Abstract
Blood Disorders covers disorders of red blood cells, white blood cells, platelets, and coagulation including congenital and acquired disorders. Neoplastic Disorders covers both Hematopoietic and Solid Tumors with information on epidemiology, clinical presentation, diagnosis, and treatment of these disorders.
Keywords: RBCs disorders, Platelet disorders, WBCs disorders, Coagulation disorders, Neoplastic disorders
Abbreviations
- DIC
disseminated intravascular coagulation
- LCH
Langerhans cell histiocytosis
- MCV
mean cell volume
- TIBC
total iron binding capacity
- RBCs
red blood cells
- WBCS
white blood cells
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- IL
interleukin
- TNF
tumor necrosis factor
- PCR
polymerase chain reaction
- PNH
paroxysmal nocturnal hemoglobinuria
- SS
homozygous sickle cell genes
- SC
heterozygous sickle cell and C genes
- CXR
chest x-ray
- ACS
acute chest syndrome
- PK
pyruvate kinase
- EBV
Epstein-Barr virus
- SDS
-
- GCSF
granulocyte colony stimulating factor
- HSM
hepatosplenomegaly
- MDS
myelodysplastic syndrome
- AML
acute myelogenous leukemia
- ASD
atrial septal defect
- VSD
ventricular septal defect
- PDA
patent ductus arteriosus
- TOF
tetralogy of Fallot
- CoA
coarctation of the aorta
- ITP
idiopathic thrombocytopenic purpura
- HUS
hemolytic uremic syndrome
- MPV
mean platelet volume
- IVIg
intravenous immunoglobulin
- DDAVP
desmopressin
- VWD
von Willebrand disease
- PTT
partial thromboplastin time
- GI
gastrointestinal
- CMP
complete metabolic panel
- ESR
erythrocyte sedimentation rate
- CBC
complete blood count
- JPA
juvenile pilocytic astrocytoma
- CNS
central nervous system
- US
ultrasound
- KUB
kidney, ureter, and bladder x-ray
- U/A
urinalysis
Blood Disorders
Blood disorders generally fall into four categories:
-
Red cell disorders
- Anemia
- Erythrocytosis
-
White cell disorders
- Neutropenia
- Abnormal white cells
-
Platelet disorders
- Thrombocytopenia
- Abnormal platelets
Coagulation disorders
Red Cell Disorders
Anemia
-
Incidence of anemia in childhood (Fig. 1)
- Iron deficiency anemia (IDA), 60–70 %
- Hemolytic anemia, 15–20 %
- Hypoproliferative anemia, 10 %
- Maturation abnormalities, 7–8 %
Fig. 1.
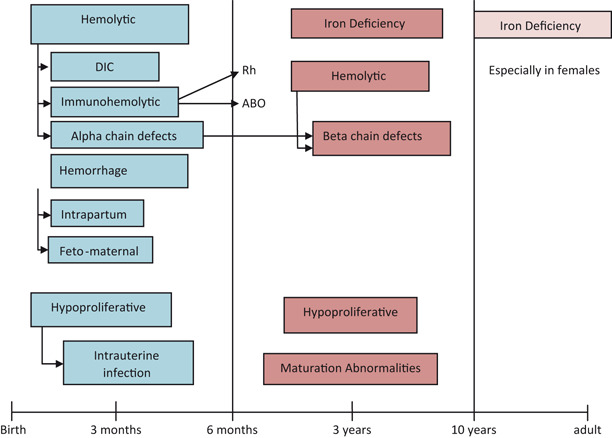
Prevalence of anemia in different age groups
Anemia in the Newborn (Fig. 2)
Fig. 2.
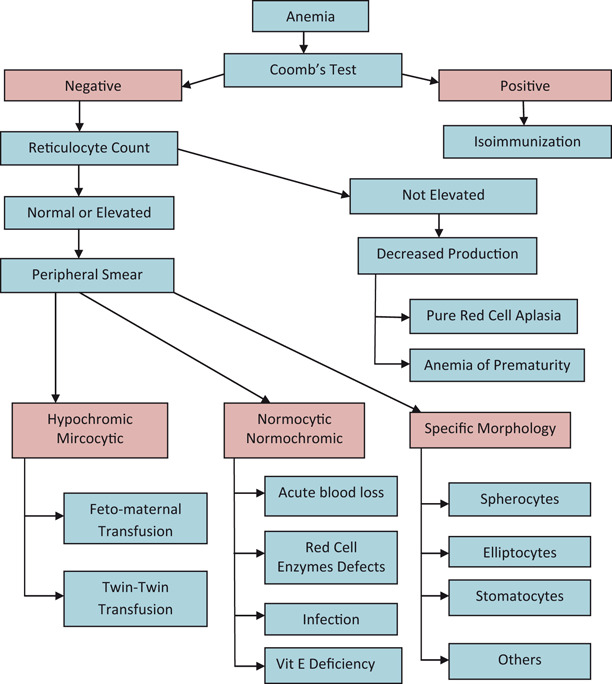
An approach to the diagnosis of anemia in the newborn infant
Hemolysis
Congenital
-
Hemoglobinopathies
- Chain defects more common
-
Red cell membrane defects
- Hereditary spherocytosis
- Hereditary elliptocytosis
- Hereditary stomatocytosis
-
Red cell enzyme defects
- G6PD
- PK
Acquired
-
Nonimmune
-
Vitamin E deficiency
- ◦ Hemolytic anemia
- ◦ Edema
- ◦ Thrombocytosis
- Infantile pyknocytosis
-
-
Immune
- ABO
- RH
-
Infections
- DIC
- Bacterial
- Viral
Blood loss
-
Prenatal
- Fetomaternal
- Twin–twin transfusion
Placental
Umbilical
-
Postnatal
- Plasma factor deficiencies
- Platelets––deficiency or dysfunction
- Abnormal platelet function
Decreased red cell production
Pure red cell aplasia
-
Anemia of prematurity
- Early
- Late
- Iatrogenic
Infection
-
Infiltration
- Congenital leukemia
- Neuroblastoma
- LCH
- Osteopetrosis
Approach to Diagnosis of Anemia in Older Child
Inadequate RBCs/hemoglobin (Hgb)
-
Size of red cells (MCV) (Fig. 3)
- Microcytic (MCV < 70 + age) (Fig. 4)
- Normocytic (MCV > 70 + age and< 100)
Macrocytic (MCV > 100)
Reticulocyte count
Fig. 3.
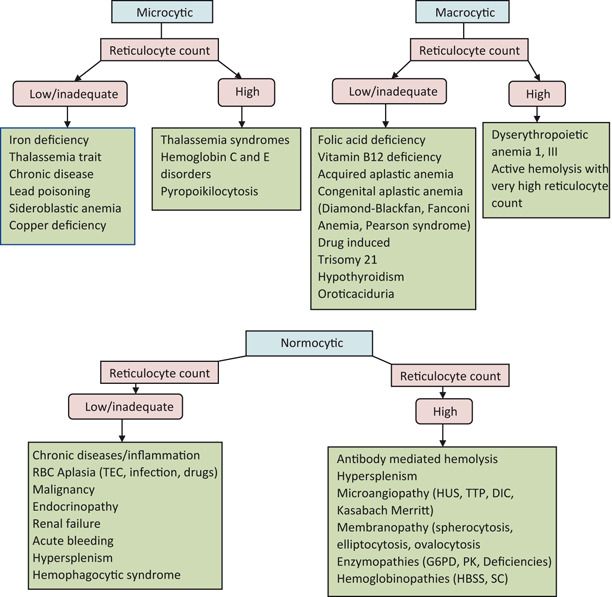
Approach to anemia in older children based on MCV
Fig. 4.
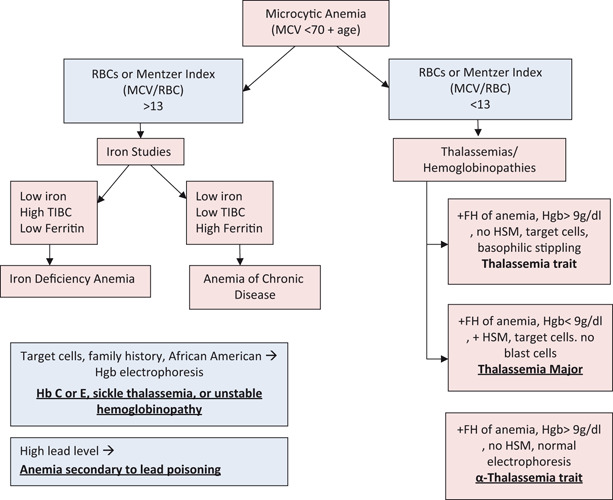
Microcytic anemia
Microcytic Anemia
Iron Deficiency Anemia (IDA)
Most common hematologic disease in infancy and childhood
Etiology
-
Nutritional
- Low birth weight
- Rapid growth
- Consumption of large amount of cow’s milk (> 32 oz whole cow’s milk/day)
-
Impaired absorption
- Primary iron deficiency
- Malabsorption syndrome
-
Blood loss
- Gastrointestinal
- Primary iron deficiency
- Cow’s milk allergy or exudative enteropathy
- Lesions: Meckel’s, vascular malformations
- Parasites: hookworms
- Genitourinary
- Menstrual
- Hemoglobinuria
- Hemosiderinuria
- Pulmonary
- Goodpasture’s syndrome
- Pulmonary hemosiderosis
Clinical Presentation
Pallor
Pagophagia: desire to eat unusual substance as ice, dirt, etc.
-
If Hgb level falls < 5 g/dL
- Irritability
- Anorexia
- Tachycardia
- Systolic murmur
Laboratory
Low serum ferritin (depleted iron stores)
Low serum iron—may fluctuate
Increased TIBC (serum transferrin)
RBCs become more microcytic, hypochromic, and increased poikilocytosis as disease progresses (Fig. 5)
Increased RBC distribution width (RDW)
Normal WBCs
Thrombocytosis; occasionally marked (600,000–1 million/mm3)
Low reticulocyte count
Mentzer index > 13 (MCV/RBCs)
Fig. 5.
