Abstract
COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and spreading worldwide has become a serious challenge for the entire health care system as regards infection prevention, rapid diagnosis, and treatment. Lung ultrasound (LUS) is a dynamically developing diagnostic method used in intensive care, cardiology and nephrology, it can also be helpful in diagnosing and monitoring pneumonia. Interstitial pneumonia appears to be the most common clinical manifestation of coronavirus infection. We present 4 case reports of COVID-19 involving the lungs, in which transthoracic lung ultrasound was successfully utilized as a constituent of bedside diagnostics and a review of the literature concerning potential use of LUS in COVID-19 diagnostics. The possibility to perform this examination repeatedly, its non-invasiveness and high sensitivity make it an important element of care provided for patients with viral pneumonia.
Keywords: Lung ultrasound, COVID-19, Coronavirus, Viral pneumonia
1. Introduction
An outbreak of epidemic caused by an unknown SARS-CoV-2 belonging to the coronavirus family began in December 2019 in China. Coronavirus disease 2019 (COVID-19) caused by this newly discovered virus has very quickly spread all over the world. Considering the rapidly increasing number of cases the World Health Organization (WHO) declared a pandemic on 11th March 2020. The incubation period of this disease transmitted by droplets ranges from 2 to 14 days, usually between 3 and 7 [1,2]. Its course is varied – the majority of patients (about 80%) are asymptomatic or present with mild symptoms of a respiratory tract infection (fever, cough, weakness) [3]. Approximately 15% of patients have severe symptoms, and 5% require treatment at Intensive Care Units due to pneumonia requiring mechanical ventilation (2.3%), the development of acute respiratory distress syndrome (ARDS), sepsis or multiple organ failure (MOF) [4,5]. COVID-19 mortality rate has been estimated as 3.4% [5,6]. Considering the epidemic context, it is essential to diagnose the disease quickly. The basic diagnostic method is a reverse transcription polymerase chain reaction (RT-PCR) test of a nasopharyngeal swab or sputum sample, with a sensitivity of 60–94%, but the result may be sometimes available only after 24 h. The infection is manifested by the following abnormalities in laboratory test results: leukopenia, lymphocytopenia, increased levels of LDH and CRP [7]. Imaging techniques are important tools in the diagnosis of SARS-CoV-2 infections - chest computed tomography (CT) is presently recommended by experts as a screening examination [8]. The hallmarks of COVID-19 revealed by CT are bilateral, mostly peripheral, lesions. Characteristic imaging features include ground glass opacities (over 50% of patients) with areas of consolidation. Lymphadenopathy, pleural effusions, and pulmonary nodules are rare [8]. CT imaging is characterized by high sensitivity, however, it has some limitations. It may be impossible to perform CT for a patient with hypoxemia, who is mechanically ventilated with high levels of oxygen or for hemodynamically unstable patients. Transthoracic lung ultrasound (LUS) is characterized with a sensitivity and specificity of 0.78–0.90 (depending on the duration of the disease) for the diagnosis of ARDS [9]. This tool appears very attractive for the implementation in the diagnosis of patients with suspicions of COVID-19, especially because it can be performed at any stage of the diagnostic and therapeutic process and does not require transportation that might be risky for the patient [10].
2. Case series
We present 4 case reports of COVID-19 involving the lungs, in which lung ultrasound was successfully utilized as a constituent of bedside diagnostics. LUS was performed on admission when real-time polymerase chain reaction results were still pending. Symptoms, laboratory results, chest X-ray and lung ultrasound parameters are summarized in Table 1 .
Table 1.
Summary of symptoms, laboratory results, chest X-ray and lung ultrasound parameters in the 4 patients with COVID-19.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age | 25 | 84 | 80 | 52 |
| Sex | M | M | F | F |
| Symptoms: | ||||
|
yes | no | yes | yes |
|
yes | yes | no | yes |
| CRP | N | N | H | H |
| Procalcitonin | N | N | H | N |
| X-ray | N | congestive lesions | inflammatory lesions | small bilateral infiltrates |
| Lung ultrasound | ||||
|
yes | yes | yes | yes |
|
no | yes | yes | yes |
|
no | no | yes | no |
|
yes | yes | no | yes |
|
no | no | no | no |
Abbreviations: CRP – C-reactive protein; N – normal; H – high.
