 (15.1-18.0) | .19 |
| aPTT, seconds | 35.0 (30.2-40.8) | 34.4 (32.6-42.9) | .71 |
| Total bilirubin, mg/dL | 0.5 (0.3-0.8) | 0.6 (0.4-1.0) | .17 |
| AST, U/L | 46.0 (31.0-71.5) | 39.0 (34.3-53.5) | .53 |
| ALT, U/L | 36.0 (19.3-55.5) | 45.0 (25.5-63.8) | .26 |
| SCr at admission, mg/dL | 1.19 (0.89-2.40) | 1.12 (0.86-1.52) | .75 |
| SCr at time of duplex, mg/dL | 1.38 (0.80-3.46) | 1.58 (1.02-2.99) | .84 |
| SOFA score | 7.0 (5.8-10.0) | 6.0 (3.0-9.0) | .52 |
| Development of AKI | 50 (43.1) | 9 (50.0) | .77 |
| MAP at time of duplex, mm Hg | 84.64 ± 12.74 | 85.39 ± 9.79 | .81 |
| Troponin, ng/mL | 0.01 (0.01-0.05) | 0.01 (0.01-0.02) | .43 |
| CPK, U/L | 157.0 (79.0-509.5) | 166.0 (64.3-232.3) | .44 |
| d-Dimer at time of duplex, μg/mL | 3.58 (2.51-9.62) | 13.61 (4.04-19.97) | .055 |
| Average in-hospital d-dimer, μg/mL | 3.54 (2.05-8.53) | 11.93 (8.25-16.97) | <.001 |
| First d-dimer, μg/mL | 2.55 (1.45-6.28) | 18.88 (7.79-20.00) | .002 |
| Fibrinogen, mg/dL | 654.5 (535.8-780.0) | 501.0 (440.0-629.0) | .002 |
| CRP, mg/dL | 10.8 (5.0-19.6) | 14.2 (5.4-26.1) | .41 |
| Lactate dehydrogenase, U/L | 501.0 (363.8-632.5) | 459.0 (399.5-533.5) | .47 |
| Ferritin, ng/mL | 1,103.0 (703.5-2,076.5) | 1,679.0 (1,168.0-2,577.0) | .055 |
| Interleukin-6, pg/mL | 52.9 (22.4-158.6) | 47.0 (25.3-155.0) | .98 |
| Arterial partial pressure of oxygen, mm Hg | 97.0 (77.5-129.0) | 77.0 (67.0-123.0) | .23 |
| Fraction of inspired oxygen | 42.5 (21.0-80.0) | 50.0 (40.8-76.3) | .09 |
| P:F ratio | 175.0 (121.7-248.8) | 135.0 (102.7-258.0) | .46 |
| Glasgow Coma Score | 15.0 (13.0-15.0) | 15.0 (15.0-15.0) | .25 |
| Mechanical ventilation at time of duplex | 41 (35.0) | 10 (55.6) | .16 |
| Anticoagulant at time of duplex | .29 | ||
| None | 22 (18.8) | 0 (0.0) | |
| Low molecular weight heparin prophylaxis | 28 (23.9) | 5 (35.7) | |
| Subcutaneous heparin prophylaxis | 36 (30.8) | 7 (50.0) | |
| Therapeutic anticoagulation (UH, DOAC) | 19 (16.2) | 2 (14.3) | |
| Therapeutic bivalirudin | 2 (1.7) | 0 (0.0) | |
| Prophylactic apixaban | 10 (8.5) | 0 (0.0) | |
| COVID-19 therapy | |||
| Hydroxychloroquine | 107 (91.5) | 15 (83.3) | .51 |
| Azithromycin | 26 (22.2) | 3 (16.7) | .82 |
| Leronlimab | 0 (0.0) | 2 (11.8) | .01 |
| Sarilumab | 6 (5.1) | 3 (16.7) | .19 |
| Remdesivir | 2 (1.7) | 1 (5.6) | .86 |
| Lopinavir/ritonavir combination | 2 (1.7) | 0 (0.0) | 1 |
| Glucocorticoid | 36 (30.8) | 9 (50.0) | .18 |
| ICU LOS | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | .77 |
| Hospital LOS | 8.0 (5.0-12.8) | 6.0 (5.0-11.5) | .69 |
| Days from COVID-19 symptom to duplex | 7.5 (2.6-10.5) | 7.55 (4.0-8.6) | .66 |
| Days from admission to duplex | 7.0 (2.6-10.4) | 7.3 (3.5-8.4) | .69 |
| Days from SARS-Cov-2 test to duplex | 7.0 (2.6-10.0) | 7.6 (6.1-9.2) | .49 |
| PE | .29 | ||
| Negative CT or VQ scan | 4 (3.4) | 0 (0.0) | |
| Confirmed by CT or VQ scan | 3 (2.6) | 2 (11.1) | |
| High clinical suspicion or highly suggestive TTE findings | 6 (5.1) | 1 (5.6) | |
| Not tested by CT or VQ scan | 104 (88.9) | 15 (83.3) | |
| Mortality | 18 (16.4) | 2 (11.1) | .83 |
