To the Editor,
Previous studies have shown that common comorbidities were significantly associated with the increased risk of adverse outcomes in patients with coronavirus disease 2019 (COVID-19)1. As we know, hypertension was the most common comorbidities among COVID-19 patients2. Recently, a paper in the Journal of Infection by Zheng et al.3 has reported that the proportion of hypertension was significantly higher in critical/mortal patients compared to the non-critical patients (odds ratio (OR) = 2.72, 95% confidence interval (CI) [1.60–4.64], P = 0.0002). However, the findings were based on unadjusted effect estimates. It was worth mentioning that the data based on unadjusted effect estimates indicated hypertension was an important risk factor for the adverse outcomes of COVID-19 patients, but the pooled effects based on adjusted effect estimates were significantly reduced or even disappeared in several studies4, 5, 6, 7, 8. For instance, in the study of Wang et al., univariate analysis showed that hypertension was a risk factor for death in patients with COVID-19 (OR = 5.000, 95% CI [1.748–14.301]), while multivariate analysis showed that hypertension was not significantly associated with the risk of mortality (OR= 1.099, 95% CI [0.264–4.580])7. Similarly, univariate analysis in Cummings et al. indicated that hypertension was significantly associated with patients’ death (hazard ratio (HR) = 2.24, 95% CI [1.40–3.59]), but this association disappeared in the multivariate analysis (HR = 1.58, 95% CI [0.89–2.81])5. The same findings were also observed in Wang et al.’s study8. This meant that the association of hypertension with the adverse outcomes of COVID-19 patients might be affected by various factors such as age, gender and other comorbidities. Therefore, it is urgently required to clarify the association between hypertension and the adverse outcomes of COVID-19 patients by a systematically quantitative meta-analysis on the basis of the published studies reporting the adjusted effect estimates.
Therefore, we systematically searched the electronic databases, including Web of Science, Chinese National Knowledge Infrastructure (CNKI) and PubMed to identify all observational studies published between January 1, 2020 and June 15, 2020 that compared outcomes in hospitalized COVID-19 patients with and without hypertension. These search engines used the following two sets of keywords to capture available literature: "Coronavirus 2019, 2019-nCoV, SARS-CoV-2, COVID-19″ and "Hypertension”. Only articles that reported adjusted effect estimates of hypertension and adverse outcomes (severity including severe and critical, and mortality) in patients with COVID-19 were qualified. All calculations were implemented with Stata 11. 2 software. The pooled OR and pooled HR with their corresponding 95% CI were used to evaluate the risk of adverse outcomes in patients with COVID-19 and hypertension. The degree of heterogeneity between studies was tested using I2 statistics. The I2 values were 25%, 50%, and 75%, indicating low, medium, and high heterogeneity, respectively9. If there was no evidence of between-studies heterogeneity (I2 ≤ 50%), a fixed-effects model was used to calculate the combined effects. Otherwise, a random-effects model was selected10. The sensitivity analysis was used to evaluate the robustness of the results. Both Begg's test and Egger's test were used to evaluate publication bias.
Overall, 521 documents were initially identified according to our search criteria, and the final analysis included 19 studies of 15,302 patients4, 5, 6, 7, 8 , 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. As shown in Table 1 , the median age of COVID-19 patients ranged from 43.9 to 71 years, of which 38.2% had hypertension. The sample size ranged from 63 to 2877. Seventeen studies were retrospective and two prospective.
Table 1.
Characteristics of the included studies.
