Abstract
Objective:
Investigate the spectrum of radiographic patterns of radiation pneumonitis (RP) in lung cancer patients and identify imaging markers for high-grade RP and RP-related death.
Methods:
Eighty-two patients with lung cancer treated with conventional chest radiotherapy who had symptomatic RP were identified from the radiation oncology database. The imaging features of RP were studied for association with high-grade RP (Grade ≥3) and RP-related death (Grade 5).
Results:
RP was Grade 2 in 60 (73%), Grade 3 in 15 (18%), and Grade 5 in 7 patients (9%). Lower performance status (p=0.04), squamous cell histology (p=0.03), and FEV1≤2 (p=0.009) were associated with high-grade pneumonitis. Older age (p=0.03) and squamous cell histology (p=0.03) were associated with RP-related death. The CT findings included ground-glass and reticular opacities in all patients, with traction bronchiectasis in 77 (94%) and consolidation in 74 (90%). The most common radiographic pattern of RP was cryptogenic organizing pneumonia (COP) pattern (n=54), followed by acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS) pattern (n=10). Higher extent of lung involvement, diffuse distribution, and AIP/ARDS pattern were associated with high-grade pneumonitis and RP-related death. AIP/ARDS pattern was a significant factor for high-grade pneumonitis (OR:12.62, p=0.01) in multivariable analyses adjusting for clinical variables.
Conclusion:
COP pattern was the most common radiographic pattern for symptomatic RP in lung cancer patients. AIP/ARDS pattern was significantly associated with high-grade RP and RP-related deaths, and was an independent marker for high-grade RP. The recognition of the radiographic patterns of RP can help to effectively contribute to patient management.
Keywords: radiation, pneumonitis, lung cancer, imaging, computed tomography
INTRODUCTION
Radiation therapy has been used in oncology for more than a century and currently it plays an important role in the management of a wide variety of tumors1–3. For non-small cell lung cancer (NSCLC), radiation therapy is used as primary treatment in patients with unresectable disease or in poor surgical candidates, or as an adjuvant treatment for those with positive surgical margins4,5. Likewise, patients with limited stage small-cell lung cancer (SCLC) are treated with a combination of chemotherapy and radiotherapy6,7. Additionally, radiotherapy is also used for palliative management of metastases in lung cancer patients8.
Radiation-induced toxicity remains the biggest challenge to radiotherapy and the procedure has evolved over the years to minimize injury to non-target tissues9–11. Understandably, the organs immediately adjacent to the tumor receive higher radiation dose, and therefore radiation pneumonitis (RP) is the most common dose-limiting toxic effect in patients receiving chest radiotherapy for lung cancer12,13. Of the toxicity criteria used to evaluate RP, the Radiation Therapy Oncology Group (RTOG) criteria and the Common Terminology Criteria for Adverse Events (CTCAE) are most commonly used14–16. The CTCAE can be applied to evaluate adverse effects to any type of cancer therapy beyond radiotherapy, and thus provide more comparable grading across different treatments.
The incidence rates of symptomatic RP varies across different series with multiple factors, and in general this is less than 10%17,18. However, at least a certain degree of lung parenchymal changes are noted on computed tomography (CT) in majority of the patients after chest radiotherapy18–20, making it difficult to distinguish between clinically insignificant post radiation changes and clinically significant, symptomatic RP. A detailed imaging review focusing on a patient cohort with clinical diagnosis of symptomatic RP is needed to identify imaging features that are predictive of higher grade RP and RP-related death.
The purpose of the present study is to investigate the spectrum of imaging features and radiographic patterns of RP in patients with lung cancer treated with high-dose radiation therapy, and identify imaging markers that are associated with high-grade RP and RP-related death.
MATERIALS AND METHODS
Patients
A total of 815 patients with lung cancer were treated with conventional chest radiotherapy between January 2005- January 2014 in the Radiation Oncology Data Repository at our institution. Among them, 82 patients were clinically diagnosed as having developed symptomatic radiation pneumonitis (CTCAE grade ≥2) and had chest CT scans at the time of diagnosis of radiation pneumonitis available for review, which were included in the study population of the present study. The medical record and the imaging studies of these patients were retrospectively reviewed with the Institution Review Board (IRB) approval in this Health Insurance Portability and Accountability Act (HIPAA) compliant study. Informed consent was waived by the IRB.
Image analysis
Chest CT scans performed at the time of diagnosis of radiation pneumonitis were reviewed in consensus by two thoracic radiologists (MN and HH, with 14 and 30 years of experience respectively). The radiologists were informed of the planning target volume (PTV) of the radiation and had access to a series of prior imaging and prior lung surgery details, if applicable. This was done to ensure correct demarcation of the radiation field and to distinguish radiation-related lung changes from preexisting lung abnormalities. However, the radiologists did not have access to the RP grades at the time of CT scoring.
Imaging features were scored according to the methods used in the prior studies of treatment-related pneumonitis in cancer patients21–24. The items of evaluation included: i) extent in terms of upper, middle and lower lungs using a 6-point scale (0: none, 1: 1–5%, 2: 6–25%, 3: 26–50%, 4: 51–75%, 5: 76–100%); ii) distribution in terms of diffuse, peripheral, multifocal, focal, and geographic; iii) distribution in relation to the expected radiation field (1: definitely confined to the radiation field, 2: probably confined to the radiation field, 3: equivocal, 4: probably beyond the radiation field, 5: definitely beyond the radiation field); and iv) lobar involvement. The presence or absence of the specific CT findings including ground-glass opacities (GGO), reticular opacities, consolidation, centrilobular nodularity, traction bronchiectasis, and honeycombing was also recorded21–24. In each case, radiographic patterns of radiation pneumonitis were classified referring to ATS/ERS international multidisciplinary classification of interstitial pneumonias and the related conditions as described previously25,26 as 1) acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS) pattern, 2) cryptogenic organizing pneumonia (COP) pattern, 3) non-specific interstitial pneumonia (NSIP) pattern, 4) hypersensitivity pneumonitis (HP) pattern, and 5) indistinguishable from post radiation changes21–24. Pneumonitis related to cancer treatment represents a manifestation of the lung’s responses to injury, and the lung’s response patterns to injuries demonstrate several radiographic patterns on CT. These patterns are originally described in the setting of idiopathic interstitial pneumonias, however, can also be noted in the setting of interstitial lung diseases with underlying causes, and thus have been applied to describe treatment-related pneumonitis in cancer patients as previously published21–24,27,28.
Data analysis
Descriptive statistics were used to describe clinical characteristics and imaging features of radiation pneumonitis. Differences in distributions of categorical variables and continuous variables between patients with and without high-grade pneumonitis (defined as CTCAE grade ≥3) were evaluated using Fisher’s exact test and Wilcoxon rank-sum test, respectively. Logistic regression models were used to assess the associations between high-grade pneumonitis and clinical characteristics as well as imaging features. Covariates with p values less than 0.05 in the univariable analysis were included in the multivariable models. Among the imaging features, only AIP/ARDS pattern was included in the multivariable models as all other imaging characteristics were strongly associated with AIP/ARDS pattern. All p values were two-sided and a p value of less than 0.05 was considered significant.
RESULTS
Clinical characteristics of patients with radiation pneumonitis
Table 1 lists the demographics and clinical characteristics of the 82 patients with symptomatic RP, for all patients in the cohort, and according to the RP grades. Thirty-five patients were men and 47 were woman, with a median age of 68 years. Median time from the time of initiation of radiation therapy to RP was 4.2 months (interquartile range 3.0–5.6 months). RP grade was Grade 2 in 60 (73%), Grade 3 in 15 (18%), and Grade 5 (lethal) in 7 patients (9%).
