Abstract
Background
In February and March 2020, healthcare providers and citizens in Daegu, South Korea, experienced the onslaught of a large-scale community epidemic of COVID-19. This had a profound impact on patients who experienced out-of-hospital cardiac arrest (OHCA).
Methods
We conducted a retrospective observational study of 171 OHCA patients based on the multicenter WinCOVID registry. Demographic and clinical characteristics, overall survival, COVID-19 related data, as well as personal protective equipment (PPE) and resuscitation techniques used during the COVID-19 outbreak were evaluated and compared with outcomes from a 2018 historical cohort (n = 158).
Results
Among the interventions, high-level PPE was introduced and standard cardiopulmonary resuscitation was changed to chest compressions using mechanical devices. All OHCA patients were treated as confirmed or suspicious for COVID-19 regardless of symptoms. Furthermore, complete or partial closures of emergency centers and the number of medical personnel requiring self-isolation decreased in response to the introduction of isolated resuscitation units. However, the adjusted odds ratio and 95% confidence intervals for survival discharge and favorable neurologic outcome were 0.51 (0.25–0.97) and 0.45 (0.21–1.07) compared with those in the 2018 historical cohort.
Conclusions
Responses to the COVID-19 pandemic included changes to current PPE strategies and introduction of isolated resuscitation units; the latter intervention reduced the number of unexpected closures and quarantines of emergency resources early on during the COVID-19 outbreak. Given the possibility of future outbreaks, we need to have revised resuscitation strategies and the capacity to commandeer emergency resources for OHCA patients.
Keywords: Heart arrest, Prognosis, Coronavirus disease, Standard precautions, Cardiopulmonary resuscitation, Emergency department
Introduction
Healthcare workers who perform cardiopulmonary resuscitation (CPR) are vulnerable to dangerous infectious diseases spread by aerosols or respiratory droplets.1, 2, 3, 4 The current COVID-19 pandemic has highlighted issues associated with safety of team members during CPR.5 Guidance from the Centers for Disease Control and Prevention (CDC),6,7 the American Heart Association (AHA),8 the European Resuscitation Council (ERC)9 and several national organizations focused on CPR and emergency cardiac care (ECC) of both in-hospital or out-of-hospital cardiac arrests (OHCA) have been updated continuously in response to the current pandemic situation.5,10 However, at the early stages of the COVID-19 outbreak, there were considerable gaps in our knowledge as reflected in the recommendations and the state of preparedness at the national, regional and individual hospital levels.2,11
Healthcare providers may be unable to distinguish between confirmed/suspected patients, asymptomatic carriers of COVID-19 and those who remain uninfected in an acute setting. For OHCA events, it is clinically impossible to determine the risk of infection before performing immediate CPR. Moreover, given that infection with SARS-CoV-2 is associated with few pathognomonic characteristics and has many asymptomatic carriers, it will be difficult for emergency medical personnel to perform any sort of meaningful screening before arriving at the hospital. As a result, emergency physicians were forced to work on the assumption that all patients with OHCA were at high risk for COVID-19.
On February 18, 2020, with the increased number of confirmed cases in Daegu, we performed CPR with insufficient preparation and clinical information. As such, we were required to close several regional emergency departments (EDs) after unexpected confirmation of COVID-19 in patients after resuscitation. Many EDs were closed both consecutively and repeatedly; medical staff on duty at the time as well as inpatients were quarantined in the ED. ED closures can have a serious impact on the treatment of severely ill, emergency patients; among these are patients with myocardial infarction, acute stroke and severe trauma.
This is the first study to evaluate the impact of multiple, consecutive changes in resources and procedures used to treat OHCA patients in the setting of an emerging infectious disease. We investigated the current status of gradually changing levels of preparedness and resuscitation strategies. Furthermore, we aimed to propose new regional resuscitation strategies to be used in the COVID-19 era. In addition, we analyzed the citywide patterns of OHCA that took place during the peak of the COVID-19 outbreak and compared the results with a historical cohort.
Methods
Study setting and timeline
Daegu, South Korea, was one of the regions that were most heavily affected by COVID-19. The first patient diagnosed with this pandemic disease was confirmed on February 18, 2020; infection rapidly spread through the entire city. The number of confirmed patients increased rapidly, reaching 6000 people within a period of 1 month (240 cases per 100,000 population). One hundred and seventeen people died in Daegu city alone, accounting for 70% of all deaths in South Korea.12,13
On February 22, 2020, the first postmortem confirmation of COVID-19 in a patient who had experienced OHCA was performed in South Korea; emergency medical services (EMS) and the EMCs were cohort-isolated for >48 h. Soon after, the second and third COVID-19 confirmations after OHCA resuscitation occurred on February 26 and 27, 2020 and two more emergency centers were closed in Daegu. As OHCA is by its nature an unexpected emergency situation, ED shutdowns and temporary closures occurred repeatedly.