| Author | Location | Case | Age (years) | Male (%) | Study design | HTN | Adjusted effect estimate(95%CI) | Confounders |
|---|---|---|---|---|---|---|---|---|
| Chen C [12] | China | 150 | 59(16) | 84(56) | R | 49(32.6) | OR 2.586 (0.609–10.980) |
Age, gender, NT-proBNP, cTnI, hs-CRP, creatinine, CHD |
| Wang D [7] | China | 107 | 51(31–65) | 57(53.3) | R | 26(24.3) | OR 1.099 (0.264–4.580) |
Age, gender, CVD, creatinine concentration |
| Sun H [21] | China | 244 | NR | 137(54.5) | R | 138(56.6) | OR 0.82 (0.24–2.75) |
Age, gender, vital signs, previous respiratory diseases, laboratory values |
| Shi S [20] | China | 671 | 63(50–72) | 322(48) | R | 199(29.7) | HR 1.07 (0.46–2.53) |
Age, gender, diabetes, CHD, chronic renal disease, chronic heart failure, atrial fibrillation, CVD, COPD, procalcitonin, CRP |
| Yan X [23] | China | 1004 | NR | 493(49.1) | R | 235(23.4) | OR 2.606 (0.988–6.870) |
NLR, hs-CRP, NT-proBNP, BUN, respiratory failure, digestive system disease, CVD |
| Wang G [8] | China | 209 | NR | 105(50.2) | R | 27(12.9) | OR 0.357 (0.078–1.639) |
Age, gender, creatine kinase, lymphocyte, AST, CRP |
| Cummings MJ [5] | America | 257 | 62(51–72) | 171(67) | P | 162(63) | HR 1.58 (0.89–2.81) |
Age, gender, symptom duration before hospital presentation, chronic cardiac disease, COPD or interstitial lung disease, diabetes, interleukin-6, d-dimer |
| Phipps MM [6] | America | 2273 | 65(52–76) | 1297(57) | R | 1375(60) | OR 1.15 (0.85–1.56) |
Age, peak ALT, BMI >35, diabetes, intubation, renal replacement therapy |
| Galloway JB [14] | UK | 1157 | 71(57–82) | 666(57.6) | R | 611(52.9) | HR 1.53 (1.24–1.90) |
Age, gender |
| Huang S [16] | China | 310 | 62(40–70) | 174(56.1) | R | 113(36.5) | OR 1.562 (0.929–2.625) |
Age, gender |
| Escalera-Antezana JP [13] | Bolivia | 107 | 43.9(17.6) | 55(51.4) | R | 10(9.35) | OR 3.284 (1.276–6.291) |
Age |
| Gao C [15] | China | 2877 | NR | 1479(51.1) | R | 850(29.5) | HR 2.06 (1.10–3.83) |
Age, gender, medical history of diabetes, insulin-treated diabetes, myocardial infarction, underwent PCI/CABG, renal failure, stroke, heart failure, COPD |
| Zhao M [24] | China | 1000 | 61(46–70) | 466(46.6) | R | 282(28.2) | HR 1.974 (1.297–3.003) |
Age |
| Sabri A [19] | Iran | 63 | 54.1(15.5) | NR | R | 15(23.8) | OR 1.42 (1.13–1.71) |
History of heart disease, pericardial effusion, blood oxygen saturation |
| Lim JH [18] | Korea | 160 | NR | 86(53.8) | R | 77(48.1) | HR 1.34 (0.71–2.52) |
Acute kidney injury network, age, gender, diabetes |
| Chen F [4] | China | 660 | 55(34–68) | 295(44.7) | R | 230(34.8) | OR 0.920 (0.420–2.016) |
Age, cerebral infarction, SOFA, CRP, LDH |
| Targher G [22] | China | 310 | 47 | 149(48.1) | R | NR | OR 2.68 (1.20–5.98) |
Age, gender |
| Lala A [17] | America | 2736 | 66.40(15.8) | 1630(59.6) | R | 1065(38.9) | OR 0.99 (0.79–1.23) |
Age, gender, troponin strata, race, ethnicity, coronary artery disease, diabetes, heart failure, atrial fibrillation, chronic kidney disease |
| Cen Y [11] | China | 1007 | 61 (49–68) | 493(49.0) | P | 270(26.8) | HR 1.442 (1.109–1.876) |
Age, gender, smoking, diabetes, chronic obstructive lung disease, coronary artery disease, duration of anti-viral therapy |
All values are n (%), mean (SD) or median (IQR); NR, not reported; HTN, hypertension; P, prospective; R, retrospective; HR, hazard ratio; OR, odds ratio; NT-proBNP, amino-terminal pro-brain natriuretic peptide; cTnI, cardiac troponin I; hs-CRP, high-sensitivity C-reactive protein; CHD, Coronary heart disease; CVD, cardiovascular or cerebrovascular disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; SOFA, Sequential Organ Failure Assessment; PCI/CABG, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG); LDH, lactate dehydrogenase.