AKI, Acute kidney injury; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CPK, creatine phosphokinase; CRP, C-reactive protein; CT, computed tomography; DOAC, direct oral anticoagulant; ICU, intensive care unit; LOS, length of stay; MAP, mean arterial pressure; PaO2, partial pressure of oxygen; PE, pulmonary embolism; P:F, ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; TTE, transthoracic echocardiogram; UH, unfractionated heparin; VQ, ventilation-perfusion scan; WBC, white blood cell.
Acute DVT in patients with COVID-19 was associated with elevations in the first d-dimer level obtained, the average d-dimer throughout hospitalization, and a less elevated fibrinogen level. There were no significant differences in inflammatory markers such as CRP or neutrophil-to-lymphocyte ratio. The severity of illness at the time of data abstraction and analysis was similar including SOFA score, AKI, and subsequent mortality. There was a suggestion of an association with ferritin levels and d-dimer levels at the time of the duplex in patients with COVID-19 with acute DVT compared with those without acute DVT.
Values are median (interquartile range), mean ± standard deviation, or number (%). Boldface entries indicate statistical significance.
Only fibrinogen and the average d-dimer were found to be significantly different between the two groups. Compared with patients with COVID-19 without duplex evidence of acute DVT, COVID-19 patients with ultrasound-confirmed acute DVT had a significantly elevated median first d-dimer (18.88 μg/mL [IQR, 7.79-20.00 μg/mL] vs 2.55 μg/mL [IQR, 1.45-6.28 μg/mL]; P = .002) and average in-hospital d-dimer (median, 11.93 μg/mL [IQR, 8.25-16.97 μg/mL] vs 3.54 μg/mL [IQR, 2.05-8.53 μg/mL]; P < .001; reference range, d-dimer <0.5 μg/mL). In patients with COVID-19 with duplex evidence of acute DVT the median fibrinogen level was increased to 501.0 mg/dL (IQR, 440.0-629.0 mg/dL) compared with a level in patients without DVT of 654.5 mg/dL (IQR, 535.8-780.0 mg/dL) (P = .002, reference range, fibrinogen 187-502 mg/dL). There was a trend to significance for patients with COVID-19 and with DVT compared with patients with COVID-19, but without DVT in median d-dimer levels at the time of the duplex (13.61 μg/mL [IQR, 4.04-19.97 μg/mL] vs 3.58 μg/mL [IQR, 2.51-9.62 μg/mL]; P = .055) and median ferritin levels (1679.0 ng/mL [IQR, 1168.0-2577.0 ng/mL] vs 1103.0 ng/mL [IQR, 703.5-2076.5 ng/mL]; P = .055) (Table II).
A sensitivity analysis was conducted to further characterize the d-dimer results using an analysis of the variable as categorical. Categories included d-dimer of <0.5, 0.5-3.0, 3-10, 10-15, 15-20, and >20 μg/mL. Although the d-dimer on the day of the duplex examination was not found to be significantly different using this method, the average d-dimer and the first d-dimer obtained on hospitalization were both significant (P = .004 and P < .001, respectively). This finding was consistent with the imputed analysis. Several patients did not have a d-dimer on the day of duplex examination (4 in the acute DVT positive group and 72 in the acute DVT negative group), which likely decreased the power of the primary and sensitivity analyses in comparison to the statistically significant results for average d-dimer and first d-dimer obtained during hospitalization, where there were 20 (DVT negative) and 10 (DVT negative) missing, respectively.