As such, resuscitation procedures were then carried together with infectivity screening of patients with OHCA. Similarly, given the prevalence of asymptomatic COVID-19 carriers, almost all unexpected sudden cardiac deaths were classified as suspected COVID-19 patients.14,15 In an unprecedented epidemic situation, our researchers needed to implement new strategies for resuscitation of patients who experienced OHCA that would likewise confer protection for clinicians.
Enrolled OHCA patients
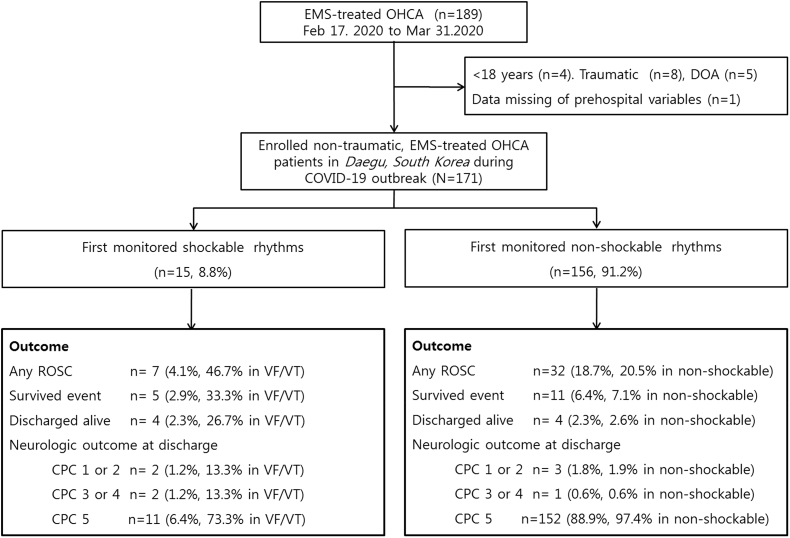
Daegu is a metropolitan city with a population of 2.44 million, and there were 1897 adult OHCA cases in 2018. Daegu includes two regional EMCs, four local EMCs and 19 emergency facilities or clinics. Of note, there are six EMCs where advanced life support, post-cardiac arrest care and cardiovascular intervention are available. Patients who were aged 18 years or older with OHCA of presumed medical aetiology and who used the EMS system in Daegu were included in our study. Patients who did not undergo resuscitative attempts and cases in which cardiac arrest occurred in a primary care clinic or long-term care hospital were excluded from the analysis. From the observation period of February 17, 2020 to March 31, 2020, a total of 189 adult OHCAs occurred in the community; five were untreated because they were dead on arrival and 13 cases were excluded (Fig. 1), and thereby 171 cases were included in the final analysis dataset of this study.
Fig. 1.
Study flow diagram. The number of out-of-hospital cardiac arrest patients in the community during COVID-19 peak outbreaks and the characteristics of enrolled patients by monitored electrocardiograph rhythms were described. EMS, emergency medical service; OHCA, out-of-hospital cardiac arrest; DOA, death on arrival; ROSC, return of spontaneous circulation; VF/VT, ventricular fibrillation/ventricular tachycardia; CPC, Cerebral Performance Category.
Study design and variables
This was a before-and-after observational cohort study to examine the changes in the characteristics and survival outcomes of adult OHCA following the COVID-19 outbreak in Daegu Metropolitan city. For comparison with previous citywide OHCAs, we analyzed the Korean OHCA Registry of the Korea Centers for Disease Control and Prevention (KCDC), which captured 158 cases of OHCA in the metropolitan city during the same period of February 17 to March 31, 2018.16
We investigated the demographics, including sex, age, medical history; CPR-related prehospital factors such as the presence of a witness, CPR by a bystander, location of the arrest, and initial electrocardiographic rhythms that were obtained before hospital arrival; EMS resuscitation care, including prehospital AED or defibrillation, intravenous adrenaline administration and invasive airway interventions; and CPR-related time variables, such as the response time interval (time from call to arrival of the ambulance at the scene) and scene time interval (time from arrival of the ambulance at the scene to departure from the scene), which were obtained as basic epidemiologic and EMS-related variables. The treatment and survival outcomes (including prehospital return of spontaneous circulation [ROSC] and neurologic outcome at discharge) after cardiac arrest were recorded as hospital-level variables.17
At the hospital level, the type of personal protective equipment (PPE) used for each CPR technique, the location of the resuscitation room, the length of stay in the ED after CPR, post-resuscitation or postmortem chest radiography, and the results of the COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test were collated. To evaluate COVID-related variables, we obtained data from the electronic medical records on admission with regard to the clinical symptoms or signs, vital signs (fever [defined as a temperature of 37.5 °C or higher], sore throat, and cough), recent exposure history and chest radiographic findings of patients who were confirmed to have COVID-19 Moreover, to compensate for the weaknesses of the cost-analysis, we investigated the ED shutdown and closures during the days of the study period. Complete shutdown was defined as a period in which the entire area of the ED was blocked off, thus prohibiting the influx of new patients (typically >48 h). Temporary ED closure was defined as the closure of areas used for OHCA patient resuscitation for 6–12 h.18
Outcome measures
The primary outcomes were descriptive analyses of the clinical outcomes as well as the changes in CPR strategies used to treat cardiac arrest patients during the outbreak period. Secondary outcomes included before-and-after comparative analyses focusing on the outbreak period and the analogous pre-epidemic time period in 2018.
