Abstract
Ambulatory monitoring devices are enabling a new paradigm of health care by collecting and analyzing long-term data for reliable diagnostics. These devices are becoming increasingly popular for continuous monitoring of cardiac diseases. Recent advancements have enabled solutions that are both affordable and reliable, allowing monitoring of vulnerable populations from comfort of their homes. They provide early detection of important physiological events, leading to timely alerts for seeking medical attention. In this review, the authors aim to summarize the recent developments in the area of ambulatory and remote monitoring solutions for cardiac diagnostics. We cover solutions based on wearable devices, smartphones, and other ambulatory sensors. The authors also present an overview of the limitations of current technologies, their effectiveness, and their adoption in the general population, and discuss some of the recently proposed methods to overcome these challenges. Last, we discuss the possibilities opened by this new paradigm, for the future of health care and personalized medicine.
Keywords: ambulatory, cardiac monitoring, mobile, telemetry, wireless
Remote and ambulatory monitoring is becoming increasingly popular among health care practitioners and patients for the long-term continuous monitoring and diagnosis of cardiac diseases (1). Hardware and software advancements have led to the development of novel devices, which are both practical and affordable, and enable monitoring of vulnerable populations from the comfort of their homes, while at the same time providing critical alerts for events requiring prompt medical attention or hospitalization (2). Wearable devices can also enable virtual or remote care, which can help bridge the health care divide between urban and rural settings.
Today, terminologies such as remote health care, virtual care, mobile health, or e-health all essentially refer to the range of solutions and paradigms that are enabled by ambulatory devices for continuous and remote monitoring. These technologies have the potential to provide reliable clinical diagnosis by collecting physiological health data over long periods of time and reduce hospitalization expenses for vulnerable patients by making it possible to monitor for critical health conditions at home (2). Furthermore, they are opening the door for personalized health care and medicine by enabling a deeper understanding of an individual patient’s (patho)physiological state and daily activities.
Cardiologists in the United States are increasingly utilizing technology to provide remote health care–based solutions and diagnostics made partly possible through implantable or wearable devices. These devices provide early detection of critical physiological events, giving patients more time to seek medical help (2). Remote monitoring and management techniques can further lead to optimization of implantable cardioverter-defibrillator leads as well as reduce the chances of any inappropriate shock related to such devices (3).
Furthermore, there is a shifting trend toward wearable and noninvasive devices, which have the potential of capturing multiple data streams of vital physiological parameters while being easy to wear, operate, and maintain and also affordable (2). This is because developments in both hardware and software technology have enabled development of sensors and algorithms that can acquire and process high-quality data noninvasively and through minimal skin contact.
In the United States alone, up to 200,000 patients are estimated to be living with stage D heart failure (4). As U.S. health care system is already over-burdened and is bracing for an aging population, high-quality yet affordable health care options for both diagnostic and preventive medical care (1) become an ever-increasing necessity. With a huge potential to significantly reduce health care costs and improve diagnostics and preventive care, it is expected that the trend will continue to shift toward remote and ambulatory monitoring enabled by technologies such as wearables, smartphones, and other mobile devices. The market size for wearable devices alone is expected to rise to around $70 billion by the year 2025, with the health care sector leading the way (5).
The 3 main components of a remote monitoring health care system are: 1) a wearable sensor collecting the data on physiological parameters; 2) a network and communications interface that enables transfer of these data to a remote monitoring station such as nurse or physician’s terminal or a smartphone; and 3) a remote cloud analytics platform that enables integration of large volumes of data, mines useful information, identifies key patterns and parameters critical for patient’s health, and recommends most optimal ways of treatment (1).
In this paper, we aim to summarize recent developments and challenges in the area of ambulatory and remote monitoring solutions for cardiac diagnostics. We cover solutions based on wearable, smartphones, and other ambulatory sensors. The paper focuses on technologies for which detailed information on both technical and clinical aspects has been available and that either have been or could potentially be developed into commercial products, rather than being mere proof of concept or an early-stage academic research endeavor. We also present an overview of the effectiveness and adoption of devices in the general population and the limitations of current technologies; we also discuss some of the methods proposed in recent literature that can help overcome those challenges. Although our review is mainly focused on literature published during the last 5 years (2013 to 2018), we have also included some of the most important techniques proposed in the preceding years.
AMBULATORY CARDIAC MONITORING TECHNOLOGIES
Holter monitors have long been the devices of choice for cardiac monitoring over long periods of time for diagnosing a large variety of cardiac disorders. However, owing to their size and bulkiness, their use in continuous monitoring has been limited, which in turn has resulted in limitations when it comes to detecting rarely occurring events of diagnostic importance (6).
Wearable ambulatory sensors have the potential to provide critical monitoring and diagnostic insights to prevent and treat such health problems, while also being small in size and comfortable to use on a long-term basis. Many wearable devices have been introduced for remote and long-term cardiac monitoring. Some of these devices work in a standalone fashion, whereas others work in conjunction with other compact and portable equipment such as smartphones.
Understanding these technologies is important not only to appreciate their potential clinical utility, but also to identify potential sources of error, as well as to understand the limitations and challenges posed by them and how they can be overcome. One of the more common sensors in ambulatory monitoring is the accelerometer. This is a device capable of detecting small movements and changes in motion based on inertial principles. Accelerometer-based sensing systems and wearables devices have been proposed for cardiac monitoring. Some of these systems are worn as fitness bands, whereas others have the sensors embedded in textiles. These wearable systems are capable of collecting accelerometer data from all 3 axes of movements and infer important physiological parameters, including the heart rate (HR) using ballistocardiogram (BCG)-based approaches. The BCG is a technique that measures the repetitive movement of the human body caused by the heartbeat and the blood ejection (7).