Three patients without evidence of acute DVT on lower extremity ultrasound examination developed PE as identified on chart review. Because our study data were derived from patients undergoing lower extremity duplex for DVT, these patients were analyzed as no DVT and we did not specifically review the imaging that may have confirmed PE for all COVID-19 admissions. A sensitivity analysis performed evaluating any DVT or PE compared with no DVT or PE did not reveal any difference in results.
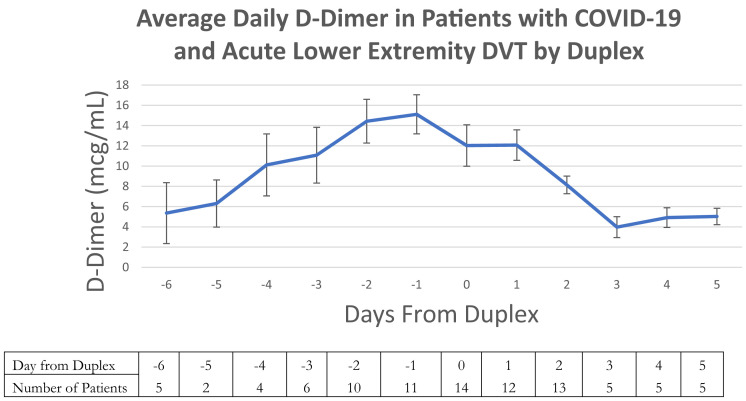
Details regarding the patients with COVID-19 who were diagnosed by venous duplex with acute DVT are displayed in Table III . In patients with a DVT, the median time from symptoms, SARS-CoV-2 testing and admission to DVT diagnosis was 7.6, 7.3, and 7.6 days, respectively. No patient required thrombolysis or surgical management of DVT. Only two patients (11.1%) had isolated below-the-knee acute DVT. When patients found to have DVT or PE on presentation were excluded, 12 SARS-CoV-2-positive patients (85.7%) developed acute DVT despite conventionally adequate chemical thromboprophylaxis and 2 developed DVT despite therapeutic anticoagulation (14.3%). None of these patients had catheter-associated DVT or extracorporeal membrane oxygenation requirements. An analysis of the individual patient-level d-dimer values of patients with COVID-19 diagnosed with DVT was subsequently performed. Fig 2 shows an increase in the average daily d-dimer for these patients leading up to the day of the duplex followed by a decrease thereafter.
Table III.
Specific characteristics of patients with coronavirus disease 2019 (COVID-19) with an acute deep venous thrombosis (DVT) noted on duplex imaging at the completion of data abstraction and at the time of analysis
| Patient No. | DVT location | PE | Days from COVID-19 symptoms to duplex | Days from admission to duplex | Anticoagulation before DVT | DVT treatment | ICU LOS, days | Hospital LOS, days | Discharge status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | LLE-CFV, FV, PV | Not tested | 9.5 | 3.5 | Enoxaparin 40 mg SQ daily | Apixaban 10mg BID for 7 days then 5 mg BID | 0 | 6 | Improved |
| 2 | LLE-GaV | Not tested | 3.6 | 3.6 | Enoxaparin 40 mg SQ daily | Apixaban 10mg BID for 7 days then 5 mg BID | Hospitalization ongoing | ||
| 3 | LLE-GaV | None by TTE | 8.6 | 8.6 | SQH 5000 IU q8h | UH infusion | 13 | 13 | Deceased |
| 4 | LLE-PV, GSV | None by TTE | 7.6 | 7.6 | SQH 5000 IU q12h | UH infusion | 0 | 10 | Deceased |
| 5 | RLE-PV | Confirmed by VQ | 1.4 | 1.4 | Therapeutic AC for PE, DVT positive the next day | Apixaban 10mg BID for 7 days then 5 mg BID | 0 | 4 | Improved |
| 6 | LLE-PV | Not tested | 8.4 | 8.4 | SQH 5000 IU q12h | Enoxaparin 1 mg/kg BID SQ | 0 | 13 | Improved |
| 7 | RLE-PV | Confirmed by CT 8 days after DVT diagnosis | 7.5 | 7.5 | SQH 5000 IU q8h | Enoxaparin 1 mg/kg BID SQ | Hospitalization ongoing | ||
| 8 | RLE-FV, PV, GaV LLE-FV, PV, GaV |
