Abstract
Background
The coronavirus disease 2019 (COVID-19) outbreak has unfavorably influenced solid organ donation activity.
Aim
The aim of this study is to investigate the effect of COVID-19 on transplantation in the North Italy Transplant program (NITp).
Material and Methods
This cross-sectional study included all consecutive potential deceased donors proposed in the NITp in 6 weeks after February 21, 2020 (period A) compared to all potential donors during the same time frame of the previous years (period B) and all potential donors 6 weeks before February 20, 2020 (period C).
Results
Fifty-eight deceased donors were proposed during period A, 95 were proposed during period B, and 128 were proposed during period C. After the evaluation process, 32 of 58 (55.2%), 60 of 95 (63.2%), and 79 of 128 (61.7%) donors were used for organ donation in periods A, B, and C, respectively (P value = .595). We observed a 47% donation reduction in period A compared to period B and a 60% reduction compared to period C. There was a reduction of 44% and 59% in transplantation comparing period A with period B and period C, respectively.
Conclusions
This study showed an important reduction of donations and transplants during the COVID-19 pandemic.
Highlights
-
•
Coronavirus disease 2019 (COVID-19) outbreak has unfavorably influenced the overall donation activity.
-
•
We observed a 47% to 60% donation reduction during the outbreak period.
-
•
Donors' decrease led to a 44% to 59% reduction in transplant activity.
Coronavirus disease 2019 (COVID-19) refers to severe respiratory infection caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The infection originated in Wuhan, China, in December 2019 and has been rapidly spreading all over the world. The World Health Organization (WHO) declared the COVID-19 outbreak a pandemic on March 11, 2020 [2]. The first case of COVID-19 in Italy was reported on February 20, 2020, with a rapid spread of the infection throughout the country. As of April 5, 2020, data from the Italian National Institute of Health showed that a total of 124,527 Italian people have tested positive for SARS-CoV-2, 3994 (3.2%) have been admitted to intensive care units (ICU), and 15,362 (12.3%) have died [3]. The Northern Italian regions were mostly affected, and this dramatic scenario changed the physiognomy of hospitals that became COVID-19 centers and created a burden on the health and economic system [4]. The initial data of the COVID-19 outbreak show that around 3% to 4% of infected patients need ICU management to overcome the acute respiratory distress syndrome. Transplantation is the treatment of choice for several end-stage diseases. Because of the high number of infected patients, the risk of ICU saturation with COVID-19 patients may unfavorably influence overall donation activity because ICU beds may be unavailable for both donors and organ recipients [5].
With this background, we investigated the effect of COVID-19 on the solid organ transplantation system from donors referred to the North Italy Transplant program (NITp).
Materials and Methods
Study design and population
This cross-sectional study included all consecutive potential deceased donors proposed by any NITp ICUs during the 6 weeks after February 21, 2020 (period A) compared to all potential donors during the same time frame of 2019 (6 weeks after February 21, 2019; period B) and during the 6 weeks before February 20, 2020 (period C). The 3 periods were compared to investigate any change in the total number of potential and eligible donations, donors’ procurement, and total transplants by solid organ and priority reason for assignment. Living donors and donors outside the NITp regions were excluded.
NITp is a transplantation program involving 6 Italian regions including Lombardy, Veneto, Friuli Venezia Giulia, Liguria, Marche, and Provincia Autonoma di Trento (PA Trento). The ICUs usually report the presence of any potential donor to the operative reference center that evaluates clinical data and establishes a risk profile as standard, nonstandard, or unacceptable, according to Centro Nazionale Trapianti guidelines [6].
General and clinical donors’ characteristics, organ allocation, and recovery were collected for each donor. Organ allocation was classified as urgent or not urgent according to recipients’ status at the moment of transplantation.
All consecutive deceased donors referred after February 20, 2020 were tested for SARS-CoV-2 real-time reverse transcription polymerase chain reaction. At the beginning of the COVID-19 outbreak, Centro Nazionale Trapianti recommended the nasopharyngeal swab and bronchoalveolar lavage (BAL) might be used as alternative in all potential donors. Subsequently, BAL became mandatory and was performed the day of procurement or within 24 hours before the recovery. In all cases, the test result had to be available before the recovery [7]. All these procedures were performed to ensure safety for all recipients to avoid donor-related infection transmission.
Statistical analysis
Descriptive analyses were performed. Continuous variables were expressed as mean values and standard deviation and compared using the two-tailed Student t test and Kruskal-Wallis test where appropriate. A P value < .05 was considered statistically significant. Categorical variables were expressed as frequencies and percentage values and compared by χ2 test. Statistical analyses were performed with SPSS version 23.0 (IBM Corp, Armonk, NY, United States).
