Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) has a high death rate in patients with comorbidities or in an immunocompromised state. We report a mild and attenuated SARS CoV-2 infection in a patient who is 17 months post stem cell transplantation and maintained on the JAK/STAT inhibitor ruxolitinib, a proposed novel therapy for SARS CoV-2 pneumonia.
Keywords: Allogeneic stem cell transplantation, COVID-19, Graft-versus-host disease, Immunocompromised, JAK inhibitor
Introduction
Severe acute respiratory syndrome (SARS) CoV-2 is an enveloped, positive-sense, single-stranded RNA β-coronavirus homologous to the sSARS and Middle East respiratory syndrome viruses.1, 2, 3 Upon infection, SARS CoV-2 enters respiratory epithelial cells and type-2 pneumocytes and induces a secondary immune response that may be associated with cytokine storm, leading to an acute respiratory distress syndrome-like picture.4, 5, 6, 7, 8 Strategies to attenuate the severity of the inflammatory response have included the use of the interleukin-6 receptor monoclonal antibody (ie, tocilizumab).6, 7, 8 Owing to the signal transduction role that the Janus-associated kinase (JAK)-signal transducer and activator of transcription (STAT) pathway plays in mediating immune-effector cell activation, there has been interest in pursuing inhibitors of this pathway as potential therapeutic agents in mitigating coronavirus 2019 (COVID-19)–associated lung inflammation.9 , 10 We recently diagnosed COVID-19 infection in a patient who was on oral ruxolitinib for management of graft-versus-host disease (GVHD) after allogeneic stem cell transplant and report on his presentation and the evolution of his clinical course.
Presentation
A 47-year-old male with a past medical history of allogeneic stem cell transplant was evaluated for fever and cough. His history is notable for angioimmunoblastic T cell lymphoma treated initially with EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) chemotherapy, resulting in a complete remission. He then underwent autologous stem cell transplant (October 2017). He relapsed in June 2018 and was treated with tipifarnib on a phase II clinical trial. He attained a second complete clinical remission and underwent an allogeneic stem cell transplant from a 10/10 matched unrelated donor in October 2018. Five months later (March 2019), he developed chronic GVHD with skin and myofascial involvement and was treated with prednisone and extracorporeal photopheresis. He continued to be symptomatic, and ruxolitinib was added in October 2019. Steroids were tapered after 2 months, and ruxolitinib 10 mg twice daily (BID) was continued in combination with photopheresis every other week (last session prior to admission). The most recent CD4+ cell count was 593/μL. He had recently been admitted (3 weeks prior to admission) for human metapneumovirus infection and had recovered. He denied exposure to anyone with COVID-19.
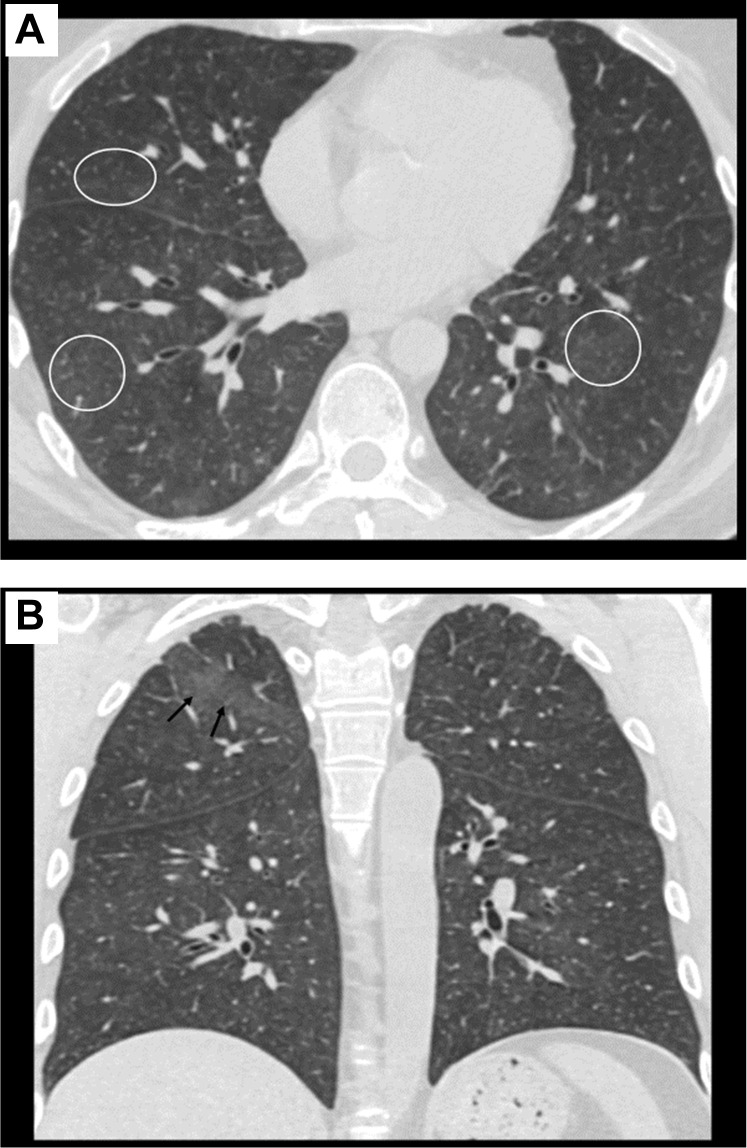