Statistical analysis
Data were analyzed by using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA) and MedCalc version 17 (MedCalc Software, Mariakerke, Belgium). The chi-square or Fisher’s exact tests were used to compare categorical variables. The normality of the variables was determined by using the Shapiro–Wilk test. A t-test or one-way analysis of variance and the Mann–Whitney U test were used to compare continuous variables. The associations between the study phase and outcomes were assessed using multivariable logistic regression analysis. We analyzed the data to identify the changes in the CPR characteristics and survival outcomes of adult OHCA patients following the COVID-19 outbreak. The adjusted odds ratios (ORs) were calculated after adjustment for sex, age, comorbidities, location of event, witness status, bystander CPR, and shockable rhythm. The characteristics of the adjusted ORs were described by using forest plots, and the OR with 95% confidence intervals (CI) were estimated. All tests were two-tailed, and a p-value of <0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Kyungpook National University Hospital (no. 2020–04032), which waived the requirement for informed consent.
Results
Epidemic characteristics of the COVID-19 outbreak in Daegu
The trend of the outbreak of confirmed COVID-19 cases in Daegu, South Korea, and the number of deaths in the community are shown in Supplement 1. Moreover, an ED shutdown was seen in the early stages of the explosive peak that was caused by CPR at 10 days immediately after the first community-confirmed patient. During the period from February 21 to February 28, the number of confirmed cases increased sharply; findings associated with OHCA patients with post-resuscitation or postmortem confirmed COVID-19 initiated a series of complete ED shutdowns throughout the city.
In total, ten of the 171 cases of CPR performed during the study period were confirmed as COVID-19-positive (5.9%). However, only two of these individuals were aware of this status prior to the need for CPR; the others were confirmed by examination after the event. The differences between the two groups based on whether or not they are confirmed COVID-19 cases, including differences in relative symptoms and infection risks are as described in Table 1. There were 100 cases (58.8%) of suspected pneumonic consolidation on post-resuscitation chest radiography. Among those who tested positive, 20–40% had no fever or respiratory symptoms prior to cardiac arrest.
Table 1.
General characteristics of the COVID-19 OHCA study population.
| Overall (n = 171) | COVID-19(+) (n = 10) | COVID-19(−) (n = 161) | p-value | |
|---|---|---|---|---|
| Age (years), median [IQR] | 74 [62–80] | 75 [64–79] | 74 [61–80] | 0.707 |
| Male sex | 108 (63.2) | 4 (40.0) | 104 (64.6) | 0.175 |
| Comorbidities | ||||
| Hypertension | 59 (34.5) | 5 (50.0) | 54 (33.5) | 0.316 |
| Diabetes mellitus | 52 (30.4) | 4 (40.0) | 48 (29.8) | 0.494 |
| Heart failure, ischemic heart disease | 26 (15.2) | 21 (10.0) | 25 (15.5) | 0.998 |
| Chronic renal disease | 8 (4.7) | 2 (20.0) | 6 (3.7) | 0.072 |
| Malignancy, cancer | 28 (16.4) | 3 (30.0) | 25 (15.5) | 0.212 |
| Ischemic or hemorrhage stroke | 16 (9.4) | 1 (10.0) | 15 (9.3) | 0.999 |
| Location of OHCA, public place | 50 (29.2) | 3 (30.0) | 47 (29.2) | 0.998 |