Table 1:
Patient Demographics, Disease and Treatment Characteristics by Radiation Pneumonitis Grades
| All patients | Grade 2 RP (%) | Grade 3–5 RP (%) | p value | Non-lethal RP (%) | Lethal RP (%) | p-value | |
|---|---|---|---|---|---|---|---|
| Age (year) | 0.25 | 0.03 | |||||
| Median | 67.5 | 65 | 73 | 66 | 76 | ||
| Range | 38–83 | 46–83 | 38–82 | 38–83 | 60–82 | ||
| Gender | 1.00 | 0.69 | |||||
| Female | 47 | 34 (72%) | 13 (28%) | 42 (89%) | 5 (11%) | ||
| Male | 35 | 26 (74%) | 9 (26%) | 33 (94%) | 2 (6%) | ||
| Race | 1.00 | 0.24 | |||||
| White | 79 | 58 (73%) | 21 (27%) | 73 (92%) | 6 (8%) | ||
| African American | 3 | 2 (67%) | 1 (33%) | 2 (67%) | 1 (33%) | ||
| Performance Status | 0.04 | 0.43 | |||||
| 0/1 | 76 | 58 (76%) | 18 (24%) | 70 (92%) | 6 (8%) | ||
| 2/3 | 6 | 2 (33%) | 4 (67%) | 5 (83%) | 1 (17%) | ||
| Smoking Status | 0.70 | 1.00 | |||||
| Never Smoker | 9 | 6 (67%) | 3 (33%) | 9 (100%) | 0 | ||
| Former/Current Smoker | 73 | 54 (74%) | 19 (26%) | 66 (90%) | 7 (10%) | ||
| Pack-Years | 0.26 | 0.22 | |||||
| Median | 40 | 50 | 53.5 | 40 | 57 | ||
| Histology | 0.03 | 0.03 | |||||
| Squamous Cell Carcinoma | 25 | 14 (56%) | 11 (44%) | 20 (80%) | 5 (20%) | ||
| Other | 57 | 46 (81%) | 11 (19%) | 55 (97%) | 2 (3%) | ||
| Clinical Stage | 0.80 | 0.69 | |||||
| I/II | 29 | 22 (75.9%) | 7 (24.1%) | 26 (89.7%) | 3 (10.3%) | ||
| III/IV | 53 | 38 (71.7%) | 15 (28.3%) | 49 (92.5%) | 4 (7.5%) | ||
| FEV1 | 0.009 | 0.24 | |||||
| ≤2 | 32 | 17 (53%) | 15 (47%) | 27 (84%) | 5 (16%) | ||
| >2 | 36 | 30 (83%) | 6 (17%) | 34 (94%) | 2 (6%) | ||
| Missing | 14 | 13 | 1 | 14 | 0 | ||
| Predicted FEV1 (%) | 0.30 | 0.43 | |||||
| ≤80 | 35 | 22 (63%) | 13 (37%) | 30 (86%) | 5 (14%) | ||
| >80 | 33 | 25 (76%) | 8 (24%) | 31 (94%) | 2 (6%) | ||
| Missing | 14 | 13 | 1 | 14 | 0 | ||
| History of COPD | 0.12 | 0.20 | |||||
| No | 56 | 44 (79%) | 12 (21%) | 53 (95%) | 3 (5%) | ||
| Yes | 26 | 16 (62%) | 10 (38%) | 22 (85%) | 4 (15%) | ||
| History of Fibrosis | 0.07 | 0.16 | |||||
| No | 80 | 60 (75%) | 20 (25%) | 74 (93%) | 6 (7%) | ||
| Yes | 2 | 0 | 2 (100%) | 1 (50%) | 1 (50%) | ||
| RT Technique | 1.00 | 0.67 | |||||
| 3DCRT | 60 | 44 (73%) | 16 (27%) | 54 (90%) | 6 (10%) | ||
| IMRT or 3DCRT+IMRT | 22 | 16 (73%) | 6 (27%) | 21 (96%) | 1 (4%) | ||
| Gross Tumor Volume | 0.22 | 0.70 | |||||
| Primary Tumor, Hilar and Mediastinal Nodes | 39 | 26 (67%) | 13 (33%) | 35 (90%) | 4 (10%) | ||
| Other | 43 | 34 (79%) | 9 (21%) | 40 (93%) | 3 (7%) | ||
| RT Dose | 1.00 | 0.69 | |||||
| <66 Gy | 48 | 35 (73%) | 13 (27%) | 43 (90%) | 5 (10%) | ||
| ≥66 Gy | 34 | 25 (75%) | 9 (25%) | 32 (94%) | 2 (6%) | ||
| Mean Lung Dose# | 0.79 | 1.00 | |||||
| ≤17 | 44 | 33 (76%) | 11 (25%) | 40 (91%) | 4 (9%) | ||
| >17 | 30 | 21 (70%) | 9 (30%) | 28 (93%) | 2 (7%) | ||
| Missing | 8 | 6 | 2 | 7 | 1 | ||
| Lung V20† | 0.79 | 1.00 | |||||
| ≤30 | 47 | 35 (75%) | 12 (24%) | 43 (92%) | 4 (8%) | ||
| >30 | 27 | 19 (70%) | 8 (30%) | 25 (93%) | 2 (7%) | ||
| Missing | 8 | 6 | 2 | 7 | 1 |
p-value based on Fisher’s exact for categorical variables and Wilcoxon rank sum test for continuous variables
Mean Lung Dose of patients with lethal RP had a median of 15.0 Gy (range: 6.2 – 18.2).
Lung V20 of patients with lethal RP had a median of 25.75% (range: 9.0 – 33.8)
RP – radiation pneumonitis, FEV1 – forced expiratory volume 1, COPD – chronic obstructive pulmonary disease, RT – radiation therapy, 3DCRT – three-dimensional conformal radiation therapy, IMRT – intensity modulated radiation therapy, lung V20 – volume of normal lung receiving 20 Gy
Seven patients were treated with radiotherapy alone, whereas 75 patients also received chemotherapy prior to the development of RP, including induction chemotherapy alone (n=2), concurrent chemotherapy alone (n=32), adjuvant therapy alone (n=5), induction and concurrent therapy (n=7), concurrent and adjuvant therapy (n=25), and induction, concurrent plus adjuvant therapy (n=4). The induction regimen was platinum-based using carboplatin or cisplatin, in combination with etoposide, paclitaxel, or pemetrexed. Concurrent regimen was also platinum-based as in the induction therapy, except for one patient who received navelbine alone. The adjuvant regimen was similarly platinum-based for most patients, except for 5 patients who received paclitaxel or docetaxel single-agent therapy.
Among the clinical characteristics, poor performance status at diagnosis of lung cancer (PS of 2 or above; p=0.04), squamous cell histology (p=0.03), and FEV1 ≤2 (p=0.009) were associated with high-grade (Grade 3 or above) RP compared to Grade 2 RP. Older age (p=0.03) and squamous cell histology (p=0.03) were also associated with RP-related death (Grade 5) (Table 1).