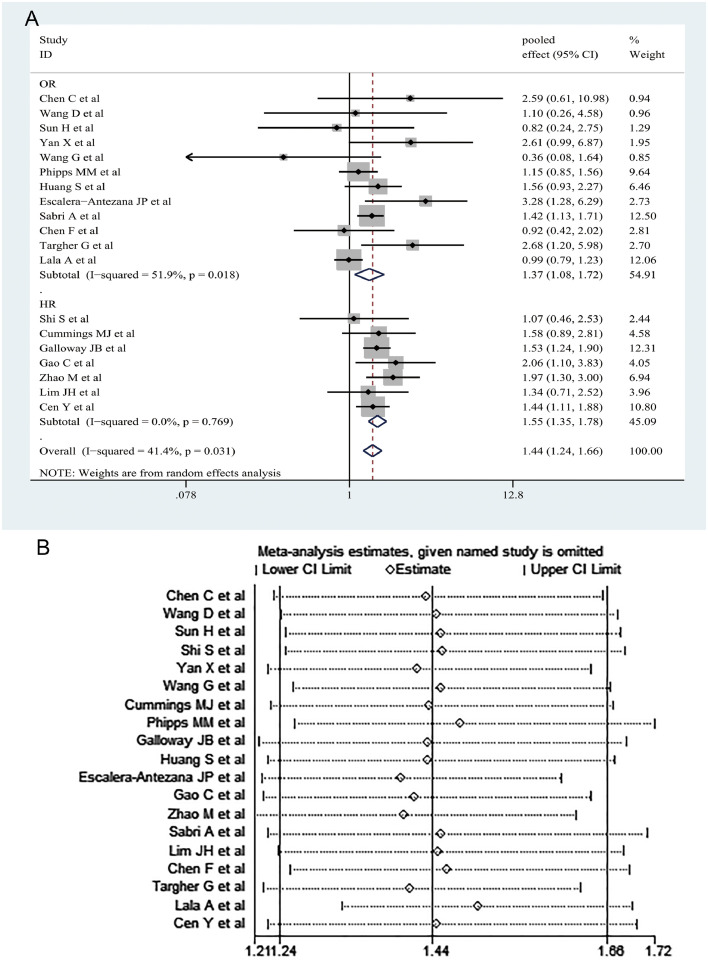
Totally, our meta-analysis showed that hypertension was significantly associated with the increased risk of adverse outcomes in COVID-19 patients on the basis of 19 studies with 15,302 cases (OR= 1.44, 95% CI [1.24–1.66]; I2 = 41.4%, random-effects model) (Fig. 1 A). Of the 19 studies, 12 reported adjusted OR and 7 reported adjusted HR. Therefore, we conducted a subgroup analysis based on the adjusted OR and adjusted HR. We also found a significant correlation between hypertension and adverse outcomes on the basis of both 12 OR-adjusted studies with 8173 cases (OR= 1.37, 95% CI [1.08–1.72]; I2 = 51.9%) and 7 HR-adjusted studies with 7129 cases (HR= 1.55, 95% CI [1.35–1.78]; I2 = 0.0%) (Fig. 1A). As shown by the sensitivity analysis, none of the studies had a significant impact on the overall results, which proves the robustness of our results (Fig. 1B). No publication bias was detected in Begg's test (P = 0.889) or Egger's test (P = 0.432).
Fig. 1.
The pooled effects and their 95% confidence interval (CI) of the relationship between hypertension and adverse outcomes in patients with COVID-19 (A). Sensitivity analysis of the relationship between hypertension and adverse outcomes in patients with COVID-19 (B).
