Clinical Practice Points.
-
•
Coronavirus disease 2019 (COVID-19) has swept the globe and poses unique treatment challenges for immunocompromised patients with hematologic malignancies.
-
•
Here, we present the case of a 54-year-old man with follicular lymphoma, recently completing treatment with maintenance rituximab, who endured a protracted course of COVID-19. The patient’s case was complicated by evolving hypoxic respiratory failure, marked lymphopenia, and hypogammaglobulinemia, which ultimately led to treatment with COVID-19 convalescent plasma, resulting in clinical improvement.
-
•
Presently, there is no definitive data on the efficacy of COVID-19 convalescent plasma in patients with COVID-19; however, many trials are ongoing. We propose that this may be an effective treatment in patients treated for lymphoma with subsequent lymphopenia and hypogammaglobulinemia.
Introduction
Many health care systems and clinicians have faced unprecedented challenges in the management of coronavirus disease 2019 (COVID-19) as the pandemic has expanded across the globe. Immunocompromised patients with hematologic malignancies have historically been more susceptible to viral respiratory diseases, to include less virulent strains of coronavirus.1 , 2 That risk is currently magnified, given the increased virulence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), unknown or unproven treatment options, and the development of regional systemic strains during this pandemic. One multicenter study in 105 patients with cancer and 536 age-matched controls showed that patients with cancer were indeed at higher risk for COVID-19 and also had a higher frequency of severe events. Moreover, patients with hematologic malignancies such as lymphoma were noted to have relatively higher death rates, intensive care unit admission rates, and invasive mechanical ventilation requirements.3 Here, we describe a patient with follicular lymphoma who endured a protracted course of COVID-19 and was ultimately treated with COVID-19 convalescent plasma (CCP). Of note, this patient was also the first to be treated with CCP in a Department of Defense (DoD) facility.
Case Report
On March 22, 2020, a 54-year-old man with a medical history of stage IVE follicular lymphoma was diagnosed with COVID-19. The patient’s previous lymphoma treatment included 6 cycles of bendamustine and rituximab completed in 2018. He completed 2 years of maintenance rituximab, with the last dose given just days prior to his diagnosis of COVID-19. He presented with classic symptoms of cough, shortness of breath, and subjective fever approximately 1 week prior to the diagnosis, which was initially treated as pneumonia. The patient’s symptoms worsened, resulting in admission to our institution and a nasopharyngeal real time reverse transcriptase polymerase chain reaction confirmed SARS-CoV-2. He initially improved with supportive care and was subsequently discharged on 5 days of azithromycin and hydroxychloroquine. His symptoms waxed and waned over the next 3 weeks, requiring multiple brief admissions for supportive care.
Ultimately, the patient’s clinical course acutely worsened, and he was admitted to our intensive care unit on April 13, 2020 for high-grade fevers up to 106.2°F, worsened shortness of breath, cough, and hypoxia, with oxygen saturations as low as 88% on room air. His chest x-ray confirmed worsening bilateral infiltrates involving greater than 50% of the lung parenchyma. Computed tomography angiography of the chest did not show any evidence of pulmonary embolism. He was placed on 4L nasal cannula of supplemental oxygen and received supportive care. However, the medical team became increasingly concerned that he would require intubation and invasive mechanical ventilation. His absolute lymphocyte count at this admission was 300 cells/mcL, with prior counts being in the 500s. His serum immunoglobulin (Ig)G, IgA, and IgM levels were low at 425 mg/dL, 50 mg/dL, and 23 mg/dL, respectively. He persistently tested positive over the prior 3 weeks, and during this admission, a nasopharyngeal swab was also positive for SARS CoV-2 as determined using a rapid molecular diagnostics system. By this point in time, the patient had endured symptomatic manifestations of COVID-19 for 29 days.
It was reasoned that, given his prior lymphoma treatment with prolonged lymphocyte-depleting systemic therapy, hypogammaglobulinemia, persistent COVID-19 infection, and deteriorating respiratory status, emergency treatment with CCP was warranted. Single-patient emergency Investigational New Drug approval was obtained from the United States Food and Drug Administration on April 13, 2020. Working in conjunction with the Keesler Medical Center Transfusion Services and the Armed Services Blood Program, 1 unit of matched CCP was obtained from CareBlood through the South Texas Blood and Tissue Center; it arrived frozen on April 14, 2020. Informed consent was appropriately obtained, and the patient agreed to treatment.