Suspected by POCUS 2 days after DVT diagnosis | 7 | 7 | Enoxaparin 40 mg SQ daily | Bivalirudin infusion | Hospitalization ongoing | ||
| 9 | RLE-PV LLE-FV, PV |
None by TTE | 5 | 5 | SQH 5000 IU q12h | UH Infusion | Hospitalization ongoing | ||
| 10 | RLE-PV | None by TTE | 9 | 9 | Enoxaparin 40 mg SQ daily | Enoxaparin 1 mg/kg BID SQ | Hospitalization ongoing | ||
| 11 | RLE-FV, PV | Not tested | 10.5 | 10.5 | Enoxaparin 40 mg SQ daily | UH infusion | Hospitalization ongoing | ||
| 12 | LLE-FV, PV, GaV | None by TTE | 2.4 | 2.4 | Diagnosed in ED | Apixaban 10mg BID for 7 days then 5 mg BID | 0 | 6 | Improved |
| 13 | RLE-CFV, PFV, PV, GaV, GSV LLE-PV |
Not tested | 8.4 | 8.4 | SQH 5000 IU q8h | Bivalirudin infusion | Hospitalization ongoing | ||
| 14 | RLE-CFV, PV, GaV, GSV | Not tested | 1.4 | 1.4 | Diagnosed in ED | Apixaban 10mg BID for 7 days then 5 mg BID | 0 | 4 | Improved |
| 15 | RLE-CFV LLE-FV |
Not tested | 28.3 | 28.3 | Therapeutic UH for elevated d-dimer | Bivalirudin infusion | Hospitalization ongoing | ||
| 16 | RLE-FV, PV | None by TTE | 6.5 | 6.5 | SQH 5000 IU q12h | UH infusion | Hospitalization ongoing | ||
| 17 | LLE-PV | None by TTE | 2.5 | 2.5 | Diagnosed immediately on admission | Apixaban 10mg BID for 7 days then 5 mg BID | Hospitalization ongoing | ||
| 18 | RLE-PV | Not tested | 7.6 | 7.6 | Apixaban 5mg q12h for elevated d-dimer | Bivalirudin Infusion | Hospitalization ongoing | ||
AC, Systemic therapeutic anticoagulation; BID, twice daily; CFV, common femoral vein; COVID-19, coronavirus disease 2019; CT, computed tomography; DVT, deep venous thrombosis; ED, emergency department; FV, femoral vein; GaV, gastrocnemius vein; GSV, great saphenous vein; ICU, intensive care unit; IU, international units; LLE, left lower extremity; LOS, length of stay; PE, pulmonary embolism; PeV, peroneal vein; POCUS, point-of-care ultrasound; PTV, posterior tibial vein; PV, popliteal vein; q8h, every eight hours; q12h, every twelve hours; RLE, right lower extremity; SQ, subcutaneous; SQH, subcutaneous heparin; TTE, transthoracic echocardiogram; UH, unfractionated heparin; VQ, ventilation-perfusion scan.
Fig 2.
Average d-dimer for patients with coronavirus disease 2019 (COVID-19) with acute deep venous thrombosis (DVT), suggesting that there is a peak in d-dimer level immediately preceding duplex diagnosis of a DVT. The trend is graphed over time with day 0 as the day of the duplex. Negative values represent the days prior, and positive values days after the ultrasound examination. Error bars represent standard error of the mean. The number of patients with a d-dimer value at each time point are listed below the graph in the table.
A subsequent chart review of the 18 SARS-CoV-2 positive patients with acute DVT was undertaken 6 weeks after completion of the patient accrual and data analysis to assess outcome. At that time, 10 patients (56%) had been discharged from the hospital (2 to skilled nursing facilities, 1 to rehabilitation, and 7 to home), 6 (33%) were deceased, and 2 (11%) continued to require ongoing inpatient hospitalization.