2.1. Case 1
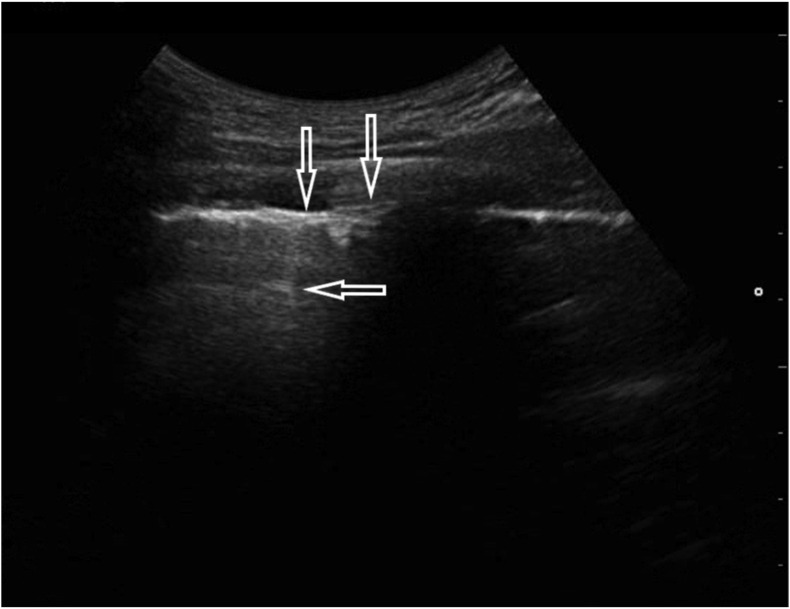
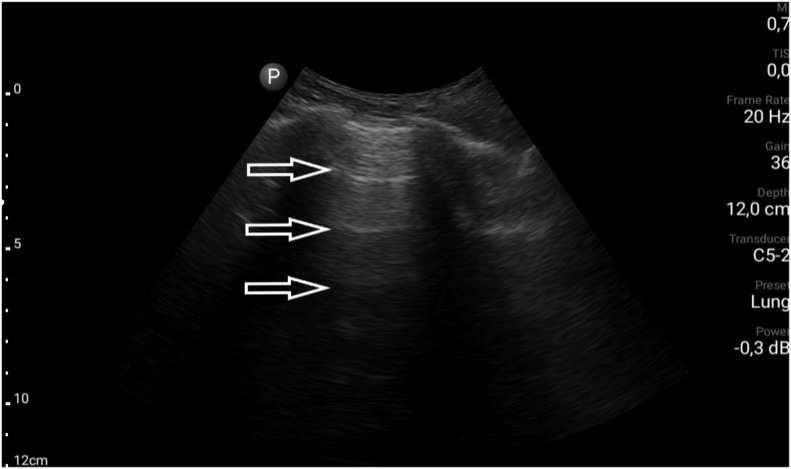
A 25-year-old male without previous medical history, complaining of fever of 39.2 °C and cough for 2 days. On admission, the patient had dyspnea with modestly lower blood saturation (SatO2 94%), but with marked hypoxemia (pO2 57 mmHg) and modest rise of blood pH (7.48) and lower pCO2 (33 mmHg). The arterial-blood gas test was performed without oxygen therapy. In other laboratory tests, CRP, procalcitonin and D-dimers measurements were normal. The chest X-ray was also normal. Because of inconclusive results of the other tests, LUS was performed, revealing multifocal minor sub-pleural consolidations accompanied by strengthening behind the lower margin of the lesion (the so-called C-line artifact), short vertical artifacts (the so-called Z-lines) and segmental pleural irregularity (Fig. 3a, Fig. 3b a and b ). Considering all information obtained and clinical data, a suspicion of COVID-19 infection was put forward, confirmed by the RT-PCR test.
Fig. 3a.
Convex transducer; irregularity of the pleural line (↓) and vertical artifacts arising from the pleural line (Z-line, ←).
Fig. 3b.
Linear transducer; small (<5 mm) sub-pleural consolidation accompanied by C-line artifact (←).