Certain conditions that are difficult to detect through traditional electrocardiogram (ECG)- or accelerometer-based approaches, such as structural abnormalities, can potentially be diagnosed by analyzing cardiac sound recordings. Such recordings can be obtained using techniques such as phonocardiography or seismocardiography (SCG), which record cardiac sound and vibrations, respectively, and have been shown to demonstrate great diagnostic potential of cardiac conditions (7).
Significant developments have also been made in the algorithmic processing of sensor data. These improvements have led to an increase in the reliability with which diagnostic information can be acquired from wearable and ambulatory devices. Furthermore, coupled with new techniques in sensor data fusion, these technologies are leading to more accurate systems that can integrate streaming data from diverse sources and extract different physiological parameters for diagnosing a particular disorder or differentiating between multiple conditions.
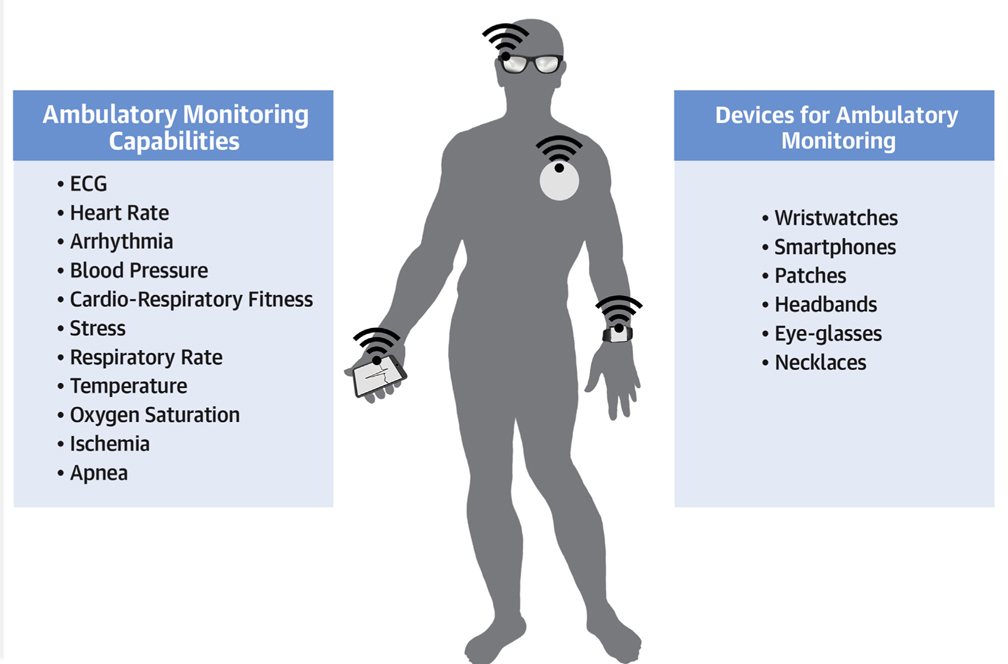
In the following subsections, we review some of the ambulatory monitoring devices based on ECG, photo-plethysmography (PPG), accelerometers, acoustics, and other techniques for developing wearable, smart-phone, or other mobile devices aiming to improve monitoring of various aspects of cardiovascular health and fitness. Table 1 presents some of the devices pertaining to the cardiovascular health monitoring that will be discussed subsequently in the subsections. A summary of the potential use cases of these tech-nologies is also presented in the Central Illustration.
TABLE 1.
Summary of Wearable Device and System Types Along With Clinical Applications
| Reference(s) | Device Name | Device Type | Clinical Applications |
|---|---|---|---|
| (8,9) | Zio patch | Patch | ECG monitoring, arrhythmia detection |
| (10) | NUVANT MCT | Patch | ECG monitoring, arrhythmia detection |
| (11) | Scanadu Scout | Handheld | HR, RR, blood pressure, temperature, oxygen saturation |
| (12) | Apple iWatch | Wristwatch | ECG monitoring, HR, AF detection |
| (14) | PulseSmart | Smartphone camera–based app | AF, PAC, and PVC detection |
| (15) | Kardia Mobile | Smartphone case | ECG monitoring, AF detection |
| (17) | ECG Check | Smartphone case | ECG monitoring |
| (18–20) | cvrPhone | Smartphone app | ECG monitoring, ischemia and apnea detection, arrhythmia susceptibility |
| (22) | N/A | Smartphone app | HR |
| (23) | N/A | Smartphone-connected device | blood pressure |
| (24) | N/A | Flexible patch | blood pressure |
| (25) | N/A | Patch and wristwatch | ECG, blood pressure, contractility |
| (26) | Google Glass | Head-mounted | HR, RR |
| (27) | Masimo Personal Health | Smartphone-connected probe | HR and SpO2 |
| (28) | N/A | Multiple wearable sensors | SpO2, HR, RR, walk speed, and acceleration |
| (31) | N/A | Patch | HR variability, skin temperature, stress diagnosis |
AF = atrial fibrillation; app = application; ECG = electrocardiography; HR = heart rate; MCT = Mobile Cardiac Telemetry; N/A =•••; PAC = premature atrial contraction; PVC = premature ventricular contraction; RR = respiratory rate; SpO2 = oxygen saturation.
CENTRAL ILLUSTRATION. Wearable Monitoring Devices.
Depiction of wearable and smartphone-based solutions for continuous ambulatory cardiac monitoring, along with a summary of potential use cases.