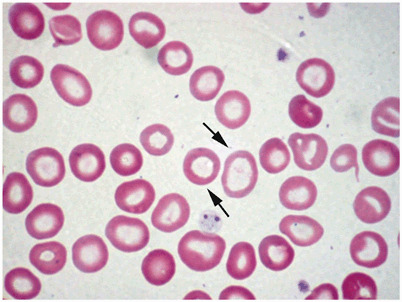
Peripheral blood smear example of hypochromic/microcytic anemia. Notice the variability in the sizes of red blood cells. The arrows point to hypochromic erythrocytes with large central hollow. (Courtesy of Dr. Nawar Hakim)
Treatment
Response to iron therapy is diagnostic and therapeutic.
-
Oral administration of ferrous salts at dose of 4–6 mg/kg of elemental iron in three divided doses .
- Very inexpensive.
- Downsides; taste, Gl irritability, and constipation (more water and fiber can solve this problem).
Rapid correction of anemia with transfusion may precipitate heart failure.
In severely anemic children (< 4 gm/dl) transfusions can be administered at a very slow rate (2–3 ml/kg).
If there is evidence of heart failure present, a modified exchange transfusion using fresh PRBCs can be considered.
-
Changes after treatment with iron.
- Within 12–24 h: irritability decreases, increased appetite.
- 36–48 h: initial bone marrow response with erythroid hyperplasia.
- 48–72 h: reticulocytosis, peaking at 5–7 days.
- 1–3 months: repletion of stores.
Hgb may increase by 0.5 g/dl/day.
Iron therapy should be continued for at least 2 months after the Hgb normalizes to replenish iron stores.
Limit cow’s milk to less than 500 cc/day .
Anemia of Chronic Disease
Associations
Chronic systemic diseases
Chronic inflammatory process, e.g., SLE, RA
Chronic pyogenic infection
Etiology
Release of inflammatory cytokines: IL-6,IL-1,TNF
Hepcidin released from the liver decreases intestinal iron absorption, also block release of iron from the macrophages
Laboratory
Hgb concentration usually 6–9 g/dL
Normal-to-low MCV
Often normochromic anemia with progression to hypochromia
Low serum iron
Normal-to-low TIBC
Elevated serum ferritin
Treatment
Treatment of the cause
Recombinant EPO may increase the Hgb level and improve well-being in patients with cancer
Lead Poisoning
High serum lead level
Markedly elevated free erythrocyte protoporphyrin
Basophilic stippling of RBCs
Ringed sideroblasts in bone marrow
Thalassemias
Alpha Thalassemia
Healthy individuals have 4 alpha globin genes, 2 on each chromosome 16
Alpha globin production is reduced to absent
Seen more frequently in those of southeast Asian and African ancestry
Diagnosis: clinically or with alpha globin chain analysis
Excess beta chains lead to beta 4 chains (Hemoglobin H, HbH)
Excess gamma chains lead to gamma 4 chains (Hemoglobin Barts, Hb Barts)
Alpha Thalassemia Syndromes
Silent trait
Deletion or dysfunction of one gene
Asymptomatic
1–2 % Hb Barts on neonatal electrophoresis
Normal Hgb electrophoresis
Alpha thalassemia trait
Deletion or dysfunction of two genes
Mild hypochromic microcytic anemia
3–10 % Hb Barts on neonatal electrophoresis
-
Laboratory
- Mentzer Index < 13
- Hgb > 9 g/dl
- Normal Hgb electrophoresis
- Often misdiagnosed as IDA
Hemoglobin H disease
Deletion of three genes
Mild-to-moderate hypochromic microcytic anemia
Splenomegaly
Jaundice
Cholelithiasis (pigment stones)
Anemia exaggerated by infection, pregnancy, exposure to oxidizing drugs
> 25 % Hgb Barts on neonatal electrophoresis
Alpha thalassemia major
Deletion of four genes
Fetal hydrops-fatal disease
Predominant Hb Barts
Beta Thalassemia
Healthy individuals have 2 beta globin genes, 1 on each chromosome 11
Beta globin production is reduced to absent
Multiple possible genetic mutations or deletions
More clinical overlap
Seen more frequently in those of Mediterranean, southeast Asian ancestry
Also seen in African Americans but generally have a milder course
Relative alpha chain excess leads to shortened red cell survival and variable splenic sequestration
Diagnosed by hemoglobin electrophoresis or beta globin chain analysis
Cannot be diagnosed by electrophoresis in the neonate
Iron, folate, and B12 must be repleted to have an accurate hemoglobin electrophoresis
Beta Thalassemia Syndromes
-
Beta thalassemia minor—silent or near silent trait (heterozygous β0 or β +)
- Asymptomatic
- Smear can be normal
- Occasional microcytosis, hypochromia, target cells, basophilic stippling
- Often normal indices or decreased MCV
- Normal to slightly elevated HgbA2 on electrophoresis
Thalassemia intermedia
More symptomatic than thalassemia trait
Refers to a clinical phenotype with diverse genetic explanations
-
Laboratory
- Microcytosis, hypochromia, target cells, and basophilic stippling on smear
- Mentzer Index < 13
- Hgb usually between 7 and 10 g/dl
- Elevated HgbA2 and HgbF on electrophoresis
Thalassemia major (Cooley’s anemia)
Variable reduction of beta globin gene production
Homozygous or double heterozygous forms (β0, β + variants)
Excess alpha globin chains result in increased destruction of RBCs and ineffective erythropoiesis
Shortened red cell life span and splenic trapping
-
Clinical presentation
-
General
- ◦ Dependent on amount of HgbF
- ◦ Severe anemia with increased iron absorption and subsequent toxicity
- ◦ Pallor, jaundice, fatigue
- ◦ Hepatosplenomegaly
-
Skeletal
- ◦ Typical facial features with maxillary hyperplasia, flat nasal bridge, frontal bossing
- ◦ Pathological bone fractures
-
Endocrine dysfunction
- ◦ Hypothyroidism
- ◦ Hypoparathyroidism
- ◦ Diabetes mellitus
-
Cardiovascular
- ◦ Congestive heart failure
- ◦ Cardiac arrhythmias
-
-
Laboratory
- Severe anemia
- Few reticulocytes < 8 % compared to degree of anemia
- Microcytosis with no normal appearing RBCs on the smear
- Numerous nucleated RBCs
- Target cells
- Mentzer index(MCV/RBCs) is < 9
- Indirect (unconjugated) bilirubin is elevated
-
Treatment
- Chronic transfusion therapy
- Before chronic transfusion is initiated diagnosis of beta thalassemia must be confirmed first
- Deferoxamine for iron chelation
- Newer chelating agent, deferasirox (Exjade, Novartis), is oral and more tolerable but long term data still being accumulated
Other Hemoglobinopathies
Hemoglobin E
Hemoglobin Lepore
Hemoglobin Koln
Rare Disorders
-
Sideroblastic anemia
- May be microcytic
- Ineffective erythropoiesis caused by iron deposition in erythroblasts
- Mild-to-moderate hemolysis
- Ringed sideroblasts in bone marrow
Protein calorie malnutrition-microcytosis without IDA
Metabolic abnormalities of iron absorption and metabolism
Macrocytic Anemia (MCV > 100 in Child Older than 2)
Folic Acid Deficiency
Etiology
-
Nutritional
- Sources—leaves; vegetable; fruits; animal organs, for example, liver and kidneys
- Body stores for folic acid is limited 2–3 months on folate-free diet
Inadequate intake—during pregnancy, growth in children, and hemolytic anemia
Goat milk consumption
Decreased folic acid absorption—removal of ileum or IBD
Anticonvulsant medication, for example, phenytoin, primidone
Congenital dihydrofolate reductase deficiency
Drug-induced abnormal metabolism—Methotrexate
Clinical presentation
Megaloblastic anemia
Irritability
Inadequate weight gain
Chronic diarrhea
Hemorrhage from thrombocytopenia in severe cases
Laboratory
Macrocytic anemia (MCV> 100; Fig. 6)
Megaloblastic changes including hypersegmented neutrophils (> 5 lobes)
Elevated LDH
Hypercellular bone marrow
Fig. 6.
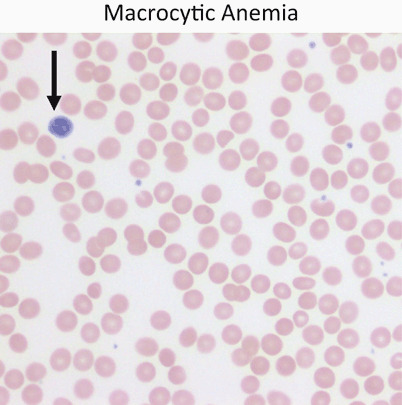
Red cells are usually approximately the size of a small lymphocyte nucleus (arrow). In this case the red cells are slightly larger than the lymphocyte nucleus on average. Macrocytic anemia is most often a result of folate or vitamin B12 deficiency
Treatment
Rule out B12 deficiency before starting folic acid therapy
Folic acid 0.5–1 mg/day IV or oral
Hematologic response can occur within 72 h (diagnostic test as well)
Treatment continued for only 3–4 weeks
Maintenance dose is 0.2 mg daily
Vitamin B12 Deficiency
Vitamin B12 stores last for 3–5 years
Sources—animal products
Etiology
Inadequate B12 intake (strict vegan)
Exclusively breast fed and maternal vegan diet
Removal of terminal ileum
Inflammatory bowel disease
Fish tapeworm (Diphylobothrium latum)
Absence of Vitamin B12 transport protein and stomach intrinsic factor (IF)
Clinical presentation
Weakness
Fatigue
Failure to thrive
Irritability
Pallor
Glossitis
Vomiting
Diarrhea
Icterus
-
Neurologic symptoms
- Paresthesias
- Developmental regression
- Neuropsychiatric changes
Laboratory
Macrocytic anemia (MCV > 100; see Fig. 6)
Megaloblastic changes including hypersegmented neutrophils (> 5 lobes)
Elevated LDH
Normal iron and folic acid levels
Increased methylmalonic acid in urine
Increased homocysteine
Low reticulocyte count for degree of anemia
-
Antiparietal cell antibody positive in pernicious anemia
- Less than 10 % of cases present under age 40
Classic Schilling test is no longer regarded as the diagnostic test
Treatment
-
Parenteral administration of Vitamin B12 1 mg daily
- With neurologic involvement continue for minimum of 2 weeks
Reticulocytosis in 2–4 days unless concurrent inflammatory disease
Maintenance of monthly IM Vitamin B12
Pearson Marrow–Pancreas Syndrome
Variant of sideroblastic anemia
Clinical presentation
Macrocytic anemia in neonatal period
Elevated level of alpha fetoprotein
Neutropenia
Thrombocytopenia
Failure to thrive
Pancreatic fibrosis
Insulin dependent diabetes mellitus
Exocrine pancreatic deficiency
Muscle and neurologic impairment
Laboratory
-
Bone marrow
- Ringed sideroblast
- Vacuolated erythroblast and myeloblast
Often confused with Diamond–Blackfan anemia and transient erythroblastopenia of childhood
Diamond–Blackfan Anemia (Congenital Hypoplastic Anemia)
Primary defect in the erythroid progenitors
Clinical presentation
Profound anemia manifested by 2–6 months of age
-
More than 50 % have congenital anomalies
- Short stature
- Craniofacial dysmorphism (snub nose, wide-set eyes, thick upper lip)
- Triphalangeal thumbs
- Bifid, subluxed, absent, or supernumerary thumbs
Laboratory
Macrocytic RBCs with no hypersegmentation of neutrophils
Normal B12 and folate
Increased adenosine deaminase activity in most patients
Decreased RBCs precursor in bone marrow
Elevated serum iron
Normal bone marrow chromosomal studies
Normal to low reticulocyte count
Negative PCR for Parvovirus B19
Treatment
Steroids
Iron chelating agents (if transfusion dependent)
Stem cell transplantation for non respondents to corticosteroids, and after several years of RBC transfusions
Prognosis
Median survival > 40 years
Normocytic Anemia (MCV > 70 + Age and < 100 in Child Older Than 2; Fig. 7)
Fig. 7.
