Abstract
Synthetic cathinones continue to proliferate in clandestine drug markets worldwide. N-ethylnorpentylone (also known as N-ethylpentylone or ephylone) is a popular emergent cathinone, yet little information is available about its toxicology and pharmacology. Here we characterize the analytical quantification, clinical presentation and pharmacological mechanism of action for N-ethylnorpentylone. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) was used to quantify N-ethylnorpentylone in blood obtained from human cases. Clinical features exhibited by the intoxicated individuals are described. The activity of N-ethylnorpentylone at plasma membrane transporters for dopamine (DAT), norepinephrine (NET) and 5-HT (SERT) was assessed using in vitro assays measuring uptake inhibition and evoked release of [3H]neurotransmitters in rat brain synaptosomes. Our LC-MS/MS method assayed N-ethylnorpentylone concentrations with limits of detection and quantification of 1 and 5 ng/mL, respectively. Quantitation was linear from 5 to 500 ng/mL, and the method displayed specificity and reproducibility. Circulating concentrations of N-ethylnorpentylone ranged from 7 to 170 ng/mL in clinical cases, and the associated symptoms included palpitations, tachycardia, agitation, hallucinations, coma and death. N-Ethylnorpentylone was a potent inhibitor at DAT (IC50=37 nM), NET (IC50=105 nM) and SERT (IC50=383 nM) but displayed no transporter releasing activity. We present a validated method for quantifying N-ethylnorpentylone in human case work. The drug is a psychomotor stimulant capable of inducing serious cardiovascular and neurological side-effects which can be fatal. In vitro findings indicate that N-ethylnorpentylone exerts its effects by potent blockade of DAT and NET, thereby elevating extracellular levels of dopamine and norepinephrine in the brain and periphery.
Keywords: N-Ethylnorpentylone, N-Ethylpentylone, Ephylone, Forensic toxicology, Liquid chromatography–tandem mass spectrometry, Monoamine transporter, New psychoactive substances, Synthetic cathinone
1. Introduction
New psychoactive substances (NPS) continue to proliferate in recreational (i.e., non-medical) drug markets worldwide.1 Synthetic cathinones are a major category of NPS that cause psychomotor stimulant effects in humans and laboratory animals.2,3 The compounds are structurally-related to the naturally-occurring compound cathinone, a main psychoactive ingredient in the Khat plant Catha edulis. After high-dose administration or repeated use, synthetic cathinones can produce serious medical complications including tachycardia, hypertension, agitation, psychosis, aggressive behavior, seizures, and in some cases, death.4–9 As such, these substances represent a serious risk to public health.
Like other stimulant drugs, synthetic cathinones exert their pharmacological effects by interacting with plasma membrane monoamine transporters proteins, thereby increasing extracellular concentrations of dopamine, norepinephrine and serotonin (5-HT) in the brain.10–12 Pyrrolidine-containing cathinones like 3,4-methylenedioxypyrovalerone (MDPV) are transporter inhibitors that block the uptake of dopamine and norepinephrine, similar to the actions of cocaine.13 Ring-substituted cathinones like 3,4-methylenedioxy-N-methylcathinone (methylone) are non-selective transporter substrates that induce the release of dopamine, norepinephrine and serotonin from neurons, similar to the mechanism of action for 3,4-methylenedioxymethamphetamine (MDMA).14 Elevations of extracellular dopamine in the brain mediate the reinforcing effects of synthetic cathinones, whereas elevations in extracellular norepinephrine are likely involved with cardiovascular side-effects.15,16
Legislation banning specific synthetic cathinones has fostered the appearance of an increasing number of new analogs in recreational drug markets. Evidence from multiple sources demonstrates that 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)pentan-1-one (N-ethylnorpentylone) is an emergent synthetic cathinone in the United States and elsewhere.17–22 N-Ethylnorpentylone is also commonly known as N-ethylpentylone or ephylone. N-Ethylnorpentylone was first described by Boehringer Pharmaceuticals in the 1960s23,24 and is structurally-related to the bath salts cathinones methylone and 1-(1,3-benzodioxol-5-yl)-2-(methylamino)pentan-1-one (pentylone) (Figure 1). According to data from the National Forensic Laboratory Information System (NFLIS), N-ethylnorpentylone was first reported in the United States during 2015.21 In Europe, the detection of the substance was first reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) in January 2016.25 In June 2018, N-ethylnorpentylone was placed into Emergency Schedule I control by the Drug Enforcement Administration (DEA) in the United States.22
Figure 1.
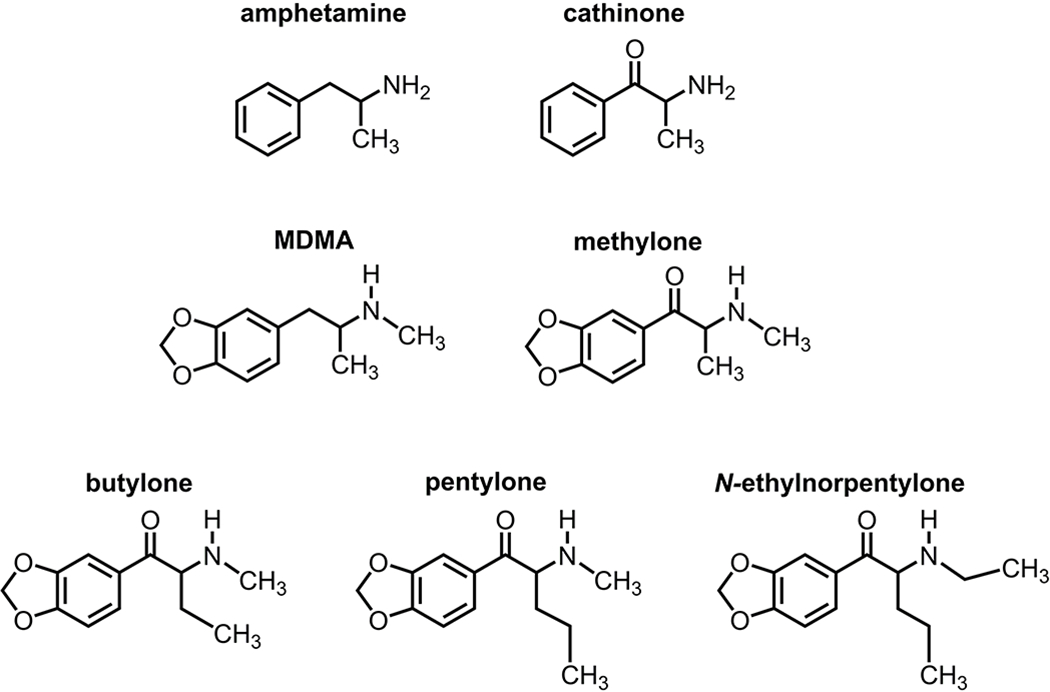
Chemical structures of N-ethylnorpentylone and related stimulant drugs.
Little is known about the toxicology or pharmacology of N-ethylnorpentylone. Thirakul et al.26 described the clinical presentation, autopsy findings and toxicology results for an acute fatality involving N-ethylnorpentylone exposure. The subject displayed tachycardia, agitation and hyperthermia prior to fatal cardiac arrest, consistent with psychostimulant intoxication. Krotulski et al.27 reported quantitative confirmation of N-ethylnorpentylone in biological specimens from 26 cases obtained through death investigations and drugged driving casework. The authors also showed that hepatic biotransformation of N-ethylnorpentylone involves β-keto reduction, N-deethylation and demethylenation to yield several unique metabolites. The molecular mechanism of action for N-ethylnorpentylone has not been investigated.