On the day of admission, he had a telehealth visit and reported 5 days of intermittent rigors with a temperature maximum of 102°F at home. He reported onset of dry cough, sweats, and myalgias without shortness of breath, and denied gastrointestinal symptoms. Owing to concern for COVID-19, he was brought to the hospital for evaluation. On arrival, his temperature was 99.6°F, his respiratory rate was 18, and his oxygen saturation was 96% on room air; he was speaking in full sentences and breathing comfortably. His lungs were clear to auscultation. A Centers for Disease Control-based reverse transcription polymerase chain reaction (RT-PCR) assay targeting N1 and N2 of SARS CoV-2 nucleocapsid gene was negative on a nasopharyngeal swab and he was admitted for fever workup. The nasopharyngeal respiratory virus swab was negative. His chest x-ray on admission demonstrated no opacities. The following day, the patient continued to have a persistent dry cough, and chest computed tomography was obtained to evaluate for possible interstitial disease or lung GVHD. Chest computed tomography (as shown in Figure 1 ) demonstrated new subtle patchy ground glass opacities and diffuse centrilobular ground glass nodules, which are a nonspecific finding, but the main differential diagnostic considerations in this clinical setting include infection (particularly viral or opportunistic) versus lung inflammation (drug toxicity or hypersensitivity pneumonitis). As per the newly described COVID-19 pneumonia imaging classification, this would be an indeterminate appearance.11
Figure 1.
Axial (A) and Coronal (B) Images From the Patient’s Chest Computed Tomography Demonstrate Diffuse Centrilobular Ground Glass Nodules (Circles) as Well as Subtle, Patchy Ground Glass Opacity in the Right Upper Lobe (Arrows). These Findings are Nonspecific but are Typically Seen in the Setting of Infectious (Such as Viral or Opportunistic Infections) or Inflammatory Disorders (Such as Drug Toxicity or Hypersensitivity Pneumonitis) and Have an Indeterminate Appearance for COVID-19 Pneumonia
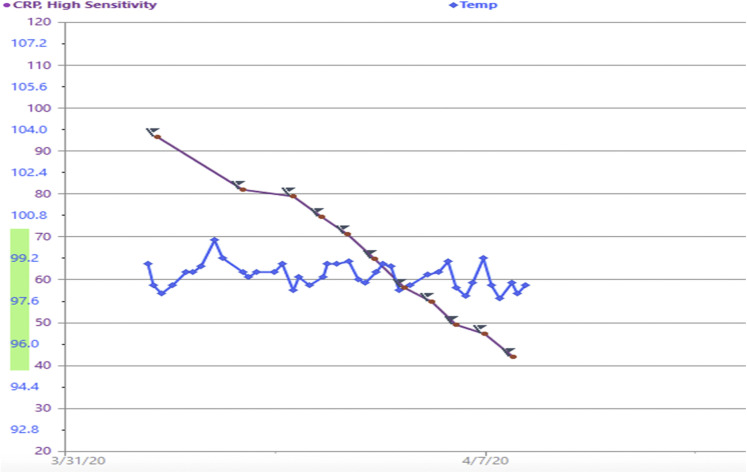
Repeat SARS CoV-2 RT-PCR using the same assay was then performed on a second nasopharyngeal swab and was positive, with a very low viral load (cycle threshold N1, 35.3/N2, 35.4). High-sensitivity C-reactive protein (CRP) was elevated at 93 mg/mL on admission, interleukin-6 was mildly elevated at 6 pg/mL (nl < 5), and interleukin-10 was in the normal range at 5 pg/mL (Figure 2 ). Fibrinogen was elevated at 655 mg/dL. His complete blood count showed white blood cells of 9.4, with absolute lymphocyte count of 1200/uL, hemoglobin 12 g/dL, and platelets 525,000/uL. Chemistries were all within normal range. The patient was treated as per our institutional COVID-19 protocol with hydroxychloroquine 400 mg BID × 1 day followed by 200 mg BID for 4 days, and he remained on ruxolitinib at a dose of 10 mg BID. His CRP declined as shown in Figure 2, and he remained afebrile without need for supplemental oxygen. He did not develop dyspnea and was discharged after 8 days of hospitalization. A week after hospital discharge, the patient remains afebrile with resolving mild cough and clinical improvement.
Figure 2.
Time Course of CRP and Temperature in Fahrenheit
Abbreviation: CRP = C-reactive protein.
