Abstract
Background
Pathological skin scars, caused by cesarean section, affected younger mothers esthetically and psychosocially and to some extent frustrated obstetricians and dermatologists. Umbilical cord mesenchymal stem cells (UC-MSCs), as a population of multipotent cells, are abundant in human tissues, providing several possibilities for their effects on skin scar tissues. Herein, we performed a randomized, double-blind, placebo-controlled, three-arm clinical trial, aiming to assess the efficacy and safety of UC-MSCs in the treatment of cesarean section skin scars among primiparous singleton pregnant women.
Methods
Ninety primiparous singleton pregnant women undergoing elective cesarean section were randomly allocated to receive placebo, low-dose (3 × 106 cells), or high-dose (6 × 106 cells) transdermal hydrogel UC-MSCs on the surface of the skin incision. The primary outcome was cesarean section skin scars followed after the sixth month, assessed by the Vancouver Scar Scale (VSS).
Results
All the participants completed their trial of the primary outcome according to the protocol. The mean score of estimated total VSS was 5.52 in all participants at the sixth-month follow-up, with 6.43 in the placebo group, 5.18 in the low-dose group, and 4.71 in the high-dose group, respectively. No significant difference was found between-group in the mean scores for VSS at the sixth month. Additional prespecified secondary outcomes were not found with significant differences among groups either. No obvious side effects or adverse effects were reported in any of the three arms.
Conclusion
This randomized clinical trial showed that UC-MSCs did not demonstrate the effects of improvement of cesarean section skin scars.
Trial registration
ClinicalTrials.gov identifier, NCT02772289. Registered on 13 May 2016.
Keywords: Umbilical cord mesenchymal stem cells, Cesarean section, Skin scars, Randomized controlled trial
Background
Cesarean delivery has been increasing worldwide over recent years, especially in China [1, 2]. It has become the most common surgical intervention in many countries for pregnant women [3]. Due to its association with adverse outcomes for mothers and babies [4], cesarean delivery has become a great concern globally. Meanwhile, pathological skin scars after cesarean section were a big problem esthetically and psychosocially among younger mothers, and to some extent, frustrating obstetricians and dermatologists.
Pathological skin scars, such as hypertrophic scar, keloids, and atrophic, are aberrant fibro-metabolic diseases resulting from severe burn injury, blunt force trauma, wound infection, or other surgical interventions [5]. They are characterized by the proliferation of fibroblasts, accumulation of collagen, and infiltration of inflammatory cells [6, 7]. Epidemiology studies indicated that the pathological skin scars were found in 33 to 91% in subjects who suffered from burn injury and 39 to 68% in cases who received surgery [8]. Moreover, numerous sufferers have different degrees of psychosocial disturbance, varying from mild to severe [9]. Currently, therapeutic strategies, such as occlusive dressings, compression therapy, surgical excision, topical or intralesional corticosteroids, radiotherapy, lasers, cryotherapy, and a combination with other adjuvant topical drugs, are commonly used for alleviating the severity of skin scars [5, 7, 10, 11]. However, most of the aforementioned therapies are ineffective or with unsatisfactory effects, including high recurrence rates, hypo- or hyper-pigmentation, telangiectasia, blisters, dermal atrophy, inflammation, erosion, superficial ulceration, crusting, erythema, edema, burning sensation, and pain at injection site [7].
Stem cells, especially mesenchymal stem cells (MSCs), representing a population of multipotent cells, are abundant in human tissues. They have aroused immense interests for clinical applications because of their easy availability and potential for recovering [12, 13]. Substantial animal and clinical studies have shown that MSCs can inhibit pathological fibrosis in many organs, such as the heart, lung, and kidney, and therefore can improve the prognosis of many diseases, such as liver injury, spinal cord injury, acute respiratory distress syndrome, blood disease, and critical limb ischemia [14–19]. These provided a possibility of skin scar tissue repair affected by MSCs.