Given the limited information available about N-ethylnorpentylone, the present study had three major aims: 1] to develop and fully validate an analytical method for determining N-ethylnorpentylone concentrations in whole blood by liquid chromatography-tandem mass spectrometry (LC-MS/MS), 2] to describe the clinical presentation of analytically-confirmed cases of N-ethylnorpentylone intoxication, and 3] to characterize the molecular mechanism of action for the drug at monoamine transporter proteins in rat brain tissue.
2. Materials and methods
2.1. Chemical and reagents
N-Ethylnorpentylone reference material was purchased from Cayman Chemical (Ann Arbor, MI, USA) and MDMA-d5, used as internal standard (IS), was purchased from Cerilliant (Round Rock, TX, USA). Methanol and formic acid (98-100%) were purchased from Scharlau (Barcelona, Spain). Ammonium formate and tert-butyl methyl ether (MTBE) were purchased from Sigma-Aldrich (St Louis, MO, USA) and sodium tetraborate from LS Chemicals (Ribeirao Preto, SP, Brazil). Ultrapure deionized water was purified by a Milli-Q RG unit from Millipore (Billerica, MA, USA). All solvents employed in the sample extractions were HPLC grade. N-Ethylnorpentylone, pentylone and methylone used for monoamine transporter assays were obtained from Cayman Chemical. [3H]Norepinephrine (60 Ci/mmol) and [3H]methyl-4-phenylpyridinium ([3H]MPP+; 80 Ci/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO, USA) while [3H]dopamine (28 Ci/mmol) and [3H]5-HT (38 Ci/mmol) were purchased from Perkin Elmer (Billerica, MA, USA). All other reagents used for monoamine transporter assays were obtained from Sigma-Aldrich unless otherwise indicated.
2.2. Standard solution preparation
For the LC-MS/MS method, a stock solution of N-ethylnorpentylone was prepared by diluting reference material in methanol. Calibrator working solutions at 0.05, 0.1, 0.5, 1, 2.5 and 5 μg/mL, as well as quality control (QC) working solutions at low (0.15 μg/mL), medium (2 μg/mL) and high (4 μg/mL) concentrations, were prepared by appropriate dilution of stock solution in methanol. Internal standard (IS) solution (MDMA-d5, 0.25 μg/mL) was prepared by dilution in methanol. All solutions were stored at −20 °C.
2.3. Sample preparation for N-ethylnorpentylone quantitation
For LC-MS/MS method development and validation, fortified whole blood was prepared by dilution of calibrator and QC working solutions in blank blood. Liquid-liquid extraction was performed in a 2 mL polypropylene tube with 100 μL of fortified whole blood (or authentic case blood samples), 25 μL of IS solution (MDMA-d5, 0.25 μg/mL), 100 μL of sodium tetraborate (saturated solution) and 900 μL of MTBE. The tubes were capped, vortexed for 5 min and centrifuged at 8,000 rpm during 5 min. The organic layer (800 μL) was dried under nitrogen stream (40 °C), reconstituted with 500 μL of methanol, transferred to LC vials and 5 μL was injected onto LC-MS/MS system
2.4. Instrumentation
The LC-MS/MS analyses were performed on a Nexera liquid chromatography system coupled to a LCMS8040 equipped with a triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan).
The chromatographic separation was performed with a Restek Force Biphenyl column (50 × 2.1 mm, 1.8 μm) (Bellefonte, PA, USA), maintained at 50 °C. The mobile phase consisted of 2 mM ammonium formate + 0.1% (v/v) formic acid in ultra-pure water (A) and 2 mM ammonium formate + 0.1% (v/v) formic acid in methanol. The gradient elution was programmed as follows: 5% B, followed by a linear change to 95% B over 2.8 min, held at 95% B for 1.5 min and returned to initial conditions over 0.1 min (total run time of 6 min). The mobile phase flow rate of 0.5 mL/min.
The mass spectrometer was equipped with an electrospray ionization (ESI) source operating in positive ionization mode. The source parameters were: heat block temperature of 400 °C; capillary voltage of 4.5 kV, nebulizer gas (N2) flow of 3 L/min, desolvation line (DL) temperature of 250 °C, drying gas (N2) flow of 15 L/min and collision induced dissociation gas (Ar) pressure of 230 kPa. The analyses were performed in multiple reaction monitoring mode (MRM), in which two MRM transitions were chosen, one for quantitation and one for confirmation. Table 1 shows the optimized conditions of Q1 pre bias (V), collision energy (V) and Q3 pre bias (V) and the retention time (min) for N-ethylnorpentylone and MDMA-d5. Data were acquired and processed by Labsolution 5.75 SP2 software (Shimadzu, Kyoto, Japan).
Table 1.
MRM optimized transitions and retention time for analysis of N-ethylnorpentylone and MDMA-d5 (IS) by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The quantifier transitions were underlined.
| Analyte | MRM transitions (m/z) | Q1 Pre bias (V) | CE (V) | Q3 Pre bias (V) | Retention time (min) |
|---|---|---|---|---|---|
| N-ethylnorpentylone |
250.2→202.2 250.2→232.2 |
11 11 |
18 13 |
24 27 |
2.25 |
| MDMA-d5 |
198.9→165.1 198.9→107.1 |
20 20 |
14 25 |
19 21 |
1.90 |
CE, collision energy
2.5. Method Validation
The LC-MS/MS method was validated according to the guidelines of Scientific Working Group for Forensic Toxicology (SWGTOX) published for quantitative analysis.28 Parameters evaluated were sensitivity and linearity, specificity, accuracy and imprecision, matrix effect, recovery, carryover, dilution integrity and stability.
Sensitivity and linearity
The criteria for analyte identification were as follows: a peak eluting within ±2% of average calibrator retention time, signal-to-noise ratio of at least 3 and qualifier/quantifier MRM peak area ratio within ±20% of the mean ratio of calibrator. The limit of detection (LOD) was defined as the lowest concentration that reached the identification criteria. The limit of quantitation (LOQ) was the lowest concentration fulfilling the identification criteria, but with signal-to-noise ratio of at least 10 and quantifying within 20% relative standard deviation (RSD) of target concentration.
Linearity was assessed over 5 days and calibration curves fit by linear least squares regression (1/x2 weighting) with concentrations ranging from 5 to 500 ng/mL. Calibrators were required to quantify within ±20% RSD of target concentration.
Specificity
Endogenous interferences were assessed by analyzing blank whole blood samples, from ten different individuals. Potential exogenous interferences by 38 commonly encountered drugs of abuse and medications (Supplementary material Table 1) were evaluated by fortifying them at 500 ng/mL into low QC and negative (IS only) samples. No interference was noted if all analytes in the low QC quantified within ±20% of target with acceptable qualifier/quantifier MRM ratios, and no peak in the negative sample satisfied LOD criteria.
Accuracy and imprecision
Intra-day and inter-day accuracy and imprecision data for N-ethylnorpentylone were evaluated with 3 replicates of each QC concentration over 5 days (ninter=15). Accuracy was defined as percent target concentration and required to be within ±20% of target concentration; imprecision was also assessed within ±20% RSD. One-way analysis of variance (ANOVA) was performed on each QC concentration to assess potentially significant inter-day variability at p<0.05.