Imaging characteristics and radiographic patterns of symptomatic radiation pneumonitis
Table 2 summarizes the imaging characteristics and radiographic patterns of symptomatic RP, in the entire population and according to the RP grades. The distribution of the lung parenchymal findings of RP on CT was diffuse in 11 (13%), peripheral in 2 (2%), multifocal in 52 (63%), focal in 2 (2%) and geographic in 15 (18%) patients. The CT findings extended beyond the expected radiation field in 67 patients (82%) and were confined to the radiation field in 15 patients (18%). One patient had undergone right lower lobectomy, one had undergone right upper lobectomy and one had undergone left pneumonectomy. Both lungs were involved in 48 patients (59%) and all lobes were involved in 28 patients (34%). Specific CT findings of GGO and reticular opacities were noted in all patients, accompanied with traction bronchiectasis in 77 (94%) and consolidation in 74 (90%) patients.
Table 2:
Patient Demographics, Disease and Treatment Characteristics by Radiation Pneumonitis Grades
| All Patients | Grade 2 RP (%) | Grade 3–5 RP (%) | p-value | Non-lethal RP (%) | Lethal RP (%) | p-value | |
|---|---|---|---|---|---|---|---|
| Extent* | 0.003 | 0.0008 | |||||
| Median | 8.5 | 8 | 12 | 8 | 15 | ||
| Range | 3–15 | 3–12 | 4–15 | 3–15 | 8–15 | ||
| Distribution | <0.0001 | 0.0003 | |||||
| Diffuse | 11 | 2 (18%) | 9 (82%) | 6 (55%) | 5 (45%) | ||
| Other | 71 | 58 (82%) | 13 (18%) | 69 (97%) | 2 (3%) | ||
| Bilateral Involvement | 0.14 | 0.23 | |||||
| Yes | 48 | 32 (67%) | 16 (33%) | 42 (88%) | 6 (12%) | ||
| No | 34 | 28 (82%) | 6 (18%) | 33 (97%) | 1 (3%) | ||
| All Lobe Involvement | 0.11 | 0.006 | |||||
| Yes | 28 | 17 (61%) | 11 (39%) | 22 (79%) | 6 (21%) | ||
| No | 54 | 43 (80%) | 11 (20%) | 53 (98%) | 1 (2%) | ||
| Out of Field | 0.75 | 0.34 | |||||
| Yes | 67 | 48 (72%) | 19 (28%) | 60 (90%) | 7 (10%) | ||
| No | 15 | 12 (80%) | 3 (20%) | 15 (100%) | 0 | ||
| Radiographic Pattern | 0.0003 | 0.0002 | |||||
| AIP/ARDS patterns | 10 | 2 (20.0%) | 8 (80.0%) | 5 (50.0%) | 5 (50.0%) | ||
| Other patterns | 72 | 58 (80.6%) | 14 (19.4%) | 70 (97.2%) | 2 (2.8%) |
RP – radiation pneumonitis, AIP/ARDS – acute interstitial pneumonia/acute respiratory distress syndrome
Extent was obtained for each patient as the sum of the scores in the upper, and middle, and lower lungs.
Of the 67 patients with the RP findings extending beyond the expected radiation field, the most common radiographic pattern of RP was COP pattern with multifocal/focal distribution (n=54; 81%) (Figure 1), followed by AIP/ARDS pattern with diffuse distribution (n=10; 15%) (Figure 2), while a few patients demonstrated NSIP pattern with peripheral distribution (n=2; 3%)(Figure 3) or HP pattern with diffuse distribution (n=1; 1%). In the remaining 15 patients (18%), the changes of RP were confined to the radiation field with a typical geographic distribution and were indistinguishable from the expected post radiation changes without symptomatic complications.
Figure 1.
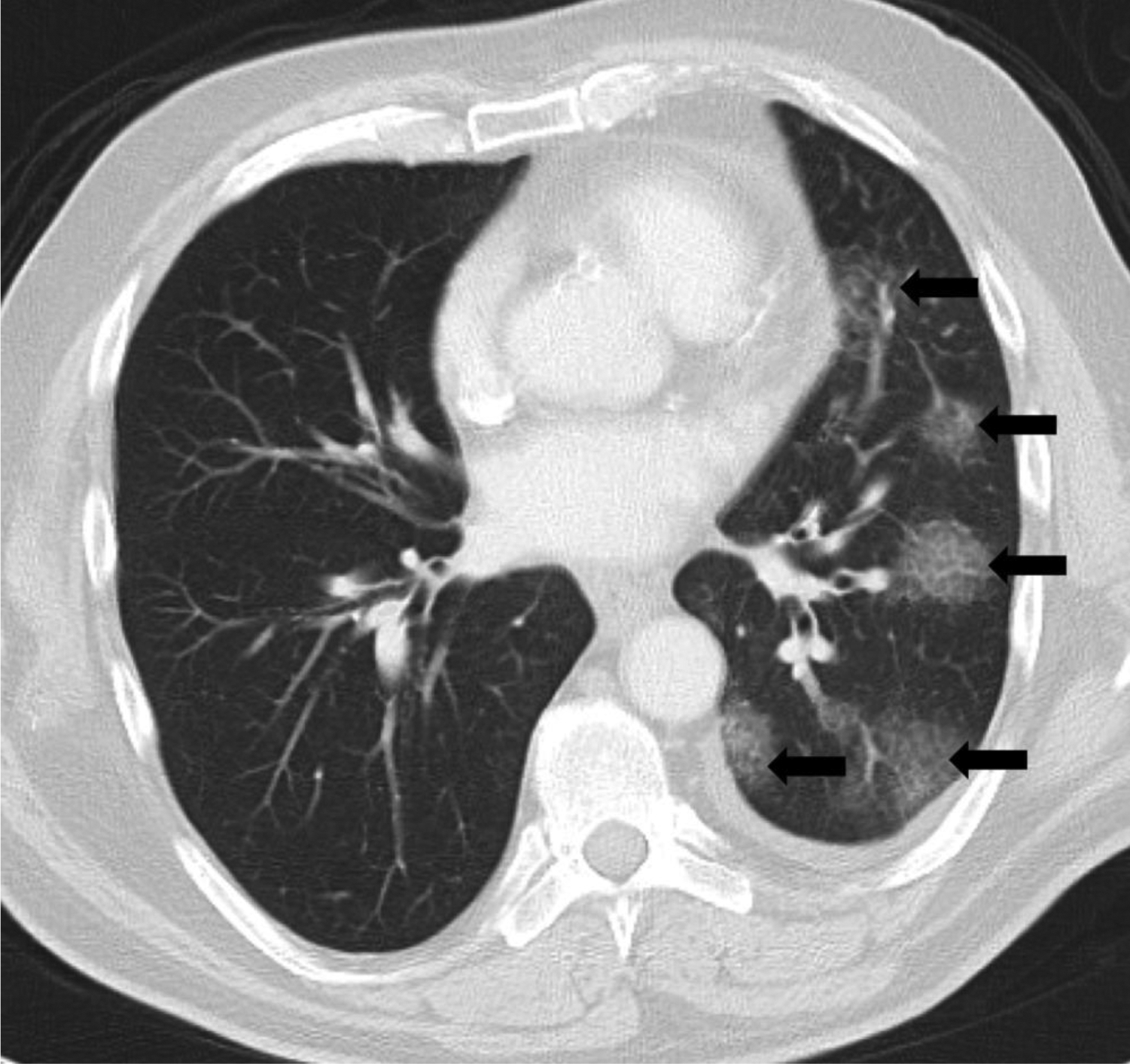
Cryptogenic organizing pneumonia (COP) pattern of radiation pneumonitis (grade 2) in a 63-year-old male with non-small cell lung carcinoma, treated with chest radiotherapy using 3-dimensional conformal radiotherapy (3DCRT). Axial chest computed tomography (CT) at the time of symptomatic radiation pneumonitis demonstrated multifocal patchy ground glass and reticular opacities in the left lung (arrows), representing a radiographic COP pattern. The patient presented with shortness of breath and wheeze which responded to steroid therapy.