Discussion
This case illustrates an attenuated COVID-19 infection in a patient who is otherwise significantly immunocompromised after allogeneic stem cell transplantation. Of interest is that the initial RT-PCR test for SARS CoV-2 RT-PCR was negative, but a repeat test returned positive 24 hours later with a low viral load. False-negative tests have been reported, and explanations include sampling error, poor test sensitivity, low viral load, or variable viral shedding in the upper airway, especially in patients who present with lower respiratory tract disease. Of note, sputum or bronchoalveolar lavage are more sensitive for diagnosing lower respiratory tract disease but are often not available for testing. This patient also demonstrated a moderate degree of diffuse lung involvement, despite being relatively asymptomatic and without hypoxia.
We speculate that the ruxolitinib or extracorporeal photopheresis may have played a role to reduce or attenuate inflammatory response, possibly in conjunction with the early administration of hydroxychloroquine after admission. In addition, potential low viral burden of infection may have impacted the severity of cytokine release syndrome and pneumonitis.
Ruxolitinib is an orally bioavailable JAK inhibitor that binds to and inhibits protein tyrosine kinases JAK 1 and 2, leading to reduction in inflammatory responses.12, 13, 14, 15 The JAK-STAT pathway is involved in cellular proliferation, hematopoiesis, and the immune responses in inflammatory diseases. Cellular targets of ruxolitinib are seen in both innate and adaptive immunity. Ruxolitinib is United States Food and Drug Administration-approved for the treatment of myelofibrosis and steroid refractory acute GVHD associated with allogeneic stem cell transplantation.16, 17, 18, 19, 20 Ruxolitinib is under study as therapy for chronic GVHD and is used commonly for this disorder owing to its tolerability and anti-inflammatory effects. In a study of hepatic inflammation, ruxolitinib was shown to repress CRP by targeting the IL6/JAK/STAT signaling pathway.21
Recent data has shown that ruxolitinib may also led to infectious complications and therefore may potentially be detrimental in the setting of COVID-19. Sylvine et al reported infectious complications with ruxolitinib from the French Pharmacovigilance Database.22 Of 30 infections reported in 26 patients, 20% were herpes zoster, 5 were mycobacterial, and 4 were fungal. Owing to its demonstrated anti-inflammatory and immunosuppressive activities, a clinical trial of ruxolitinib along with standard of care is planned to treat patients with inflammatory pneumonia owing to COVID-19 (NCT04338958). Results from the planned prospective trial should further enhance our understanding of the risks of secondary infections associated with JAK-STAT inhibitors based on their perceived myelosuppressive and immune-suppressive effects. Supplemental correlative studies will offer additional evidence on changes in host immune tolerance/effector responses and predictors of favorable outcomes with ruxolitinib in the fight against COVID-19–associated inflammation.