Matrix effects
Matrix effects were determined by fortifying two sets of samples at low and high QC concentrations. In set A, 6 blank whole blood samples were spotted and carried through the extraction procedure, and the extraction solvent was fortified with analytes and internal standard at the QC concentrations. In set B, extraction solvent was fortified with analytes and IS, and transferred directly to autosampler vials. Matrix effect was calculated as the ratio of average peak areas from set A to set B, subtracted by 1, and expressed as a percentage; a negative percentage represents peak suppression and a positive percentage represents peak enhancement.
Carryover
Blank whole blood was fortified with all analytes at the highest point of calibration curve; the extract was injected into the LC–MS/MS instrument, and then a blank sample extract was injected. Carryover was absent if no analyte peak met LOD criteria for any blank injection.
Stability
Stability of the samples was evaluated by fortifying blank whole blood with analytes at low (15 ng/mL) and high (400 ng/mL) QC concentrations (n = 3), then analyzing after storage (24 and 72 h) at refrigerator (4 °C) and freezer (−20 °C) temperatures, and after 3 freeze/thaw cycles. Additionally, processed samples were stored in the 15 °C autosampler for 24 h before being reinjected. Long-term stability (90 days) was evaluated fortifying blank whole blood at low and high QC concentrations (n = 3) and was also in one real post mortem blood sample (n = 3). The samples were stored for 90 days at refrigerator (4 °C) and freezer (−20 °C) temperatures and analyzed against a newly prepared calibration curve. In all studies, stability was observed if concentrations were ±20% of target concentrations.
2.6. Clinical Cases
For the cases reported here, intoxicated individuals were brought by ambulance to the hospital emergency department (ED), often after initial referral from the Poison Control Center (PCC) for medical and laboratory assistance. It is noteworthy that the PCC is the reference point of contact for clinical toxicology treatment and analytical toxicology diagnosis in Brazil. Thus all hospitals get in contact with the PCC to report intoxications, to carry out laboratory diagnosis, and to request assistance with treatment (use of antidotes, pharmacological treatments, etc.). All six individuals described here were suspected of exposure to drugs of abuse, including NPS, and had symptoms of acute drug intoxication. Half of the cases were examined by our medical staff in our ED, whereas the other half were examined by other regional hospitals under medical and laboratory supervision by our staff. Information about the subjects were obtained from friends and family members whenever possible, and the clinical data were generously provided by hospital staff. Patient samples were subjected to standard toxicological drug screening by gas chromatography-mass spectrometry (GC-MS) using published procedures,29,30 and blood alcohol concentration was determined by gas chromatography with flame ionization detection (GC-FID).
2.7. Monoamine Transporter Assays
Animals
Male Sprague-Dawley rats (Envigo, Frederick, MD, USA) weighing 250-350 g were housed three per cage with free access to food and water. Rats were maintained on a 12 h light/dark cycle with lights on from 7 AM to 7 PM. Animal facilities were fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee and the National Institutes of Health guidelines on care and use of animal subjects. Rats were euthanized by CO2 narcosis, and synaptosomes were prepared from brains as previously described.3,11 Briefly, rat caudate tissue was used for dopamine transporter (DAT) assays whereas whole brain minus cerebellum and caudate was used for norepinephrine transporter (NET) and 5-HT transporter (SERT) assays. Tissue was homogenized in ice-cold 10% sucrose. After 12 strokes with a Potter-Elvehjem homogenizer, the homogenates were centrifuged at 1,000 g for 10 min at 4°C, and the supernatants (i.e., synaptosomes) were retained on ice.
Transporter inhibition assays
For uptake inhibition assays, 5 nM [3H]dopamine, 10 nM [3H]norepinephrine or 5 nM [3H]5-HT was used as the radiolabeled neurotransmitter for DAT, NET or SERT, respectively. The uptake assays were optimized for the single transporter of interest by adding unlabeled blockers to prevent uptake of [3H]transmitter by competing transporters. Uptake inhibition assays were initiated by adding 100 μL of synaptosome suspension to 900 μL Krebs-phosphate buffer (126 mM NaCl, 2.4 mM KCl, 0.83 mM CaCl2, 0.8 mM MgCl2, 0.5 mM KH2PO4, 0.5 mM Na2SO4, 11.1 glucose, 0.05 pargyline, with 1 mg/mL bovine serum albumin and 1 mg/mL ascorbic acid, pH 7.4) containing the test compound and [3H]transmitter. Dose-response curves were generated by testing 8 different drug concentrations. Uptake inhibition was terminated by rapid vacuum filtration through Whatman GF/B filters and retained radioactivity in synaptosomes was quantified with liquid scintillation counting (Perkin Elmer).
Transporter release assays
For release assays, 9 nM [3H]MPP+ was used as the radiolabeled substrate for DAT and NET, whereas 5 nM [3H]5-HT was used as a substrate for SERT. All buffers used in the release assay methods contained 1 μM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for the single transporter of interest by including unlabeled blockers to prevent the uptake of [3H]MPP+ or [3H]5-HT by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h to reach steady state. Release assays were initiated by adding 850 μL of preloaded synaptosomes to 150 μL of test drug. Dose-response curves were generated by testing 8 different drug concentrations. Release was terminated by vacuum filtration, and retained radioactivity was quantified by liquid scintillation counting (Perkin Elmer). Effects of test drug concentrations were expressed as % maximum release, with maximum release (i.e., 100% Emax) defined as the release produced by 10 μM tyramine for DAT and NET assay conditions, and 100 μM tyramine for SERT assay conditions. These doses of tyramine evoke the efflux of all “releasable” tritium from synaptosomes.
Data Analysis
Dose-response data from the uptake inhibition and release assays were subjected to non-linear curve fitting using the software program GraphPad Prism 6 (GraphPad Scientific, San Diego, CA, USA). The IC50 value represents the drug concentration that yields half-maximal uptake inhibition whereas EC50 value represents the drug concentration that yields half-maximal transmitter release.
3. Results
3.1. Method validation
The limits of detection (LOD) and quantification (LOQ) for N-ethylnorpentylone were 1 and 5 ng/mL, respectively. The method was also found to be linear from 5 to 500 ng/mL, with 1/x2 weighted linear regression.
No interference was observed when the LOQ was analyzed in the presence of 1 μg/mL of 38 other drugs of abuse and pharmaceuticals, and no carryover was observed at 500 ng/mL (highest calibration curve concentration).
Inter- and intra-day imprecisions for all three QC concentrations (15, 200 and 400 ng/mL) were analyzed by one-way analysis of variance (ANOVA), with no more than 9.0% RSD. Matrix effects were minimal both at low and high QC. Recovery for the analyte and IS were higher than 81.3%. N-Ethylnorpentylone solutions were stable (less than 13% variation from the initial concentration) under diverse situations: 24 and 72 h of stability at 4 and −20 °C, three freeze and thaw cycles, and 24 h of stability in a temperature-controlled autosampler (15 °C). The long-term stability study (90 days), both QC concentrations presented −3.5% of variation of initial concentration at 4 ºC, and +6.5% at −20 ºC. For post mortem samples, the variations were −19.7% and −5.5% when stored at 4 ºC and −20 ºC, respectively. Complete N-ethylnorpentylone method validation data are summarized in Tables 2 and 3.
Table 2.