Figure 2.
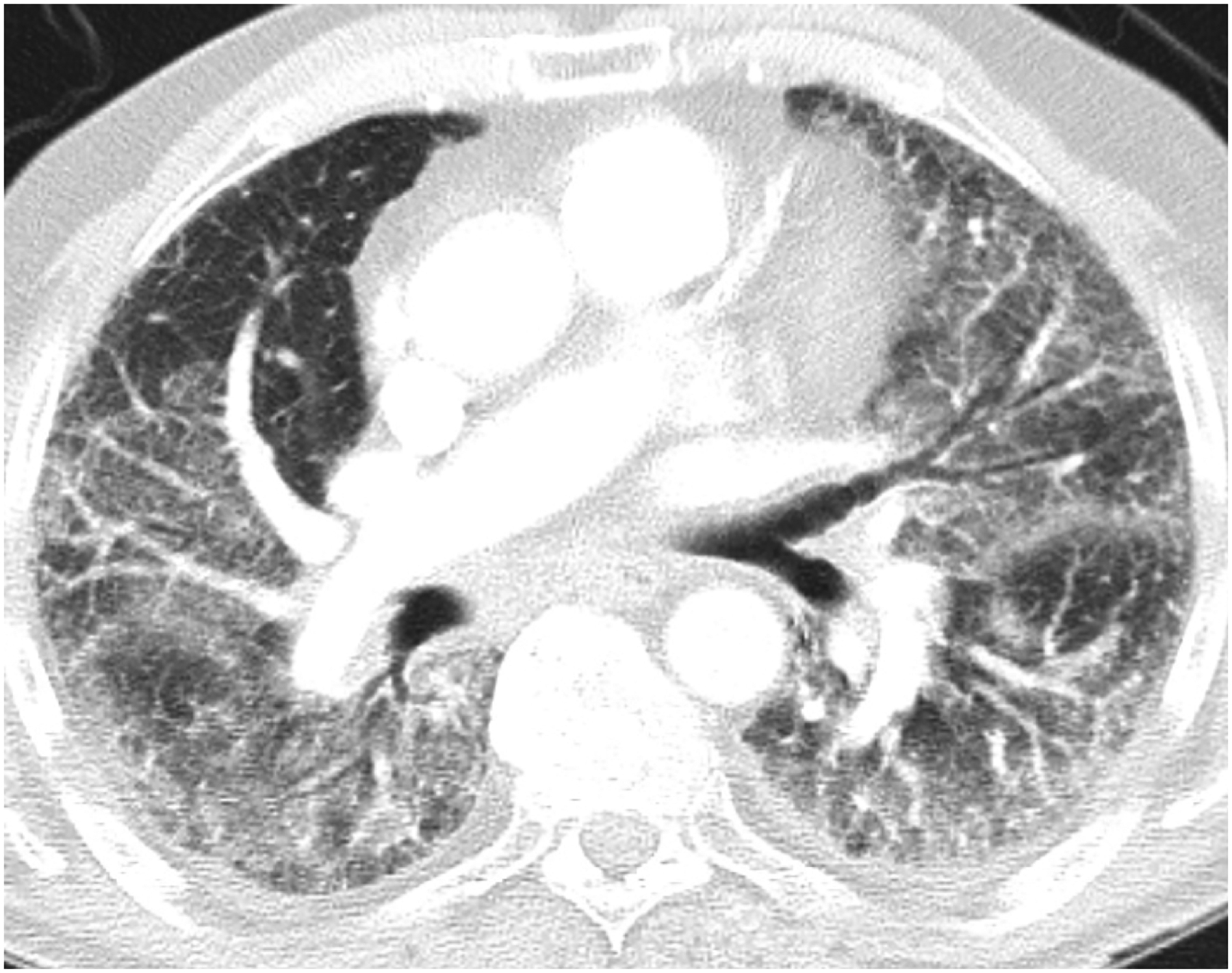
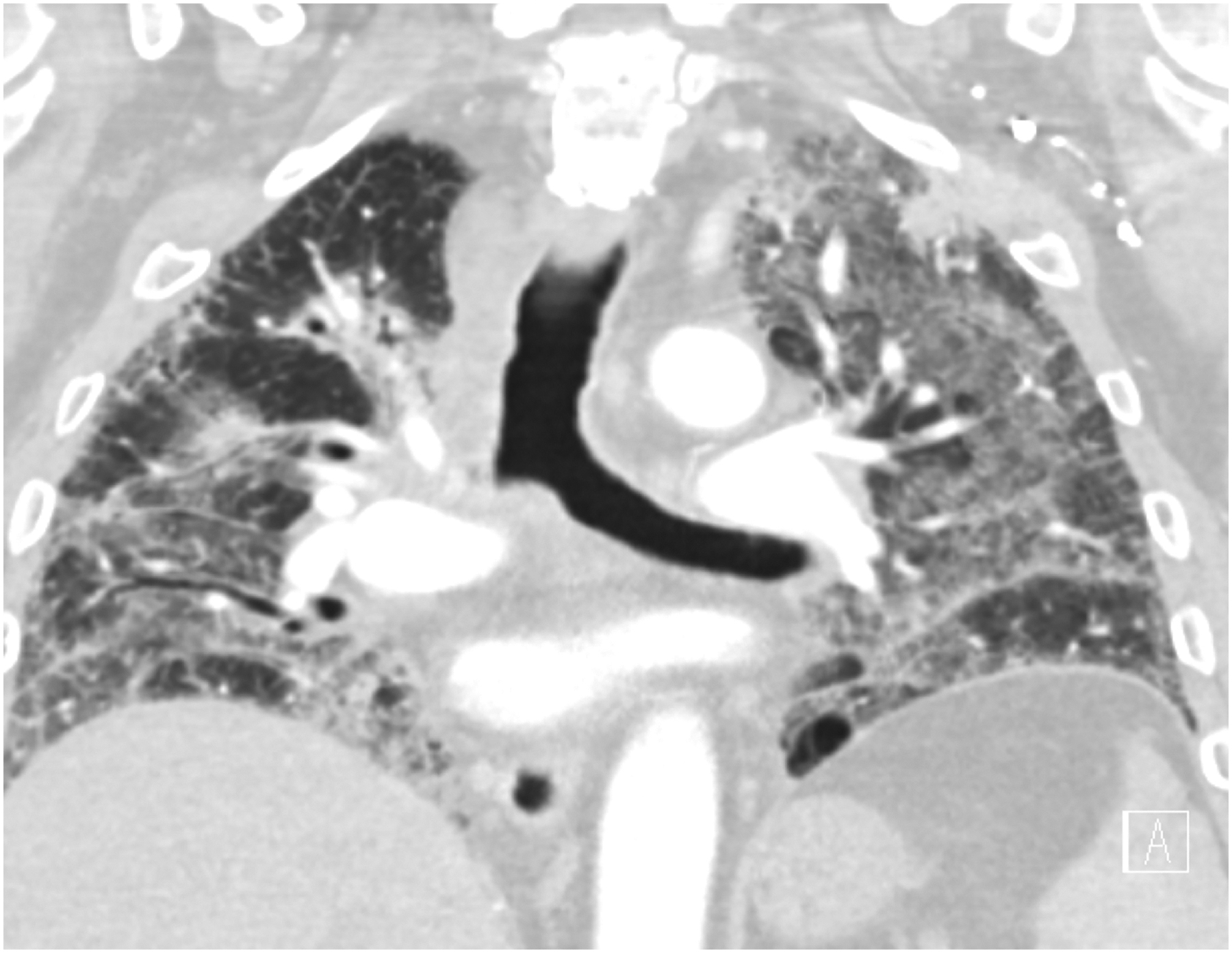
Acute interstitial pneumonia/Acute respiratory distress syndrome (AIP/ARDS) pattern of radiation pneumonitis (grade 5) in an 83-year-old male with non-small cell lung carcinoma, treated with chest radiotherapy using combined 3DCRT and intensity modulated radiotherapy (IMRT). Axial (A) and coronal (B) chest CT at the time of symptomatic radiation pneumonitis demonstrated extensive ground glass and reticular opacities throughout both lungs, with a few interspersed areas of consolidative opacities, traction bronchiectasis and loss of lung volumes, representing a radiographic AIP/ARDS pattern. The patient needed hospitalization for worsening hypoxia and passed away 2 weeks later despite supportive care.