ARRHYTHMIA DETECTION
The Zio patch (iRhythm Technologies, San Francisco, California) is a Food and Drug Administration–cleared adhesive water-proof patch, applied to the left pectoral region, which provides a single-lead ECG and is used for the continuous monitoring of cardiac rhythms. The patch can be worn for up to 14 days and provides a relatively long-term monitoring of cardiac rhythms without need of battery replacement or recharge over this time (8,9). It also includes an event marker button that can be pressed when patients are symptomatic. The Zio patch has been shown to have a higher diagnostic yield for arrhythmia detection than Holter monitoring (8). An effectiveness study was carried out for the Zio patch, in which it was applied to 174 patients discharged from emergency departments, with an average age of 52.2 ± 21.0 years, with 55% of them being women. The most common indications among these patients included palpitations (44.8%), syncope (24.1%), and dizziness (6.3%), whereas other patients indicated signs of arrhythmias, such as ventricular tachycardia (8.0%), atrial fibrillation (2.3%), bradyarrhythmias (2.9%), and unspecified arrhythmias (11.5%). These patients were asked to mail back the device at the end of 14 days or until they had symptoms to trigger an event. A total of 83 (about 48%) patients were recorded having more than 1 arrhythmia, with almost 10% being symptomatic at the time of their first arrhythmia. The median time for detection of first arrhythmia was found to be 1.0 days, with around half of the symptomatic patients not having any arrhythmia during their triggered events, exhibiting an overall diagnostic yield of 63.2% (8,9).
Although the Zio patch enables offline analysis of long-term cardiac data, another device called the NUVANT Mobile Cardiac Telemetry (MCT) (Corventis, San Jose, California) provides real-time, wireless arrhythmia monitoring and analysis (10,11). The NUVANT MCT system consists of a wearable monitoring patch and a portable data transmission device, and comes with a magnet that is used as a trigger when the patient experiences symptoms. Although the NUVANT MCT system provides real-time transmission, its data are not available to the user (patient) in real time. This feature is provided by another wearable device called the Scanadu Scout (Scanadu, Sunnyvale, California) that is based on PPG signals, and is held between the fingers while directed at the head to provide a complete range of physiological parameters and vital signs including the HR, blood pressure (BP), temperature, respiratory rate (RR), and oxygen saturation. It works with a smartphone for displaying, storing, tracking, transmitting, and analyzing data (11). Apple’s iWatch (Apple Inc., Cupertino, California) has also incorporated ECG functionality and has already been demonstrated to achieve impressive sensitivity (87%) and specificity (97%) in identifying patients with silent atrial fibrillation (12).
Several smartphone-based solutions have also been developed targeting the ambulatory diagnosis of critical cardiac events. A method that employs a smartphone app to analyze the pulsatile signal acquired by illuminating the right index fingertip using the smartphone camera light-emitting diode detects AF in real time with a reported accuracy, sensitivity, and specificity of 0.968, 0.962, and 0.975, respectively (13). A similar study analyzed the ability of a smartphone-based application (PULSESMART app) in discriminating between sinus rhythm, AF, premature atrial contractions, and premature ventricular contractions (14). Similar to the previous approach, the PULSESMART app has also showed impressive ability in real-time detection of AF, with sensitivity, specificity, and accuracy of 0.970, 0.935, and 0.951, respectively. It has also demonstrated good accuracy in premature atrial contraction (0.955) and premature ventricular contraction (0.960) discrimination.
A few notable wearable devices that work with a smartphone are already available in the market. AliveCor’s Kardia Mobile (AliveCor, Mountain View, California) is a Food and Drug Administration–cleared smartphone-based ECG event recorder capable of detecting AF in just 30 s. It integrates ECG leads in a smartphone case and allows the recording of cardiac rhythms and subsequent electronic sharing of the recordings with health care providers (15). Its accuracy and effectiveness in helping with clinical diagnosis through recordings over a long period of 14 to 30 days was compared with an external loop recorder (16). It was found that more patients had a potential diagnosis for their symptoms with the Kardia Mobile (100%) than with the external loop recorder (72.7%). Another device called ECG Check (Cardiac Designs, Park City, Utah) is a system for ECG monitoring that connects at the back of a smartphone and can provide a single-lead ECG upon finger contact. The acquired ECG signal can be viewed on the smartphone screen in real time or uploaded to a health care provider or secure server for analysis (11,17). A 12-lead ECG smartphone based system called cvrPhone, capable of identifying ischemic and apneic events using ECG signals only, was introduced by our group (18,19). It was shown that ischemic events could be detected within 2 min of occlusion, and an apneic episode could be detected within 7.9 ± 1.1 s and 5.5 ± 2.2 s using RR and tidal volume estimation algorithms, respectively. Recently, it was shown that, beyond detecting underlying ischemia, repolarization alternans, a marker of susceptibility to arrhythmias and sudden cardiac death, can be effectively estimated from body surface ECGs using cvrPhone, a user-friendly and clinically acceptable mobile platform (20).
A device that tackles similar issues of comfort in a continuous ambulatory ECG monitoring setting was introduced in 2013 (21). This device was designed with discrete electronic components and a custom printed circuit board to minimize the footprint on the user while keeping low-power operation under consideration. This wearable ECG solution, when evaluated in 10 healthy adults under different ambulatory conditions and compared with a commercially available ECG recorder, had a sensitivity of over 99% in correctly detecting heart beats under different conditions (supine, sitting, standing, hopping, walking, running, and stepping) with the exception for the case of arm movement in which the sensitivity was below 99%. The overall QRS sensitivity for the wearable monitor was 99.67%.
HEART RHYTHM AND BP MONITORING
Although the Scanadu Scout is able to provide BP measurements and Apple’s iWatch is able to provide the HR, other wearable devices are also available to provide these measurements.
Smartphone accelerometers can be used for extracting physiological parameters, such as HR, even while the phone is being carried in a bag or a pocket. One such reported technique, based on accelerometers and the BCG, was evaluated in 12 subjects (at different body postures) and reported a low mean absolute error of only 1.16 ± 3 beats/min (22).
A smartphone-based cuffless BP monitoring solution that allows the user to act as the actuator, by pressing a finger against a small device attached to the smartphone by measuring the variable-amplitude blood volume variations, provided BP estimates that were comparable with a finger cuff device, with bias and precision errors of only 3.3 and 8.8 mm Hg for systolic BP and –5.6 and 7.7 mm Hg for diastolic, respectively (23).