Method validation parameters of imprecision, bias error, recovery and matrix effect for the quantification of N-ethylnorpentylone in whole blood by LC-MS/MS.
| N-Ethyl norpentylone | Intra-day imprecision (%RSD) (n=3) | Inter-day imprecision (%RSD) (n=15) | Bias (%) (n=15) | Recovery (%) (n=6) | Matrix effect (%) (n=6) |
|---|---|---|---|---|---|
| Low QC (15 ng/mL) | 4.7 | 4.0 | 0.9 | 94.6 | −12.3 |
| Med QC (200 ng/mL) | 3.4 | 4.6 | 4.9 | n.d. | n.d. |
| High QC (400 ng/mL) | 9.0 | 8.1 | −2.4 | 86.4 | −4.8 |
n.d., not determined at this concentration.
Table 3.
Short term stability data (% difference) for N-ethylnorpentylone in whole blood after storage at two temperatures (4 °C and −20 °C) for 24 h and 72 h, and after 3 freeze-thaw cycles.
| Analyte | T (°C) | Stability (%) (n=3) | |||
|---|---|---|---|---|---|
| 24h | 72h | 3 freeze-thaw cycles | |||
| N-ethylnorpentylone | Low QC (15 ng/mL) | 4 | −2.4 | −4.9 | −11.8 |
| −20 | −1.8 | −12.6 | |||
| High QC (400 ng/mL) | 4 | 7.1 | 5.9 | −3.0 | |
| −20 | −3.3 | −2.9 | |||
To evaluate whether authentic serum samples could be effectively analyzed using the developed method, calibration curves and QCs (low and high) were prepared using blank whole blood and serum. No significant difference was observed when comparing the calibration curves and QC results in these matrices.
3.2. Case reports
Case 1
A 32-year-old man attending a rave party displayed psychomotor agitation and aggressiveness, then eventually fainted. First aid was provided by paramedics at the party, but the man died in the ambulance on route to the hospital at Aracaju city (northeast Brazil). According to friends, he consumed alcohol and synthetic drugs sold at the rave party. The body was transferred to the coroner’s laboratory the same day, where the necropsy was immediately performed. External examination of the body revealed facial swelling, cyanosis at the extremities and yellowish liquid emanating from the mouth and nostrils. Internal examination revealed generalized hemorrhage of the pulmonary alveoli, abnormal increase in liver size and absence of urine in the bladder. No traumatic lesions were found in the cranial or abdominal cavities. Post mortem whole blood was collected for toxicological analysis, and N-ethylnorpentylone (170 ng/mL) was the only psychoactive analyte detected. Figure 2 depicts a chromatogram of N-ethylnorpentylone in whole blood from this fatal case, analyzed by the LC-MS/MS method described above.
Figure 2.
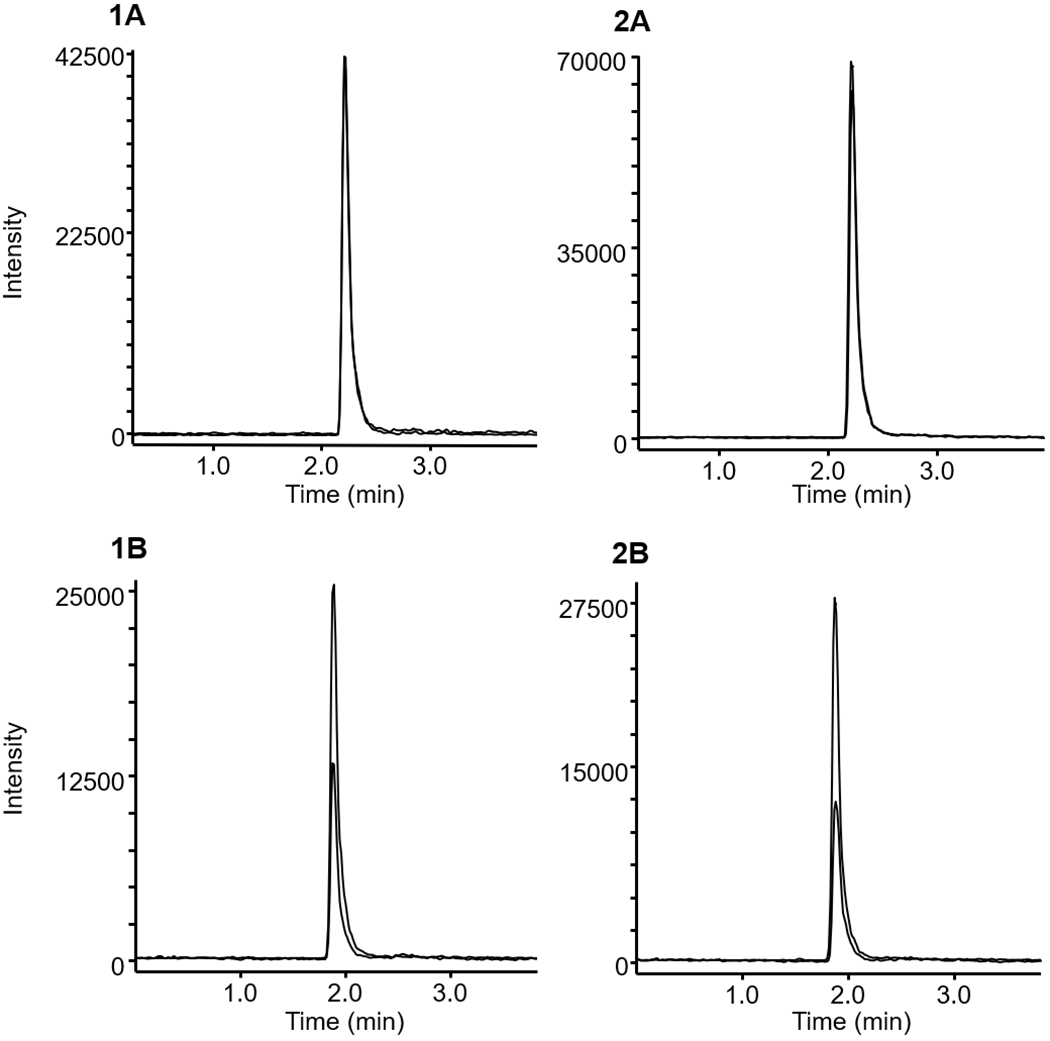
Multiple reaction monitoring (MRM) chromatograms of (1) a post mortem whole blood sample (N-ethylnorpentylone = 170 ng/mL) and (2) fortified whole blood (250 ng/mL) submitted to presented method. (A) N-ethylnorpentylone, (B) MDMA-d5 (IS). Sample preparation and analytical conditions are described in the experimental section.
Case 2
An 18-year-old man was brought by ambulance to our ED from a rave party where he displayed agitation and signs of several injuries. The patient presented with tachycardia (176 bpm) and mydriatic pupils, oscillated between psychomotor agitation and neurological depression, but maintained spontaneous ventilation. A computerized tomography (CT) scan excluded traumatic injuries. Routine laboratory screening for traditional drugs of abuse, based on liquid-liquid extraction followed by GC-MS, was negative in both urine and serum. Subsequent LC-MS/MS analysis revealed N-ethylnorpentylone in urine and serum (serum: 7 ng/mL). The patient recovered after a few hours, and by 12 h was asymptomatic and discharged.
Case 3
A 26-year-old woman was brought by ambulance to our ED after being reported to the PCC. She was initially found unconscious in her apartment with sphincter release (feces and urine were found). According to her mother and friends, the patient went to a party the previous night and used ecstasy pills and marijuana. At the ED, the patient was confused, sleepy, with several tongue injuries suggestive of intentional bite, disconnected speech, and episodes of visual hallucinations. The data in Table 4 show that creatine kinase was markedly elevated but other laboratory measures were within the normal range. Urine toxicology screening collected 48 h after the party detected MDMA and N-ethylnorpentylone. No cannabinoids, benzodiazepines or GHB were detected. Serum toxicology analysis did not detect any substances. The patient was hydrated and stayed under observation for 24 h. At discharge, she was lucid, responsive, and space and time oriented, but with anterograde amnesia.