Conclusions
The novel coronavirus SARS CoV-2 has a rising death toll, especially in patients with comorbidities and those who are immunocompromised. Our patient was 17 months post stem cell transplantation and was maintained on the JAK/STAT inhibitor ruxolitinib, a proposed novel therapy for SARS CoV-2 pneumonia, throughout his clinical course, with successful attenuation of symptoms. Although there are a number of biomodulatory agents used for immunosuppression in the setting of organ and stem cell transplantation and autoimmune disorders, there is little available data to date on their impact on coronavirus infection and inflammatory response, nor is there data regarding underlying immunosuppressed state owing to transplant or other disorders and the clinical course of COVID-19 infection.
Recommendations on how to manage immunosuppression agents in allogeneic transplant patients who are infected with SARS CoV-2 have not been clearly established. As of June 2020, the Center for International Blood and Marrow Transplant Research reported 192 cases of SARS CoV-2 in transplant recipients, 110 of which had allogeneic transplants.23 Of these, the majority occurred in the first 3 years after transplant, and 81 patients have either resolved or improved. With agents like ruxolitinib, whose mechanism of action may reduce SARS CoV-2–related complications, it may be prudent to consider continuing therapy, but alternatively, in significantly immunocompromised patients, reduction of immunosuppression may be appropriate. Until data is available regarding immunosuppression agents used and T cell subset recovery from registries such as the Center for International Blood and Marrow Transplant Research, clear guidelines regarding management of COVID-19 in post-allogeneic transplant patients on immunosuppression may remain anecdotal. As we learn more about the relevance of different pathways and host effector mechanism in COVID-19 immune response, a rational strategy for the use of agents such as ruxolitinib can be defined.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18:123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren L.L., Wang Y.M., Wu Z.Q., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian S., Xiong Y., Liu H., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceribelli A., Motta F., De Santis M., et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109:102442. doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C.C., Chen M.Y., Chang Y.L. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. 2020;83:534–536. doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell B., Moss C., George G., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W., Zhao Y., Zhang F., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson S., Kay F.U., Abarra S., et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imag. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman J.S., Scherle P.A., Collins R., et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J Invest Dermatol. 2011;131:1838–1844. doi: 10.1038/jid.2011.140. [DOI] [PubMed] [Google Scholar]
- 13.Kiu H., Nicholson S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesa R.A. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs. 2010;13:394–403. [PubMed] [Google Scholar]
- 15.Quintas-Cardama A., Vaddi K., Liu P., et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carniti C., Gimondi S., Vendramin A., et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21:3740–3749. doi: 10.1158/1078-0432.CCR-14-2758. [DOI] [PubMed] [Google Scholar]
- 17.Khandelwal P., Teusink-Cross A., Davies S.M., et al. Ruxolitinib as salvage therapy in steroid-refractory acute graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2017;23:1122–1127. doi: 10.1016/j.bbmt.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Khoury H.J., Langston A.A., Kota V.K., et al. Ruxolitinib: a steroid sparing agent in chronic graft-versus-host disease. Bone Marrow Transplant. 2018;53:826–831. doi: 10.1038/s41409-017-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffini E., Giaccone L., Festuccia M., et al. Ruxolitinib in steroid refractory graft-vs.-host disease: a case report. J Hematol Oncol. 2016;9:67. doi: 10.1186/s13045-016-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spoerl S., Mathew N.R., Bscheider M., et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 21.Febvre-James M., Lecureur V., Fardel O. Potent repression of C-reactive protein (CRP) expression by the JAK1/2 inhibitor ruxolitinib in inflammatory human hepatocytes. Inflamm Res. 2020;69:51–62. doi: 10.1007/s00011-019-01293-1. [DOI] [PubMed] [Google Scholar]
- 22.Sylvine P., Thomas S., Pirayeh E., French Network of Regional Pharmacovigilance Centers Infections associated with ruxolitinib: study in the French Pharmacovigilance database. Ann Hematol. 2018;97:913–914. doi: 10.1007/s00277-018-3242-8. [DOI] [PubMed] [Google Scholar]
- 23.Available at: https://www.cibmtr.org/Covid19/Pages/default.aspx. Accessed July 22, 2020.