A wearable and flexible pressure sensor–based on microfluidic elements has been proposed for arterial BP monitoring (24). This sensor consists of a noninvasive, ultraflexible, and transparent device that can be worn on a continuous basis. The technology can prove useful in noninvasively measure BP, thus potentially helping overcome the critical issue of flexibility and allowing for continuous monitoring, while still remaining comfortable to wear over extended periods of time.
A system based on BCG and SCG was used for monitoring relative changes in cardiac output, contractility, and BP (25). The system was based on a patch that measures the ECG, SCG, and body movement, via low-noise accelerometers and a wrist-worn component in the form of a watch that is placed against a sternum for measuring the BCG. Machine learning was used to mitigate the motion artifacts for accurate estimation of cardiac output, contractility, and BP from accelerometer data.
Google Glass (Google, Mountain View, California) is a head-mounted wearable gadget that comes equipped with accelerometer, gyroscope, and a camera. Measurements from the head-mounted gyroscope yielded more reliable results compared with those obtained with traditionally used accelerometers whereas the camera could also serve as a promising sensor for recording the physiological parameters of the individual (26). The same study showed a low mean absolute error of 1.18 beats/min for the HR and 0.94 breaths/min for the RR.
In a study comparing a camera-based app called the Pulse Oximeter and a probe-based smartphone app called Masimo Personal Health (Masimo Personal Health, Irvine, California), with standard pulse oximetry for detecting HR and peripheral capillary oxygen saturation (SpO2) (27), the smartphone camera–based pulse oximetry was found to be non-inferior to the standard, while the probe-based smartphone app was found to be superior to standard pulse oximetry in reliability.
CARDIORESPIRATORY FITNESS
A wearable and wireless multiparameter system to monitor SpO2, HR, and walking information was developed and tested for the 6-min walk test (6MWT). The 6MWT is a common criterion for evaluating an individual’s cardiopulmonary condition and has been shown to effectively estimate the HR and dynamic changes and differences in the subject’s walking speed and acceleration during the test (28). The wearable and wireless sensor was able to detect dynamic changes in the cardiac and respiratory functions of the individual during the 6MWT, so the system can potentially be used for continuous ambulatory monitoring.
The maximum oxygen uptake method is currently the gold standard for estimating the cardiorespiratory fitness (CRF) by measuring the oxygen consumption during maximal exercise. An algorithm for assessing the CRF of an individual in free living conditions instead provides an estimate of the CRF based on HR and context information provided by the accelerometer (e.g., as walking), using pattern recognition (29). It was shown to provide estimates of CRF that were closely correlated with laboratory equipment–based measurements and up to 21% lower error rates when compared with maximum oxygen uptake estimates.
An algorithm designed for wearable systems that can provide an estimate of the oxygen uptake was proposed by Beltrame et al. (30), using data derived from accelerometers, respiratory bands, and HR monitors that were incorporated into a random forest regression model. The proposed method has the potential to enable wearable devices to provide early detection of changes in the health status of a patient in a free living environmental scenario.
STRESS DIAGNOSIS
It is well known that cardiac events can also be triggered or magnified under emotionally stressful situations. A stress monitoring patch capable of monitoring the arterial pulsed wave, the skin temperature, and conductance has shown that the pulsed wave sensor could be worn comfortably, and it also provided estimates of HR variability (31).
Another wearable sensor system for monitoring stress that uses support vector machines exhibited up to 86% classification accuracy based on significant ECG features, electrodermal activity, and electroencephalography (32).
Table 2 presents a summary of benefits, reported performance and limitations for some of the most promising devices and systems discussed in the Ambulatory Cardiac Monitoring Technologies section.
TABLE 2.
Benefits, Performance, and Limitations of Wearable Devices and Systems
| Reference(s) | Device Name | Benefits | Reported Performance and Limitations |
|---|---|---|---|
| (8,9) | Zio Patch | Up to 14 days’ operation without battery replacement or recharge | Higher yield than Holter monitors (overall diagnostic yield of 63.2%); no real-time analysis or transmission |
| (10) | NUVANT MCT | Real-time analysis and transmission | Real-time information is not available to the user |
| (11) | Scanadu Scout | Real-time analysis available to the user | The device has been discontinued |
| (12) | Apple iWatch | Small, lightweight, easy to operate | Sensitivity of 87% and specificity 97% in identifying patients with silent atrial fibrillation |
| (14) | PulseSmart | Uses standard smartphone camera and flashlight, real-time analysis | Irregular pulse identification with sensitivity, specificity, and accuracy of 0.970, 0.935, and 0.951, respectively. PAC and PVC discrimination with 95.5% and 96% accuracy, respectively |
| (15) | Kardia Mobile | AF detection in 30 s | 100% diagnostic yield compared with 72.7% with ELR |
| (17) | ECG Check | Single-lead ECG, real-time analysis and transmission | Ventricular escape beat detection sensitivity of 93.91% and predictability of 94.12% |
| (18–20) | cvrPhone | Cardiorespiratory event detection from ECG signals | Ischemia detection within 2 min of occlusion. Apnea detection within 7.9 ± 1.1 s |
| (22) | N/A | HR detection with phone in pocket | Low mean absolute error of 1.16 ± 3 beats/min |
| (23) | N/A | Cuffless blood pressure measurement | Bias and precision errors of 3.3 and 8.8 mm Hg for systolic and –5.6 and 7.7 mm Hg for diastolic BP, respectively |
| (24) | N/A | Ultraflexible and transparent for continued use over long periods | Pressure sensitivity of 0.1 kPa–1, response time in tens of ms |
| (25) | N/A | BCG-based real-time monitoring of changes in cardiac output, blood pressure, contractility | More robust to motion artifact than devices based on PPG only |
| (26) | Google Glass | Higher accuracy based on gyroscope over accelerometers | Low mean absolute error of 1.18 beats/min for the HR and 0.94 breaths/min for the RR. |
| (27) | Masimo Personal Health | Portable, cost-effective, and noninvasive method to monitor oxygen saturation | Superior performance to standard pulse oximetry |
| (28) | N/A | Built to perform 6-min walk test | Detects dynamic changes in cardiopulmonary function during the 6-min walk test |
| (31) | N/A | Stamp size sensor integrating 3 sensors of skin temperature, skin conductance, and pulsed wave | Skin temperature sensitivity of 0.31 Ω/°C, skin conductance sensitivity of 0.28 µV/0.02 µS, and pulsed wave response time of 70 ms |
BCG = ballistocardiogram; ELR = electronic loop recorder; other abbreviations as in Table 1.