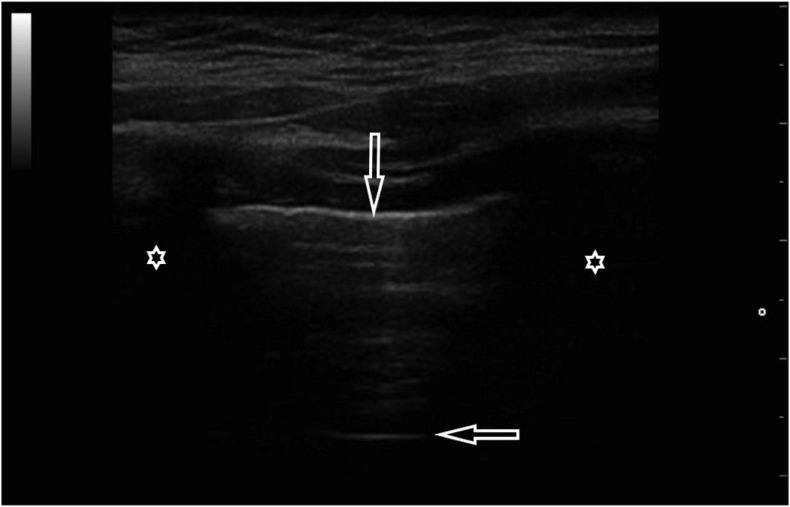
2.2. Case 2
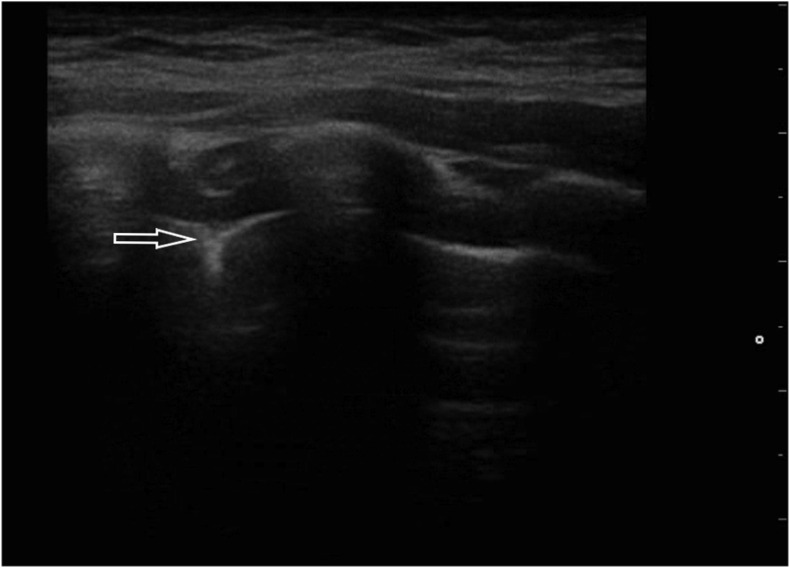
An 84-year-old man with many comorbidities involving the cardiovascular system, including persistent atrial fibrillation and pulmonary hypertension, admitted to hospital due to fever of 38.4 °C. Primary diagnosis was a urinary tract infection and concomitant respiratory infection. However, no abnormality was found in the general urine test. CRP and procalcitonin levels were not elevated; however, pancytopenia and reduced oxygen partial pressure (pO2 69 mmHg) were noteworthy. Chest X-ray revealed features of lung congestion. The results of laboratory and imaging tests did not explain the high fever, so COVID-19 was suspected, and then confirmed by RT-PCR. In the course of diagnostics, an ultrasound examination of the lungs was also performed, revealing the following abnormalities: segmentally irregular pleural line and single focally located B-lines (Fig. 4a, Fig. 4b a and b).
Fig. 4a.
Convex transducer; focally visible B-line artifacts (→, ←).
Fig. 4b.
Linear transducer; irregular pleural line (↓) accompanied by single B-line artifacts (→).
2.3. Case 3
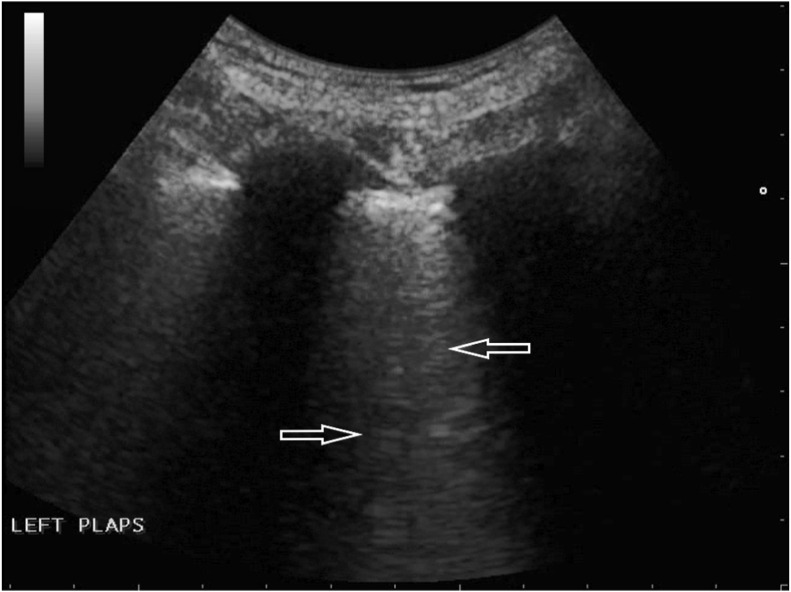
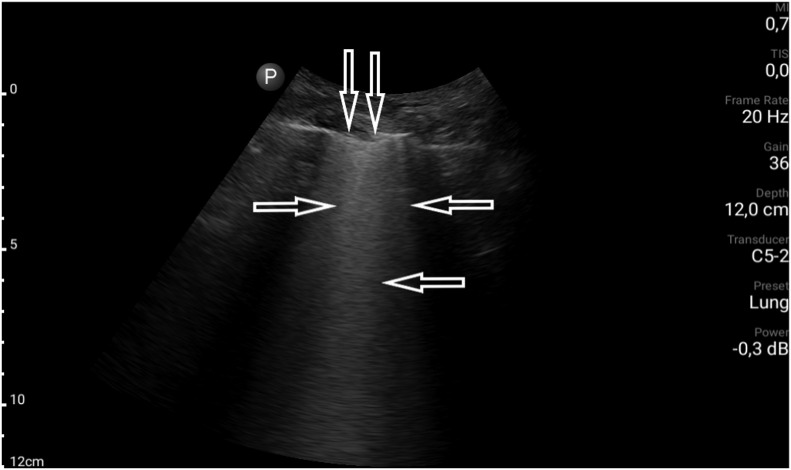
An 80-year-old female with many comorbidities, including cirrhosis and chronic kidney disease (CKD G3), and a history of ischemic stroke and episode of deep vein thrombosis, was admitted to the clinic due to acute dyspnea. On admission, the patient was in a serious condition – blood pressure was 80/50 mmHg, there was tachypnoea of 30 breaths/min., blood saturation could not by measured, and an attempt to collect arterial blood for an arterial-blood gas test was unsuccessful. Laboratory tests performed during the first hours after admission revealed: CRP 72.5 mg/l (<5.0); LDH 347 U/L (<247); procalcitonin 24.2 ng/ml (<0.5); WBC 13.1 G/L; RBC 3.49 T/l, Hb 9.9 g/dl; Ht – 30%, PLT 334 000/l. In a control peripheral blood sample, WBC decreased to 6.1 G/L; there was no neutropenia, though. Flu and RSV swabs (RT-PCR) were negative. The result of the chest X-ray examination was