Discussion
We herein describe our early experience with DVT in patients with COVID-19. The development of DVT can severely impact the outcomes of critically ill patients with increased mechanical ventilation time, increased hospital and ICU length of stay, and mortality.8 Known risk factors for DVT include a prior venous thromboembolic event (VTE), malignancy, genetic or acquired hypercoagulable states, pregnancy, smoking, long distance travel, immobility, inflammation, obesity, and importantly critical illness.9, 10, 11 Infection with novel SARS-CoV-2 seems to induce a hypercoagulable state that may not be completely explained by critical illness alone when present. A retrospective analysis of 81 patients admitted to the ICU with COVID-19 suggested a risk of VTE as high as 25% with the authors highlighting that “no preventive anticoagulant was administered.”5 Importantly, they describe elevated d-dimer levels as a marker of risk and suggest a cutoff value of 1.5 μg/mL for VTE prediction. Other investigators have found increased risk of mortality with elevated d-dimers and fibrinogen levels.12
In this study, there was evidence of an increased risk of acute lower extremity DVT in patients with COVID-19 compared with those without COVID-19, although this increase did not reach statistical significance. Acute DVT in patients with COVID-19 was significantly associated with an elevated first d-dimer level, an average d-dimer throughout hospitalization, and a less elevated fibrinogen level. When comparing patients who had confirmed COVID-19 with and without acute DVT, there were no significant differences in inflammatory markers such as CRP or neutrophil-to-lymphocyte ratio. The severity of illness at the time of data abstraction and analysis was similar as well including SOFA score, AKI, and mortality (Table II). We did not identify differences in BMI, rates of a history of congestive heart failure or pulmonary status at the time of duplex (as measured by arterial partial pressure of oxygen, fraction of inspired oxygen, the ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen, and mechanical ventilation at the time of the duplex).
There was a statistically nonsignificant trend toward elevated ferritin levels in patients with COVID-19 with ultrasound evidence of acute DVT compared with those without. Although likely not statistically significant owing to study sample size, we suspect that this difference could be clinically significant. Ferritin may be a marker of hyperinflammation with COVID-19.13 A recent metanalysis suggested that serum ferritin may serve to discriminate severe disease.14 Further study should aim to characterize this relationship, especially as it relates to the development of acute DVT with COVID-19.
We also observed an increasing d-dimer level that peaked the day before the duplex was performed and declined thereafter. Not all patients had d-dimer levels drawn daily precluding our ability to ascertain whether this overall trend was observed at the individual patient level. We did, however, observe volatility in individual patient d-dimer levels day over day where data was available (Fig 2). Upon diagnosis of acute DVT, all patients received therapeutic anticoagulation which may have played a role in the down-trending average daily d-dimer. Consequently, d-dimer seems to serve as an important biomarker of the development of DVT in patients with COVID-19, a finding that will likely be elucidated with further studies.
The clinical implications of acute lower extremity DVT in patients with COVID-19 remains unclear. Autopsy studies have identified microthrombi in pulmonary capillaries that are potentially more consistent with in situ thrombosis rather than a distal embolic phenomenon.15 An already significant respiratory illness compromised by PE may result in appreciably worse patient outcomes. Only two patients had isolated distal, below-the-knee DVT, which under other circumstances might not warrant anticoagulation.16 , 17 Still, with concerns that SARS-CoV-2 triggers a significant inflammatory response,18 it may be prudent to consider systemic therapeutic anticoagulation until the pathophysiology of the viral disease and its sequalae can be further elucidated. This is especially true given that it seems that the majority of DVT in these patients with COVID-19 occurs despite conventionally adequate DVT chemical prophylaxis.