| Prehospital parameters | ||||
| Witnessed event, anyone | 130 (76.0) | 10 (100) | 120 (74.5) | 0.120 |
| Bystander CPR | 58 (33.9) | 1 (10.0) | 57 (35.4) | 0.167 |
| Initial shockable rhythm | 15 (8.8) | 0 (0) | 15 (9.3) | 0.603 |
| Prehospital mechanical CPR | 134 (78.4) | 7 (70.0) | 127 (79.9) | 0.738 |
| Time variables, median [IQR] | ||||
| Response time interval (min) | 8 [6–10] | 8 [5–10] | 8 [6–10] | 0.758 |
| Scene time interval (min) | 19 [15–25] | 24 [17–33] | 19 [15–25] | 0.114 |
| COVID-19 related | ||||
| Previous COVID-19 dx before OHCA | 2 (1.2) | 2 (20.0) | 0 (0) | 0.003 |
| Presumed symptoms before OHCA | 20 (11.7) | 6 (60.0) | 14 (8.7) | <0.001 |
| High risk of exposure or contacta | 7 (4.3) | 2 (20.0) | 5 (3.1) | 0.024 |
| Abnormal chest x-ray findings | 100 (58.5) | 8 (80.0) | 92 (57.1) | 0.198 |
| PPE during CPR | ||||
| Level D or higher level protection | 121 (70.8) | 8 (80.0) | 113 (70.2) | 0.725 |
| Mechanical CPR in ED | 99 (59.6) | 4 (40.0) | 95/156 (60.9) | 0.205 |
| Isolated unit or NPIR | 102 (59.6) | 4 (40.0) | 98 (60.9) | 0.138 |
| Survival Outcomes | ||||
| Prehospital ROSC | 8 (4.7) | 0 (0) | 8 (5.0) | 0.996 |
| ROSC | 39 (22.8) | 3 (30.0) | 36 (22.4) | 0.697 |
| Survival admission | 16 (9.4) | 1 (10.0) | 15 (9.3) | 0.990 |
| Survival discharge | 8 (4.7) | 0 (0) | 8 (5.0) | 0.995 |
| Favorable neurologic | 5 (2.9) | 0 (0) | 5 (3.1) | 0.997 |
CPR, cardiopulmonary resuscitation; ED, emergency department; OHCA, out-of-hospital cardiac arrest; ROSC, return of spontaneous circulation; PPE, personal protective equipment; NPIR, negative pressure isolated room; IQR, interquartile ranges; dx, diagnosis.
High risk: High-risk groups (Sincheonji church members, hospital staff, or patients from community-infection areas like Cheongdodaenam Hospital), recent visit to a risk country and contact with COVID-19 patient or COVID-19 suspected person.
Changing CPR strategies and PPE levels
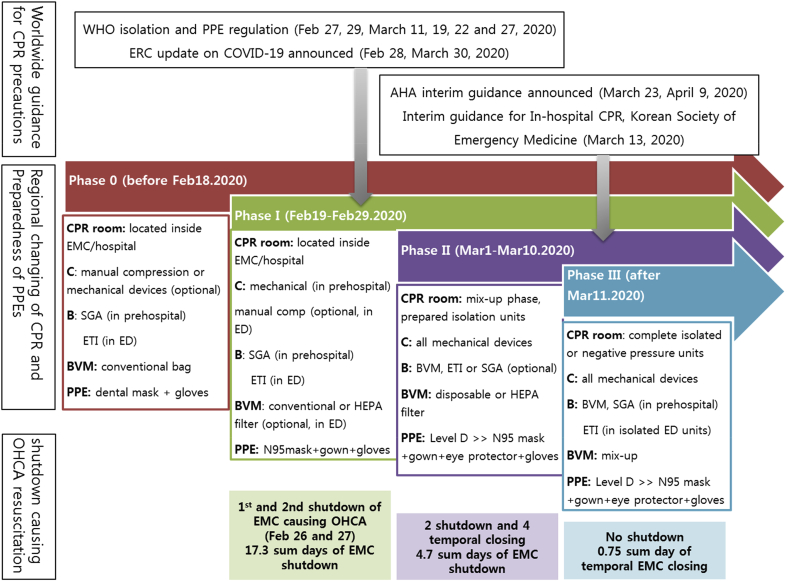
The six EMCs in Daegu have different resources, including levels of staffing and medical equipment; however, we applied various strategies that were tailored for each hospital. Fig. 2 reveals an overview of the changes in the resuscitation techniques that were introduced in stages. In Phase I, there was no provision for back-up equipment that would prevent the rapid increase of confirmed or suspected COVID-19 patients, and no provisions had been made for vacancies due to the need for self-isolation by medical staff in order to prevent the spread of infectious diseases in hospitals. As such, we experienced closing of EDs for 48 h or more. In Phase II, the PPE levels increased as adequate levels of protective equipment were supplied (Table 2), although 26% of EDs were still performing CPR in the resuscitation room inside the hospital area. Temporary ED shutdowns continued during this period. In Phase III, all emergency centers (except one introduced independent sector in ED) performed resuscitation outside the main hospital area in order to avoid the need for future shutdowns. During this period, there were no complete shutdowns of any of the EDs, and only temporary resuscitation unit closures occurred for periods of 18 h only. Fortunately, we experienced no direct infection of healthcare personnel during any of the resuscitation events that occurred during the study period.
Fig. 2.