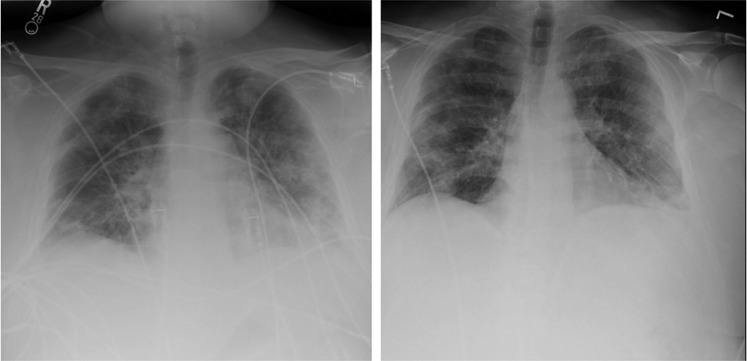
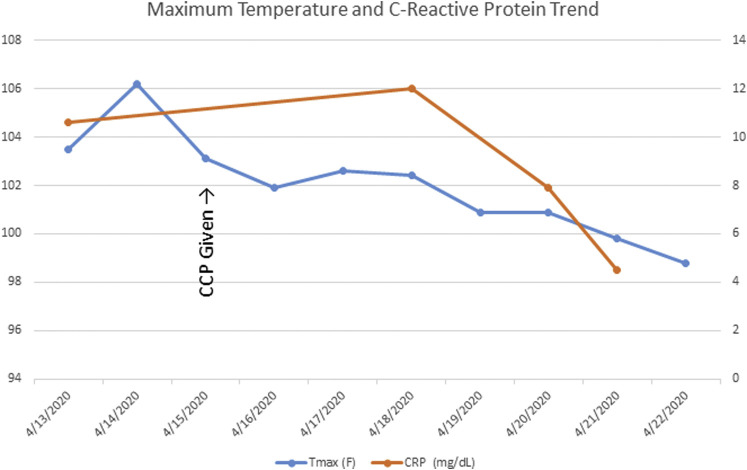
The patient was transfused the single CCP unit of 200 mLs the following morning. His fever trended downward over the next 3 days, and he became afebrile on April 19, 2020. He was completely weaned off of supplemental oxygen by his discharge on April, 22 2020, and an ambulatory challenge showed no evidence of hypoxia. Additionally, the patient’s chest x-rays showed improvement of the previously noted bilateral pulmonary infiltrates (Figure 1 ), and a downtrend of C-reactive protein was also noted (Figure 2 ). He was discharged home to remain in isolation until testing reveals a negative reverse transcriptase polymerase chain reaction for SARS-CoV-2. The patient had not been tested at the time of this manuscript submission.
Figure 1.
Portable Chest X-ray of Patient Taken 2 days Prior to CCP With Improvement Noted in Follow-up Chest X-ray 6 days after CCP Transfusion
Abbreviations: CCP = COVID-19 convalescent plasma (CCP); COVID-19 = coronavirus disease 2019.
Figure 2.
Fever and C-reactive Protein Trends Through Patient’s Hospitalization
Abbreviations: CCP = COVID-19 convalescent plasma (CCP); COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; Tmax = maximum temperature.
Conclusion
CCP is a plasma component of blood collected by apheresis. The plasma theoretically contains antibodies from the recovered donor that may aid in recovery of the patient by passive transfer of immunity. A laboratory-confirmed COVID-19 patient that is considered recovered and meets standard plasma donation requirements may become a donor 14 days after resolution of symptoms.4 In this case, CCP was procured from an outside source within 24 hours. Future cases at our institution and within the DoD network will benefit from on-site collections of CCP by the Keesler Blood Donor Center.
Although no controlled evidence for use of CCP currently exists for COVID-19 treatment, anecdotal improvement has been reported.5 In the case series from Wuhan, clinical improvement was reported in all of the patients, and radiographic improvement was noted in 5 of 6 patients. There are ongoing clinical trials investigating the efficacy and safety of CCP in patients afflicted with the infection,6 and the DoD is also establishing an expanded access program for CCP. Understandably, our experience should not be applied to all patients. However, until further evidence guides our understanding of COVID-19 treatment, it is reasonable to consider CCP in patients with severe COVID-19 disease and known B-cell deficiency with hypogammaglobulinemia.
Disclosure
Zachary Wright discloses honoraria from Merck & Co. The remaining authors have stated that they have no conflicts of interest.
References
- 1.Martino R., Ramila E., Rabella N. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003;36:1–8. doi: 10.1086/344899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakki M., Rattray R.M., Press R.D. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol. 2015;68:1–5. doi: 10.1016/j.jcv.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M., Lui D., Liu M. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration COVID-19-related guidance documents for industry, FDA staff, and other stakeholders. https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/covid-19-related-guidance-documents-industry-fda-staff-and-other-stakeholders Available at: Accessed: July 10, 2020.
- 5.Ye M., Fu D., Ren Y. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Vir 2020 Apr 15. https://doi.org/10.1002/jmv.25882 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 6.Joyner M. Expanded access to convalescent plasma for the treatment of patients with COVID-19. https://clinicaltrials.gov/ct2/show/NCT04338360 Available at: Accessed: July 10, 2020.