ADOPTION AND EFFECTIVENESS IN POPULATION
By 2016, nearly two-thirds of the U.S. adult population already owned a smartphone. Estimates from 2013 indicated that remote patient monitoring solutions have already passed the $4.9 billion annual revenue mark, with connected medical devices accounting for almost 77% of these revenues (33). There are a few technological factors that define the effectiveness and adoption of ambulatory monitoring devices, including a size and form factor that will be acceptable to and comfortable for the patients, so as to encourage use over a long period of time. Battery life also significantly impacts the length of time continuous monitoring can be provided by an ambulatory sensor (1).
Many nontechnical issues also exist that create a barrier in the more widespread utilization of ambulatory monitoring devices and are responsible for the limited success of telemonitoring systems so far. Some of these are issues related to reimbursement and insurance policies as well as to funding available to hospitals for establishing health care facilities that can leverage monitoring and diagnosis enabled through ambulatory data. According to a survey report compiled by MobileSmith, which specializes in developing apps for health care applications, a large number of health care providers are now paying more attention to mobile technologies for reducing read-mission rates, as many as 75% of which are estimated to be preventable (34). Research done by Mayo Clinic demonstrated that only 1 in 5 patients participating in a smartphone-based study for recording BP and weight on a daily basis needed to be readmitted within 3 months, whereas a staggering 60% of those who did not participate in the program required readmission (34).
However, many patients are not yet ready or familiar enough with these technologies to adopt them in their daily lives, with only half of the people who own a wearable device currently report to use it on a regular basis (35). A study that was recently conducted to assess the use of wearable devices among elderly populations (36), and also used a systematic review and a questionnaire, determined that currently very few elderly people have been using a wearable device; however, 60% of them showed interest in using a wearable device in the future. The study also suggested that more awareness should be created to inform people of the potential benefits from using wearable devices on a continuous basis, and how the same can help with early detection and prevention of medical emergencies (36).
Another survey conducted by a research unit of the cloud computing firm Salesforce in 2016 reported that Americans are open to health care options enabled through technology, with the younger generation more likely to own wearable devices and willing to share their health data with doctors and insurance companies (37). The report also identified some interesting trends, such as the fact that 62% of overall respondents would prefer a primary care physician who would use their data from wearable devices to manage their health outcomes. Moreover, an even larger majority of respondents (78%) were willing to provide their doctors access to health data from their wearable devices for most up-to-date view of their health. Also of interest is that 67% of the respondents would be willing to use a wearable tracking device provided by their insurance company if doing so would lead to lower insurance rates (37). In a systematic review carried out to determine the preferences of both patients and clinicians in using noninvasive body-worn sensor systems, it was determined that in order to be acceptable and effective for daily use, these systems should be small, compact, lightweight, and easy to operate and maintain (38).
In terms of the effectiveness of ambulatory monitoring solutions, several studies have reported significant positive impact of mobile health monitoring. One study assessed the usability and adherence of a real-time, long-term monitoring system among older adults with and without a chronic health condition. The system was used for 6 months by participants and consisted of a wireless wristwatch and other components. The findings indicated that a health monitoring system designed for older adults for use over an extended period of time may help those with chronic conditions to prevent unnecessary hospitalizations while remaining under continuous monitoring at the comfort of their homes (39). Along these lines, a screening study that was performed to examine the effectiveness of a wearable patch in detecting AF, using continuous ECG monitoring of asymptomatic patients with known risk factors, identified AF in 1 in 20 subjects with no prior AF history, a finding with significant public health impact (40). One study on the effectiveness of tele-health monitoring of elderly patients with congestive heart failure and chronic obstructive pulmonary disease, and which monitored BP, weight, and SpO2, as well as daily physiological activity, concluded that such systems have the potential to lead to more timely interventions (41). Another study explored the potential of cardiac monitoring through wearable sensors in the context of inpatient rehabilitation and found that such sensors provide an effective way for assessing the HR response to rehabilitation treat-ments while adding no adverse effects related to the sensors, to the protocol (42).
Although these studies highlight the potential benefits from these new technologies, it is important to realize the costs that will be associated with fully implementing and integrating them in mainstream medical practice. Standardization of data storage and transmission from these wearable devices needs to be implemented. These data will then need to be integrated with current electronic health record systems. Medical professionals will need to undergo training and education in order to understand the accuracy and limits of the data from these devices, especially when compared with currently standard clinical de-vices and equipment. The gap between the 2 will certainly result in at least a percentage of false flags, leading to unnecessary testing and medical in-terventions to eliminate patient concerns. Although all of these factors are certainly expected to add to direct and indirect health care costs, a gradually implemented standardized approach that adopts and makes use of these technologies is expected to provide a higher quality of health care and life, and improved patient satisfaction, in a cost-effective manner.