Changes in the preparedness of community emergency centers for cardiopulmonary resuscitation (CPR) procedures, personal protective equipment and resuscitation before and after COVID-19 outbreak, and their effects on emergency room shutdown. We divided this period into four phases; phase 0 is the pre-epidemic state, phase I is the chaotic period in the unprepared situation because of the rapid increase in COVID-19 patients and unexpected emergency department (ED) shutdown, phase II is the time to secure and apply the resuscitation strategy suitable for the hospital situation, and finally, phase III is the complete implementation of isolated resuscitation units and new CPR strategies.
Table 2.
Changes in CPR and PPE strategies and unexpected ED shutdown event causing resuscitation for OHCA patients in the emergency department during the COVID-19 outbreak.
| 2020 COVID-19 outbreak |
||||||
|---|---|---|---|---|---|---|
| Before |
Pre-prepared |
Mixed |
Implemented |
p-value | ||
| (Phase 0) |
(Phase I) |
(Phase II) |
(Phase III) |
|||
| Before Feb 19 |
Feb 19–29 |
Mar 1–10 |
Mar 11–31 |
|||
| n = 5 | n = 41 | n = 41 | n = 84 | |||
| Postmortem confirmation | RT-PCR COVID-19 (+) | 0 | 4 (9.8) | 5 (12.2) | 1 (1.2) | 0.054 |
| Resuscitation strategies | ||||||
| P: PPE for CPR team | N-95 or dental mask + gloves | 5 (100) | 16 (39.0) | 0 | 2 (2.4) | <0.001 |
| (multiple response) | N-95 + gloves + goggles + gown + etc. | 0 | 17 (41.5) | 10 (24.4) | 25 (29.8) | 0.153 |
| Level D | 0 | 14 (34.1) | 32 (78.0) | 75 (89.3) | <0.001 | |
| Level C with PAPR | 0 | 0 | 4 (9.8) | 2 (2.4) | 0.400 | |
| C: compressiona | Mechanical CPR | 3 (60.0) | 9 (22.5) | 21 (53.8) | 66 (80.5) | <0.001 |
| B: breathing and ventilation | BVM, conventional or disposable | 5 (100) | 37 (90.2) | 30 (73.2) | 60 (71.4) | 0.082 |
| BVM with HEPA filter | 0 | 4 (9.8) | 11 (26.8) | 24 (28.6) | ||
| D: defibrillation | Manual, paddle | 4 (80.0) | 39 (95.1) | 37 (90.2) | 78 (92.9) | 0.610 |
| Defibrillator patches/Pads | 1 (20.0) | 2 (4.9) | 4 (9.8) | 6 (7.1) | ||
| I: isolated CPR room | Conventional CPR room inside ED | 5 (100) | 34 (82.9) | 7 (17.1) | 12 (14.3) | <0.001 |
| Outside or isolation units | 0 | 0 | 7 (17.1) | 19 (22.6) | ||
| Negative-pressure isolated unit | 0 | 7 (17.1) | 27 (65.9) | 53 (63.1) | ||
| ED stay, median time (min) | ED length of stay (median, [IQR]) | 29 [23–60] | 68 [48–201] | 110 [67–206] | 81 [59–201] | 0.020 |
| ED shutdown event | Complete shutdown | 0 | 7 (17.1) | 2 (4.9) | 0 | 0.001 |
| Temporary closure | 0 | 1 (2.4) | 4 (6.1) | 1 (1.7) | ||
| Subtotal duration of shutdown (hour) | 0 | 415 | 142.5 | 18 | – | |
| OHCA survival outcomes | Prehospital ROSC | 0 | 3 (7.3) | 2 (4.9) | 3 (3.6) | 0.772 |
| ROSC | 1 (20.0) | 11 (26.8) | 7 (17.1) | 20 (23.8) | 0.750 | |
| Survival admission | 1 (20.0) | 6 (14.6) | 2 (4.9) | 7 (8.3) | 0.378 | |
| Survival discharge | 0 | 4 (9.8) | 1 (2.4) | 3 (3.6) | 0.347 | |
| Favorable neurologic | 0 | 2 (4.9) | 1 (2.4) | 2 (2.4) | 0.844 | |
CPR, cardiopulmonary resuscitation; ED, emergency department; OHCA, out-of-hospital cardiac arrest; PPE, personal protective equipment; PAPR, powered air-purifying respirator; BVM, bag–valve mask; HEPA, high-efficiency particulate air; IQR, interquartile range, 25th to 75th percentile; ROSC, return of spontaneous circulation.
Unknown or undetermined data: not performed chest compression because of prehospital ROSC [Phase I (n = 1), Phase II (n = 2), and Phase III (n = 2)].
OHCA during the COVID-19 epidemic outbreak in Daegu, South Korea
During the observation period, the data from a total of 189 patients with EMS-treated adult OHCA were analyzed, with 171 patients who were treated at the six EMCs. The short-term survival outcomes, including ROSC and survival to discharge rates were 22.8% and 4.7%, respectively (Table 3). The survival prognosis based on each initial ECG rhythm is shown in Fig. 1.