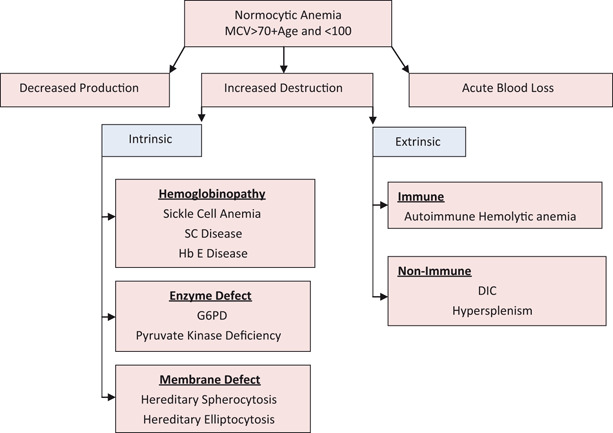
Approach to normocytic anemia
Transient Erythroblastopenia Childhood
Background
Most common acquired red cell aplasia in childhood
More common than Diamond–Blackfan anemia (congenital hypoplastic anemia)
Etiology
Transient suppression of RBC production
Often noted after a viral infection
No evidence of Parvovirus B19
Clinical presentation
Age—3 months to 3 years of age, most > 12 months
More common in males
Laboratory
MCV normal for age
Hemoglobin can be as low as 2.2 g/dl
Reticulocytes decreased
Bone marrow biopsy rarely needed but erythroid suppression seen
Normal adenosine deaminase (ADA)
Treatment
Reassurance
Recover within 2–3 months
Occasionally transfusion is necessary
Hereditary Spherocytosis
Background
-
Autosomal-dominant inheritance
- Less frequently can be autosomal recessive
25 % of patients have no family history
Most common molecular defects are in spectrin or ankyrin, major components of the RBC cytoskeleton
Clinical presentation
May be asymptomatic into adulthood
Anemia
Hyperbilirubinemia sufficient to require exchange transfusion in newborn period
Pallor
Jaundice
Fatigue
Exercise intolerance
Splenomegaly
Pigment gallstones may form as early as 4–5 years of age
-
Susceptible to aplastic crisis as a result of parvovirus B19 infections
- Erythroid marrow failure may result rapidly in profound anemia HCT < 10 %, high cardiac output failure, hypoxia, cardiovascular collapse, and death; platelet may also fall
Laboratory
Reticulocytosis
Indirect hyperbilirubinemia
High LDH
Low haptoglobin
Normal MCV
Elevated MCHC
-
High percentage of spherocytes on smear (Fig. 8)
- Can be confirmed with osmotic fragility test or flow cytometry
Fig. 8.
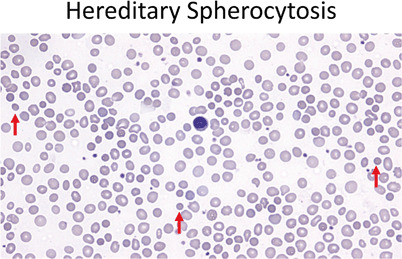
Red cells should be similar in size to the small lymphocyte nucleus (center). In hereditary spherocytosis the red cells are small and hyperchromatic, lacking central pallor (40 ×). Red arrows point out a few of the examples in this field
Treatment
Folic acid 1 mg po daily to prevent deficiency and the resultant decrease in erythropoiesis
-
Splenectomy indications:
- Hgb < 10 g/dl
- Reticulocytosis
- Aplastic crisis
- Poor growth
- Cardiomegaly
Some do not recommend splenectomy in patients with hemoglobin > 10 g/dl and reticulocytes < 10 %
Vaccination for encapsulated organism Haemophilus influenza, meningococcus, pneumococcus should be given before splenectomy, then prophylactic penicillin V 125 mg BID < 5 years and 250 BID for > 5 years
Partial splenectomy is useful in children < 5 years
Hereditary Elliptocytosis
Less common than hereditary spherocytosis (HS)
Clinical presentation
Presentation same as in HS
Laboratory
-
Red blood cells shows various degree of elongation, may be rod shaped
- Other abnormal shapes may be present microcytosis, spherocytes, poikilocytosis
Treatment
No treatment necessary unless hemolysis present
Otherwise same as in HS
Paroxysmal Nocturnal Hemoglobinuria
Background
Most often caused by an acquired (rather than inherited) intrinsic defect in the cell membrane
Deficient membrane associated protein include decay-accelerating factor, C8-binding protein
Clinical presentation
Nocturnal and morning hemoglobinuria
Thrombosis and thromboembolic phenomena is a very serious complication
Aplastic anemia may precede the episodes of PNH
Laboratory
Red blood cells shows various degree of elongation; may be rod shaped
Evidence of hemolysis—elevated LDH, elevated bilirubin, low haptoglobin
Negative direct antiglobulin test
Flow cytometry for CD55 and CD59
Positive results on acidified serum hemolysis Ham test, or sucrose lysis test (historical)
Markedly decreased acetylcholinesterase activity and decay accelerating factor is found
Treatment
-
Acute
- Transfusion to suppress production of PNH cells
- Glucocorticoids 2 mg/kg/24 h (controversial)
-
Chronic
- Eculizumab prevents complement binding and decreases hemolysis
- Warfarin to prevent thrombotic complications
- May need supplemental iron to offset losses from hemoglobinuria
Sickle Cell Disease (SCD)
Background
Hemoglobin S is the result of a mutation resulting in a substitution of valine for glutamic acid at sixth position in beta globin chain
Autosomal recessive inheritance
Clinical presentation
Usually diagnosed on neonatal screen
Manifestations of clinical symptoms can be as early as 6 months of age
-
Crises
- Splenic sequestration
- Pain crisis
- Aplastic crisis
- Parvovirus B19 frequent cause of aplastic crises
-
Infections
- Bacterial sepsis is the greatest cause of morbidity and mortality
- Bacterial infection by encapsulated organisms is the most common at all ages
Functional asplenia as early as 6 months, by age 5 in most children
Laboratory
Anemia
Sickle cells on smear (Fig. 9)
Positive sickle prep
Hemoglobin electrophoresis—SS, SC, SD
Fig. 9.
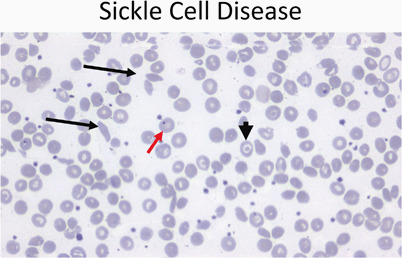
Peripheral smear (40 ×) from a patient with sickle cell disease showing sickle cells (black arrows), target cells (arrowhead), and a Howell–Joley body (red arrow)
Management
-
Fever
- Medical emergency due to high risk of severe bacterial infection and high fatality
- Parenteral IV third generation cephalosporin (Cefotaxime)
- Penicillin VK oral prophylaxis until 5 years of age
- 125 mg PO BID until 3 years
- 250 mg PO BID until 5 years
- Continue past 2 years of age if history of infection with encapsulated organism
- Osteomyelitis—frequently staph or salmonella
Pain crisis
-
Hydroxyurea
- Increases level of hemoglobin F and total hemoglobin
- Decreases the pain crises by 50 %
- Side effect is myelosuppression, but reversible
- If begun in infancy may preserve the splenic function, improve the growth, and decrease ACS
- Initial dose is 15–20 mg/kg increase gradually by 2.5–5 mg/kg up to max of 35 mg/kg/day
- Monitor for toxicity
Aplastic crisis
RBC lifespan is between 10 and 20 days in patient with SCD
Cessation of RBC production for 10–14 days can lead to profound anemia
-
Clinical presentation
- Pallor
- Fatigue
- Decreased activity
- Poor feeding
- Altered mentation
-
Laboratory
- Severe anemia
- Reticulocytopenia
- Occasional thrombocytopenia
-
Management
- Transfusion support as needed until reticulocyte recovery has occurred.