inconclusive: congestive lesions to be differentiated with inflammatory lesions were described. In the physical examination, massive crackles and rales on both sides of the chest were revealed. To complete the diagnostic process, a bedside LUS examination was performed. Profile A (Fig. 5 a) was found in the upper fields of both lungs; in the middle field on the right side, the alveolar-interstitial syndrome (the white lung) and the blurred pleural line (Fig. 5 b) were visualized. Considering all obtained information and clinical data (including the fact that the patient lives in a nursing home), a suspicion of COVID-19 infection was put forward, confirmed by the RT-PCR test of the nasopharyngeal swab.
Fig. 5a.
Convex transducer; normally aerated upper field of the lung (visible A-line artifacts, →).
Fig. 5b.
Convex transducer; irregular pleural line (↓) and B-line artifacts visible in the middle field on the right (→, ←).
2.4. Case 4
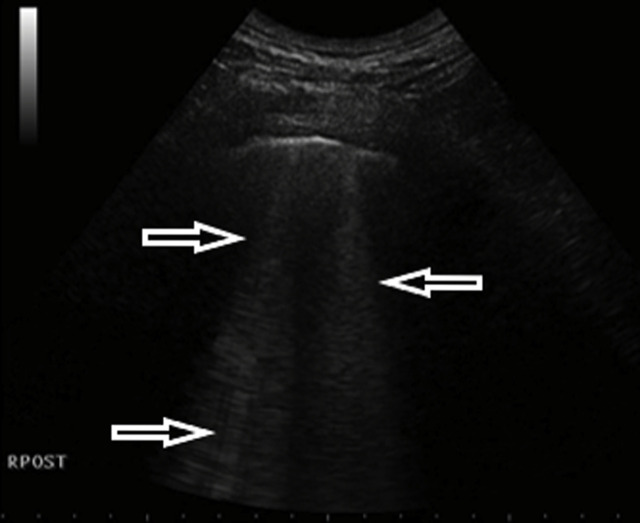
A 52-year-old female admitted to the emergency department due to fever, cough and dyspnea. The patient had no previous medical history of comorbidities. Fever and cough started 4 days earlier. On admission, desaturation was noticed – SatO2 was 90% without oxygen therapy. An arterial-blood gas test revealed pO2 decreased to 58 mmHg and pCO2 to 32 mmHg, pH was 7.41. Additionally, leukopenia was found in a peripheral blood sample. Moreover, LDH activity was markedly elevated to 350 U/L (normal activity < 246 U/L) and so was PCR concentration. Procalcitonin concentration was normal. The chest X-ray examination showed small bilateral infiltrates. In the subsequent days of hospitalization, LUS was performed, revealing confluent B-lines in anterior, lateral and posterior regions in the lower and upper fields and small consolidations in both lower fields (Fig. 6a, Fig. 6b a and b). Unfortunately the patient's condition worsened, in a control arterial-blood gas test pO2 was low (approximately 60 mmHg) despite the oxygen therapy (FiO2 40%). The patient was transferred to an intermediate care unit in order to obtain better monitoring.