LIMITS, CHALLENGES, AND FUTURE OUTLOOK
Wearable sensor systems can help reduce the costs associated with high-quality and continuous health care monitoring by reducing unnecessary hospital admissions and length of stay (43). These sensors also enable better self-management interventions by helping patients pay more attention to their vital health parameters, daily routines and exercise, nutrition, and medications (44). Although recent developments in hardware and software algorithms have enabled great progress in the reliability, affordability, and effectiveness of continuous ambulatory monitoring devices, certain challenges remain that need to be carefully accounted when developing technologies and devices for clinically effective diagnosis. Vital sign signals such as the PPG and ECG, and recently, BP, are being actively incorporated into ambulatory monitoring devices such as fitness bands and smart watches. However, at present, there are very limited data on the clinical accuracy of such wearable devices (12).
As a notable example, a study recently analyzed the accuracy of the most prominent wrist-worn wearable devices (45). The devices included Apple iWatch, Fitbit Charge HR (Fitbit Inc., San Francisco, California), Samsung Gear S (Samsung, Seoul, South Korea), and Mio Alpha (Mio Labs, Portland, Oregon). The goal was to compare the HR and energy spent in different conditions such as resting, walking, running, and cycling with that measured using standard ECG and indirect calorimetry, respectively. It was found that none of the 4 devices was better than the ECG or indirect calorimetry for estimating the HR and energy spent. However, a recent Apple iWatch version has been reported to successfully provide reliable alerts for the detection of AF, with multiple reports of iWatch data resulting in timely intervention and diagnosis (46).
Although PPG signals can provide information such as the HR and have even become standard of care in some clinical settings, there does exist some controversy regarding their accuracy and reliability, particularly when considering diverse demographic and environmental factors (47). Changes in temperature, body movements, hairs, skin color, and even tattoos can have an impact on the readings via PPG (12). Similarly, accelerometers can provide a wealth of in-formation by monitoring not only the overall body movement, but also small motions of the skin surface caused by heart beats and blood circulation. However, much of the reliability from accelerometers is dependent on the algorithms designed for processing raw accelerometer data, which are greatly affected by motion artifacts (48). ECG signals too can be corrupted by noise and motion artifacts in ambulatory conditions.
It is therefore important to take into account the quality of the estimates and reliability of alerts generated from data collected through an ambulatory monitoring device before any critical step is taken in evaluating, diagnosing, or treating a person’s health condition. There are techniques that have been developed in this area that can help overcome or assess the limitations of current ambulatory solutions. For example, numerous signal quality assessment algorithms have been proposed, which can help assess the quality of ECG signals used for ambulatory purposes (49). In order to conquer the technological barriers and improve the reliability of ambulatory monitoring systems, several signal processing and machine learning techniques have been proposed that can lead to enhanced sensor performances as well as enable identification of key underlying patterns in a patient’s vital signs parameters and overall health (1). For example, an innovative technique to overcome the limitations of PPG signals discussed previously utilizes data from multiple individuals to train an algorithm to account for variations and dependencies related to human skin tone in order to enable more accurate BP measurement using the reflective PPG technique (50). An artificial intelligence–based approach (51) has been used to help develop an automated algorithm for arrhythmia detection by training a convolutional neural network on both real normal and synthesized abnormal beats. The synthesized abnormal beats were generated by modeling the common causes of arrhythmias and how they cause degradation in ECG beats. This approach was shown to detect abnormal ECG beats from the first 3 occurrences with a probability of 99.4%.
However, when it comes to adopting signal processing, machine learning, and similar approaches in health care and critical medical applications, one should take into account the high variability of physiological signals and parameters between patients. Even for patients under normal health conditions, ECG signals can vary significantly, and it can be very difficult to model or differentiate normal from abnormal. This is a situation where patient specific data and care methodologies can be of significant assistance.
As wearables and nonwearables sensors become more reliable and affordable, their ubiquitous integration into our daily lives will lead to continuous ambulatory monitoring by a combination of technologies (52). These multimodal signals will be able to collect and provide a wealth of diverse data from the daily routine of a person as well as information of clinical relevance. The Internet-of-Things is one framework that will make such realm possible by providing the framework for exchange and communication of data between sensors and ultimately with health care providers and caregivers. These connected sensors will be able to provide critical event detection and alerts and also offer better insights for improved health care and personalized medicine and therapy (53). Coupled with the power of social networks, this can further lead to more insights for both patients and health care providers helping identify effective approaches in dealing with diseases. Online platforms such as PatientsLikeMe are already enabling such advancements by letting people with rare diseases connect with one another to learn about the best possible therapies for themselves (53). Yet, a recent survey indicated that the vast majority of Americans are currently not aware of this platform and would like to learn more about it, although they are concerned about the insurance coverage, costs, and potential use of their data for discrimination (54). This indicates that more awareness is needed to educate the public of how their data collected through ambulatory monitoring devices can potentially benefit them, while also maintaining their privacy and interests. Technological developments need to be looked into carefully from the perspective of privacy, security, and data ownership to ensure that patients feel comfortable and confident in sharing such significant amount of data regarding their daily lives with data analytic companies, health care providers, and insurance companies (53,55). For that matter, transparency is an important issue that needs to be considered to ensure that data are collected and used with the consent of the people the data have been collected from, and only after they have been fully briefed of how their data will be utilized. The recent General Data Protection Regulation, enacted by European Union, aims at regulating some of the most important issues in this space, including those related to algorithms designed to make decisions without human intervention (56). However, as the opportunities and impact of such vast amount of data collected from our daily lives, laws, and frameworks is realized, regulations will need to evolve continuously in order to ensure the best interests of the general population.
Finally, when using off-the-shelf smartphone-based sensors, such as the camera or accelerometers, it is important to remember that different smart-phones have different levels of sensor quality and specifications. Although tests may provide optimal performances using high-end devices, the reliability may not be the same with some more low-cost affordable smartphones, which have generally been adopted in the broader and global population.