Results
Potential donors
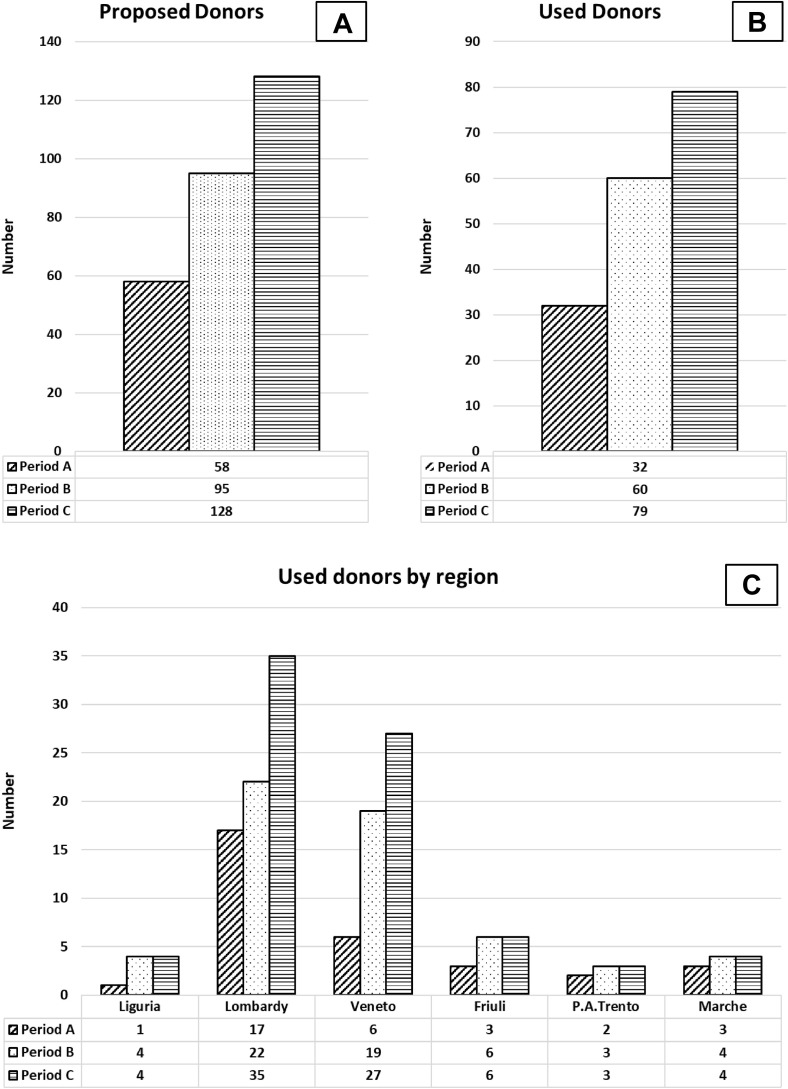
From February 21 to April 3, 2020, a total of 58 deceased donors were proposed, that is, 2 (3.4%) in Liguria, 25 (43.1%) in Lombardy, 20 (34.5%) in Veneto, 4 (6.9%) in Friuli, 2 (3.4%) in PA Trento, and 5 (8.6%) in Marche.
Ninety-five donors were evaluated during the same time frame during 2019 (period B) and 128 during the 6 weeks before February 20, 2020 (period C).
During period A, 26 donors (45%) were excluded, mainly because of objection for organ donation (17/26, 65.4%); of these, 6 were excluded for unacceptable risk (23%), of whom half (3/6) were excluded for the presence of an active cancer and half because of COVID-19 positive BAL (3/6). Organs from 3 (11.5%) potential donors were considered unsuitable for donation during clinical evaluation and/or surgical procedures. We did not observe any difference in terms of consent rate or any other reason for excluding donors across the 3 periods (P value = .956).
After the evaluation process, 32 of 58 (55.2%), 60 of 95 (63.2%), and 79 of 128 (61.7%) donors were used in period A, B, and C, respectively (P value = .595). No differences were found in used donors’ proportion across the regions by the considered time frames (P value = .902) (Table 1 ).
Table 1.