Table 3.
Comparison of survival outcomes and related factors of out-of-hospital cardiac arrest before and after the COVID-19 outbreak.
| Non-traumatic, EMS-treated OHCA |
p-value | |||
|---|---|---|---|---|
| 2018.02.17–3.31 Citywide, in Daegu |
2020.2.17–3.31 COVID-19 outbreak |
|||
| N = 158 | N = 171 | |||
| Basic epidemiology | male, sex | 103 (65.2) | 108 (63.2) | 0.608 |
| Age, median [IQR] | 74.3 [61.8–82.2] | 74.0 [62.0–80.0] | 0.559 | |
| private or nursing home | 112 (70.9) | 121 (70.8) | 0.907 | |
| Any witnessed event | 88 (55.7) | 130 (76.0) | <0.001 | |
| Bystander CPR | 50 (31.6) | 87(50.9) | <0.001 | |
| prehospital VF/VT | 19 (12.0) | 15 (8.8) | 0.218 | |
| Prehospital AED applied | 30 (19.0) | 22 (12.9) | 0.040 | |
| EMS-related time interval (min) | Response time interval | 6 [5–8] | 8 [6–10] | 0.009 |
| Scene time | 13 [10–17] | 19 [15–25] | <0.001 | |
| Prehospital CPR | Mechanical CPR | no data available | 134 (78.4) | |
| Epinephrine, intravenous | 6 (3.8) | 63 (36.8) | <0.001 | |
| Prehospital airwaya | BVM, only others | 45 (29.0) | 61 (36.7) | 0.116 |
| SGA | 87 (56.1) | 89 (53.6) | 0.426 | |
| Endotracheal intubation | 23 (14.8) | 16 (9.6) | 0.051 | |
| Prehospital ROSC | 15 (9.5) | 8 (4.7) | 0.036 | |
| Survival outcomes | Survival events | 49 (31.0) | 39 (22.8) | 0.023 |
| Survival discharge | 14 (8.9) | 8 (4.7) | 0.065 | |
| Favorable neurologic | 9 (5.7) | 5 (2.9) | 0.095 | |
VF, ventricular fibrillation; VT, ventricular tachycardia; AED, automated external defibrillator; EMS, emergency medical services; BVM, bag–valve mask; SGA, supraglottic airway; ROSC, return of spontaneous circulation.
3 missing values in 2018 citywide data.
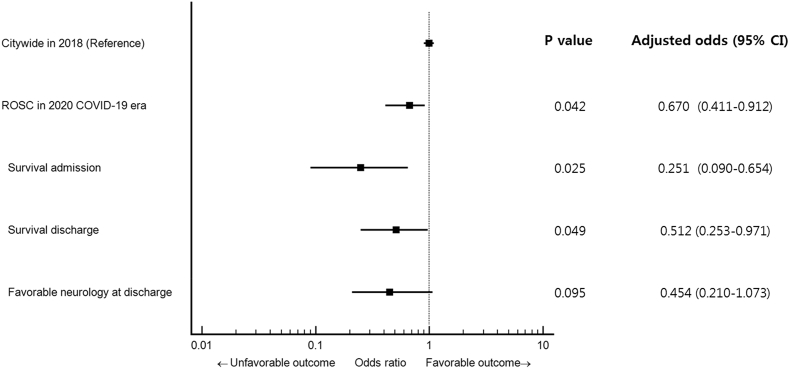
Survival outcomes during the COVID-19 outbreak were significantly decreased compared to the same period in 2018 (Fig. 3). After adjustment for patient- and community-associated variables, the adjusted OR and 95% CI for survival to discharge and favorable neurologic outcomes were 0.51 (0.25–0.97) and 0.45 (0.21–1.07), respectively, compared with the 2018 historical patient cohort.
Fig. 3.
Forest plot of survival outcomes for out-of-hospital cardiac arrest in COVID-19 era. Data are adjusted for sex, age, location of event, witness status, bystander CPR and any shockable rhythm.
Discussion
During the COVID-19 epidemic outbreak, we changed the procedure for standard CPR as performed on patients who experience OHCA. As we needed to maintain a high suspicion of infectivity, the level of PPE used was raised for those participating in CPR. The most important implementation strategy was the introduction of an isolated resuscitation unit within an area that was separated from the ED. As a result of this intervention, frequent ED closure as observed in the early stages of the epidemic was dramatically reduced. However, the survival outcomes deteriorated during the COVID-19 outbreak.