There are several limitations inherent to this study. The actual number and incidence of all VTEs may be underestimated for several reasons. Hospital policies aimed at decreasing disease spread and minimizing staff risk has led to a more focused approach to duplex testing, and no screening studies in any patient population during this time period were performed. In addition, many patients deemed terminal may not have been considered candidates for duplex evaluation. Moderately symptomatic and/or critically ill patients already on therapeutic anticoagulation and in whom a duplex evaluation would not lead to a change in management may not have undergone studies. Hence, it is likely that the threshold to order a duplex scan to assess for DVT was higher than in the COVID-19-negative patients. Furthermore, patients with PE and without DVT, or those with upper extremity DVT, were not captured in this study, especially given similar restrictions on computed tomography and ventilation-perfusion testing, as well as potential patient instability precluding further imaging. Twenty-one patients (19.9%) of the DVT-negative group were also prescribed therapeutic anticoagulation at the time of the duplex examination. Conceivably, this factor may have served to prevent some instances of DVT. Last, inpatient treatment is ongoing for many patients in this study with some data not reflecting a complete hospital course.
This study is limited by the false-negative rate of the PCR test for SARS-CoV-2 as well. Although the exact false-positive rate is not known, it is conceivable that many of the patients who tested negative despite a strongly suggestive clinical picture had COVID-19. Unfortunately, there is no gold standard test; however, data from the radiology literature comparing PCR with computed tomography scans of the chest at presentation estimate a false-negative rate of PCR as high as 20%.19 , 20
Additionally, although patients routinely received DVT prophylaxis with either enoxaparin or subcutaneous heparin, the choice was left to the treating providers. Some providers chose to prioritize enoxaparin for patients with COVID-19 given once daily dosing to decrease nursing exposure. Others preferred prophylactic heparin, given the perceived ease of cessation in the setting of bleeding. To spare the necessary patient contact involved, a preference developed for apixaban, which has been used as prophylaxis for patients with cancer, in acute infectious diseases, and in orthopedic thromboprophylaxis with success.21, 22, 23 Decision support in our electronic medical record for prophylactic subcutaneous heparin dosing follows Micromedex recommendation, which refers to a study by Reynolds et al24 that found no difference in every 12 hour and every 8 hour dosing. Our institution practice, however, is to use every 8 hours dosing for patients with a BMI of greater than 30. Additionally, the decision to initiate therapeutic anticoagulation evolved during the study period. With increasing anecdotal reports of thrombotic events worldwide, our center moved to establishing a threshold d-dimer value of 3 mg/dL to initiate anticoagulation, because the risks of not doing so were deemed potentially too great. These issues may also have impacted the likelihood of developing DVT in our patient sample.
Issues surrounding infection containment and patient stability for transportation and testing may have impacted the diagnosis of PE as well. It is for this reason that PE was not included in the outcome definition in this study, given concerns of significant underestimation. In this study, we elected to focus solely on lower extremity acute DVT as one of the most common manifestations of venous thromboembolic disease. Additionally, DVT is easily evaluated with duplex examination, which can be a portable bedside study. Other manifestations of hypercoagulability are also possible, such as arterial thrombosis or mesenteric venous thrombosis. This is factor of special interest given reports documenting the early and severe occurrence of in situ pulmonary thrombosis and evidence of damage to the pulmonary alveoli and the vascular endothelium in the lungs.15 However, to decrease heterogeneity in the sample, we elected not to include patients with these conditions. It was our goal that the current study provide an early confirmation of the anecdotal findings of physicians globally that there is a potentially increased likelihood of acute DVT with COVID-19 and that d-dimer testing is indispensable.
Given these considerations, there is both a positive and negative selection bias in this study because duplex testing was only obtained for patients where clinical management may be altered by the result. Consequently, decreased testing with more stringent criteria requiring a greater clinical degree of suspicion may have skewed the degree of DVT frequency. Although our data and early published case reports/series suggest a potential increase in the risk of acute DVT in patients with COVID-19, the true incidence cannot be estimated by our case-control study design and would best be assessed using a cohort design. Certainly, a prospective study with widespread screening would most optimally quantify the risk of acute DVT in this disease for both hospitalized patients as well as all those afflicted including those undergoing conservative supportive symptomatic therapy at home. Based on all of these limitations, we suspect that the true rate of DVT in our population may be underestimated, the degree of which we are unable to determine at present.