General Characteristics of Used Donors in the 3 Evaluated Periods
| Period A |
Period B |
Period C |
P Value | |
|---|---|---|---|---|
| (6 weeks after February 21, 2020) | (6 weeks after February 21, 2019) | (6 weeks before February 20, 2020) | ||
| Total | 32 | 60 | 79 | |
| Reason for hospitalization∗(%) | ||||
| Brain hemorrhage | 17 (53.1) | 25 (43.1) | 40 (50.6) | |
| Ischemic stroke | 3 (9.4) | 5 (8.6) | 5 (6.3) | |
| Postanoxic injury | 10 (31.3) | 16 (26.7) | 22 (27.8) | |
| Trauma | 2 (6.3) | 9 (15.5) | 11 (13.9) | |
| Meningitis | 0 (0) | 2 (3.4) | 0 (0) | |
| Suicide | 0 (0) | 1 (1.7) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 1 (1.3) | .708 |
| Sex (%) | ||||
| Female | 19 (59.4) | 24 (40.0) | 39 (49.4) | |
| Male | 13 (40.6) | 36 (60.0) | 40 (50.6) | .196 |
| Blood group (%) | ||||
| 0 | 14 (43.8) | 24 (40.0) | 29 (36.7) | |
| A | 14 (43.8) | 22 (36.7) | 35 (44.3) | |
| B | 2 (6.3) | 8 (13.39 | 10 (12.7) | |
| AB | 2 (6.3) | 6 (10.0) | 5 (6.3) | .864 |
| Risk profile (%) | ||||
| Standard | 20 (62.5) | 36 (60.0) | 56 (70.9) | |
| Nonstandard | 12 (37.5) | 24 (40.0) | 23 (29.1) | .316 |
| Region (%) | ||||
| Liguria | 1 (3.1) | 4 (6.89) | 4 (5.1) | |
| Lombardia | 17 (53.1) | 22 (37.9) | 35 (44.3) | |
| Veneto | 6 (18.8) | 19 (32.8) | 37 (34.2) | |
| Friuli | 3 (9.4) | 6 (10.3) | 6 (7.6) | |
| Provincia Autonoma di Trento | 2 (6.3) | 3 (5.2) | 3 (3.8) | |
| Marche | 3 (9.4) | 4 (6.9) | 4 (5.1) | .902 |
| Age (years) | ||||
| Mean (SD) | 60 (14.4) | 58 (19.2) | 60 (17.2) | |
| Median (min-max) | 62 (20-85) | 61 (1-85) | 62 (3-86) | .844 |
Abbreviation: SD, standard deviation.
Spontaneous cerebrovascular events were classified as brain hemorrhages. Traumatic events were classified as trauma.
During period A, the mean donors’ age was 60 years (standard deviation ± 14.4), and female sex was prevalent (19/32, 59.4%). Brain hemorrhage and postanoxic injuries were the main causes for hospitalization (17 [53.1%] and 10 [31.3%], respectively). More than half (20/32, 63%) had a standard risk profile, whereas no standard risk profile was attributed to the remaining 37% (12/32) of donors. No differences were found in age (P value = .844), sex (P value = .196), cause of hospitalization (P value = .682), and risk profile (P value = .316) distribution among the 3 periods. Furthermore, no difference was observed between ABO group distribution (P value = .864) (Table 1).
During period A, a 39% reduction of total deceased donation rate was observed as compared to period B and a 55% reduction as compared to period C. Considering used donors, the reduction was 47% and 60%, respectively. Compared to period B, the highest reductions were observed in Liguria and Veneto (75% and 68%, respectively), and the lowest were in Lombardy and Marche (23% and 25%, respectively). The reduction is higher when period C was considered, 75% in Liguria, 51% in Lombardy, 78% in Veneto, 50% in Friuli, 33% in PA Trento, and 25% in Marche (Fig 1 ).
Fig 1.
Trend of donors during the 3 evaluated periods. The figure plotted numbers of total potential donors (A), used donors (B), and used donors by regions (C) during the considered periods: period A (6 weeks after February 21, 2020, included); period B (6 weeks after February 21, 2019, included); and period C (6 weeks before February 20, 2020, included).
Transplantation rate
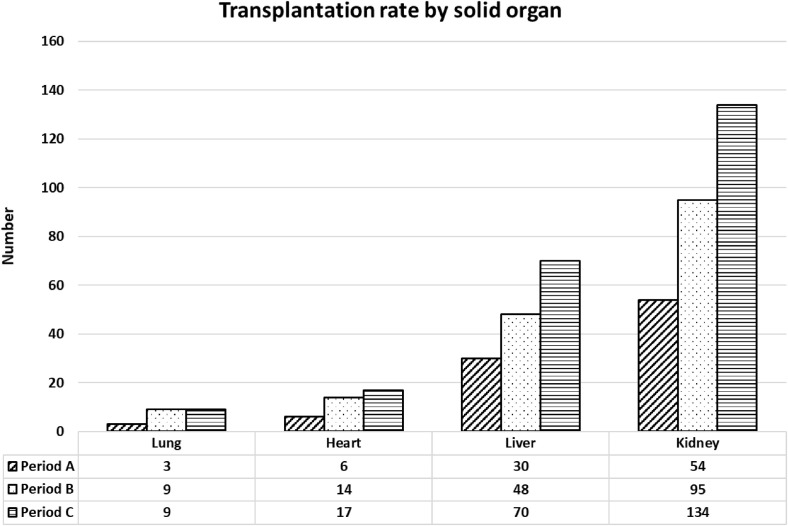
During period A, 95 solid organ transplants were performed (ie, 54 kidney, 32 liver [including 2 left split-livers], 3 lung, and 6 heart transplants). Urgent liver, heart, and lung transplants were 5/32 (16%), 0 (0), and 3/6 (50%), respectively. Globally, a 44% and 59% reduction of total solid organ transplantation rate was observed compared to period B and period C, respectively.