-
Dactylitis (hand–foot disease)
- Often the first manifestation of pain in children
- Occurs in 50 % of children by 2 years of age
- Unilateral can be confused with osteomyelitis
-
Treatment
- ◦ Pain medications (e.g.,acetaminophen with co-deine)
Splenic sequestration
Etiology unknown
30 % of children with sickle cell anemia have episodes of significant sequestration
-
Clinical presentation
- Increase in size of spleen
- Evidence of hypovolemia
- Decline in hemoglobin of at least 2 g/dl from the base line
-
Treatment
- Maintenance of hemodynamic stability
- Isotonic fluid
- Blood transfusion
-
Prognosis
- Repeat sequestration is very common
- Parents should be taught how to palpate the spleen
Vaso-occlusive crisis
Disruption of blood flow in microvasculature by sickle cells
Risk factors exposure to cold, hypoxia, and acidosis
-
Clinical presentation
- Pain which can affect any part of the body
- Pain most often in chest, back, abdomen, and extremities
-
Management
- Pain medications
- Acetaminophen up to IV morphine depending on the severity
- IV hydration does not relieve the pain
- Blood transfusion does not prevent or relieve the pain
- Concern about opioids dependency must not be a reason not treat a child with pain
Priapism
-
Penile erection lasts > 30 min
- Pain medication
- Sitz bath
-
Penile erection lasts > 4 h
- May result in sexual dysfunction
- Aspiration of blood from corpora cavernosa
- Followed by irrigation with diluted epinephrine will cause immediate relief
Neurological complications
-
11–20 % will have either overt or silent stroke
- Overt stroke means presence of focal neurological deficit > 24 h and or cerebral infarct by T2-weighted MRI
- Silent stroke means absence of focal neurological lesions > 24 h with cerebral infarct on T2-weighted MRI
-
Clinical presentation
- Headache
- Seizures
- Cerebral venous thrombosis
- Reversible posterior leukoencephalopathy syndrome (RPLS)
-
Treatment
- Oxygen to maintain saturation > 96 %
- Transfusion within 1 h to increase Hgb level to max of 11 g/dL
- CT to exclude cerebral hemorrhage
-
Primary prevention of stroke
- TCD (transcranial Doppler) to measure blood velocity
- If blood velocity is > 200 cm/s prophylactic transfusion is indicated to decrease the Hgb S to < 30 %
- Can start as early as 2–3 years of age
-
Secondary prevention
- Transfusion therapy after initial stroke
- Maintain the Hgb S < 30 %
- Complications:
20 % have second stroke in the first year after first stroke
-
Iron overload (200 mg of iron/unit RBCs):
- Iron-chelating agents
- Phlebotomy
- Erythrocytapheresis (expensive and complicated)
Acute chest syndrome
-
Clinical presentation
- Fever
- Respiratory distress
- Chest pain
- New radiodensity on CXR
- All patients with fever should have CXR even in absence of respiratory symptoms
-
Treatment
- Oxygen
-
Simple exchange transfusion indications:
- ◦ Decreasing oxygen saturation
- ◦ Increasing work of breathing
- ◦ Rapid change in respiratory effort
- Most common episode preceding ACS is pain crisis treated with opioids, especially morphine
- Overlap between pneumonia and ACS requires use of macrolide and third-generation cephalosporin
- Most common organism in ACS: S. pneumoniae,Mycopl asma, Chlamydia pneumoniae
-
Pulmonary hypertension
- PH is a major risk of death in adult with sickle cell anemia
Renal disease
Gross hematuria
Papillary necrosis
Nephritic syndrome
Renal infarcts
Pyelonephritis
Renal medullary necrosis
-
Treatment
- ACE inhibitors beneficial for patients with proteinuria
General considerations
High risk of academic failure, poor high school graduation rate
1/3 of children have cerebral infarcts
-
Other complication of sickle cell anemia
- Delayed puberty
- Vascular necrosis of femoral head
- Retinopathy
- Surgical procedures—complications include pain and ACS post operatively
- Blood transfusion before surgery to keep the hemoglobin approximately 10 g/dl
Methemoglobinemia (congenital or acquired)
Decrease ability to release o2 to tissues
Methemoglobin of 15 % associated with visible cyanosis
Methemoglobin of 70 % is lethal
Methemoglobin colors the blood brown
Exposure to 100 % oxygen will change the color
-
Triggers
- Rotavirus infection
- Gastroenteritis
- Water high nitrites
- Aniline teeth gel
Treatment: methylene blue
Pyruvate Kinase Deficiency
Background
Active enzyme in Embden–Meyerhof pathway
Deficiency leads to defective red cell glycolysis and decrease ATP production
Red cells are rigid and deformed, metabolically and physically vulnerable with decreased red cell survival
Clinical presentation
Varies from severe neonatal hemolytic anemia to mild well compensated hemolysis
Severe jaundice and anemia and can occur during neonatal period
Splenomegaly
Aplastic crisis with parvovirus B19 infection
Laboratory
Reduced RBC PK enzyme level
Elevated reticulocyte count
Smear with polychromatophilia, macrocytosis, ovalocytes, acanthocytes, or pyknocytes
Treatment
Exchange transfusion may be indicated for hyperbilirubinemia in newborn
Blood transfusion as necessary
Folic acid supplementation
Splenectomy should be performed if frequent transfusion after age 5–6 years
Glucose-6-Phosphate Dehydrogenase
Pathophysiology
-
First enzyme in the pentose phosphate pathway of glucose metabolism
- Activity falls rapidly as red cell ages
- Decreased glucose metabolism with impaired elimination of oxidants and subsequent loss of red cell membrane integrity
Severity of hemolysis depends on the quantity and type of G6PD deficiency and nature of hemolytic agent (usually an oxidation mediator) (Table 1)
Table 1.
WHO classification of G6PD deficiency
| Class | Level of deficiency | Enzyme activity | Prevalence |
|---|---|---|---|
| I | Severe | < 10 % enzyme activity; chronic non-spherocytic hemolytic anemia in the presence of normal erythrocyte function | Uncommon; occurs across all population |
| II | Severe | < 10 % enzyme activity with intermittent hemolysis | Varies; more common in Asian and Mediterranean populations |
| III | Moderate |
10–60 % enzyme activity Hemolysis with stressor only |
10 % of black males in USA |
| IV | Mild to none |
60–150 % enzyme activity No clinic sequelae |
Rare |
| V | None |
> 150 % enzyme activity No clinic sequelae |
Rare |
Genetics
X-linked recessive
Variable intermediate expression shown by heterozygous females
More common in African American and Mediterranean ancestry
Clinical presentation: episodes of hemolysis produced by:
-
Drugs
-
Antioxidant drugs include:
- ◦ Aspirin
- ◦ Sulfonamides
- ◦ Antimalarials
- Usually 24–48 h after exposure
- Hemoglobin usually normal between episodes
- Occasionally need additional stress of infection or neonatal state
-
-
Fava beans
- Acute life-threatening, often leading to acute renal failure
- Associated with Mediterranean and Canton varieties
- Blood transfusions usually required
Infection
-
Neonatal jaundice
- Associated with Mediterranean and Canton varieties
- Occasional exposure to naphthalene, aniline dyes, marking ink, or other drug
- Infants may present with pallor, jaundice, dark urine
- Jaundice may be hepatic in origin
- Often no known exposure to drugs
-
Chronic nonspherocytic hemolytic anemia
- Mainly in northern Europeans
- Reticulocytosis
- Increased autohemolysis with only partial correction by glucose
- Slight jaundice
- Mild splenomegaly
Laboratory
Anemia
Heinz bodies seen in unstained red blood cells due to hemoglobin precipitation
Diagnosis demonstrated by reduced G6PD activity in RBCs should be few weeks after the hemolytic episode
Treatment
Avoidance of agents
Transfusion as needed
Folic acid supplementation
-
Chronic nonspherocytic hemolytic anemia
- Consider chronic transfusion to keep Hgb at approximately 8 g/dl
- Iron chelation as needed
-
Splenectomy
- Severe chronic anemia
- Hypersplenism
- Splenomegaly with physical impediment
Other Enzyme Deficiencies
Hexokinase deficiency
Glucose phosphate isomerase deficiency
Aldolase deficiency
Diphosphoglycerate deficiency
Adenosine triphosphate deficiency
Enloase deficiency
-
Phosphofructokinase deficiency
- Myopathy
- Associated with type VII glycogen storage disease
- Common in Ashkenazi Jews
-
Triosephosphate isomerase deficiency
- Cardiac anomalies
- Recurrent infections
- Progressive neuromuscular disease with generalized spasticity
-
Phosphoglyercate kinase deficiency
- First ATP generating enzyme
- Sex-linked recessive
- Intellectual disability (ID)
- Seizures
- Behavioral disorders
Autoimmune Hemolytic Anemia
Etiology
Antibodies against antigens on RBCs surface
IgG against Rh complex is the most common in children
IgM cold antibodies usually associated with infections, for example, Mycoplasma and EBV
Clinical presentation
Pallor
Jaundice
Pyrexia
Hemoglobinuria
Splenomegaly
Laboratory
Profound anemia
Reticulocytosis
Positive direct antiglobulin (Coombs) test
Polychromasia
Spherocytosis
High cold agglutinin titre
Treatment
Supportive treatment for mild cases
Corticosteroids for IgG mediated disease
Blood transfusion (blood unit with the least reaction by Coomb’s technique)