The interim CPR guidelines published by the AHA and ERC as well as the KCDC that were developed based on experience with MERS were distributed on site4,19, 20, 21; however, at the actual site of the primary emergency, appropriate equipment and PPE were not adequate. Of note, the previously published 2015 CPR Guidelines do not cover adjustments that might be made in response to an infectious epidemic.22,23 Revised CPR recommendations were issued on March 11, 2020; these included the use of an isolation room, additional precautionary equipment for bag-valve mask and ventilation, mechanical compression and PPE enhancement. Most of the previous studies on PPE during CPR were based on results obtained during the SARS and MERS epidemics.4,19,20 It is also critical to recognise that there is a very high rate of asymptomatic carriers of SARS-CoV-224,25; the rate of virus transmission has far exceeded that of previous virus pathogens and has already spread worldwide.26 Accordingly, we urgently needed to make changes to the level of protective equipment and standard CPR strategies used during resuscitation events.
As we progressed to Phase III, it was necessary to set up an isolated resuscitation unit that functioned independently of the existing space in the hospital in order to reduce ED closures. Currently, ED regulations in Korea are designated by the Emergency Medical Service Act.27 Almost resuscitation areas are located inside the ED, at a site near to the entrance for easy ambulance access. We were quite dismayed to find that, until the isolated areas were available, some OHCA patients were unable to enter the resuscitation room inside the ED and inside the hospital to protect the ED. Indeed, five OHCA patients were resuscitated in the parking lot near the ED or in the EMS ambulance. We realised that there was a significant knowledge gap with respect to our desire to provide high-quality CPR and likewise our concerns regarding preventing of in-hospital contamination by COVID-19. Finally, five EMCs (all except one independent sector in ED), became capable of operating a resuscitation room with a mobile intensive care unit or a negative pressure isolation unit. Over the course of 2 weeks, independent isolated CPR areas specialised for individual hospitals were set up; this became a decisive factor in our efforts to reduce or eliminate ED shutdowns after treatment of patients who present as OHCA.
With respect to our additional findings, we determined whether the COVID-19 epidemic crisis had an impact on survival of OHCA patients using a before-and-after analysis. We note that survival after OHCA during the epidemic period was lower than in 2018. While our findings indicate that increasing the level of PPE and performing CPR in an independent unit ultimately prevented the loss of emergency medical resources during early phase of COVID-19 outbreak, we do note the significantly lower overall survival during the epidemic period. Additional research is needed in order to generate a better understanding of this outcome. Although this low survival rate may be a direct effect of COVID-19 infection itself, we also need to consider the possibilities of significant delays in EMS transfer time in the prehospital setting or at the scene of the primary event. We were unable to describe the reasons behind differential survival and the potential negative impact of our interventions with respect to the performance of high-quality CPR; the pandemic has most likely changed the risk-benefit balance for CPR.5,9,10
Our study has some limitations. First, it is difficult to generalize our findings because each country, community and hospital maintains different resources. Second, during the study period, only one medical physician was infected at an emergency centre during a physical examination; however, he was not providing CPR. As such, the new and enhanced PPE strategy can be viewed as excessive by some communities or by individual regions or countries. However, given the current global epidemic, we recognise that CPR involves emergency procedures associated with a high risk of infection and typically no information regarding a given patient’s COVID-19 status. Furthermore, upon identifying a patient as SARS-CoV-2-positive, EDs are often forced to close for extended periods of time. Third, the observation period of this study was 2 months; as such, the impact on long-term survival outcomes, which are among the most important for OHCA research, have not been fully considered. We will continue to conduct further studies on this subject, including those focused on long-term outcomes. As a final point, the lower survival rate may be due to a lower quality of resuscitation, prolonged EMS time at the scene, as well as transport time delays. Some researchers have suggested that policies on do-not-resuscitate or termination of resuscitation may have affected these results during the COVID-19 outbreak.5,28
In conclusion, when faced with an emerging infectious disease, it is important to revise CPR strategies and protective protocols so that conditions for all are optimised. As such, we recommend the use of isolated resuscitation units as new means to conserve emergency resources during this pandemic; this strategy will provide important benefits even in those areas that have seen reductions OHCA associated survival. For the community, efforts to prevent ongoing ED closures, acquisition of PPE with full level D protection and design and implementation of isolated resuscitation units were essential strategies in the fight against COVID-19.
Sources of funding
None.
Declaration of competing interestCOI
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2020.100015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zuo M., Huang Y., Ma W., Xue Z., Zhang J., Gong Y. Expert recommendations for tracheal intubation in critically ill patients with novel Coronavirus Disease 2019. Chin Med Sci J. 2020 doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giwa A.L., Desai A. Novel coronavirus COVID-19: an overview for emergency clinicians. Emerg Med Pract. 2020;22(2 Suppl 2):1–21. [PubMed] [Google Scholar]
- 3.Wong J., Goh Q.Y., Tan Z. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anesth. 2020;67(6):732–746. doi: 10.1007/s12630-020-01620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam H.S., Yeon M.Y., Park J.W., Hong J.Y., Son J.W. Healthcare worker infected with Middle East Respiratory Syndrome during cardiopulmonary resuscitation in Korea, 2015. Epidemiol Health. 2017;39 doi: 10.4178/epih.e2017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFilippis E.M., Ranard L.S., Berg D.D. Cardiopulmonary resuscitation during the COVID-19 pandemic. Circulation. 2020;141:1833–1835. doi: 10.1161/circulationaha.120.047260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance. 2020. https://apps.who.int/iris/handle/10665/331498 19 March.