Fig. 6a.
Convex transducer; focal B lines origin from the pleural line (→, ←).
Fig. 6b.
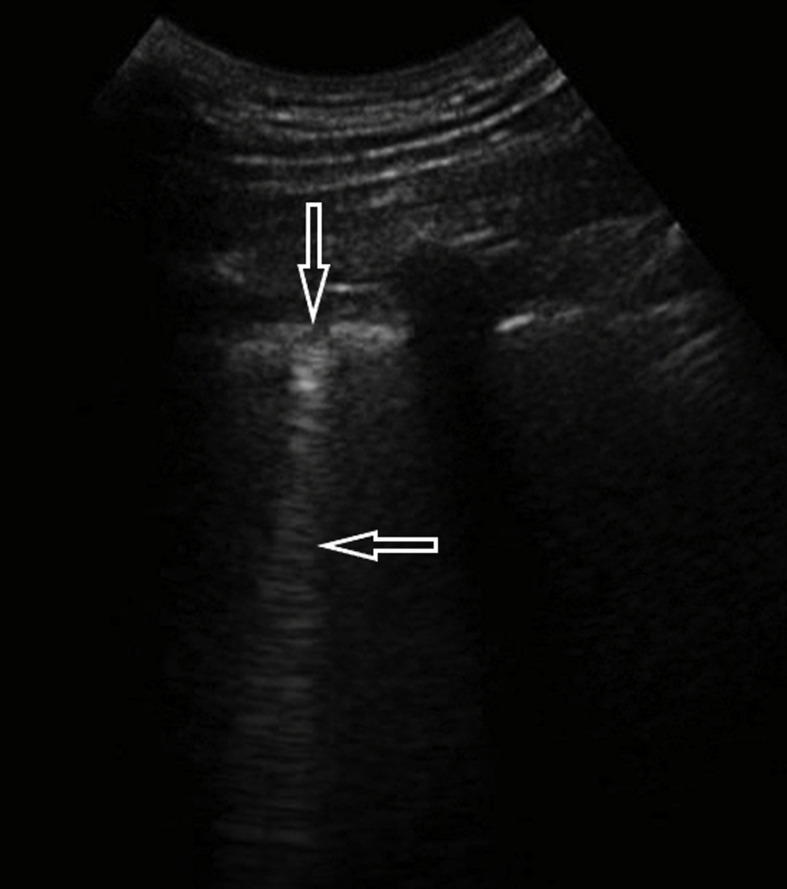
Convex transducer; small subpleural consolidation (↓) and a vertical artifact behind the bottom of the lesion (the so-called: C line, ←).
3. Review
3.1. LUS examination technique and equipment requirements
LUS may be performed with any ultrasound device available at the work place. Miniaturized hand-held ultrasound devices, the size of smart phone or tablet, with a transducer merit attention. The small size of the ultrasound device facilitates performing the examination at the patient's bedside, in ambulatory care conditions, or even during a home visit. Convex and linear transducers are used to perform a classical LUS. However, for patients suspected of COVID-19 or diagnosed with COVID-19 pneumonia, one transducer should be used. Convex or micro-convex transducers seem to be optimal. Irrespective of the size of the device available, it should be protected against contamination with pathogens. To this end, a foil sleeve is placed over the transducer and the cable. Small, pocket-sized devices can be placed inside the foil sleeve (Fig. 1a). For full size ultrasound machines transparent protective foil is useful (Fig. 1b). It allows for changing the settings during the examination depending on the prevailing needs.
Fig. 1a.
Ultrasound transducer in a foil sleeve protecting it against contamination.
Fig. 1b.
Folic cover for ultrasound device and protective clothing for the person performing lung ultrasound exam.
Due to multiple lesions caused by COVID-19 infection it is recommended to perform the examination in the sitting position, scanning the chest from the apex to the base of the lungs, in consecutive body lines.