CONCLUSIONS
Remote and ambulatory monitoring devices for cardiac disorders are showing great promise for the early detection of life-threatening conditions and critical events through long-term continuous monitoring during routine daily lives of individuals. They also show a significant potential of reducing health care costs by preventing unnecessary hospitalizations as well as enabling better self-management and intervention approaches. Moreover, great new paradigms are enabled through these technologies, such as personalized medicine, which can help the diagnosis and develop treatments for previously less well understood diseases and conditions. Although technological advancements are progressing at a rapid pace, nontechnical barriers to widespread adoption and regulatory and privacy concerns need to be addressed carefully in order to fully leverage the potential of ambulatory monitoring devices in providing high-quality, reliable, and affordable health care.
HIGHLIGHTS.
Ambulatory cardiac monitoring devices allow collection of long-term data for diagnostics of clinical relevance.
Recent advancements have enabled affordable and reliable devices for early detection of important physiological events.
Improving adoption in general population is leading to new paradigms of health care and personalized medicine.
Acknowledgments
The work was supported by an American Heart Association Grant-in-Aid (#15GRNT23070001), the American Heart Association Institute of Precision Medicine (17UNPG33840017), the RICBAC Foundation, and the National Institutes of Health (1 R01 HL135335-01, 1 R21 HL137870-01, and 1 R21EB026164-01). Dr. Singh has served as a consultant for Abbott, Boston Scientific, Medtronic, Biotronik, EBR, Toray, Impulse Dynamics, MicroPort, and BackBeat. Dr. Heist ••• with Biotronik, Boston Scientific, Abbott, and Medtronic. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- BCG
ballistocardiogram
- BP
blood pressure
- ECG
electrocardiogram
- HR
heart rate
- MCT
Mobile Cardiac Telemetry
- PPG
photoplethysmography
- RR
respiratory rate
- SpO2
peripheral capillary oxygen saturation
REFERENCES
- 1.Patel S, Park H, Bonato P, Chan L, Rodgers M. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil 2012;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehn BM. Telemedicine helps cardiologists extend their reach. Circulation 2016;134:1189–91. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee NA, Singh JP. Making sense of remote monitoring studies in heart failure. Eur Heart J 2017;38:2361–3. [DOI] [PubMed] [Google Scholar]
- 4.Berg DD, Vaduganathan M, Upadhyay GA, Singh JP, Mehra MR, Stewart GC. Cardiac implantable electronic devices in patients with left ventricular assist systems. J Am Coll Cardiol 2018; 71:1483–93. [DOI] [PubMed] [Google Scholar]
- 5.Ajami S, Teimouri F. Features and application of wearable biosensors in medical care. J Res Med Sci 2015;20:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonato P Wearable sensors and systems. From enabling technology to clinical applications. IEEE Eng Med Biol Mag 2010;29:25–36. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Kim EG, Cao G, Liu S, Xu Y. Physiological acoustic sensing based on accelerometers: a survey for mobile healthcare. Ann Biomed Eng 2014; 42:2264–77. [DOI] [PubMed] [Google Scholar]
- 8.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127:95.e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014;15:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel JM, Mehta V, Fogoros R, Chavan A. Study of arrhythmia prevalence in NUVANT Mobile Cardiac Telemetry system patients. Conf Proc IEEE Eng Med Biol Soc 2012;2012:2440–3. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JA, Topol EJ, Steinhubl SR. Novel wire-less devices for cardiac monitoring. Circulation 2014;130:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter A, Frontera A. Smart-watches: a potential challenger to the implantable loop recorder? Europace 2016;18:791–3. [DOI] [PubMed] [Google Scholar]
- 13.McManus DD, Lee J, Maitas O, et al. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 2013;10:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McManus DD. PULSESMART: Pulse-based arrhythmia discrimination using a novel smart-phone application. J Cardiovasc Electrophysiol 2016;48:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelboom G, Camacho E, Abraham ME, et al. Smart wearable body sensors for patient self-assessment and monitoring. Arch Public Health 2014;72:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018;41:487–94. [DOI] [PubMed] [Google Scholar]
- 17.Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–4. [DOI] [PubMed] [Google Scholar]
- 18.Sohn K, Merchant FM, Sayadi O, et al. A novel point-of-care smartphone based system for monitoring the cardiac and respiratory systems. Sci Rep 2017;7:44946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn K, Merchant FM, Abohashem S, et al. Utility of a smartphone based system (cvrphone) to accurately determine apneic events from electrocardiographic signals. PLoS One 2019;14: e0217217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn K, Dalvin SP, Merchant F, et al. Utility of a smartphone based system (cvrPhone) to predict short-term arrhythmia susceptibility. Sci Rep 2019;9:14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eric SW, Maggie KD, Charles GS. A wearable cardiac monitor for long-term data acquisition and analysis. IEEE Trans Biomed Eng 2013;60:189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez J, McDuff DJ, Picard RW. BioPhone: Physiology Monitoring from Peripheral Smart-phone Motions. Paper presented at: International Conference of the IEEE Engineering in Medicine and Biology Society; August 25–29, 2015; Milan, Italy. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar A, Kim C-S, Naji M, Natarajan K, Hahn J-O, Mukkamala R. Smartphone-based blood pressure monitoring via the oscillometric finger-pressing method. Sci Transl Med 2018;10: eaap8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Digiglio P, Li R, Wang W, Pan T. Microflotronic arterial tonometry for continuous wearable non-invasive hemodynamic monitoring. Ann Biomed Eng 2014;42:2278–88. [DOI] [PubMed] [Google Scholar]