Previous studies have suggested that hypertension was a risk factor for adverse outcomes of COVID-19 patients, but the studies did not consider the effects of confounding factors on the findings2 , 25, 26, 27, 28. Presently, our results showed that hypertension was significantly associated with the increased risk of adverse outcomes in COVID-19 patients on the basis of the adjusted effect estimates, which suggests that hypertension is an independent risk factor for predicting the severity and mortality of COVID-19 patients. Thus, COVID-19 patients with hypertension deserve more clinical attention. It should be acknowledged that some limitations existed in our study. Firstly, the judgment criteria of adverse results in the included studies were not uniform. Secondly, all the included studies reported the adjusted effect estimates, but the confounding factors adjusted in each study were not entirely consistent. Thirdly, the stage of hypertension and whether it is controlled or poorly controlled are also unknown. The included studies did not adequately report data on chronic hypertension medications and therefore these could not be analyzed.
In summary, our meta-analysis demonstrated for the first time that hypertension was an independent risk factor for predicting the adverse outcomes of patients with COVID-19. Further well-designed studies with larger sample sizes are required to verify the findings of our present study.
Declaration of Competing Interest
All authors report that they have no potential conflicts of interest.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81973105).
Contributor Information
Haiyan Yang, Email: yhy@zzu.edu.cn.
Yadong Wang, Email: wangyd76@163.com.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis IJID. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. official publication of the International Society for Infectious DiseasesMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen F., Sun W., Sun S., Li Z., Wang Z., Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin Transl Med. 2020 doi: 10.1002/ctm2.40. Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/s0140-6736(20)31189-2. Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phipps M.M., Barraza L.H., LaSota E.D., Sobieszczyk M.E., Pereira M.R., Zheng E.X. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. 2020 doi: 10.1002/hep.31404. May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G., Wu C., Zhang Q., Wu F., Yu B., Lv J. C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation. Open Forum Infect Dis. 2020;7(5) doi: 10.1093/ofid/ofaa153. Mayofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 11.Cen Y., Chen X., Shen Y., Zhang X.H., Lei Y., Xu C. Risk factors for disease progression in mild to moderate COVID-19 patients- a multi-center observational study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.041. the official publication of the European Society of Clinical Microbiology and Infectious DiseasesJun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E008. doi: 10.3760/cma.j.cn112148-20200225-00123. Mar 6. [DOI] [PubMed] [Google Scholar]
- 13.Escalera-Antezana J.P., Lizon-Ferrufino N.F., Maldonado-Alanoca A., Alarcon-De-la-Vega G., Alvarado-Arnez L.E., Balderrama-Saavedra M.A. Risk factors for mortality in patients with Coronavirus Disease 2019 (COVID-19) in Bolivia: an analysis of the first 107 confirmed cases. Le infezioni in Medicina. 2020;28(2):238–242. Jun 1. [PubMed] [Google Scholar]
- 14.Galloway J.B., Norton S., Barker R.D., Brookes A., Carey I., Clarke B.D. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.064. May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020:1–8. doi: 10.1038/s41440-020-0485-2. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.06.007. Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J.H., Park S.H., Jeon Y., Cho J.H., Jung H.Y., Choi J.Y. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9(6) doi: 10.3390/jcm9061718. Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabri A., Davarpanah A.H., Mahdavi A., Abrishami A., Khazaei M., Heydari S. Novel coronavirus disease 2019: predicting prognosis by using a computed tomography severity score and clinicolaboratory data. Polish Arch Internal Med. 2020 doi: 10.20452/pamw.15422. Jun 5. [DOI] [PubMed] [Google Scholar]
- 20.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun H., Ning R., Tao Y., Yu C., Deng X., Zhao C. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16533. May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targher G., Mantovani A., Byrne C.D., Wang X.B., Yan H.D., Sun Q.F. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.06.001. Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan X., Li F., Wang X., Yan J., Zhu F., Tang S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J Med Virol. 2020 doi: 10.1002/jmv.26061. May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M., Wang M., Zhang J., Gu J., Zhang P., Xu Y. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12 doi: 10.18632/aging.103298. Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuin M., Rigatelli G., Zuliani G., Rigatelli A., Mazza A., Roncon L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J Infect. 2020;81(1):e84–e86. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320926899. Apr-Jun1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/aging.103000. Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Wu J., Sun X., Xue H., Shao J., Cai W. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: a meta-analysis. Epidemiol Infect. 2020;148:e106. doi: 10.1017/s095026882000117x. May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]