Treatment recommendations for patient with COVID-19 are evolving. In general, the clinical approach to treatment in the United States for has been informed largely by the global experience with this disease. Late during the study period, concerns regarding hypercoagulability from the global experience resulted in a consideration for therapeutic anticoagulation when the d-dimer increased to more than 3.0 mg/mL. It is therefore possible that potentially incident thrombotic events were prevented as a result, or at least prevented the ordering of confirmatory imaging. Similarly, patients who were on therapeutic anticoagulation for alternative reasons (such as atrial fibrillation) may have avoided the development or diagnosis of an acute DVT.
Conclusions
Overall, we suspect that these results reflect the real-world experience of acute DVT diagnosis and treatment in patients with COVID-19. A less elevated fibrinogen and an increase in d-dimer were associated with DVT diagnosis, and the d-dimer curve may signal acute DVT development. However, the clinical significance of the fibrinogen finding is unclear because levels in the patients with COVID-19 with acute DVT were increased at the upper limit of normal. The clinical usefulness of these laboratory trends in guiding the workup and treatment of patients with COVID-19 requires further study. These early data describe the clinical characteristics of acute DVT in COVID-19 patients and may provide a framework for further study.
Author contributions
Conception and design: IK, BG
Analysis and interpretation: IK, BG, KC, AH, DJ, HB, JI, EL
Data collection: BG, KC, AH, DJ
Writing the article: IK, BG, EL
Critical revision of the article: IK, BG, KC, AH, DJ, HB, JI, EL
Final approval of the article: IK, BG, KC, AH, DJ, HB, JI, EL
Statistical analysis: IK
Obtained funding: Not applicable
Overall responsibility: IK
IK and BG contributed equally to this article and share co-first authorship.
Acknowledgments
The authors thank Ana Garcia, RVT, for her help in obtaining the data.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1702–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombos Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S., Chen S., Li S., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald C.F. Low-flow oxygen: how much is your patient really getting? Respirology. 2014;19:469–470. doi: 10.1111/resp.12290. [DOI] [PubMed] [Google Scholar]
- 8.Malato A., Dentali F., Siragusa S., Fabbiano F., Kagoma Y., Boddi M. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13:559–568. doi: 10.2450/2015.0277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook D., Douketis J., Crowther M.A., Anderson D.R., VTE in the ICU Workshop Participants The diagnosis of deep venous thrombosis and pulmonary embolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20:314–319. doi: 10.1016/j.jcrc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Ejaz A., Ahmed M.M., Tasleem A., Rafay Khan Niazi M., Ahsraf M.F., Ahmad I. Thromboprophylaxis in intensive care unit patients: a literature review. Cureus. 2018;10:e3341. doi: 10.7759/cureus.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox E.A., Kahn S.R. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–365. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- 12.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 15.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. [A pathological report of three COVID-19 cases by minimally invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 16.Righini M., Galanaud J.P., Guenneguez H., Brisot D., Diard A., Faisse P. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016;3:e556–e562. doi: 10.1016/S2352-3026(16)30131-4. [DOI] [PubMed] [Google Scholar]
- 17.Utter G.H., Dhillon T.S., Salcedo E.S., Shouldice D.J., Reynolds C.L., Humphries M.D. Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surg. 2016;151:e161770. doi: 10.1001/jamasurg.2016.1770. [DOI] [PubMed] [Google Scholar]
- 18.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020 Apr 3 doi: 10.1002/jmv.25819. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long C., Xu H., Shen Q., Zhang X., Fan B., Wang C. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana A.A., Soff G.A., Kakkar A.K., Vadhan-Raj S., Riess H., Wun T. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Rde A., Yoshida W.B., Maffei F.H., El Dib R., Nunes R., Rollo H.A. Systematic review of randomized controlled trials of new anticoagulants for venous thromboembolism prophylaxis in major orthopedic surgeries, compared with enoxaparin. Ann Vasc Surg. 2013;27:355–369. doi: 10.1016/j.avsg.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Carrier M., Abou-Nassar K., Mallick R., Tagalakis V., Shivakumar S., Schattner A. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds P.M., Van Matre E.T., Wright G.C., McQueen R.B., Burnham E.L., Ho P.J.M. Evaluation of prophylactic heparin dosage strategies and risk factors for venous thromboembolism in the critically ill patient. Pharmacotherapy. 2019;39:232–241. doi: 10.1002/phar.2212. [DOI] [PubMed] [Google Scholar]