- 25.Etemadi M, Inan OT. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J Appl Physiol (1985) 2018;124: 452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez J, Li Y, Rehg JM, Picard RW. Cardiac and respiratory parameter estimation using head-mounted motion-sensitive sensors. EAI Endorsed Trans Pervasive Health Technol 2015;1:e2. [Google Scholar]
- 27.Tomlinson S, Behrmann S, Cranford J, Louie M, Hashikawa A. Accuracy of smartphone-based pulse oximetry compared with hospital-grade pulse oximetry in healthy children. Telemed J E Health 2017;24:527–35. [DOI] [PubMed] [Google Scholar]
- 28.Li SH, Lin BS, Wang CA, Yang CT, Lin BS. Design of wearable and wireless multi-parameter monitoring system for evaluating cardiopulmonary function. Med Eng Phys 2017;47:144–50. [DOI] [PubMed] [Google Scholar]
- 29.Altini M, Casale P, Penders J, ten Velde G, Plasqui G, Amft O. Cardiorespiratory fitness estimation using wearable sensors: Laboratory and free-living analysis of context-specific submaximal heart rates. J Appl Physiol 2016;120:1082–96. [DOI] [PubMed] [Google Scholar]
- 30.Beltrame T, Amelard R, Wong A, Hughson RL. Extracting aerobic system dynamics during unsupervised activities of daily living using wearable sensor machine learning models. J Appl Physiol (1985) 2018;124:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon S, Sim JK, Cho YH. A flexible and wearable human stress monitoring patch. Sci Rep 2016; 6:23468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betti S, Molino Lova R, Rovini E, et al. Evaluation of an integrated system of wearable physiological sensors for stress monitoring in working environments by using biological markers. IEEE Trans Biomed Eng 2017;65:1748–58. [DOI] [PubMed] [Google Scholar]
- 33.Fagerberg J, Kurkinen L. mHealth and Home Monitoring M2M Research Series. Gothenburg, Sweden: Berg Insight, 2012. [Google Scholar]
- 34.Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc 2015;90:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MobileSmith. How Mobile Apps Can Reduce Preventable Readmissions. 2015. Available at: https://www.beckershospitalreview.com/pdfs/white-papers/How%20Mobile%20Apps%20Can%20Reduce%20Preventable%20Readmissions%20-%20MobileSmith%202015.pdf. Accessed •••.
- 36.Kekade S, Hseieh CH, Islam MM, et al. The usefulness and actual use of wearable devices among the elderly population. Comput Methods Programs Biomed 2018;153:137–59. [DOI] [PubMed] [Google Scholar]
- 37.Salesforce. 2015 State of the Connected Patient Report. 2016. Available at: https://a.sfdcstatic.com/content/dam/www/ocms-backup/assets/pdf/industries/2015-State-of-the-Connected-Patient.pdf. Accessed •••.
- 38.Bergmann JHM, Chandaria V, McGregor A. Wearable and implantable sensors: the patient’s perspective. Sensors (Basel) 2012;12:16695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans J, Papadopoulos A, Silvers CT, et al. Remote health monitoring for older adults and those with heart failure: adherence and system usability. Telemed J E Health 2016;22:480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the screening study for un-diagnosed atrial fibrillation (STUDY-AF). Clin Cardiol 2015;38:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gokalp H, de Folter J, Verma V, Fursse J, Jones R, Clarke M. Integrated telehealth and telecare for monitoring frail elderly with chronic disease. Telemed J E Health 2018. August 21 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 42.Weeks DL, Sprint GL, Stilwill V, Meisen-Vehrs AL, Cook DJ. Implementing wearable sensors for continuous assessment of daytime heart rate response in inpatient rehabilitation. Telemed J E Health 2018. April 2 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 43.Mortara A, Pinna GD, Johnson P, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure). Eur J Heart Fail 2009;11:312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piwek L, Ellis DA, Andrews S, Joinson A. The rise of consumer health wearables: promises and barriers. PLoS Med 2016;13:e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallen MP, Gomersall SR, Keating SE, Wisløff U, Coombes JS. Accuracy of heart rate watches: implications for weight management. PLoS One 2016;11:e0154420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American College of Cardiology. Apple Heart Study Identifies AFib in Small Group of Apple Watch Wearers. 2019. Available at: https://www.acc.org/latest-in-cardiology/articles/2019/03/08/15/32/sat-9am-apple-heart-study-acc-2019. Accessed •••.
- 47.Pan T, Xu Y. Mobile medicine: can emerging mobile technologies enable patient-oriented medicine? Ann Biomed Eng 2014;42:2203–4. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Silva I, Wu D, Zheng J, Wu H, Wang W. Adaptive motion artefact reduction in respiration and ECG signals for wearable health-care monitoring systems. Med Biol Eng Comput 2014;52:1019–30. [DOI] [PubMed] [Google Scholar]
- 49.Satija U, Ramkumar B, Manikandan MS. A review of signal processing techniques for electrocardiogram signal quality assessment. IEEE Rev Biomed Eng 2018;11:36–52. [DOI] [PubMed] [Google Scholar]
- 50.Mukherjee R, Ghosh S, Gupta B, Chakravarty T. A universal noninvasive continuous blood pressure measurement system for remote healthcare monitoring. Telemed J E Health 2018;24:803–10. [DOI] [PubMed] [Google Scholar]
- 51.Kiranyaz S, Ince T, Gabbouj M. Personalized monitoring and advance warning system for cardiac arrhythmias. Sci Rep 2017;7:9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health 2017;23:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wicks P, Stamford J, Grootenhuis MA, Haverman L, Ahmed S. Innovations in e-health. Qual Life Res 2014;23:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress and Outlook Report. 2018. Available at: http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PM_at_FDA_A_Progress_and_Outlook_Report.pdf. Accessed •••.
- 55.Soh PJ, Vandenbosch GAE, Mercuri M, Schreurs DMMP. Wearable wireless health monitoring: current developments, challenges, and future trends. IEEE Microw Mag 2015;16: 55–70. [Google Scholar]
- 56.Olhede SC, Wolfe PJ. The growing ubiquity of algorithms in society: implications, impacts and innovations subject areas. Philos Trans A Math Phys Eng Sci 2018;376:20170364. [DOI] [PMC free article] [PubMed] [Google Scholar]