3.2. Normal image in a LUS scan
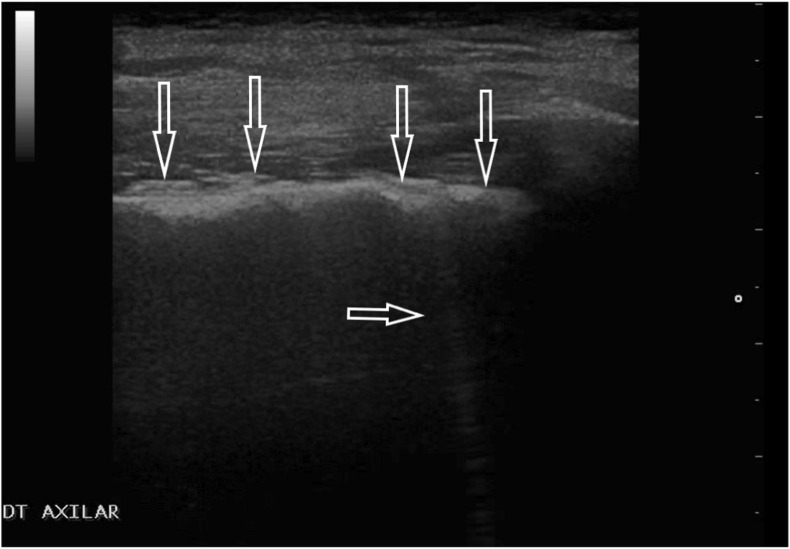
A normally aerated lung constitutes a barrier that strongly reflects the ultrasound beam. Consequently, although ultrasonography has been used in clinical diagnostics for many years, it has not been employed for the assessment of the lungs [[10], [11], [12], [13]]. A normal ultrasonographic image is characterized by a smooth, regular, echogenic, continuous pleural line, the presence of lung sliding (the movement of the pleural line consistent with the respiratory activity), A-line artifacts (horizontal artifacts that occur beneath the pleural line at multiples of the distance between the ultrasound transducer and the pleural line) [[10], [11], [12], [13]] (Fig. 2.) It should be noted, however, that although these features indicate a normally aerated lung, this is not equivocal with the exclusion of some pulmonary diseases, for instance, asthma, chronic obstructive pulmonary disease (COPD), and pulmonary embolism without subpleural consolidations. Consequently, it is essential to refer the result of the ultrasound examination to clinical information (medical interview, other additional examinations) [[10], [11], [12], [13]].
Fig. 2.
Normal ultrasound image: regular and echoic pleural line (↓) and A-line (←), shadow behind the ribs (*); linear transducer.
3.3. Ultrasonographic features in COVID-19 infections
Lesions found in LUS in the course of COVID-19 infections constitute features of interstitial pneumonia, including: irregular pleural line, B-lines (multifocal, discrete or confluent), small (centomeric) consolidations, both non-translobar and translobar consolidations, multilobar distribution of abnormalities. Fluid in the pleural cavity is rare and occurs is small volumes [[14], [15], [16]]. In the early phase of the disease and in a mild infection, focal B-line artifacts are visible. As the disease progresses, the number of B-lines increases and they begin to constitute the interstitial syndrome, subsequently the alveolar-interstitial syndrome and finally the white lung. The artifacts are accompanied with small, round subpleural consolidations and abnormalities within the pleural line [14,15]. As the inflammation progresses, ARDS develops that, apart from the enumerated ultrasonographic features, is characterized by spared areas, large-size consolidations and the absence or significant limitation of lung sliding [17]. Pathologies are most frequently localized in the middle and lower lung fields at the lateral-posterior chest. In the healing phase, interstitial lesions disappear gradually, which is presented in the ultrasound examination as a reduction of the number of B-lines and consolidations. Instead of pathological lesions, A-line artifacts appear (found in a normally aerated lung) and lung sliding improves (lung movement consistent with the respiratory activity). The fibrotic process may constitute the adverse outcome of the healing of inflammatory lesions. Ultrasonographic features of pulmonary fibrosis are comprehensively described in medical literature and involve characteristic abnormalities within the pleural line (irregular, coarse in appearance, fragmented, or blurred) and B-line artifacts [[18], [19], [20]]. In this case, similar to interstitial pneumonia of a viral etiology, the more B-line artifacts are visible, the more significantly the interstitial tissue is affected [20].