Table 4.
Laboratory results for analytically-confirmed N-ethylnorpentylone cases.
| Laboratory Test Results | Case Number | |||
|---|---|---|---|---|
| 3 (female) | 4 (male) | 5 (male) | 6 (male) | |
| pH (7.35 - 7.45) |
n.a. | 7.46 | 7.41 | n.a. |
| pCO2 (35 - 45 mmHg) |
n.a. | 29.4 | 31 | n.a. |
| pO2 (80 - 100 mmHg) |
n.d. | 72.5 | 86 | n.a. |
| HCO3 (22 - 26 mmol/L) |
n.a. | 20.5 | 19 | n.a. |
| base excess (−2 - +2 mmol/L) |
n.a. | −1.6 | −3.8 | n.a. |
| Sodium (Adult < 65 y: 136 - 145 mEq/L) |
140 | 137 | 137 | 137 |
| Potassium (Adult < 65 y 3.3 - 5.1 mEq/L) |
3.6 | 3.4 | 3.7 | 3.8 |
| Calcium (8.5 - 10.1 mg/dL) |
n.a. | n.a. | 1.1 | n.a. |
| Magnesium (1.6 - 2.4 mg/dL) |
n.a. | n.a. | 1.6 | n.a. |
| Lactate (0.5 - 1.6 mmol/L) |
n.a. | n.a. | 11.0 | n.a. |
| Hb (Male.: 13.2 - 18.0 g/dl) (Fem.: 11.5 - 16.5 g/dl) |
13.1 | 15.5 | 13.0 | 13.0 |
| Hematocrit (Male.: 39.0 - 51.0%) (Fem.: 36.0 - 48.0 %) |
38.4 | 43.0 | 43.0 | 39.9 |
| serum creatinine (Male.: 0.8 - 1.3 mg/dL) (Fem.: 0.6 - 1.0 mg/dL) |
0.5 | 0.9 | 0.7 | 0.7 |
| BUN (15 - 39 mg/dL) |
14 | 14 | 28 | 31 |
| creatine kinase (Male.: 39 - 308 U/L) (Fem.: 26 - 192 U/L) |
7,151 | 605 | 1,260 | 713 |
| creatine kinase MB isoenzyme (7 - 25 U/L) |
n.a. | 21 | n.a. | n.a. |
| alanine aminotransferase (12 - 78 U/L) |
35 | 25 | n.a. | 27 |
| aspartame aminotransferase (10 - 40 U/L) |
93 | 40 | n.a. | 24 |
| phosphatase alkaline (Adult: 50 - 136 U/L) |
53 | n.a. | n.a. | n.a. |
| Glucose (70 - 100 mg/dL) |
n.a. | 104 | n.a. | 101 |
n.a., not available
Case 4
A 19-year-old man without previous illness attended a rave party and stayed 14 h consuming various drugs and alcohol. In total, he reportedly consumed five ecstasy tablets, ingested one LSD blotter hit, smoked two packs of cigarettes and drank an undetermined amount of the Brazilian alcoholic beverage “catuaba”. The patient reported having palpitations at the end of the party and decided to get help in a hospital 41-miles from our location, and then the PCC was called. At physical examination he appeared conscious and oriented, but was agitated, with palpitations and tachycardia (180 bpm). The patient exhibited normal blood pressure (BP 123/89 mmHg), ECG and axillary temperature (37.3°C); pulmonary and abdominal examination revealed nothing abnormal. Creatine kinase was greater than normal but other laboratory test results were generally unremarkable as summarized in Table 4. Serum and urine samples were collected and sent under refrigerated conditions via ambulance to the PCC analytical toxicology laboratory. Urine toxicology screening detected MDMA and N-ethylnorpentylone, caffeine and cotinine (nicotine metabolite). No LSD was detected. Serum toxicology analysis detected alcohol (0.8 g/L), N-ethylnorpentylone (19 ng/mL) and MDMA (54 ng/mL). The patient was treated symptomatically with diazepam and metoclopramide until remission of symptoms and discharged 24 h later.
Case 5
The PCC was contacted by the ED of a hospital 43-miles away, requesting clinical and analytical toxicology support for a 35-year-old man who had been consuming alcohol and other drugs of abuse in his own house for two consecutive days. He was found unconscious, with neurological depression (Glasgow 5) and anisocoria. At admission to the ED, the patient received fentanyl, midazolam for orotracheal intubation, tramadol and dipyrone. A CT scan was performed but showed no brain abnormalities. According to his family, the patient did not have a history of psychiatric problems, use of continuous medications or morbid antecedents. Serum and urine samples were collected and sent under refrigerated conditions via ambulance to the PCC analytical toxicology laboratory. Laboratory test results at admission are shown in Table 4. Creatine kinase and lactate levels were markedly elevated. After 6 h of admission, the patient evolved to neurogenic shock, and decerebration. CT remained unchanged and no blood alcohol was detected. Routine laboratory screenings for traditional drugs of abuse in urine and serum were negative. N-Ethylnorpentylone was detected in urine and serum (serum: 149 ng/mL). Clinical symptoms remained unchanged and stable after 6 days of hospitalization. CT at 6 days detected vertebral artery dissection that affected the brainstem and cerebrovascular hemorrhage of the brainstem was diagnosed. Thirty-five days later the patient was discharged in a vegetative state with neurological damage to the third cranial nerve (oculomotor nerve) which made it impossible for the patient to open his eyes.
Case 6
A 26-year-old man with a previous history of mental disorders was admitted to our psychiatry emergency unit for differential diagnosis of drug misuse. He presented with symptoms of psychosis, paranoia, sleeplessness and inconsistent speech (talking about wars, guns, Nazism). Clinical laboratory results are shown at Table 4. Increased creatine kinase (713 U/L) was observed but other measures were within normal limits. Routine laboratory screening for traditional drugs of abuse in serum was negative. Subsequent LC-MS/MS analysis revealed N-ethylnorpentylone in serum at a concentration of 61 ng/mL. The patient was restrained by the psychiatry medical staff, sedated with midazolam, intravenously hydrated and stayed under observation for 24 h. He subsequently started psychiatric therapy with valproic acid, risperidone, haloperidol, and levomepromazine.
3.3. Monoamine transporter assays
It is well established that psychomotor stimulants interact with monoamine transporter proteins in nervous tissue to achieve their pharmacological effects.10–14 As a first step in characterizing the mechanism of action for N-ethylnorpentylone, we examined the ability of the drug to inhibit [3H]neurotransmitter uptake at DAT, NET and SERT in rat brain synaptosomes. The effects of N-ethylnorpentylone were compared to the structural analogs pentylone and methylone (see Figure 1). Figure 3 depicts the effects of test drugs on inhibition of transporter-mediated uptake and Table 5 summarizes potency estimates expressed as IC50 values. N-Ethylnorpentylone was a potent and efficacious inhibitor at DAT (IC50 = 37±2), NET (IC50 = 105± 4) and SERT (IC50 = 383±27), but effects on DAT and NET were predominant. The DAT/SERT ratio for N-ethylnorpentylone was >10 indicating substantial selectivity for DAT. The structurally-related analog, pentylone, also displayed efficacious uptake inhibition at DAT, NET and SERT but was much less potent when compared to N-ethylnorpentylone at all sites. Finally, the bath salts cathinone, methylone, exhibited weak but efficacious uptake inhibition at DAT, NET and SERT with IC50 values around 1 μM, consistent with our previously published results.3,11 Methylone was essentially non-selective in its effects on uptake with a DAT/SERT ratio close to unity (i.e., 0.83). The findings from the uptake inhibition experiments demonstrate the N-ethylnorpentylone is a transporter blocker with greater potency and DAT-selectivity when compared to methylone or pentylone.