MSCs, owing to their multifunctional roles, can migrate to the wound sites directionally. They formed a part of microenvironment, improved wound healing, and inhibited pathological skin scars [20]. Among them, UC-MSCs are most easily accessible, with low immunogenicity, and can be expanded in vitro [21]. UC-MSCs can be easily isolated and collected from the umbilical cord by using an accessible procedure [22]. Previous researches have shown that MSCs can suppress proliferation and activation of keloid fibroblasts and inhibit extracellular matrix synthesis through a paracrine signaling mechanism [23, 24], and thus may be a novel topical agent for pathological skin scar treatment. Liu and his colleges [16] found that transplantation of MSCs via the ear artery can inhibit the hypertrophic scarring in a rabbit ear hypertrophic scar model significantly, indicating that MSCs may have potential clinical applications in modulating the process of wound healing. Similarly, another research in a rabbit model has demonstrated that local injection of MSCs efficiently prevented hypertrophic scar formation by regulating inflammation [15]. In a case report, researchers found that hydrogel with MSCs can accelerate wound healing and ameliorate the condition of the wound in a diabetic patient within only 3 weeks [25]. Taken together, these studies suggested that MSCs can regulate the process of wound healing and prevent pathological skin scar formation.
In our present work, we conducted a randomized, double-blind, placebo-controlled clinical trial in order to assess the efficacy and safety of umbilical cord mesenchymal stem cells (UC-MSCs) in the treatment of cesarean section skin scars among primiparous singleton pregnant women. The effectiveness of UC-MSCs on treating cesarean section skin scars, to our knowledge, has not been reported yet in the literature. Our hypothesis was that UC-MSCs can improve the degree of scar repairing and increase the satisfaction of patients.
Methods
Primiparous singleton pregnant women undergoing elective cesarean section in the Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University, a tertiary university-affiliated hospital, were screened for participation in this double-blind, three-arm, randomized, controlled trial. The number of deliveries in our hospital has stabilized at approximately 12,000 per year [26]. This trial has been registered at the ClinicalTrials.gov (registration number NCT02772289) and approved by the Ethics Committee of the Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University.
The trial protocol was designed by the trial executive committee and further approved by the institutional review board of the hospital. The trial protocol has been published in an international peer-reviewed journal in the field of clinical trials [27] and presented at an international conference on stem cell and regenerative medicine [28]. The trial UC-MSCs (1 × 106 cells each pump) and matching placebo were donated from the Health-Biotech Pharmaceutical Company (Beijing, China). The company was not involved in the trial design, data analysis, manuscript preparation, publication, or presentation of the trial.
The UC-MSCs were isolated from the umbilical cord of a healthy donor after informed consent by the method described above [22, 25]. Briefly, the umbilical cord tissue was washed with phosphate-buffered saline (PBS) and then minced into 1–2 mm3 fragments. These tissue pieces were then incubated with 0.075% collagenase (Sigma, St Louis, MO, USA) for 30 min and then 0.125% trypsin (Gibco, Grand Island, NY, USA) for 30 min with gentle agitation at 37 °C. Fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) was added to neutralize the excess trypsin. The digested mixture was then passed through a 100-μm filter to obtain cell suspensions. The cells were plated at a density of 1 × 106 cells/cm2 in non-coated T-25 or T-75 cell culture flasks. Growth medium consisted of Dulbecco’s modified Eagle’s medium with low glucose (DMEM-LG; Gibco) and 5% fetal bovine serum, supplemented with 10 ng/mL vascular endothelial growth factor (VEGF; Sigma), 10 ng/mL epidermal growth factor (EGF; Sigma), 100 U penicillin/streptomycin (Sigma), and 2 mM l-glutamine (Gibco). Cell cultures were maintained in a humidified atmosphere with 5% CO2 at 37 °C. After 3 days of culture, the medium was replaced and non-adherent cells were removed. The medium was then changed twice weekly thereafter until 60–80% confluence had been reached. The obtained adherent cells were replated at a density of 1 × 104/cm2.
The UC-MSCs released from the cell banking were cultured and expanded in the good manufacturing practice (GMP) laboratory to prepare final cell products. They were checked to be sterile and free from human immunodeficiency virus, cytomegalovirus, syphilis, mycoplasma, hepatitis B virus, hepatitis C virus, Epstein–Barr virus, and endotoxins. The CD73, CD105, CD90, CD151, CD166, Oct4, nestin, and Sox2 are highly expressed (> 95%), and CD45, CD34, CD31, CD11b, CD184, and HLA-DR are negative. The final UC-MSCs were activated and mixed with sodium alginate powder, resulting in a gelatinous UC-MSC complex. The UC-MSCs can be stored for a year in a − 80 °C refrigerator, and the post-transplantation cell survival should be more than 80%.