IVIg
Splenectomy in persistent cases
Prognosis of acute form
Response to glucocorticoids
Low mortality rate
Full recovery
Hemolytic Anemia Secondary to Extracellular Factors
-
Mechanical injury
- HUS
- Kasabach-Merrit syndrome: hemangioma and thrombocytopenia
Thermal injury
Renal disease
-
Liver disease
- Change in cholesterol to phosphlipid level which affects the membrane of RBC
-
Toxins and venom
- Streptococcus, haemophilus influenzae, staphylococcus and clostridium infection
- Cobras, rattlesnakes, have phospholipids in their venom—cause spherocytic hemolysis
Erythrocytosis
Definition
RBCs 25 % > upper normal value
Clinical presentation
Hypertension, headache , shortness breath, neurologic symptoms, thrombocytosis may cause hemorrhage and thrombosis
Primary (polycythemia vera)
-
Major criteria
- Increased red cell mass
- Arterial oxygen saturation > 92 %
- Palpable spleen
-
Minor criteria
- Platelet count > 400,000
- Leukocytosis > 12,000
- Increased leukocyte alkaline phosphatase
- Increased vitamin B12 > 900 pg/ml, binding capacity > 2200 pg/ml
Secondary
Increase HCT > 65 %
-
Clinical presentation
- Hyperviscosity, headache, hypertension
-
Etiology
-
Familial
- ◦ Hemoglobinopathy
-
Hypoxia
- ◦ Altitude
- ◦ Cardiac disease
- ◦ Lung disease
- ◦ Central hypoventilation
-
Hormonal
- ◦ Adrenal
- ◦ Anabolic
-
Renal
- ◦ Tumor/cysts
- ◦ Renal artery stenosis
- ◦ Hydronephrosis
-
Liver
- ◦ Dysfunction
- ◦ Hepatoma
-
Metabolic
- ◦ 2,3 diphosphoglycerate deficiency
-
Neonatal
- ◦ Normal intrauterine environment
- ◦ Twin-Twin transfusion
- ◦ Diabetic mother
- ◦ IUGR
- ◦ Trisomies
- ◦ Congenital adrenal hyperplasia
- ◦ Thyrotoxicosis
-
Treatment
Periodic phlebotomy for hematocrit > 65–70 % or hemoglobin > 23 g/dl
Fanconi Anemia
Genetics
Autosomal recessive
Clinical presentation
-
Skin abnormalities in 65 % of cases
- Hyperpigmentation of the trunk and intertriginous areas, café-au-lait spots, vitiligo
Short stature—60 %
-
Upper limb anomalies—50 %
- Absent thumbs
- Triphalangeal thumbs
- Congenital hip dysplasia
-
Male genitalia—40 %
- Underdeveloped penis
- Undescended testes
- Hypogonadism
-
Female genitalia
- Malformation of vagina, uterus
-
Facial anomalies
- Microcephaly , small eyes, epicanthal folds, abnormal shape ears, or absent ears
Intellectual disability (ID)—10 %
-
Kidney abnormalities
- Horseshoe kidney, absent, or duplicate kidney
Laboratory
Macrocytic anemia
Variable progression to full-blown pancytopenia due to aplasia
Complications
Acute leukemia
Carcinoma of head and neck, and upper esophagus
Shwachman–Diamond Syndrome
Rarest form of pancytopenia
Genetics
Autosomal recessive
Clinical presentation
Failure to thrive
-
Exocrine pancreatic insufficiency—50 %
- Fat malabsorption—absence of steatorrhea does not exclude SDS
-
Skeletal abnormalities
- Short stature—Metaphyseal chondrodysplasia
- Abnormal digits—syndactyly, clinodactyly, or supernumerary metatarsals
-
Abnormal facies
- Bifid uvula, short, or cleft palate
- Dental dysplasia
- Hypertelorism
- Microcephaly
Retinitis pigmentosa
Recurrent bacterial infections
Laboratory
Abnormal pancreatic enzymes and steatorrhea
Neutropenia
Bone marrow showing myeloid hypoplasia
Pancytopenia—60 %
Diagnosis
Mutation analysis for SBDS is definitive in 90 %
Complications —increase with age, usually after 10 years of age
Aplastic anemia
Myelodysplastic syndrome
Acute myelogenous leukemia
Treatment
Androgen with low-dose prednisone
White Cell Disorders
Neutropenia
Acute
-
Viral infection
- Epstein–Barr virus
- Respiratory syncytial virus
- Influenza A and B
- Hepatitis
- Human herpesvirus 6 (HHV 6) infections
Bacterial infection
Hypersplenism
-
Drug-induced—recovery after medication cessation
- Antimicrobials—sulfonamides, penicillin
- Antirheumatics—gold, phenylbutazone, penicillamine
- Anticonvulsants—phenothiazine
- Analgesic and anti-inflammatory—ibuprofen
Chronic
-
Cyclic neutropenia
-
Clinical presentation
- ◦ Approximately 21-day cycles with changing neutrophil counts with neutropenia spanning 3–6 days
- ◦ Nadir may be in severe range
- ◦ Fever and oral ulceration often during nadir
- ◦ Gingivitis, pharyngitis, skin infections during nadir
- ◦ Occasionally more serious infections—pneumonia, necrotizing enterocolitis with peritonitis, and Escherichia coli or Clostridium sepsis.
- ◦ Count may be recovering when brought to medical attention
-
Laboratory
- ◦ Counts 2–3/week for 6 weeks
Treatment- ◦ Prophylactic GCSF during nadir in some cases
- ◦ Immediate attention with fevers
-
-
Chronic benign neutropenia
- No specific abnormality found
- No serious infections
- No treatment necessary except attention for fevers
Congenital
-
Kostmann syndrome (severe congenital neutropenia)
- Autosomal recessive
-
Clinical presentation
- ◦ Mouth ulcers
- ◦ Gingivitis
- ◦ Otitis media
- ◦ Cellulitis
- ◦ Respiratory infections
- ◦ Skin infections and abscesses—most common
- ◦ Pneumonia and deep tissue abscesses—often life threatening
- ◦ Mild HSM
- ◦ Progress to MDS/AML
-
Treatment
- ◦ GCSF
- ◦ Stem cell transplant for MDS/AML
Cartilage hair hypoplasia
Chédiak–Higashi syndrome
Fanconi anemia
Immune
Autoimmune neutropenia
Neonatal alloimmune neutropenia
Dysgammaglobulinemia
Hyper IgM syndrome
HIV
PNH
Nutritional
B12 and folic acid deficiency—ineffective erythropoiesis with neutropenia
Bone marrow infiltration
Malignancy
MDS
Lymphoproliferative disorders
Platelet Disorders (Fig. 10)
Fig. 10.
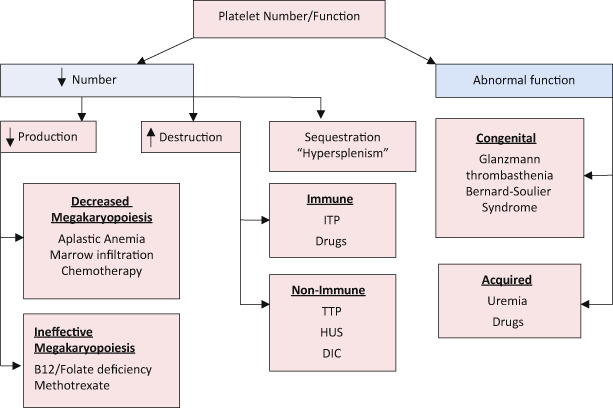
Approach to platelet disorders
Thrombocytopenia
Decreased Production
Amegakaryocytic Thrombocytopenia
Genetics
Autosomal recessive
Clinical presentation
Rash, bruising, or bleeding at birth
-
Most common anomalies:
- Neurologic—cerebellar and cerebral atrophy are frequent
- Cardiac findings—ASD, VSD, PDA, TOF, CoA
-
Other anomalies
- Abnormal hips, feet, kidney, eye, and palate malformation
Diagnosis
Initially absent megakaryocytes then pancytopenia
If beyond neonatal periods, bone marrow aspirate, and biopsy will confirm the diagnosis
Thrombocytopenia Absent Radius Syndrome (TARS)
Clinical presentation
Thrombocytopenia
Absent radius
Congenital heart disease—TOF, ASD, VSD
-
Others
- Eosinophilia
- Leukemoid reaction
- Intellectual disability (ID)
Increased Destruction
Normal to increased megakaryocytes in bone marrow
-
Platelet destruction
-
Immune
- ◦ ITP
- ◦ Drugs
-
Non-Immune
- ◦ TTP
- ◦ HUS
- ◦ DIC
- ◦ Infection
- ◦ Cardiac
-
Idiopathic Thrombocytopenic Purpura (ITP)
Etiology
Antiplatelet antibody
Often a few weeks after infection
Clinical presentation
Petechiae, ecchymoses, epistaxis
Variable symptoms, but usually healthy appearing child
Laboratory
Thrombocytopenia
Normal to increased size of platelets (MPV)
Normal RBCs and WBCs
Treatment
Observation
IVIg
Steroids
WinRho
Platelet transfusion is contraindicated unless life threatening bleeding is present
Splenectomy if > 4 years of age with severe ITP longer than 1 year
-
Neonatal immune thrombocytopenias
- Autoimmune
- Alloimmune
- Erythroblastosis fetalis
-
Secondary
- Viral
- Bacterial
- Drug induced
- Posttransfusion purpura
- SLE
- Hyperthyroidism
- Lymphoproliferative disorders
Hemolytic uremic syndrome
Background
Non-immune
Microangiopathic hemolytic anemia
E. coli O157:H7 is a very common cause
Shigella dysenteriae type I is a another cause
Clinical presentation
Usually children between 4 months and 2 years
Infection with gastrointestinal symptoms—vomiting and often bloody diarrhea
Development of oliguria, hypertension, renal failure
Laboratory
Thrombocytopenia
Microangiopathic hemolytic anemia
Helmet cells, schistocytes, burr cells, spherocytes
Elevated BUN and creatinine
Reduced large multimers of von Willebrand factor (VWF)
Decreased immunoglobulins in some patients
Decreased prostaglandin 12 (PG12) in some patients
Treatment
Aggressive management of renal failure
Correction of anemia with transfusion
Avoid platelet transfusion if possible
Thrombotic Thrombocytopenic Purpura (TTP)
Background
Nonimmune
Microangiopathic hemolytic anemia
Etiology
-
Idiopathic
-
Acute
- ◦ Autoantibody, ADAMTS13 IgG inhibitor
-
Chronic
- ◦ ADAMTS13 mutation
- ◦ Mutation of HF gene
-
Sporadic
- ◦ Gene mutations may be less severe
-
Secondary
- Autoimmune disease
- Malignancy
- Infection
- Drugs
- Stem cell transplantation
- Bacterial endocarditis
-
Clinical presentation
Fever
Headache
Malaise
Abdominal/chest pain
Arthralgia/myalgia
Nausea/vomiting
Pallor
Purpura
Jaundice
Fluctuating neurologic signs and symptoms
Progressive renal failure
Laboratory
Thrombocytopenia
DIC
Blood smear with polychromasia, basophilic stippling, schistocytes, microspherocytes, and nucleated RBCs (Fig. 11)
Elevated VWF antigen
Reduced haptoglobin
Hemoglobinuria and hemosiderinuria
Increased unconjugated bilirubin
Increased LDH
Widespread hyaline microthrombi in the microvasculature in biopsy specimens
-
Other disorders with consumption thrombocytopenia
- DIC
- Virus associated hemophagocytic syndrome
- Hemangioma (Kasabach–Merritt syndrome)
- Cyanotic heart disease
Fig. 11.