Figure 3.
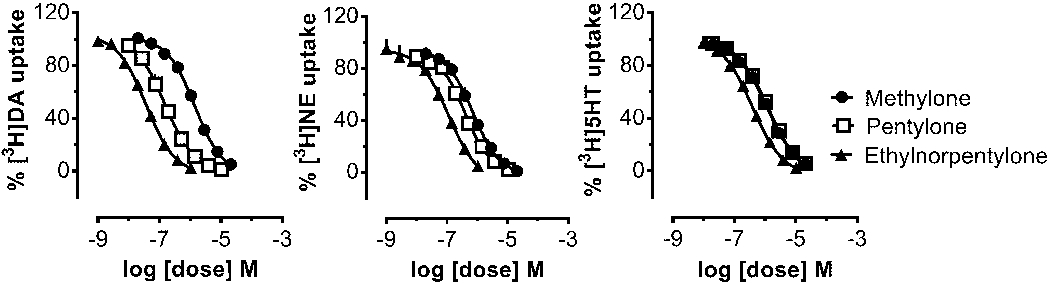
Dose-response effects of N-ethylnorpentylone and related drugs on the inhibition of [3H]neurotransmitter uptake at DAT, NET and SERT in rat brain synaptosomes. Data are mean ± SD for N=3 separate experiments performed in triplicate.
Table 5.
Dose-response effects for N-ethylnorpentylone and related synthetic cathinones on inhibition of [3H]neurotransmitter uptake at DAT, NET or SERT in rat brain synaptosomes.
| Compound | Inhibition of [3H]dopamine Uptake at DAT IC50 (nM) |
Inhibition of [3H]norepinephrine Uptake at NET IC50 (nM) |
Inhibition of [3H]5-HT Uptake at SERT IC50 (nM) |
DAT/SERT ratio |
|---|---|---|---|---|
| N-Ethyl Norpentylone | 37 ± 2 | 105 ± 14 | 383 ± 27 | 10.4 |
| Pentylone | 154 ± 11 | 401 ± 27 | 1198 ± 59 | 7.8 |
| Methylone | 1389 ± 81 | 712 ± 53 | 1149 ± 65 | 0.8 |
Data are mean ± SD for n=3 experiments performed in triplicate.
DAT/SERT ratio is [DAT IC50]−1/[SERT IC50]−1, where higher value indicates greater DAT selectivity
Uptake inhibition assays are critical for identifying drugs which target monoamine transporter proteins, but these assays cannot distinguish between drugs acting as non-transported uptake inhibitors versus those acting as transportable substrates. Thus, we next carried out release assays to determine whether N-ethylnorpentylone could act as a transporter substrate inducing release of [3H]substrates at DAT, NET and SERT. Figure 4 depicts the effects of test drugs on the release of [3H]MPP+ via DAT and NET or [3H]5-HT via SERT, while Table 6 summarizes potency estimates expressed as EC50 values. The data in Figure 3 show that N-ethylnorpentylone was unable to evoke release at DAT, NET or SERT, which indicates the drug is not a transporter substrate. Pentylone displayed an interesting profile in the release assay, where it acted as a substrate-type releaser at SERT but was inactive at DAT and NET. By contrast, methylone displayed efficacious releasing activity at DAT, NET and SERT, with similar potency at each site, as previously reported.3,11 The combined findings from the uptake and release assays clearly demonstrate that N-ethylnorpentylone is a “pure” uptake inhibitor devoid of substrate activity at monoamine transporters.
Figure 4.
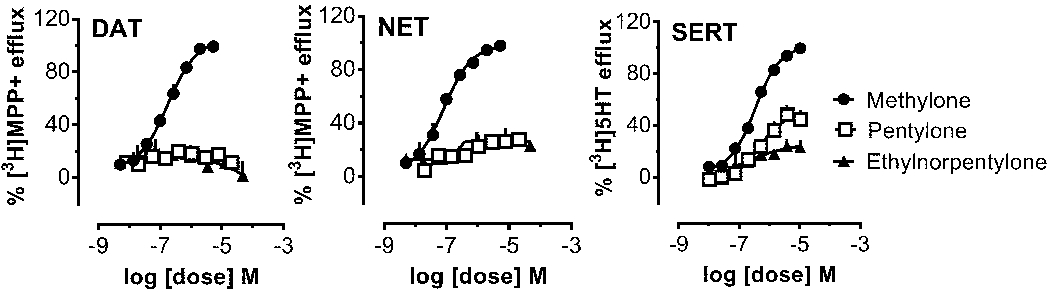
Dose-response effects of N-ethylnorpentylone and related drugs to induce the release of [3H]MPP+ via DAT and NET or [3H]5-HT via SERT in rat brain synaptosomes. Data are mean ± SD for N=3 separate experiments performed in triplicate.
Table 6.
Dose-response effects of N-ethylnorpentylone and related synthetic cathinones on release of [3H]MPP+ via DAT and NET or [3H]5-HT via SERT from rat brain synaptosomes.
| Compounds | Release of [3H]MPP+ via DAT EC50 (nM) [%Emax] |
Release of [3H]MPP+ via NET EC50 (nM) [%Emax] |
Release of [3H]5-HT via SERT EC50 (nM) [%Emax] |
DAT/SERT ratio |
|---|---|---|---|---|
| N-EthylNorpentylone | inactive | inactive | inactive | --- |
| Pentylone | inactive | Inactive | 476 ± 98[54%] | --- |
| Methylone | 178 ± 17[96%] | 81 ± 8[94%] | 342 ± 20[99%] | 1.9 |
Data are mean ± SD for n=3 experiments performed in triplicate.
DAT/SERT ratio is [DAT EC50]−1/[SERT EC50]−1, where higher value indicates greater DAT selectivity.
Inactive indicates release efficacy<25% of Emax.
4. Discussion
N-Ethylnorpentylone is a synthetic cathinone that is being encountered in recreational drug markets worldwide, yet little is known about its toxicology or pharmacology.26,27 Numerous analytical methods are published describing analyses of synthetic cathinones in biological samples,31–37 but only two articles have reported quantitation of N-ethylnorpentylone.26,27 Here we present a fully validated method to detect and quantify this substance in whole blood. Our method displays linearity from 5 to 500 ng/mL and exhibits LOD and LOQ of 1 and 5 ng/mL, respectively. The method is characterized by suitable specificity since no interference was observed when the analysis was carried out in the presence of 38 other drugs of abuse and pharmaceuticals, including structurally similar cathinone compounds, such as methylone, 4-fluoromethcathinone, mephedrone, butylone and pentylone, which might interfere with detection of N-ethylnorpentylone.