From November 2016 to September 2018, pregnant women were referred to the study team for screening. The eligibility of enrollment was confirmed by investigators. Primiparous singleton pregnant women aged 21 to 35 years between the 37th and 42nd weeks of gestation were eligible for enrollment if they were going to have a programmed cesarean delivery. Pregnant women with a gel allergy were excluded because of the difficulty to complete the trial in women with the symptom. Women who were planning to have any other cosmetic procedure to the skin incision during the study period and who had a history of smoking, wounds or local disease in the treatment area, recent or current cancer history, any systemic uncontrolled disease, or presented with a keloid formation were excluded. Women unwilling to adhere to the trial protocol were also excluded. The supplementary appendix provided the trial inclusion and exclusion criteria. Written informed consent was obtained from all the included subjects.
Routine blood examinations for aspartate aminotransferase (AST), alanine aminotransferase (ALT), uric acid (UA), blood urea nitrogen (BUN), and tumor markers (CA125, CA153, CA199, and CEA) were carried before skin incision. Meanwhile, physical examinations, past medical histories, and pigmentation and erythema around the skin incision were subsequently measured and recorded.
Eligible participants who signed informed consent forms were then randomly assigned in a 1:1:1 ratio, by means of a computer algorithm, to allocate placebo, low-dose (3 × 106), or high-dose (6 × 106) groups. Transdermal hydrogel was occupied immediately after skin incision suturing. The participants received a total of six hydrogels on the surface of the skin incision, once a day. Participants in the placebo group received placebo hydrogel for 6 days; those in the low-dose group will receive one dose of hydrogel with cells for three consecutive days and then placebo hydrogel for the next three consecutive days; those in the high-dose group will receive cells for 6 days.
The primary outcome of cesarean section skin scars was assessed at 6 months after enrollment by using Vancouver Scar Scale (VSS). Four parameters were used in the evaluation of the severity of cesarean section skin scars: pigmentation (normal, hypopigmented, mixed, or hyperpigmented), vascularity (normal, pink, red, or purple), pliability (normal, supple, yielding, firm, ropes, or contracture), and height (flat, < 2 mm, 2–5 mm, or > 5 mm) [29]. Each parameter contained ranked subscales that may be summed to obtain a total score ranging from 0 to 14, with 0 representing normal skin. The higher the total score, the more the severity of the scarring. The outcome at the first and third months after enrollment was also assessed.
Additional prespecified secondary outcomes included adverse effects, wound healing at 14 days, erythema and pigmentation around the skin incision, skin scar area and volume, immunoglobulins (IgG, IgA, IgM) and complement component (C3, C4) in milk, and satisfaction of treatment (an overall assessment of the treatment, as measured on a 5-point scale [with “very good,” “good,” “moderate,” “slight,” or “none”] assessed by the questionnaire of “would you recommend this method of treatment to a friend?”).
Statistical analysis
The sample size was calculated according to the primary outcome of VSS. The score of VSS was evaluated according to professor Yu-Chen Huang’s preliminary trial in Taipei Medical University WanFang Hospital [30]. The sample size of this anticipated trial was 74, and the final sample size was increased to 90 (n = 30 in each group) to achieve 90% power to detect 1.5 difference of the VSS in the MSC group and the placebo group. Data analysis was performed using R 3.1 software.
All the results were analyzed according to the intention-to-treat principle. Standard descriptive methods were used to summarize the trial participants overall and by intervention group. Continuous variables were demonstrated by mean ± standard deviation and calculated by one-way analysis of variance (ANOVA). Categorical variables were demonstrated by a relative number and calculated by chi-square test. The primary outcome was assessed among all women who had the follow-up on the sixth month according to the intention-to-treat principle. After homogeneity test of characteristics of the participants at baseline among groups, the difference (with 95% confidence interval) of VSS of women between the MSC group and placebo group was calculated using two-sided t test and F test. After correcting of multiple comparisons by Bonferroni correction, the differences were considered significant at α = 0.017.