3.4. Clinical value of ultrasound
In the context of the SARS-CoV-2 pandemic, a rapid diagnosis of COVID-19 is particularly significant since the identification and isolation of infected patients limits the pace of spreading the infection in the general population [7,21]. The epidemiological interview, important when the first cases occurred in a given area, presently, with more than 7.8 million of confirmed cases (data as of 15th June 2020), loses its significance considering the likelihood of getting infected [7,22]. The clinical picture – fever, cough, dyspnea, and weakness – may indicate the coronavirus infection, but similar symptoms may appear also in common acute conditions (including viral infections of a different etiology), or during exacerbation of chronic diseases. Commonly used diagnostic procedures for SARS-CoV-2 infection, i.e., CT and the RT-PCR test, are available in hospital conditions, yet the waiting time for the result, especially as regards the genetic test, may be prolonged [[23], [24], [25]]. It appears that the use of transthoracic LUS would be an ideal solution since this examination can be performed at the patient's bedside at any stage of the diagnostic and therapeutic process. The presence of specific (but not pathognomic) abnormalities in the LUS scan, combined with concordant clinical information obtained during the medical and epidemiologic interview, may estimate with a high probability the risk of COVID-19 in the examined patient and customize further treatment [26,27]. Due to the common use of portable ultrasound devices, point of care diagnostics is available at the emergency departments, in the emergency medical services, in GP surgeries, or at a patient's home [28,29]. The advantages of ultrasonography, apart from the possibility of the bedside examination without the necessity of transporting the patient, include non-invasiveness and lack of exposure to X-rays. Consequently, this examination can be performed as often as is clinically necessary. This may be particularly important for patients in very serious clinical conditions, who require advanced therapeutic techniques at Intensive Care Units (invasive mechanical ventilation, renal replacement therapy, extracorporeal membrane oxygenation - ECMO). Transportation of such patients to tomography units to assess the development of pulmonary lesions and its pace may be risky or actually impossible. In this patient group, chest ultrasound, extended with basic echocardiography, assessment of the abdominal cavity and inferior vena cava (IVC) may be very useful in treatment monitoring and optimization. It should also be stressed, that multiple organ ultrasound assessment is performed by one operator thus minimizing the contamination of the equipment and exposure of additional medical staff to infection. An additional advantage, in the group of most critically ill patients, particularly those mechanically ventilated, may be the use of ultrasound to monitor the efficiency of recruitment maneuvers and to detect complications such as pneumothorax or pleural effusion [[30], [31], [32], [33], [34], [35]].
4. Conclusions
LUS may be useful in the diagnosis of patients suspected of COVID-19 (at the department, in a tent adjacent to the department, at a patient's home, etc.). Moreover, it may be utilized for monitoring of the disease at Intensive Care Units. An ultrasound examination is relatively uncomplicated and can be performed repeatedly at any place, depending on clinical needs. The low cost and safety of an ultrasound examination (absence of ionizing radiation) encourages clinicians to utilize ultrasonography in the diagnosis of pulmonary diseases. We believe, that in condition of inconclusive clinical data, incorrect LUS results may sensitize us to the possibility of COVID-19 coexistence. Moreover, if a person has negative RT-PCR test and positive transthoracic ultrasound it seems reasonable to repeat the RT-PCR test and then, if negative, to search for causes of interstitial changes other than COVID-19. However, in order to prove the efficiency of this imaging technique for the diagnosis of COVID-19 further multi-center studies are necessary.
Financial disclosure
The authors have no funding to disclose.
The author contribution
Study Design: Natalia Buda.
Data Collection: Jolanta Cylwik, Elena Segura-Grau, Marcin Wełnicki, Natalia Buda.
Statistical Analysis: none.
Data Interpretation: Natalia Buda, Elena Segura-Grau, Marcin Wełnicki, Jolanta Cylwik.
Manuscript Preparation: Natalia Buda, Jolanta Cylwik, Marcin Wełnicki, Elena Segura-Grau.
Literature Search: Natalia Buda, Jolanta Cylwik, Marcin Wełnicki.
Funds Collection: n/a.