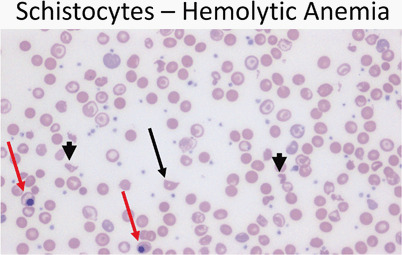
Peripheral smear (40 ×) from a patient with hemolytic anemia showing a schistocyte (arrow) as well as fragmented cells (arrowheads). Note the presence of nucleated red cells (red arrows)
Abnormal Platelets
Wiskott–Aldrich Syndrome
Thrombocytopenia
Tiny platelet
Eczema
Recurrent infection
Bernard–Soulier Syndrome
Absence or deficiency of VWF receptors on the platelet membrane
Markedly prolonged bleeding time
Glanzmann’s Thrombasthenia
Severe platelet dysfunction that yield prolonged bleeding time
Normal platelet count
Aggregation studies show abnormal or absent aggregation
Prolonged bleeding time
Coagulation Disorders
Hemophilia
-
X-linked recessive
- Factor VIII (hemophilia A)—85 %
- Factor IX (hemophilia B)—10–15 %
Bleeding may start from birth or even fetus
Clinical presentation
Easy bruising
Intramuscular (deep) hematomas—localized pain and swelling
-
Hemarthroses
- Hallmark of hemophilia
- Ankle most common
- Knee and elbow increasing frequency with age
Laboratory
PTT is usually 2–3 times upper limit of normal
PT, bleeding time, platelet count normal
Specific assay for factor VIII or IX will confirm the diagnosis
Classifications
Severe hemophilia < 1 %
Moderate hemophilia 1–5 %
Mild hemophilia > 5 %
Treatment
-
Factor replacement
- Mild to moderate bleeding—raise factor to 35–50 %
- Severe or life threatening hemorrhage—raise level to 100 %
Lifelong prophylaxis usually started with first joint hemorrhage
DDAVP may be sufficient in mild forms of hemophilia
Avoidance of high risk behavior
Complications
Severe hemorrhage
Arthropathy
Von Willebrand Disease
Etiology
VWF is a carrier protein for factor VIII
VWF stored in platelets and endothelial cell
VWF adheres to exposed the subendothelial matrix after vascular damage causing platelets to adhere via glycoprotein IB receptors on the VWF
Clinical presentation
VWD usually have symptoms of mucocutaneous hemorrhage
Excessive bruising, epistaxis, menorrhagia, post-operative bleeding (e.g., tonsillectomy, wisdom teeth extraction)
-
Females more commonly diagnosed than males secondary to menorrhagia
- Any menstruating female with iron deficiency, should have a detailed history of bruising and other bleeding symptoms
- Stress doubles or triples level of VWF
Laboratory
-
No single assay to rule out or diagnose VWF
- Bleeding time or PFA
- PTT—often prolonged but frequently normal in type 1 VWD
- VWF antigen
- VWF Ristocetin cofactor activity
- Plasma factor VIII activity
- VWF multimers
- Platelet count
Treatment
-
Based on subtype and trial of DDAVP
- Type 1 usually treated with DDAVP
- DDAVP 0.3 microgram/kg increases the level of VWF and factor VIII 3–5 fold
- Type 2B and 3 primarily treated with FVIII:VWF concentrates
- Platelet type treated with platelet transfusions
Disseminated Intravenous Coagulopathy
Etiology
Widespread intravascular consumption of platelets and plasma clotting factors and deposition of fibrin
Clinical presentation
Bleeding (e.g., from venipuncture sites)
Petechiae, ecchymoses
Clot formation
-
Associated conditions
-
Tissue injury
- ◦ Trauma, especially cranial
- ◦ Burns
- ◦ Venom
- ◦ Malignancy
- ◦ Obstetric emergencies
-
Endothelial cell injury or abnormal vascular surfaces
- ◦ Infection/sepsis
- ◦ Immune complexes
- ◦ Eclampsia
- ◦ Oral contraceptives
- ◦ Giant hemangioma
- ◦ Respiratory distress syndrome (ARDS)
- ◦ Malignancy
-
Platelet, leukocyte, or red cell injury
- ◦ Incompatible blood transfusion
- ◦ Infection
- ◦ Allograft rejection
- ◦ Hemolytic syndromes
- ◦ Drug hypersensitivity
- ◦ Malignancy
-
Laboratory
Prolonged PT and PTT
Decreased fibrinogen
Decreased platelets
Increased fibrin degradation products and D-dimers
Presence of helmet cells, schistocytes
Increased PF4 (platelet factor 4)
Increased FPA (fibrinopeptide A)
Decreased factor V, VIII, XIII
Treatment
Treatment of underlying disorder
Replacement therapy of components as indicated
Neoplastic Disorders
Acute Leukemia
Epidemiology (Table 2 )
Table 2.
Prevalence of leukemia
| Type | Prevalence (%) |
|---|---|
| Acute lymphoblastic | 75–80 |
| • Pre B cell | 80 |
| • Mature B cell (Burkitt) | 1–2 |
| • T cell | 15–20 |
| Acute myeloblastic | 20 % |
| Acute undifferentiated | < 0.5 % |
| Acute mixed lineage | |
|
Chronic Myeloid • Philadelphia chromosome positive • Juvenile myelomonocytic |
3 % |
25–30 % of all childhood cancer
Peak age 2–5 years
Clinical presentation
Anorexia
Fatigue
Fever
Bone and joint pain (especially lower extremities)
Pallor
Petechiae, ecchymoses, epistaxis
-
Extramedullary spread
- Lymphadenopathy
- Hepatosplenomegaly
- Cough, orthopnea
- CNS disease—5 %—cranial nerve palsies
- Testicular involvement—20 %—testicular enlargement
- Ovarian involvement—30 %
- Skin lesions
- Gingival hypertrophy
Laboratory
-
Cytopenias
- Thrombocytopenia—90 %
- Anemia—80 %
- Neutropenia
- 95 % have two cytopenias
- 4 % have only one cytopenia
- 1 % have a normal CBC
-
50 % with elevated WBC
- Usually see blasts if WBC > 5000
Flow cytometry diagnosis
Peripheral blood
ALL: Peripheral blood usually shows leukocytosis with a population of large mononuclear cells (Fig. 12)
AML: Peripheral blood usually shows myeloblasts with a high ratio of nucleus to cytoplasm (Fig. 13)
Fig. 12.
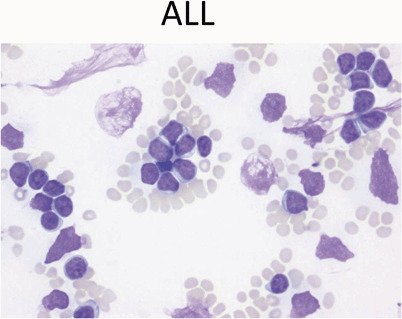
Peripheral blood showing leukocytosis with a population of large mononuclear cells with high nuclear-cytoplasmic ratio, scant blue cytoplasm, and fine chromatin with occasional nucleoli. These are features of lymphoblasts. Note scattered smudge cells, another feature often seen in peripheral smears with leukemia
Fig. 13.
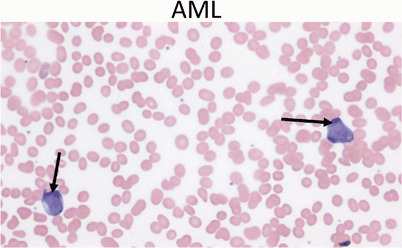
Peripheral blood showing two myeloblasts with a high ratio of nucleus to cytoplasm, finely dispersed chromatin and one or more large nucleoli (arrows). Acute myeloid leukemia represents only 20 % of childhood leukemia
Treatment
Per local or national protocols
Associated syndromes/risk factors
-
ALL
-
Down’s syndrome.
- ◦ Acute leukemia is 34 times more common in children with Down’s syndrome.
- ◦ 20–30 % will develop leukemia by age 3 years.
- ◦ Ratio of ALL and AML is the same as the general population.
- ◦ AML has a better outcomes in children with Down’s syndrome.
-
◦ 10 % of neonates with Down’s syndrome may develop a transient leukemia or myelodysplastic syndrome.
- ▪ Characterized by a high leukocyte count, blast cells, anemia , thrombocytopenia and hepatosplenomegaly.
- ▪ Resolve within days to weeks from initial presentation.
- Ataxia-telangiectasia.
-
Bloom’s syndrome.
- ◦ Immunodeficiency, progeria, growth retardation.
- ◦ Chromosome fragility/breakage.
- ◦ Predisposition to cancer.
-
Fanconi anemia.
- ◦ Pancytopenia, radial bone abnormalities, kidney, skin, or GI abnormalities .
- ◦ Chromosome fragility/breakage.
-
-
AML
- Ionizing radiation
- Organic solvents
- Paroxysmal nocturnal hemoglobinuria
- Down’s syndrome
- Fanconi anemia
- Bloom’s syndrome
-
Kostmann syndrome
- ◦ Severe congenital neutropenia
- ◦ High mortality rate—70 %
-
Shwachman–Diamond syndrome
- ◦ Congenital neutropenia
- ◦ Metaphyseal chondrodysplasia
- ◦ Exocrine pancreatic deficiency
-
Diamond–Blackfan syndrome
- ◦ Congenital pure red cell aplasia
- ◦ Increased erythrocyte adenosine deaminase
- ◦ Short stature
- ◦ Developmental delay
- ◦ Thumb malformations
- ◦ Craniofacial anomalies
- ◦ Urogenital anomalies
- ◦ Increased MCV on CBC
-
Neurofibromatosis
- ◦ Bone marrow failure
- ◦ Predisposition to cancers, especially AML and neuroblastoma
-
Chronic myelogenous leukemia (CML)
- 99 % characterized by specific translocation known as the Philadelphia chromosome t(9;22)
Lymphadenopathy
Causes of lymphadenopathy according to location
-
Cervical
- Oropharyngeal infections, for example, EBV
- Mycobacterial lymphadenitis
- Cat scratch disease
- Kawasaki disease
-
Supraclavicular
- Right side—Malignancy or infection in the mediastinum
- Left side—Malignancy or infection from the abdomen
- Lymphoma
- Tuberculosis
-
Hilar
- Tuberculosis
- Histoplasmosis
- Leukemia
- Lymphoma
- Sarcoidosis
-
Axillary
- Cat scratch disease
- Arm or chest infection
- Leukemia
- Lymphoma
-
Abdominal
- Malignancy
- Mesenteric adenitis
Clinical approach to lymphadenopathy
-
History
- Associated other systemic symptoms
-
Age
- Lymph node enlargement in children less than 5 years most likely infectious
- Histiocytosis can cause lymphadenopathy in children < 3 years
- Large lymph node in neonate most likely related to congenital infection
- Likelihood of malignant lymphoma increases in adolescents
-
Location
- Supraclavicular lymphadenopathy is always abnormal and the chances of malignancy are high
-
Size
- Size of the enlarged lymph node aids in determining the need for further evaluation
- Axillary and cervical > 1 cm
- Inguinal > 1.5 cm
- Epitrochlear > 0.5 cm
- Anywhere > 2 cm
-
Characteristics
- Usually develops over weeks or months.