Figure 3.
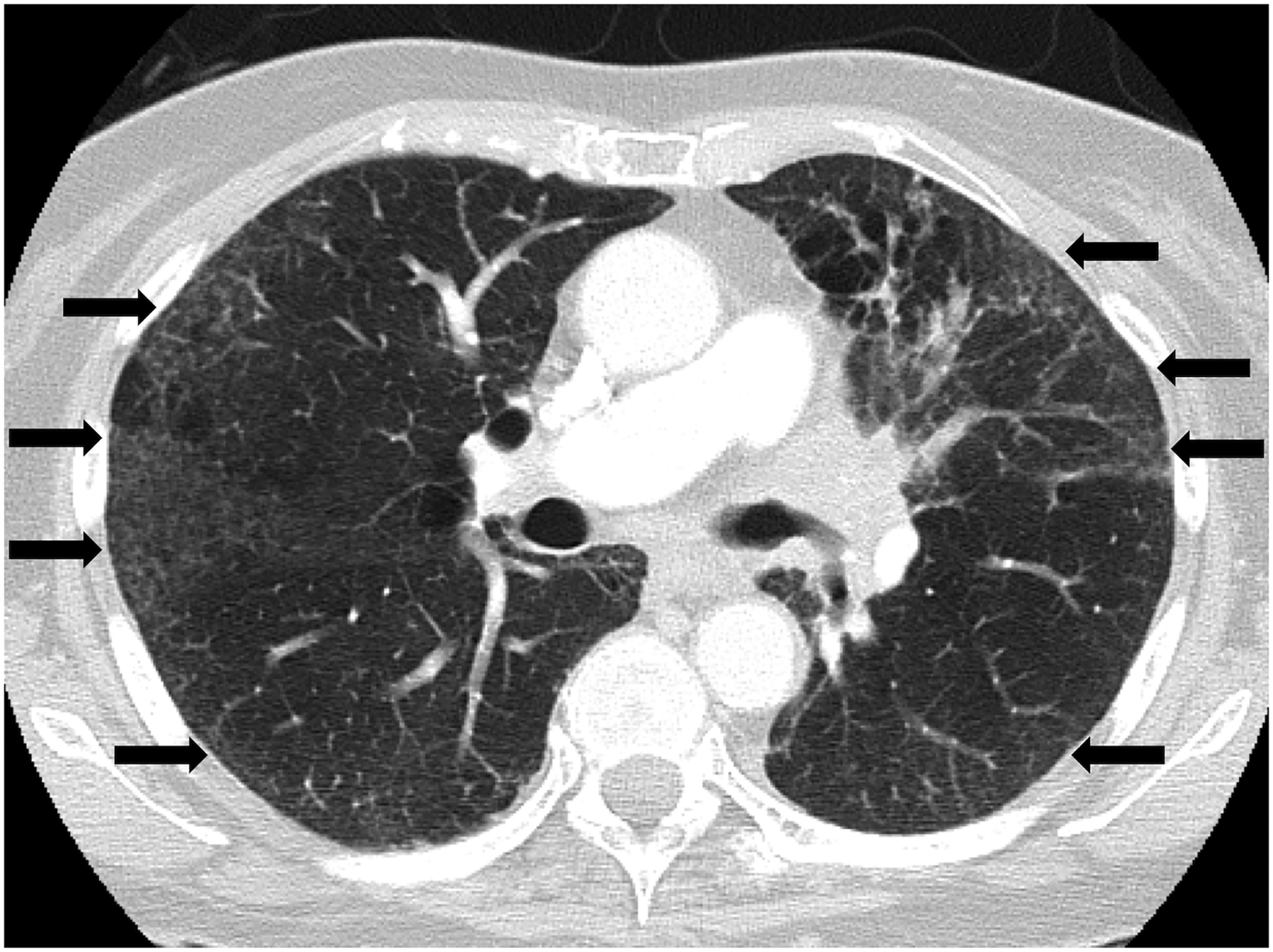
Nonspecific interstitial pneumonia (NSIP) pattern of radiation pneumonitis (grade 2) in a 74-year-old female with non-small cell lung carcinoma, treated with chest radiotherapy using 3DCRT. Axial CT at the time of symptomatic radiation pneumonitis demonstrated ground glass and reticular opacities in a peripheral distribution in both lungs (arrows), representing a radiographic NSIP pattern. The patient presented with cough and shortness of breath that responded to steroid therapy.
The imaging findings associated with high-grade RP included a higher extent of lung involvement on CT (p=0.003), diffuse distribution (p<0.0001), and AIP/ARDS pattern (p=0.0003). Imaging findings associated with RP-related death included a higher extent of lung involvement on CT (p=0.0008), diffuse distribution (p=0.0003), all lobe involvement (p=0.006), and AIP/ARDS pattern (p=0.0002).
Univariable and multivariable analyses for factors associated with RP grades
To further analyze the clinical and imaging characteristics associated with high-grade RP, univariable and multivariable logistic regression analyses were performed (Tables 3 and 4). In the univariable analyses, the clinical characteristics associated with high-grade RP included older age (age >67; OR: 2.80, p=0.0504), lower performance status (PS 2 or 3; OR: 6.44, p=0.04), squamous cell histology (OR: 3.29, p=0.02), and FEV1 ≤2 (OR: 4.41, p=0.008). Older age (OR: 6.86, p=0.08) and squamous cell histology (OR: 6.88, p=0.03) were also associated with pneumonitis-related death (Table 3). The imaging characteristics that are associated with high-grade RP included a higher extent of lung involvement (Extent ≥10 using the sum of the extent scores of all three levels of the lungs for each patient; OR: 5.22, p=0.002), diffuse distribution (OR: 20.07, p=0.004), and AIP/ARDS pattern (OR: 16.57, p=0.0009). The imaging characteristics associated with RP-related death were higher extent (OR: 16.50, p=0.01), all lobe involvement (OR 14.45, p=0.02), diffuse distribution (OR: 28.75, p=0.003), and AIP/ARDS pattern (OR: 35.0, p=0.0002) (Table 4). In 7 patients with lethal RP, the median time between the CT diagnosis of RP to death was 3.0 weeks (range: 0.6 – 43.3 weeks).