- 7.World Health Organization Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance. 2020. https://apps.who.int/iris/handle/10665/331215 27 February.
- 8.Edelson Dana P., Sasson Comilla, Chan Paul S. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or Confirmed COVID-19. Circulation. 2020 doi: 10.1161/circulationaha.120.047463. [DOI] [PubMed] [Google Scholar]
- 9.Savary D., Morin F., Fadel M., Metton P., Richard J., Descatha A. Considering the challenge of the Covid-19 pandemic, is there a need to adapt the guidelines for basic life support resuscitation? Resuscitation. 2020;152:50–51. doi: 10.1016/j.resuscitation.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz Z., Perkins G.D. Cardiopulmonary resuscitation after hospital admission with covid-19. BMJ. 2020;369:m1387. doi: 10.1136/bmj.m1387. [DOI] [PubMed] [Google Scholar]
- 11.Spina S., Marrazzo F., Migliari M., Stucchi R., Sforza A., Fumagalli R. The response of Milan’s emergency medical system to the COVID-19 outbreak in Italy. Lancet. 2020;395:e49–e50. doi: 10.1016/s0140-6736(20)30493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Kor Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.-W., Lee K.S., Kim K., Lee J.J., Kim J-y. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z., Liu S., Xiang M. Protecting healthcare personnel from 2019-nCoV infection risks: lessons and suggestions. Front Med. 2020;14:229–231. doi: 10.1007/s11684-020-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y.T., Do Shin S., Hong S.O. Effect of national implementation of Utstein recommendation from the global resuscitation alliance on ten steps to improve outcomes from Out-of-Hospital cardiac arrest: a ten-year observational study in Korea. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D.E., Lee M.J., Ahn J.Y. New termination-of-resuscitation models and prognostication in out-of-hospital cardiac arrest using electrocardiogram rhythms documented in the field and the emergency department. J Kor Med Sci. 2019;34 doi: 10.3346/jkms.2019.34.e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung H.S., Lee D.E., Kim J.K. Revised triage and surveillance protocols for temporary emergency department closures in tertiary hospitals as a response to COVID-19 crisis in Daegu Metropolitan City. J Kor Med Sci. 2020;35:e189. doi: 10.3346/jkms.2020.35.e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mejicano G.C., Maki D.G. Infections acquired during cardiopulmonary resuscitation: estimating the risk and defining strategies for prevention. Ann Intern Med. 1998;129:813–828. doi: 10.7326/0003-4819-129-10-199811150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Chiang W.-C., Wang H.-C., Chen S.-Y. Lack of compliance with basic infection control measures during cardiopulmonary resuscitation—are we ready for another epidemic? Resuscitation. 2008;77:356–362. doi: 10.1016/j.resuscitation.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vindigni S.M., Lessing J.N., Carlbom D.J. Hospital resuscitation teams: a review of the risks to the healthcare worker. J Intensive Care. 2017;5:59. doi: 10.1186/s40560-017-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truhlář Anatolij, Deakin Charles D., Soar Jasmeet. European resuscitation Council guidelines for resuscitation 2015: section 4. Cardiac arrest in special circumstances. Resuscitation. 2015;95:148–201. doi: 10.1016/j.resuscitation.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.J., Rho T.H., Kim H. Part 3. Advanced cardiac life support: 2015 Korean guidelines for cardiopulmonary resuscitation. Clin Exp Emerg Med. 2016;3:S17. doi: 10.15441/ceem.16.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parodi S.M., Liu V.X. From containment to mitigation of COVID-19 in the US. J Am Med Assoc. 2020 Mar 13 doi: 10.1001/jama.2020.3882. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y., Nie Y., Penny M. Transmission dynamics of the COVID-19 outbreak and effectiveness of government interventions: a data-driven analysis. J Med Virol. 2020 doi: 10.1002/jmv.25750. 10.1002/jmv.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M.J., Hwang S.O., Cha K.C., Cho G.C., Yang H.J., Rho T.H. Influence of nationwide policy on citizens’ awareness and willingness to perform bystander cardiopulmonary resuscitation. Resuscitation. 2013;84:889–894. doi: 10.1016/j.resuscitation.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Curtis J.R., Kross E.K., Stapleton R.D. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19) J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4894. Mar 27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.