Subgroup analysis of the primary outcome according to participants’ demographics and clinical characteristics was prespecified. The analyses were stratified according to gestational age and maternal age. Two-sided t test, F test, chi-square test, or Fisher’s exact test was used to compare the groups in all secondary analyses; the results were presented without adjustment for multiplicity and should be considered exploratory. All the data was analyzed by SPSS statistical software.
Results
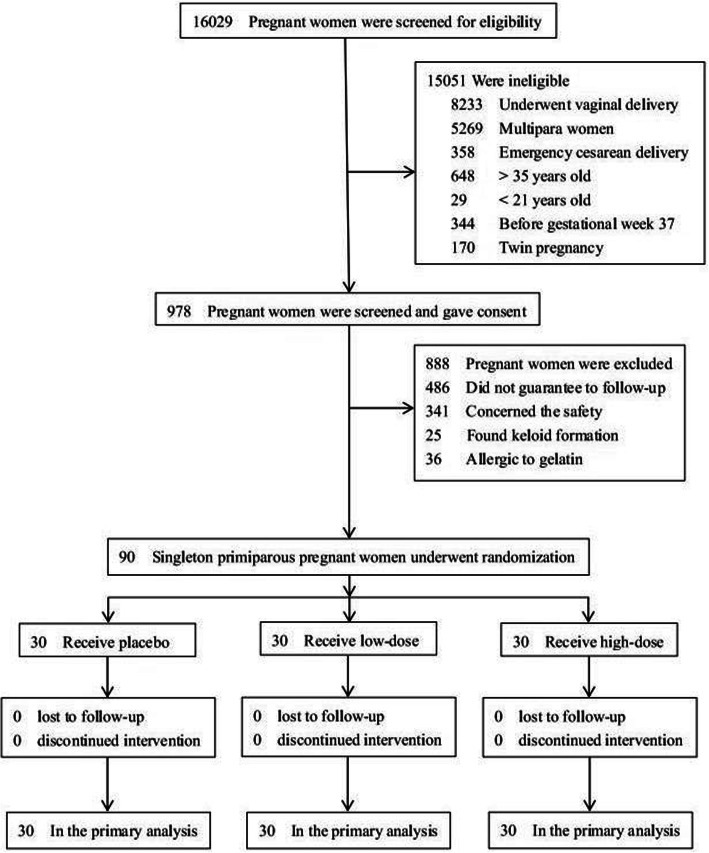
From November 2016 to September 2018, a total of 16,029 pregnant women gave birth in the department and 7796 (48.64%) women underwent cesarean delivery. In these cesarean births, there were 5269 multipara women, 358 emergency cesarean sections, 677 too old (> 35 years) or too young (< 21 years), 344 preterm delivery (before gestational week 37), and 170 twin pregnancy. Of the 978 potential eligible participants, 486 pregnant women planned to go to other cities after delivery, 25 pregnant women were found with keloid formation, 36 women were allergic to gelatin, and 341 women declined to participate because of safety concerns. Finally, 90 eligible singleton primiparous pregnant women were enrolled and underwent randomization, with 30 women in each group (Fig. 1). Participant recruitment closed when the sample size reached ninety.
Fig. 1.
Enrollment and outcomes
All the participants were occupied to the trial, and the follow-ups of the primary outcome were finished according to the protocol. The mean age and gestation were 28.04 years and 39.47 weeks, respectively. The mean time of operation and the length of incision were 39.20 min and 14.53 cm, respectively. The mean value of pigmentation and erythema was 165.37 and 265.43, respectively. No abnormal liver and kidney indexes were found in all participants. Women randomized to each of the three groups were well balanced regarding demographic and delivery characteristics (Table 1).
Table 1.