Table 3:
Logistic Regression for the association between Radiation Pneumonitis Grades and Clinical Characteristics
| Grade 3–5 RP vs. Grade 2 RP | Lethal RP vs. Non-Lethal RP | |||
|---|---|---|---|---|
| OR3 (95% CI) | p-value | OR3 95% CI) | p-value | |
| Age at RT Start (year) | 0.0504 | 0.08 | ||
| ≤67 | Ref. | Ref. | ||
| >67 | 2.80 (0.998, 7.98) | 6.86 (0.79, 59.8) | ||
| Gender | 0.84 | 0.44 | ||
| Female | Ref. | Ref. | ||
| Male | 0.91 (0.34, 2.44) | 0.51 (0.09, 2.79) | ||
| Race | 0.80 | 0.16 | ||
| White | 0.72 (0.06, 8.41) | 0.16 (0.01, 2.09) | ||
| African American | Ref. | Ref. | ||
| Performance Status | 0.04 | |||
| 0/1 | Ref. | Ref. | ||
| 2/3 | 6.44 (1.09, 38.13) | 2.33 (0.23, 23.4) | ||
| Smoking Status | 0.64 | |||
| Never Smoker | Ref. | - | - | |
| Former/Current Smoker | 0.70 (0.16, 3.10) | - | - | |
| Histology | 0.02 | 0.03 | ||
| Squamous Cell Carcinoma | 3.29 (1.18, 9.18) | 6.88 (1.23, 38.31) | ||
| Other | Ref. | Ref. | ||
| Clinical Stage | 0.68 | 0.67 | ||
| I/II | Ref. | Ref. | ||
| III/IV | 1.24 (0.44, 3.51) | 0.71 (0.15, 3.40) | ||
| FEV1 | 0.008 | 0.42 | ||
| ≤2 | 4.41 (1.44, 13.50) | 3.15 (0.57, 17.51) | ||
| >2 | Ref. | Ref. | ||
| Missing1 | 0.39 (0.04, 3.52) | - | ||
| Predicated FEV1 (%) | 0.13 | 0.55 | ||
| ≤80 | Ref. | Ref. | ||
| >80 | 0.54 (0.19, 1.55) | 0.39 (0.07, 2.15) | ||
| Missing1 | 0.13 (0.02, 1.11) | - | ||
| History of COPD | 0.11 | 0.15 | ||
| No | Ref. | Ref. | ||
| Yes | 2.29 (0.83, 6.33) | 3.21 (0.66, 15.55) | ||
| RT Technique | 0.96 | |||
| 3DCRT | 0.97 (0.32, 2.91) | 2.33 (0.27, 20.56) | ||
| IMRT or 3DCRT+IMRT | Ref. | Ref. | ||
| GTV | 0.21 | 0.60 | ||
| Primary Tumor, Hilar and Mediastinal Nodes | 1.89 (0.70, 5.09) | 1.52 (0.32, 7.28) | ||
| Other/Missing | Ref. | Ref. | ||
| RT Dose | 0.95 | 0.47 | ||
| <66 Gy | Ref. | Ref. | ||
| ≥66 Gy | 0.97 (0.36, 2.62) | 0.54 (0.10, 2.95) | ||
| Mean Lung Dose | 0.63 | 0.71 | ||
| ≤17 | Ref. | Ref. | ||
| >17 | 1.29 (0.46, 3.63) | 0.71 (0.12, 4.17) | ||
| Lung V20 | 0.70 | 0.87 | ||
| ≤30 | Ref. | Ref. | ||
| >30 | 1.23 (0.43, 3.53) | 0.86 (0.15, 5.04) | ||
RP – radiation pneumonitis, RT – radiation therapy, OR – odds ratio, CI – confidence interval, FEV1 – forced expiratory volume 1, COPD – chronic obstructive pulmonary disease, 3DCRT – three-dimensional conformal radiation therapy, IMRT – intensity modulated radiation therapy, GTV – gross tumor volume, lung V20 – volume of normal lung receiving 20 Gy
Missing category with greater than 10 patients was included in the model.
Odds ratio
Variables with p<0.05 in the univariable analysis were included in the multivariable analysis.
Table 4.
Logistic Regression for the association between Radiation Pneumonitis grades and Radiographic Characteristics
| Grade 3–5 RP vs. Grade 2 RP | Lethal RP vs. Non-Lethal RP | |||
|---|---|---|---|---|
| OR3 (95% CI) | p-value | OR3 (95% CI) | p-value | |
| Extent* | 0.002 | 0.01 | ||
| <10 | Ref. | Ref. | ||
| ≥10 | 5.22 (1.83, 14.9) | 16.50 (1.87, 145.61) | ||
| Distribution | 0.0004 | 0.0003 | ||
| Not Diffuse | Ref. | Ref. | ||
| Diffuse | 20.07 (3.87, 104.1) | 28.75 (4.57, 180.99) | ||
| Bilateral | 0.12 | 0.16 | ||
| No | Ref. | Ref. | ||
| Yes | 2.33 (0.80, 6.78) | 4.71 (0.54, 41.11) | ||
| All Lobes | 0.07 | 0.02 | ||
| No | Ref. | Ref. | ||
| Yes | 2.53 (0.92, 6.92) | 14.45 (1.64, 127.14) | ||
| Out of Field | 0.51 | |||
| No | Ref. | - | - | |
| Yes | 1.58 (0.40, 6.24) | - | - | |
| Pattern | 0.0009 | 0.0002 | ||
| AIP/ARDS pattern | 16.57 (3.16, 86.74) | 35.0 (5.37, 228.04) | ||
| Other patterns | Ref. | Ref. | ||
RP – radiation pneumonitis, OR – odds ratio, CI – confidence interval, AIP/ARDS – acute interstitial pneumonia/acute respiratory distress syndrome
Extent was obtained for each patient as the sum of the scores in the upper, and middle, and lower lungs.
Multivariable analyses were performed for the association between AIP/ARDS pattern and high-grade RP, adjusting for clinical factors that were significant in the univariable analyses. Other imaging characteristics were not included in the multivariable analyses, as they were strongly associated with AIP/ARDS pattern. AIP/ARDS pattern remained as a significant risk factor for high-grade RP (OR: 12.62 [95%CI: 1.73 – 91.92], p=0.01), after adjusting for performance status (PS 2–3 vs. PS 0–1, OR: 3.94 [95%CI: 0.41, 37.49], p=0.23), squamous cell histology (OR: 1.98, [95%CI: 0.54 – 7.30], p=0.31), and FEV1 (FEV1 ≤2 vs. FEV>2, OR: 5.27 [95%CI: 1.25 –22.14], p=0.03).
Discussion
The present study has characterized the spectrum of imaging findings and radiographic patterns of symptomatic RP that has been clinically diagnosed in patients with lung cancer treated with conventional chest radiotherapy, and identified imaging markers for high-grade RP and RP-related death. The AIP/ARDS pattern on chest CT is predictive for both high-grade RP and RP-related death, and was an independent marker for high-grade RP after adjusting for other significant clinical variables. The results of the study call for awareness of the radiographic patterns of RP, which can help to predict the clinical severity and outcome of RP and to contribute to optimize patient management.