In heart transplantation, a reduction of 57% and 65% was observed compared to periods B and C, respectively; in lung transplantation the reduction was 67% compared to both periods; in liver transplantation, a reduction of 38% and 57%, respectively, was observed; and in kidney transplantation, a reduction of 43% and 60%, respectively, was observed (Fig 2 ).
Fig 2.
Trend of solid organ transplants during the 3 evaluated periods. The figure plotted numbers of total transplants by solid organs during the 3 considered periods: period A (6 weeks after February 21, 2020, included); period B (6 weeks after February 21, 2019, included); and period C (6 weeks before February 20, 2020, included).
No differences were observed in single organ discharge rate and reason for discharging across the 3 periods (P value > .05) with the exclusion of lung discharge reason (P value .01) (Table 2 ).
Table 2.
Transplantation Rate and Rule Out Reason by Solid Organs
| Period A |
Period B |
Period C |
P Value | |
|---|---|---|---|---|
| (6 weeks after February 21, 2020) | (6 weeks after February 21, 2019) | (6 weeks before February 20, 2020) | ||
| Used lung (%) | ||||
| Yes | 3 (9.4) | 9 (15.0) | 9 (11.4) | |
| No | 29 (90.6) | 51 (85.0) | 70 (88.6) | .697 |
| Rule out reason (%) | ||||
| Donor age | 7 (24.1) | 23 (45.1) | 26 (37.1) | |
| Lung disease | 16 (55.2) | 24 (47.1) | 36 (51.4) | |
| Ex vivo perfusion | 0 (0) | 1 (2.0) | 2 (2.9) | |
| Function | 6 (20.7) | 0 (0) | 1 (1.4) | |
| No recipient | 0 (0) | 3 (5.9) | 5 (7.1) | .01 |
| Used heart (%) | ||||
| Yes | 6 (18.8) | 14 (23.3) | 17 (21.5) | |
| No | 26 (81.3) | 46 (76.7) | 62 (78.5) | .878 |
| Rule out reason (%) | ||||
| Donor age | 7 (26.9) | 22 (47.8) | 26 (41.9) | |
| Hear disease | 17 (65.4) | 15 (32.6) | 24 (38.7) | |
| DCD | 2 (7.7) | 3 (6.5) | 3 (4.8) | |
| Function | 0 (0) | 5 (10.9) | 6 (9.7) | |
| No recipient | 0 (0) | 1 (2.2) | 3 (4.2) | .208 |
| Used liver (%) | ||||
| Yes | 32 (94.1) | 50 (80.6) | 73 (90.1) | .109 |
| No | 2 (5.9) | 12 (19.4) | 8 (9.9) | |
| Rule out reason (%) | ||||
| Biopsy | 0 (0) | 2 (16.7) | 4 (50.0) | |
| Hypoperfusion | 0 (0) | 4 (33.3) | 0 (0) | |
| Liver disease | 1 (50.0) | 5 (41.7) | 3 (37.5) | |
| DCD lung | 1 (50.0) | 1 (8.3) | 1 (12.5) | .469 |
| Used kidney (%) | ||||
| Yes | 54 (84.4) | 134 (84.8) | 95 (79.2) | |
| No | 10 (15.6) | 24 (15.2) | 25 (20.8) | .657 |
| Rule out reason (%) | ||||
| Kidney disease | 4 (40.0) | 4 (16.0) | 9 (37.5) | |
| Score | 4 (40.0) | 5 (20.0) | 1 (4.2) | |
| Vascular anomalies | 0 (0) | 7 (28.0) | 4 (16.7) | |
| Macroscopic aspect | 0 (0) | 4 (16.0) | 2 (8.3) | |
| DCD lung | 2 (20.0) | 2 (8.0) | 2 (8.3) | |
| No recipient | 0 (0) | 3 (12.0) | 6 (25.0) | .589 |
Abbreviations: DCD, donation after circulatory death; DCD lung, lung donation after circulatory death.