- Nontender, discrete, firm, rubbery, often immobile
Biopsy criteria
-
Size
- > 2 cm
- Increasing over 2 weeks
- No decrease in size after 4 weeks
-
Location
- Supraclavicular
-
Consistency
- Hard
- Matted
- Rubbery
-
Associated features
- Abnormal CXR
- Fever
- Weight loss
- Hepatosplenomegaly
Hodgkin Lymphoma
Hodgkin disease (HD)
Rare in children < 10 years
15 % of cancers in persons between 15 and 19 years
Bimodal peaks of incidence from 15–35 years of age and at 55 years of age
-
Infectious agents may be involved
- EBV
- HHV6
- CMV
Reed–Sternberg cell is the hallmark of HD (Fig. 14)
Fig. 14.
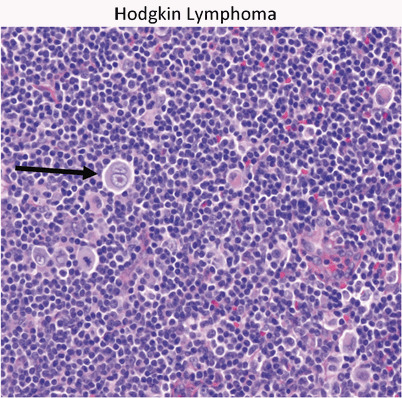
Hodgkin’s lymphoma presents as a localized or regional lymphadenopathy. The characteristic cell in Hodgkin’s is the Reed–Sternberg cell (arrow)
Clinical presentation
Painless lymphadenopathy
Airway obstruction
Pleural dysfunction
Pericardial dysfunction
Hepatocellular dysfunction
Bone marrow infiltration
-
Systemic symptoms (B symptoms)
- Fever > 39 C
- Weight loss > 10 % of body weight
- Night sweats
Diagnosis
CXR
CT abdomen and pelvis
PET scan
CBC, CMP, ESR, ferritin
Treatment
Chemotherapy and radiotherapy are very effective
-
Chemotherapy regimens
- COPP (cyclophosphamide, vincristine, procarbazine, and prednisone)
- ABVD (Doxorubicin (adriamycin) bleomycin, vinblastine, and dacarbazine)
Prognosis
Early stage disease have event free survival 85–90 %, overall survival at 5 years of 95 %
-
Poor prognostic features
- Bulky tumor
- Advanced stage at diagnosis
- B symptoms
Patient who relapse > 12 months after chemotherapy alone or combined modality have good retrieval response
Non-Hodgkin Lymphoma
60 % of all lymphomas in children
Burkitt lymphoma is the most common
Most children have de novo disease (no underlying condition)
-
Related diseases
- Severe combined immunodeficiency (SCID)
- Wiskott–Aldrich syndrome
- Ataxia telangiectasia
- Bloom’s syndrome
- HIV
- EBV
Clinical presentation
Rapidly growing tumors with symptoms based on size and location
Burkitt lymphoma of abdomen (sporadic type) more common in the USA
Burkitt lymphoma of head and neck (endemic type) more common in Africa
Superior vena cava (SVC) syndrome—chest involvement
Intestinal obstruction—abdominal mass
Paraplegia with spinal cord involvement
-
Tumor lysis syndrome
- Hyperkalemia, hyperuricemia, hyperphosphatemia, hypocalcemia
Diagnosis
CXR
CT abdomen and pelvis
CBC, CMP, Mg, Phos, Uric Acid, LDH
EBV
Biopsy
Classic “starry sky” appearance of Burkitt lymphoma (Fig. 15)
Fig. 15.
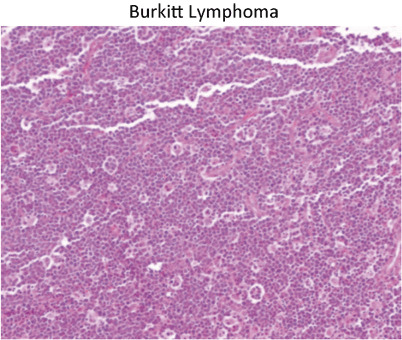
Classic “starry sky” appearance of Burkitt lymphoma. The stars are actually macrophages that are phagocytosing apoptotic Burkitt cells. This example presented as a colonic mass with intussusception
Treatment
Chemotherapy
Prognosis
Excellent in most of children
90–100 % survival rate with localized disease
Brain Tumors
Epidemiology
Almost 20 % of all pediatric cancers
Peak age 0–4 years
-
Most common cancer mortality in children
- 25 % of all deaths from cancer
Clinical presentation
Based on location, size, growth rate and age
-
Increased intracranial pressure
- Headache
- Vomiting (often mornings)
- Mental changes, irritability
-
Visual disturbances
- ◦ Diplopia
- ◦ Papilledema
- ◦ Parinaud’s
- Gait disturbances
Failure to thrive
Cranial nerve abnormalities
Focal neurologic deficits
Seizures
Pathologic diagnosis
Based on cell of origin
-
Can occur at multiple locations in the CNS
- Infratentorial—60 %
- Supratentorial—40 %
-
Common in children
-
Astrocytoma (Fig. 17)
- ◦ 40 % of all CNS tumors
- ◦ Juvenile pilocytic astrocytoma—most common subtype in children
- ◦ Classic site for JPA is cerebellum, but can occur anywhere in CNS
-
Treatment
- ◦ Surgery—primary treatment
- ◦ Chemotherapy
- ◦ Radiation therapy
-
Medulloblastoma (Fig. 16)
- ◦ 20 % of all brain tumors (second most common)
- ◦ 90 % of embryonal tumors
- ◦ Arises in cerebellum and fourth ventricle
- ◦ May metastasize down spinal cord and rarely outside CNS
-
◦ Similar cell type to primitive neuroectodermal tumor (PNET)Treatment
- ▪ Surgery—prognosis based on extent of resection
- ▪ Chemotherapy
- ▪ Radiation therapy
-
Ependymoma
- ◦ Derived from the ependymal lining of the ventricles
- ◦ 70 % occur in the posterior fossa
-
Pineal tumors
-
◦ Germ cell tumors
- ▪ Germinoma
- ▪ Yolk sac tumor
- ▪ Mixed germ cell tumor
- ◦ Pineoblastoma
- ◦ PNET
-
-
Craniopharyngioma
- ◦ 7–10 % of childhood brain tumors
- ▪ Suprasellar location
- ◦ Solid and cystic components
-
◦ Associated with panhypopitutarism and visual loss
- ▪ Tumor related
- ▪ Treatment related
-
-
Syndromes associated with brain tumors
- Neurofibromatosis type 1: optic glioma, astrocytoma, neurofibroma, malignant nerve sheath tumor
- NF type 2: vestibular schwannomas, meningiomas, spinal cord ependymoma, spinal cord astrocytoma
- Von Hippel–Lindau: Hemangioblastoma, angiomatosis, pheochromocytoma, renal cell carcinoma, pancreatic cyst
- Li–Fraumeni: astrocytoma
- Cowden syndrome: multiple hamartomas including the brain; dysplastic gangliocytoma of the cerebellum
- Turcot syndrome: medulloblastoma and colon polyps
Fig. 17.
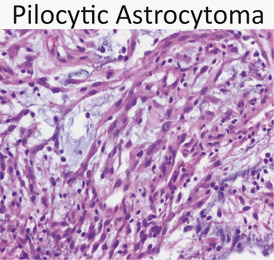
Pilocytic astrocytoma is composed of bipolar cells with frequent microcystic spaces. Juvenile pilocytic astrocytoma is the most common childhood primary brain tumor
Fig. 16.
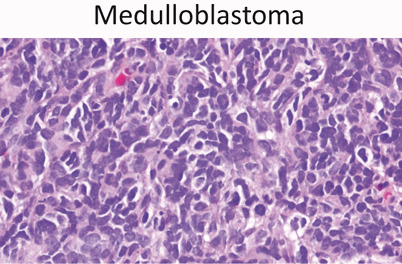
Medulloblastoma (40x) is a so-called “small round blue” cell tumor of childhood. Medulloblastoma is a posterior fossa tumor and the second most common brain tumor of childhood
Neuroblastoma (Fig. 18)
Fig. 18.
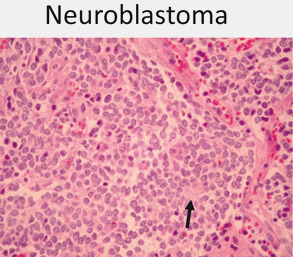
Neuroblastoma is one of the small round blue cell tumors of childhood. A majority are at least poorly differentiated with the presence of some neuropil (black arrow) often in association with Homer- Wright rosettes (10x)
Epidemiology
Third most common pediatric cancer
8 % of childhood malignancy
Most commonly diagnosed neoplasm in infants (28–39 % of neonatal malignancies)
Mean age is 2 years
Clinical presentation
Fever, failure to thrive
-
Paraneoplastic symptoms
- Secretory diarrhea
- Increased sweating
- Hypertension
- Opsoclonus, myoclonus (dancing eyes and dancing feet)
-
Most cases arise in abdomen
- Abdominal pain
- Distended abdomen, mass
-
Thoracic tumors
- Occasional Horner’s syndrome
-
Spinal tumors
- Paraplegias
-
Metastatic disease
- Bone pain (bone mets)
- Cytopenias (bone marrow infiltrate)
- Orbital proptosis and ecchymosis-“raccoon eyes” (retro-orbital soft tissue infiltrate)
- Bluish subcutaneous nodules (skin infiltrate)
Diagnosis
CT/MRI scans often show calcifications
-
Tumor markers
- Urine homovanillic acid (HVA), vanillylmandelic acid (VMA)
-
Poor prognostic factors on pathology
- N-myc proto-oncogene (MYCN) amplification
- DNA hyperdiploidy (if less than 1 year of age)
Treatment
Chemotherapy
Radiation therapy
Stem cell transplant
New vaccines/antibodies
Retinoic acid
Associated syndromes/risk factors
Hirschsprung’s disease
Pheochromocytoma in family
Fetal hydantoin syndrome
Fetal alcohol syndrome
Nesidioblastosis
Wilms Tumor (Fig. 19)
Fig. 19.