Among the clinical characteristics, older age, lower performance status, squamous cell histology and FEV1 ≤2 were associated with high-grade RP, and older age and squamous cell histology were also associated with RP-related death. Patients with lower performance status due to neoplastic disease or other co-morbidities understandably have a lesser ability to tolerate additional lung injury, and therefore are at an increased risk of developing higher grade symptomatic RP or lethal RP. In our study, patients with performance status of 2 or 3 had 6.4 times increased risk of developing high-grade RP compared to those with performance status of 0 or 1. Likewise, FEV1 ≤2 was also associated with increased risk of high-grade RP. The result is similar to that seen by Robnett et al where patients with lower performance status had 7.8 times increased risk of severe RP, and no patient with FEV1>2 developed high-grade pneumonitis29. The older age of greater than 67 years was associated with an increased risk of both high-grade RP and RP-related death (OR 2.8 and 6.86, respectively), which is similar to the study by Kharofa et al that showed the age greater than 70 years was associated with increased risk of grade ≥3 pneumonitis30. The association of squamous cell histology with high-grade and lethal pneumonitis as seen in our study may be related to the fact that patients with squamous cell carcinoma of the lung are more likely to be elderly and smokers with a worse overall prognosis31,32. Unlike earlier studies12,33, we did not find a significant association between mean lung dose and RP grades, which may be due to a small number of patients, but could also be related to the fact that we focused on patients with symptomatic RP, and did not include asymptomatic RP with imaging abnormalities alone. History of chronic obstructive pulmonary disease also did not increase risk of severe RP, as previously reported by Kasymjanova et al34. Only two patients had a positive history for pulmonary fibrosis and thus the variable was insignificant as a risk factor for RP in the present cohort.
The detailed evaluation of the CT findings of symptomatic RP demonstrated that the CT findings extend beyond the expected radiation field in majority of the patients (82%), which may be an important indicator when making radiological diagnosis of RP. However, the remaining patients (18%) had CT findings that are confined to the expected radiation field in a geographic distribution that is indistinguishable from the post radiation changes, which highlights the limitation of the imaging assessment alone for the diagnosis of RP and emphasizes the multidisciplinary approach for the entity. Among those with CT findings beyond the radiation field, COP pattern which is characterized by multifocal consolidative and GGO was the most frequent radiographic pattern, and was seen in 81% of patients. Multiple prior articles have also described organizing pneumonia as a common manifestation of RP35–38. It is also well known that organizing pneumonia is the most common manifestation of lung injury to a wide variety of insults including radiation pneumonitis39–41.
Among the imaging features, the higher extent of lung involvement on CT, diffuse distribution, and AIP/ARDS pattern were associated with both high-grade RP and lethal RP. Among these factors, AIP/ARDS pattern had the highest odds of 16.57 for high-grade RP, and 35.0 for lethal RP. Several previously reported cases of high-grade and lethal RP have described similar imaging findings of extensive, diffuse, consolidative and ground glass opacities in both lungs, associated with reticular opacities and traction bronchiectasis42–44. These observations suggest that, as the severity of radiation pneumonitis increases, its effects evolve from localized lung injury to a more extensive inflammatory response manifesting as extensive pulmonary opacities45–48. Moreover, after adjusting for other significant clinical factors on multivariable analysis, the AIP/ARDS pattern remained as a significant predictor for high-grade RP with 12.6 times increased risk, indicating the importance of the recognition of this radiographic pattern when assessing the chest CT scans in patients treated with chest radiotherapy.
It is also interesting to note the similarity of the findings in our study to that of multiple prior studies on drug-related pneumonitis21,23,24,49,50. For example, a prior study of the imaging of PD-1 inhibitor-related pneumonitis reported that 65% of the patients demonstrated COP pattern. AIP/ARDS pattern was also associated with high-grade pneumonitis23,49. Likewise, two prior studies on mTOR inhibitor-related pneumonitis showed that COP pattern is the most common radiographic pattern, noted in up to 70% of the patients21,24. These observations further confirm that pneumonitis is a manifestation of lung’s response to injury, including radiation and anti-cancer agents22.
The spectrum of imaging features and radiographic patterns of RP characterized in the present study also has implications on the toxicity assessment from emerging anti-cancer therapy using a combination of radiotherapy and immune-checkpoint inhibitor therapy. The recently concluded PACIFIC trial reported significant survival benefits of consolidation therapy using PD-L1 inhibitor, durvalumab, in patients with stage III NSCLC treated with platinum-based chemoradiotherapy51,52. In this trial, pneumonitis, including both drug-related pneumonitis and RP, was noted in 33.9% patients receiving durvalumab and was the most frequent adverse effect leading to discontinuation of therapy51. The detailed description of RP in lung cancer patients treated with conventional radiotherapy forms a basis to further understand pneumonitis in patients receiving combination therapy with radiation and immune-checkpoint inhibitors, which is a new area of clinical challenges and discoveries with emerging biomarkers53.
The limitations of the study include a retrospective nature in patients treated at a single institution and the relatively small number of lethal RP. However, we identified the radiographic patterns that are associated with high-grade RP using the comprehensive clinical and imaging data in the patients with symptomatic RP identified in the consecutive radiation oncology database. Given a small number of lethal RP, multivariable analyses for the risk factors of RP-related death were not performed. The study focused on symptomatic RP (Grade ≥2), and did not include Grade 1 RP, because the diagnosis of Grade 1 pneumonitis exclusively depends on radiographic findings, which is extremely challenging in the setting of chest radiotherapy that is almost always associated with a certain degree of lung parenchymal changes on imaging. Identification of early radiographic makers of high-grade RP is an important next step to optimize patient management and mitigate the consequence of RP, however, the availability of diagnostic chest CT prior to the development of RP is limited and variable in this retrospective cohort. This important question needs to be further addressed in a cohort with prospective study design.
In conclusion, symptomatic RP in lung cancer patients demonstrated several characteristic radiographic patterns, with COP pattern being the most common. AIP/ARDS pattern should raise a concern for high-grade RP and potentially lethal RP. The observations in the present study can be applied to detect, evaluate, and monitor RP in patients treated with chest radiotherapy, and also can form a basis for further studies of pneumonitis from combination radiotherapy and immune-checkpoint inhibitor therapy.
Supplementary Material
Highlight.
Radiographic patterns of symptomatic radiation pneumonitis (RP) were characterized
AIP/ARDS pattern is a predictive marker for high-grade RP and RP-related death
Radiographic patterns of RP help to predict the clinical severity and outcome
ACKNOWLEDGEMENT
The investigators, MN and HH, are supported by R01CA203636 and U01CA209414 (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Thomas, Chen, Mak: Nothing to disclose
Hatabu: Reserch funding from Canon Inc., Canon Medical Systems, and Konica-Minolta; Consultant to Canon Medical Systems, and Mitsubishi Chemical Inc.