Discussion
Our study shows that during the first 6 weeks of the COVID-19 outbreak, there was an important reduction of potential donors and solid organ transplantation compared to the same period in 2019 in the regions referred to the NITp. This decrease is even higher when the 6 weeks before February 20, 2020 were considered. As expected, the trend of reduction was higher in the most highly affected regions.
Transplantation is the treatment of choice for several end-stage diseases, but during the COVID-19 spread, the lower number of potential donors and ICU bed availability limited the access to this treatment. Our study observed a more severe reduction of organ donations compared to a previous paper [5]. This difference reflects the dramatically higher spread in the NITp regions than in the rest of Italy.
Despite the Transplantation Society’s recommendations to consider the temporary suspension of both deceased and living related donors [8], transplant activities continued in the NITp area, satisfying not only the emergency list (8 transplants), but also the standard list. Our experience shows the utility and importance of continuing donation and transplant process if procurement and transplant procedures follow a COVID-19–free pathway for both donors and recipients.
Aside from the limited donor numbers, a potential donor-related COVID-19 transmission could have been a risk for transplanted patients; thus extensive COVID-19 testing of donors has been considered mandatory from the beginning [6]. We conducted a survey of patients transplanted after February 21 and found that 5 patients developed COVID-19 after more than 10 days from transplantation. All these patients were transplanted from different donors, and no other recipients transplanted with an organ from these 5 donors developed the infection. Furthermore, we found a significantly lower incidence of COVID-19–related diseases in transplanted recipients compared to recently published papers where previously transplanted patients and/or waiting list patients were included [[9], [10], [11]]. In line with a previous paper [12], those data may support the hypothesis that the COVID-19 transmission should not be considered donor related and the extensive procedure of testing guarantees a safe transplantation.
The main limitation of this study was the limited extension of transplanted follow-up to assess the actual incidence of post-transplant COVID-19 infection. This was not the aim of this paper, but the follow-up was long enough to be confident in excluding a donor-related transmission.
Conclusions
In conclusion, Italy has been one of the first countries dealing with the spread of COVID-19. This dramatic scenario changed the physiognomy of our hospitals and negatively impacted the management of the donation process. On the other hand, we did not find any absolute concern in the safety of solid organ transplantation. BAL monitoring seemed to be safe in preventing COVID-19 donor-related transmission. However, the need for shifting resources from transplantation to COVID-19 emergency and the risk of hospital-related transmission inevitably raise questions regarding the selection of recipients.
References
- 1.Zhang X. Epidemiology of Covid-19. N Engl J Med. 2020;382:1869. doi: 10.1056/NEJMc2005157. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Novel coronavirus (COVID-19) situation. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd [accessed 05.05.20]
- 3.Italian National Institute of Health Report of COVID-19 patients. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5351&area=nuovoCoronavirus&menu=vuoto [accessed 05.05.20]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelico R., Trapani S., Manzia T.M., Lombardini L., Tisone G., Cardillo M. The COVID-19 outbreak in Italy: initial implications for organ transplantation programs. Am J Transplant. 2020;20:1780–1784. doi: 10.1111/ajt.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CNT (2017) general criteria for evaluation of donor suitability adopted in Italy. Versione 1.0 23 febbraio 2017, http://www.trapianti.salute.gov.it/trapianti/archivioProtocolliCnt.jsp; [accessed 16.07.20].
- 7.Italian National Transplant Centre Information for transplant programs regarding novel coronavirus 2019. http://www.trapianti.salute.gov.it/trapianti/homeCnt.jsp [accessed 05.05.20]
- 8.The Transplantation Society An update and guidance on 2019 novel coronavirus (2019-nCov) pretransplant ID clinicians. https://tts.org/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians [accessed 05.05.20]
- 9.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A. Management of patients on dialysis and with kidney transplant during SARS-COV-2 (COVID-19) pandemic in Brescia. Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato M.F., Invernizzi F., Lampertico P., Rossi G. Health status of patients who underwent liver transplantation during the coronavirus outbreak at a large center in Milan, Italy. Clin Gastroenterol Hepatol. 2020;S1542-3565:30538-3. doi: 10.1016/j.cgh.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauterio A, De Carlis R, Belli L, De Carlis L. How to guarantee liver transplantation in the north of Italy during the COVID-19 pandemic. A sound transplant protection strategy [e-pub ahead of print]. Transpl Int https://doi.org/10.1111/tri.13633, accessed April 29, 2020. [DOI] [PubMed]