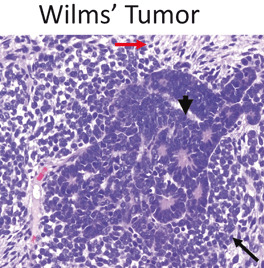
Wilms’ Tumor is a triphasic tumor composed of blastemal (black arrow), epithelial (arrowhead) and mesenchymal components (red arrow). Most are diagnosed before 6 years of age
WT-1 gene located on 11p13
Epidemiology
Peak incidence 2–5 years of age
8 cases/million children < 15 years
Clinical presentation
Abdominal mass often noted first by parents
Abdominal pain, vomiting, hematuria in 12–25 %
Hypertension
-
Anomalies and syndromes associated with Wilms tumor
- Beckwith-Wiedemann (organomegaly, macroglossia, omphalocele, hemihypertrophy)
- WAGR (aniridia, genitourinary abnormalities, intellectual disability (ID), del 11p13)
- Denys-Drash (early onset renal failure with renal mesangial sclerosis, male pseudohermaphroditism)
Diagnosis
US, KUB, CT, and/or MRI
U/A
Treatment
Surgery, chemotherapy, and radiotherapy
-
Poor prognostic factor
- Large tumor > 500 g
- Advanced stage (III or IV)
- Unfavorable histologic type
Rhabdomyosarcoma
Epidemiology
Most common soft tissue sarcoma
3.5 % of childhood tumors
Increased frequency with neurofibromatosis
Peak incidence 1–5 years
10 % occur in the first year of life
70 % appear within first decade
Clinical presentation
-
Anatomic distribution
- Head and neck—40 %
- GU—20 %
- Trunk—10 %
- Retroperitoneal and others
Specific histologic types
Embryonal: 60 %, intermediate prognosis
Alveolar type: 15 %, most in trunk and extremeties, poor prognosis (Fig. 20)
Botryoid type: 6 %, “bunch of grapes”, most in vagina, uterus, bladder, nasopharynx, and middle ear, good prognosis
Pleomorphic form: 1 %, adult type
Fig. 20.
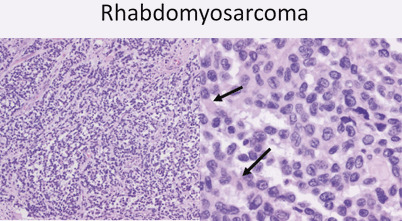
At low power (10x) alveolar rhabdomyosarcoma has a vaguely alveolar growth pattern with neoplastic cells lining thin fibrous septae. At higher power pink cytoplasmic material is evident (arrows) showing early myogenic differentiation
Osteosarcoma
Epidemiology
Most common primary malignant bone tumor in children
Most present in second decade
More common in males
Clinical presentation
Local pain, swelling, often history of injury
-
Associated syndromes/risk factors
- Retinoblastoma , Li–Fraumeni syndrome, Paget disease, radiotherapy
Diagnosis
-
Pathologic findings (Fig. 21)
- Spindle to epithelioid cells producing osteoid (bone forming)
-
Radiologic findings
- Scelerotic destruction (sunburst)
- Lytic lesion less common
Fig. 21.
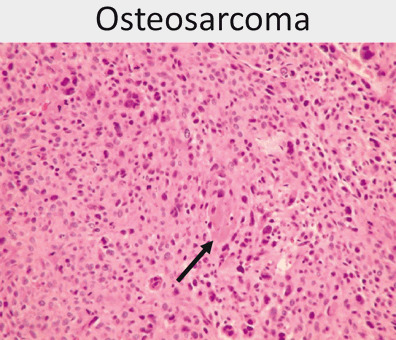
Osteosarcoma is composed of a pleomorphic cell population of ovoid and frankly bizarre cells with focal osteoid formation (black arrow)
Differential diagnosis
Ewing sarcoma
Osteomyelitis
Metastasis
Lung and bone
Treatment
Chemotherapy
-
Surgical resection
- Amputation
- Prosthesis
Ewing Sarcoma
Epidemiology
Second decade
More common in males
Clinical presentation
Local pain, swelling, fever
-
Location
- Diaphysis of long bone, flat bones
Diagnosis
-
Pathology
- Undifferentiated small round cell tumor
-
Radiologic findings
- Primarily lytic lesions (onion ring appearance)
Treatment
Chemotherapy
Radiation therapy
+/ − surgery
Prognosis
Localized—60 % survival
Metastatic—20–30 % survival
Osteoid Osteoma
Small benign bone tumor
Epidemiology
Occurs in patients from 2–50 years of age
Male are more common than females
Clinical presentation
-
Gradually increasing pain
- Often worse at night and relieved by aspirin
Lower extremity lesion may develop limp, atrophy, or weakness
Palpation and range of motion may not alter the discomfort
Vertebral lesions may cause scoliosis
Most common in proximal tibia and femur, can involve any bone
Diagnosis
-
Radiologic findings
- Round, oval metaphyseal or diaphyseal lucency surrounded by sclerotic bone
- Central lucency or nidus shows intense uptake of bone scan
- 25 % only visualized by CT
- Not seen on MRI
Treatment
Removal of the lesion and ablation of nidus
Treat pain with aspirin
Retinoblastoma
Epidemiology
Arises following mutation of both Rb genes at 13q14
-
Hereditary form associated with germline inactivating mutation of one copy of RB1 gene
- Need “second hit”, somatic mutation to second RB1 gene to develop tumor
- 80–90 % with germline mutation get a second hit and develop retinoblastoma
Sporadic cases involve 2 somatic mutations to RB1 gene
60 % sporadic, 40 % familial
-
30 % bilateral
- 90 % of familial tumors are bilateral
May be present congenitally
Most present between 6 months and 2 years of age
Clinical presentation
Leukocoria—white pupillary reflex
Strabismus —usually the initial presenting complaint
Orbital inflammation, proptosis, hyphema , irregular pupils with advanced disease
Pain if secondary glaucoma develops
Diagnosis
Exam by ophthalmologist under anesthesia
CT or MRI
Metastatic workup for larger lesions
Treatment
Chemotherapy
Focal laser photocoagulation
Radiation therapy in severe cases
Enucleation of unresponsive cases, especially if loss of vision (Fig. 22)
Fig. 22.
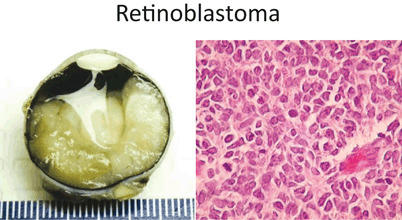
Left – Gross photo showing the white tumor mass filling twothirds of the posterior chamber of the eye. Right – Retinoblastoma is another “small round blue” cell tumor of childhood
Prognosis
95 % cure rate in US
Hepatoblastoma (Fig. 23)
Fig. 23.
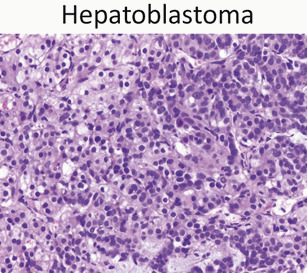
Hepatoblastoma is composed of epithelial components – fetal, embryonal, or a mixture of the two – and occasionally mesenchymal components. The image here is of fetal epithelial type hepatoblastoma with a classic “light and dark” appearance
Epidemiology
Children < 3 years
Can be congenital
90 % occur by age of 5 years, 70 % by age of 2 years
Male predominance
Prevalence of 1 per 120,000 (1 per 1 million children under age 15 years)
-
Associated syndromes/risk factors
- Familial adenomatous polyposis (APC gene mutation)
- Glycogen storage disease
- Beckwith–Wiedemann syndrome
- Li–Fraumeni syndrome
- Low birth weight infants
- Wilms tumor
Clinical presentation
Large asymptomatic mass
Right lobe more common
Weight loss, anorexia, vomiting, or abdominal pain
Diagnosis
US, KUB, CT, and/or MRI
Bilirubin and liver enzymes are usually normal
Alpha-fetoprotein is elevated in all hepatoblastomas
Anemia and thrombocytosis are common
Hepatitis B and C serologies {usually negative}
Treatment
Chemotherapy
Tumor resection
As much as 85 % of liver can be resected
Hepatic regeneration noted within 3–4 months of surgery
Contributor Information
Osama Naga, Email: osamanaga@yahoo.com.
Staci Bryson, Email: staci.bryson@ttuhsc.edu.
Arlynn F. Mulne, Email: lynne.mulne@ttuhsc.edu.
Suggested Readings
- 1.Boxer LA. Neutrophil abnormalities. Pediatr Rev. 2003;24:52–62. doi: 10.1542/pir.24-2-52. [DOI] [PubMed] [Google Scholar]
- 2.Donadieu J, Fenneteau O, Beaupain B, Mahlaoui N, Chantelot CB. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J Rare Dis. 2011;6:26. doi: 10.1186/1750-1172-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaston MH, Verter JI, Woods G. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314:1593–9. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 4.Knight PJ, Mulne AF, Vassy LE. When is lymph node biopsy indicated in children with enlarged peripheral nodes? Pediatrics. 1982;69:391–6. [PubMed] [Google Scholar]
- 5.Rogers ZR. Priapism in sickle cell disease. Hematol Oncol Clin N Am. 2005;19:917–28. doi: 10.1016/j.hoc.2005.08.003. [DOI] [PubMed] [Google Scholar]