Nishino: Consultant to Daiichi Sankyo, AstraZeneca; Research grant from Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo; Honorarium from Roche
REFERENCES:
- 1.Lederman M The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol 7, 639– 648 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Gianfaldoni S et al. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci 5, 521–525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney G, Jacob S, Featherstone C & Barton M The role of radiotherapy in cancer treatment. Cancer 104, 1129–1137 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS et al. Non–Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw 15, 504–535 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Howington JA, Blum MG, Chang AC, Balekian AA & Murthy SC Treatment of Stage I and II Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST 143, e278S–e313S (2013). [DOI] [PubMed] [Google Scholar]
- 6.Kalemkerian GP et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J. Natl. Compr. Canc. Netw 16, 1171–1182 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Ren D, Liu H & Chen J Comparison and discussion of the treatment guidelines for small cell lung cancer. Thorac. Cancer 9, 769–774 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairchild A et al. Palliative Thoracic Radiotherapy for Lung Cancer: A Systematic Review. J. Clin. Oncol 26, 4001–4011 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Haasbeek CJA, Slotman BJ & Senan S Radiotherapy for lung cancer: Clinical impact of recent technical advances. Lung Cancer 64, 1–8 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Bucci MK, Bevan A & Roach M Advances in Radiation Therapy: Conventional to 3D, to IMRT, to 4D, and Beyond. CA. Cancer J. Clin 55, 117–134 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Connell PP & Hellman S Advances in Radiotherapy and Implications for the Next Century: A Historical Perspective. Cancer Res. 69, 383–392 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Kim TH et al. Dose-volumetric Parameters for Predicting Severe Radiation Pneumonitis after Three-dimensional Conformal Radiation Therapy for Lung Cancer. Radiology 235, 208–215 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues G, Lock M, D’Souza D, Yu E & Van Dyk J Prediction of radiation pneumonitis by dose–volume histogram parameters in lung cancer—a systematic review. Radiother. Oncol 71, 127–138 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J & Pajak TF Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int. J. Radiat. Oncol 31, 1341–1346 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Tucker SL et al. Impact of Toxicity Grade and Scoring System on the Relationship Between Mean Lung Dose and Risk of Radiation Pneumonitis in a Large Cohort of Patients With Non–Small Cell Lung Cancer. Int. J. Radiat. Oncol 77, 691–698 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
- 17.Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J. Clin. Oncol [DOI] [PubMed] [Google Scholar]
- 18.Palma DA et al. Predicting Radiation Pneumonitis after Chemoradiotherapy for Lung Cancer: An International Individual Patient Data Meta-analysis. Int. J. Radiat. Oncol. Biol. Phys 85, 444–450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig TR et al. Radiation Injury of the Lung After Three-Dimensional Conformal Radiation Therapy. Am. J. Roentgenol 178, 1383–1388 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Ikezoe J et al. CT appearance of acute radiation-induced injury in the lung. Am. J. Roentgenol 150, 765–770 (1988). [DOI] [PubMed] [Google Scholar]
- 21.Nishino M et al. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: a radiographic pattern-based approach. Eur. J. Cancer 53, 163–170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishino M, Hatabu H, Sholl LM & Ramaiya NH Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care. Radiographics 37, 1371–1387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishino M et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res 22, 6051–6060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishino M, Boswell EN, Hatabu H, Ghobrial IM & Ramaiya NH Drug-Related Pneumonitis During Mammalian Target of Rapamycin Inhibitor Therapy: Radiographic Pattern-Based Approach in Waldenström Macroglobulinemia as a Paradigm. The Oncologist 20, 1077–1083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sverzellati N et al. American Thoracic Society–European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. RadioGraphics 35, 1849–1871 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Travis WD et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med 188, 733–748 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller NL, White DA, Jiang H & Gemma A Diagnosis and management of drug-associated interstitial lung disease. Br. J. Cancer 91, S24–S30 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min JH et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother. Pharmacol 68, 1099–1109 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Robnett TJ et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int. J. Radiat. Oncol. Biol. Phys 48, 89–94 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Kharofa J & Gore E Symptomatic radiation pneumonitis in elderly patients receiving thoracic irradiation. Clin. Lung Cancer 14, 283–287 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Kawase A et al. Differences Between Squamous Cell Carcinoma and Adenocarcinoma of the Lung: Are Adenocarcinoma and Squamous Cell Carcinoma Prognostically Equal? Jpn. J. Clin. Oncol 42, 189–195 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Cooke DT et al. Survival Comparison of Adenosquamous, Squamous Cell, and Adenocarcinoma of the Lung After Lobectomy. Ann. Thorac. Surg 90, 943–948 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Claude L et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother. Oncol 71, 175–181 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kasymjanova G et al. Does the presence of emphysema increase the risk of radiation pneumonitis in lung cancer patients? Curr. Oncol 25, e610–e614 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oie Y et al. Relationship between radiation pneumonitis and organizing pneumonia after radiotherapy for breast cancer. Radiat. Oncol. Lond. Engl 8, 56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crestani B et al. Migratory bronchiolitis obliterans organizing pneumonia after unilateral radiation therapy for breast carcinoma. Eur. Respir. J 8, 318–321 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Crestani B et al. Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. The Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM”O”P). Am. J. Respir. Crit. Care Med 158, 1929–1935 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Otani K, Seo Y & Ogawa K Radiation-Induced Organizing Pneumonia: A Characteristic Disease that Requires Symptom-Oriented Management. Int. J. Mol. Sci 18, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasu TS et al. Clinical and Radiologic Distinctions Between Secondary Bronchiolitis Obliterans Organizing Pneumonia and Cryptogenic Organizing Pneumonia. Respir. Care 54, 1028–1032 (2009). [PubMed] [Google Scholar]
- 40.Drakopanagiotakis F et al. Cryptogenic and Secondary Organizing Pneumonia: Clinical Presentation, Radiographic Findings, Treatment Response, and Prognosis. Chest 139, 893–900 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Epler GR Bronchiolitis obliterans organizing pneumonia, 25 years: a variety of causes, but what are the treatment options? Expert Rev. Respir. Med 5, 353–361 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Keffer S, Guy CL & Weiss E Fatal Radiation Pneumonitis: Literature Review and Case Series. Adv. Radiat. Oncol (2019) doi: 10.1016/j.adro.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgiou A & Farmer A Severe and Fatal Multilobar Nonclassic Radiation Pneumonitis following Stereotactic Body Radiation Therapy (SBRT) for Treatment of Inoperable Non-Small-Cell Lung Cancer: A Report of Two Cases and Possible Enhancement by Concurrent Amiodarone. Case Reports in Pulmonology https://www.hindawi.com/journals/cripu/2019/8754951/ (2019) doi: 10.1155/2019/8754951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onishi H et al. Case Series of 23 Patients Who Developed Fatal Radiation Pneumonitis After Stereotactic Body Radiotherapy for Lung Cancer. Technol. Cancer Res. Treat 17, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang JH et al. Extensive acute lung injury following limited thoracic irradiation: radiologic findings in three patients. J. Korean Med. Sci 15, 712–717 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byhardt RW, Abrams R & Almagro U The association of adult respiratory distress syndrome (ARDS) with thoracic irradiation (RT). Int. J. Radiat. Oncol. Biol. Phys 15, 1441–1446 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Fulkerson WJ, McLendon RE & Prosnitz LR Adult respiratory distress syndrome after limited thoracic radiotherapy. Cancer 57, 1941–1946 (1986). [DOI] [PubMed] [Google Scholar]
- 48.Ozawa Y et al. Impact of Preexisting Interstitial Lung Disease on Acute, Extensive Radiation Pneumonitis: Retrospective Analysis of Patients with Lung Cancer. PLOS ONE 10, e0140437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishino M, Sholl LM & Hodi FS Anti–PD-1–Related Pneumonitis during Cancer Immunotherapy. N. Engl. J. Med 373, 288–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishino M & Hatabu H Programmed Death-1/Programmed Death Ligand-1 Inhibitor–Related Pneumonitis and Radiographic Patterns. J. Clin. Oncol 35, 1628–1629 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Antonia SJ et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med 377, 1919–1929 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Antonia SJ et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med 379, 2342–2350 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Schoenfeld JD et al. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J. Immunother. Cancer 7, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