Declaration of competing interest
The authors declare no conflict of interests.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Mar;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb15;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Report of the WHO . WHO; 2020. China joint mission on coronavirus disease 2019 (COVID-19) pp. 1–40.https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020 Feb 6;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 Jun;295(3):200463. doi: 10.1148/radiol.2020200463. Epub Feb 20 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soldati G., Demi M., Demi L. Ultrasound patterns of pulmonary edema. Ann Transl Med. 2019;7(Suppl 1):S16. doi: 10.21037/atm.2019.01.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo P.H., Copetti R., Feller-Kopman D., Mathis G., Maury E., Mongodi S. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019 Sep;45(9):1200–1211. doi: 10.1007/s00134-019-05725-812. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein D., Mézière G., Biderman P., Gepner A., Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997 Nov;156(5):1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 12.van Sloun R.J.G., Demi L. Localizing B-lines in lung ultrasonography by weakly supervised deep learning, in-vivo results. IEEE J Biomed Health Inform. 2020;24(4):957–964. doi: 10.1109/JBHI.2019.2936151. [DOI] [PubMed] [Google Scholar]
- 13.Bekgoz B., Kilicaslan I., Bildik F., Keles A., Demircan A., Hakoglu O. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am J Emerg Med. 2019 Nov;37(11):2020–2027. doi: 10.1016/j.ajem.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Peng Q.Y., Wang X.T., Zhang L.N. Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849‐850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39:1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moro F., Buonsenso D., Moruzzi M.C., Inchingolo R., Smargiassi A., Demi L. How to perform lung ultrasound in pregnant women with suspected COVID-19. Ultrasound Obstet Gynecol. 2020 May;55(5):593–598. doi: 10.1002/uog.22028. [DOI] [PubMed] [Google Scholar]
- 17.Copetti R., Soldati G., Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008 Apr 29;6:16. doi: 10.1186/1476-7120-6-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buda N., Piskunowicz M., Porzezińska M., Kosiak W., Zdrojewski Z. Lung ultrasonography in the evaluation of interstitial lung disease in systemic connective tissue diseases: criteria and severity of pulmonary fibrosis - Analysis of 52 patients. Ultraschall Med. 2016 Aug;37(4):379–385. doi: 10.1055/s-0041-110590. [DOI] [PubMed] [Google Scholar]
- 19.Soldati G., Demi M., Inchingolo R., Smargiassi A., Demi L. On the physical basis of pulmonary sonographic interstitial syndrome. J Ultrasound Med. 2016 Oct;35(10):2075–2086. doi: 10.7863/ultra.15.08023. [DOI] [PubMed] [Google Scholar]
- 20.Buda N., Kosiak W., Smoleńska Z., Zdrojewski Z. Transthoracic lung ultrasound in the monitoring of interstitial lung disease: a case of scleroderma. Pol Arch Med Wewn. 2013;123(12):721–722. doi: 10.20452/pamw.2021. [DOI] [PubMed] [Google Scholar]
- 21.Luan R.S., Wang X., Sun X., Chen X.S., Zhou T., Liu Q.H. Vol. 51. 2020 Mar. pp. 131–138. ([Epidemiology, treatment, and epidemic prevention and control of the coronavirus disease 2019: a review]. Sichuan Da Xue Xue Bao Yi Xue Ban). 2. Chinese. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Q&A on coronaviruses (COVID-19). How does COVID-19 spread? https://www.who.int/news-room/q-a-detail/q-a-coronaviruses
- 23.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 Apr;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., Kang S., Tian R., Zhang X., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol. 2020 May;75(5):341–347. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections — the state of the art. Emerg Microb Infect. 2020 Mar 20:1–26. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonsenso D., Piano A., Raffaelli F., Bonadia N., de Gaetano Donati K., Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020 Mar;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 27.Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020 Mar 20 doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schieb E., Greim C.A. Notfallsonographie [emergency sonography] Anaesthesist. 2015 Apr;64(4):329–342. doi: 10.1007/s00101-015-0019-5. quiz 343-4. German. [DOI] [PubMed] [Google Scholar]
- 29.Becker T.K., Martin-Gill C., Callaway C.W., Guyette F.X., Schott C. Feasibility of paramedic performed prehospital lung ultrasound in medical patients with respiratory distress. Prehosp Emerg Care. 2018 Mar-Apr;22(2):175–179. doi: 10.1080/10903127.2017.1358783. [DOI] [PubMed] [Google Scholar]
- 30.Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D.A., Mathis G., Kirkpatrick A.W. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012 Apr;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein D.A., Mezière G., Lascols N., Biderman P., Courret J.P., Gepner A. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005 Jun;33(6):1231–1238. doi: 10.1097/01.ccm.0000164542.86954.b4. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein D.A. Lung ultrasound in the critically ill. Ann Intensive Care. 2014 Jan 9;4(1):1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenstein D. Springer; 2010. Whole body ultrasonography in the critically ill. eBook. [DOI] [Google Scholar]
- 34.Tusman G., Acosta C.M., Costantini M. Ultrasonography for the assessment of lung recruitment maneuvers. Crit Ultrasound J. 2016 Dec;8(1):8. doi: 10.1186/s13089-016-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess D.R. Recruitment maneuvers and PEEP titration. Respir Care. 2015 Nov;60(11):1688–1704. doi: 10.4187/respcare.04409. [DOI] [PubMed] [Google Scholar]