The serum concentrations of N-ethylnorpentylone that we measured agree with those reported by Krotulski et al.27 who examined drug concentrations in 26 cases obtained through death investigations and drugged driving casework. The two highest concentrations of N-ethylnorpentylone that we measured were found in a fatal overdose case (i.e., 170 ng/mL) and a serious intoxication with lasting cerebrovascular complications (i.e., 149 ng/mL). Importantly, N-ethylnorpentylone was the only psychoactive compound detected in our fatal case, confirming the drug can have lethal consequences, as originally reported by Thirakul and colleagues.26 We found much lower drug concentrations in the cases where symptoms were transient and medical recovery was complete. Our findings could be interpreted to suggest that blood concentrations of N-ethylnorpentylone exceeding 100 ng/mL are indicative of life-threatening medical consequences. On the other hand, Krotulski et al. showed that blood concentrations of N-ethylnorpentylone in fatal overdose cases ranged from 12 to 50,000 ng/mL, illustrating the difficulty in relating post mortem drug concentrations with clinical presentation and long-term outcomes.
The symptoms produced by N-ethylnorpentylone intoxication were consistent with the reported effects of other synthetic cathinones, including the bath salts cathinones MDPV and methylone.38,39 Specifically, our cases displayed sympathomimetic symptoms such as tachycardia and palpitations, along with agitation, aggression and hallucinations. All of the cases with accompanying laboratory results exhibited high creatine kinase levels, a symptom which is often associated with rhabdomyolysis after synthetic cathinone intoxication. O’Conner et al.40 showed that the risk for rhabdomyolysis is greater after synthetic cathinone administration when compared to other stimulant drugs of abuse. It is noteworthy that the fatal case of N-ethylnorpentylone overdose described here had diffuse alveolar hemorrhage which likely contributed to death. This syndrome has not been reported for synthetic cathinones but is sometimes observed in cocaine overdose.41 In our other serious N-ethylnorpentylone intoxication, we report a cerebrovascular hemorrhage that left the individual with lasting brain damage. Although our data with N-ethylnorpentylone are observational, and no cause and effect relationships can be gleaned, other psychomotor stimulants are known to produce cerebrovascular complications including stroke and intracerebral hemorrhage.42 Preclinical studies in rats show that cardiovascular effects of synthetic cathinones involve stimulation of centrally-mediated sympathetic outflow.16
Synthetic cathinones are known to exert their pharmacological effects by interacting with plasma membrane monoamine transporters in the brain and periphery.10–14 Drugs that interact with monoamine transporters can be divided into two types based on their specific mechanism of action: 1) transporter inhibitors and 2) transporter substrates.13 Transporter inhibitors bind to the extracellular face of the transporter protein to block uptake of neurotransmitters. Transporter substrates also bind to the transporter, but these drugs are subsequently translocated through the transporter channel to the inside of the cell where they evoke neurotransmitter release via reverse transport (i.e., transporter-mediated release). Here we show for the first time that N-ethylnorpentylone is a potent uptake inhibitor at DAT, NET and SERT, with preference for the dopamine system. In contrast to methylone, N-ethylnorpentylone is devoid of substrate activity. It seems probable that the α-carbon propyl chain and amine ethyl chain render N-ethylnorpentylone unable to act as a transporter substrate. Stated more simply, N-ethylnorpentylone is sterically too large to fit through the transporter channel, so can only function as a non-transportable inhibitor. In this regard, the mechanism of N-ethylnorpentylone is more akin to MDPV than to methylone and other ring-substituted cathinones.13,14
Four of the 6 cases described here attended rave parties where they ingested ecstasy tablets, other drugs of abuse, and alcohol. Recent evidence suggests that ecstasy pills can contain synthetic cathinones, including N-ethylnorpentylone, alone or in combination with MDMA.43 Ecstasy users should be aware that effects of N-ethylnorpentylone may not mimic the effects of MDMA. Our in vitro findings suggest that the presence of N-ethylnorpentylone in ecstasy tablets might present a substantial risk since this compound has more potent effects at DAT when compared to MDMA. Preclinical studies carried out in mice implicate DAT activity in mediating the lethal effects of methylone,44 and potent DAT inhibitors like MDPV have an increased propensity for inducing dangerous cardiovascular and neurological complications in human users.4,38,39 The in vivo pharmacological effects of N-ethylnorpentylone alone or in combination with MDMA are not known and deserve further investigation.
5. Conclusions
To summarize, we developed and validated an analytical method for the quantitation of N-ethylnorpentylone in human casework and describe the clinical symptoms of intoxication in 6 individuals. We found that N-ethylnorpentylone concentrations in human blood ranged from 7 to 170 ng/mL, and subjects intoxicated with the drug displayed a variety of symptoms including palpitations, tachycardia, agitation, aggression, hallucinations, coma and, in one case, death. The in vitro transporter assays revealed that N-ethylnorpentylone is a potent uptake inhibitor at DAT, NET and SERT, with strong preference for the dopamine system. Importantly, N-ethylnorpentylone is not a transporter substrate and does not induce neurotransmitter release. Taken together, the findings demonstrate that N-ethylnorpentylone is a psychomotor stimulant drug of abuse capable of inducing life-threatening medical consequences.
Supplementary Material
Acknowledgments
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (process number 2015/10650-8 and 2018/00432-1) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (process number 830525/1999-8). This research was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, USA (grant DA 00523 to MHB).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Madras BK. The growing problem of new psychoactive substances (NPS) In: Baumann M, Glennon R, Wiley J. Neuropharmacology of New Psychoactive Substances (NPS). Springer; 2016;1–18. doi: 10.1007/7854_2016_34 [DOI] [Google Scholar]
- 2.Baumann MH, Solis E, Watterson LR, et al. Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci. 2014;34(46):15150–15158. doi: 10.1523/JNEUROSCI.3223-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann MH, Partilla JS, Lehner KR, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karch S Cathinone neurotoxicity (“The “3Ms”). Curr Neuropharmacol. 2015;13(1):21–25. doi: 10.2174/1570159X1366614121022500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrkkö E, Andersson M, Kronstrand R. The toxicology of new psychoactive substances. Ther Drug Monit. 2016;38(2):190–216. doi: 10.1097/FTD.000000000000026 [DOI] [PubMed] [Google Scholar]
- 6.Wright TH, Cline-Parhamovich K, Lajoie D, et al. Deaths involving methylenedioxypyrovalerone (MDPV) in Upper East Tennessee. J Forensic Sci. 2013;58(6):1558–1562. doi: 10.1111/1556-4029.1226 [DOI] [PubMed] [Google Scholar]
- 7.Wyman JF, Lavins ES, Engelhart D, et al. Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts.” J Anal. Toxicol 2013;37(3):182–185. doi: 10.1093/jat/bkt00 [DOI] [PubMed] [Google Scholar]
- 8.Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176–180. doi: 10.3109/00952990.2011.643999 [DOI] [PubMed] [Google Scholar]
- 9.Diestelmann M, Zangl A, Herrle I, et al. MDPV in forensic routine cases: psychotic and aggressive behavior in relation to plasma concentrations. Forensic Sci Int. 2018;283:72–84. doi: 10.1016/j.forsciint.2017.12.00 [DOI] [PubMed] [Google Scholar]
- 10.Simmler L, Buser T, Donzelli M, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann MH, Ayestas MA, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97(1):20–26. doi: 10.1016/j.lfs.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann MH, Bukhari MO, Lehner KR, et al. Neuropharmacology of 3,4-methylenedioxypyrovalerone (MDPV), its metabolites, and related analogs. Curr Top Behav Neurosci. 2016;32:93–117. doi: 10.1007/7854_2016_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshleman AJ, Wolfrum KM, Reed JF, et al. Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J Pharmacol Exp Ther. 2016;360(1):33–47. doi: 10.1124/jpet.116.236349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler CW, Thorndike EB, Goldberg SR, et al. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl). 2016;233(10):1981–1990. doi: 10.1007/s00213-015-4057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindler CW, Thorndike EB, Suzuki M, Rice KC, Baumann MH. Pharmacological mechanisms underlying the cardiovascular effects of the “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV). Br J Pharmacol. 2016;173(24):3492–3501. doi: 10.1111/bph.13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug Enforcement Administration (DEA). Emerging threat report (mid-year) https://ndews.umd.edu/sites/ndews.umd.edu/files/dea-emerging-threat-report-2017-mid-year.pdf. Accessed March, 201
- 18.Forum Bluelight http://www.bluelight.org/vb/threads/774023-Novel-stimulant-Ephylone-(N-Ethyl-Pentylone-k-Ethyl-K-k-EBDP) Accessed March, 2018.
- 19.Drug Enforcement Administration (DEA) 2018. N-Ethylpentylone https://www.deadiversion.usdoj.gov/drug_chem_info/n-ethylpentylone.pdf Accessed August, 2018.
- 20.Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) [Accessed August, 2018];N-Ethylpentylone monograph. 2016 http://www.swgdrug.org/Monographs/N-Ethylpentylone.pdf.
- 21.National Forensic Laboratory Information System (NFLIS) 2016. Synthetic cannabinoids and synthetic cathinones reported in NFLIS, 2013-2015 https://www.deadiversion.usdoj.gov/nflis/SR-SynthCannabinoidCathinone.pdf#search=ethylpentylon Accessed August, 2018.
- 22.Drug Enforcement Administration (DEA) 2018. Schedules of controlled substances: temporary placement of N-ethylpentylone in schedule I https://www.gpo.gov/fdsys/pkg/FR-2018-06-13/pdf/2018-12669.pdf Accessed August, 2018. [PubMed]
- 23.Aryl- aminoketone derivatives 1967. https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=GB&NR=1085135A&KC=A&FT=D&ND=3&date=19670927&DB=EPODOC&locale=en_EP#. Accessed August, 2018.
- 24.Verfahren zur Herstellung von substituierten Phenyl-alpha-aminoketonen und deren Saeureadditionssalzen bzw. deren optischen Antipoden 1967. https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=DE&NR=1242241B&KC=B&FT=D&ND=3&date=19670615&DB=EPODOC&locale=en_EP#. Accessed August, 2018.
- 25.European Monitoring Center for Drugs and Drug Addiction (EMCDDA) 2017. EMCDDA - Europol 2016 Annual report on the implementation of council decision 2005/387/JHA http://www.emcdda.europa.eu/system/files/publications/4724/TDAN17001ENN_PDFWEB.pdf Accessed August, 2018.
- 26.Thirakul P,S Hair L,L Bergen K,M Pearson J Clinical presentation, autopsy results and toxicology findings in an acute N-ethylpentylone fatality. J Anal Toxicol. 2017;41(4):342–346. doi: 10.1093/jat/bkx00 [DOI] [PubMed] [Google Scholar]
- 27.Krotulski AJ, Papsun DM, De Martinis BS, Mohr ALA, Logan BK. N-ethyl pentylone (ephylone) intoxications: quantitative confirmation and metabolite identification in authentic human biological specimens. J Anal Toxicol. 2018;42(7):467–475. doi 10.1093/jat/bky025. [DOI] [PubMed] [Google Scholar]
- 28.Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology. J Anal Toxicol. 2013;37(7):452–474. doi: 10.1093/jat/bkt05 [DOI] [PubMed] [Google Scholar]
- 29.Maurer HH, Pfleger K, Weber AA. Mass Spectral and GC Data of Drugs, Poisons, Pesticides, Pollutants, and Their Metabolites - Volume 1: methods and tables. 4th ed. Germany: Wiley-VCH; 2011. [Google Scholar]
- 30.Grapp M, Maurer HH, Desel H. Systematic forensic toxicological analysis by GC-MS in serum using automated mass spectral deconvolution and identification system. Drug Test Anal. 2016;8(8):816–825. doi: 10.1002/dta.1848 [DOI] [PubMed] [Google Scholar]
- 31.de Castro A, Lendoiro E, Fernández-Vega H, et al. Liquid chromatography tandem mass spectrometry determination of selected synthetic cathinones and two piperazines in oral fluid. Cross reactivity study with an on-site immunoassay device. J Chromatogr A. 2014;1374:93–101. doi: 10.1016/j.chroma.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 32.Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem. 2013;405(29):9437–9448. doi: 10.1007/s00216-013-7386- [DOI] [PubMed] [Google Scholar]
- 33.Alsenedi KA, Morrison C. Determination and long-term stability of twenty-nine cathinones and amphetamine-type stimulants (ATS) in urine using gas chromatography–mass spectrometry. J Chromatogr B. 2018;1076:91–102. doi: 10.1016/j.jchromb.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 34.Alsenedi KA, Morrison C. Comparison of six derivatizing agents for the determination of nine synthetic cathinones using gas chromatography-mass spectrometry. Anal Methods. 2017;9:2732–2743. doi: 10.1039/C7AY00597K [DOI] [Google Scholar]
- 35.Neifeld JR, Regester LE, Holler JM, et al. Ultrafast screening of synthetic cannabinoids and synthetic cathinones in urine by RapidFire-tandem mass spectrometry. J Anal Toxicol. 2016;40(5):379–387. doi: 10.1093/jat/bkw025 [DOI] [PubMed] [Google Scholar]
- 36.Hong W-Y, Ko Y-C, Lin M-C, et al. Determination of synthetic cathinones in urine using gas chromatography–mass spectrometry techniques. J Anal Toxicol. 2015;40(1):12–16. doi: 10.1093/jat/bkv10 [DOI] [PubMed] [Google Scholar]
- 37.Glicksberg L, Bryand K, Kerrigan S. Identification and quantification of synthetic cathinones in blood and urine using liquid chromatography-quadrupole/time of flight (LC-Q/TOF) mass spectrometry. J Chromatogr B. 2016;1035:91–103. doi: 10.1016/j.jchromb.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 38.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812 [DOI] [PubMed] [Google Scholar]
- 39.Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8(1):33–42. doi: 10.1007/s13181-011-0193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor AD, Padilla-Jones A, Gerkin RD, Levine M. Prevalence of rhabdomyolysis in sympathomimetic toxicity: a comparison of stimulants. J Med Toxicol. 2015;11(2):195–200. doi: 10.1007/s13181-014-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng W, Sutter ME, Albertson TE. Stimulants and the lung. Clin Rev Allergy Immunol. 2014;46(1):82–100. doi: 10.1007/s12016-013-8376- [DOI] [PubMed] [Google Scholar]
- 42.Buttner A Neuropathological alterations in cocaine abuse. Curr Med Chem. 2012;19(33):5597–5600. doi: 10.2174/09298671280398894 [DOI] [PubMed] [Google Scholar]
- 43. [Accessed March, 2018]; Ecstasydata.org https://www.ecstasydata.org/results.php?start=0&search_field=all&s=ethylpentylone.
- 44.Piao Y-S, Hall FS, Moriya Y, et al. Methylone-induced hyperthermia and lethal toxicity. Behav Pharmacol. 2015;26(4):345–352. doi: 10.1097/FBP.0000000000000135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