Characteristics of the participants at baseline [mean ± SD or n (%)]
| All participants | Placebo group | Low-dose group | High-dose group | F/χ2 | p value | |
|---|---|---|---|---|---|---|
| Age (years) | 28.04 ± 3.39 | 28.01 ± 3.82 | 28.34 ± 3.05 | 27.79 ± 3.34 | 0.197 | 0.821 |
| Age group | ||||||
| 21–27 | 45 (50.0) | 17 (56.7) | 12 (40.0) | 16 (53.3) | 1.867 | 0.500 |
| 28–35 | 45 (50.0) | 13 (43.3) | 18 (60.0) | 14 (46.7) | ||
| Gestation (week) | 39.47 ± 1.10 | 39.50 ± 1.07 | 39.30 ± 1.25 | 39.61 ± 0.97 | 0.603 | 0.550 |
| Gestation group | ||||||
| 37–39 week | 59 (65.6) | 20 (66.7) | 20 (66.7) | 19 (63.3) | 0.098 | 0.999 |
| 40–42 week | 31 (34.4) | 10 (33.3) | 10 (33.3) | 11 (36.7) | ||
| Operation time (min) | 39.20 ± 10.45 | 39.17 ± 12.48 | 38.48 ± 7.87 | 39.93 ± 10.81 | 0.137 | 0.873 |
| Incision length (cm) | 14.53 ± 1.07 | 14.36 ± 1.23 | 14.73 ± 0.93 | 14.52 ± 1.05 | 0.881 | 0.418 |
| Pigmentation | 165.37 ± 73.15 | 167.99 ± 69.86 | 160.07 ± 81.14 | 168.13 ± 70.00 | 0.116 | 0.890 |
| Erythema | 265.43 ± 77.03 | 264.43 ± 77.75 | 271.99 ± 84.63 | 259.69 ± 69.93 | 0.188 | 0.829 |
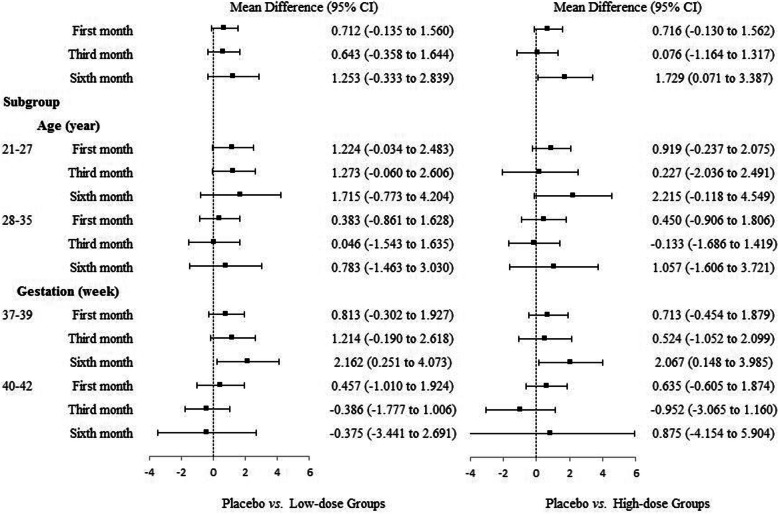
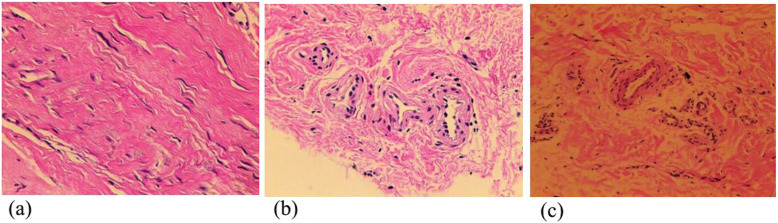
The mean total VSS score was 5.52 in all participants at the sixth month, with 6.43 in the placebo group, 5.18 in the low-dose group, and 4.71 in the high-dose group, respectively. No significant difference was found between-group in the mean scores of VSS at the sixth month. Similar results were also shown among groups at the first and third months (Table 2 and Fig. 2). Representative hematoxylin-eosin (H&E) staining pictures are shown in Fig. 3.
Table 2.
Vancouver Scar Scale and satisfaction outcomes among participants
| All participants | Placebo group | Low-dose group | High-dose group | F/χ2 | p value | |
|---|---|---|---|---|---|---|
| VSS | ||||||
| First month | 4.68 ± 1.49 | 5.17 ± 1.40 | 4.46 ± 1.53 | 4.46 ± 1.47 | 1.852 | 0.164 |
| Third month | 5.20 ± 1.74 | 5.48 ± 1.69 | 4.83 ± 1.63 | 5.40 ± 1.96 | 0.899 | 0.413 |
| Sixth month | 5.52 ± 2.70 | 6.43 ± 2.48 | 5.18 ± 2.79 | 4.71 ± 2.66 | 2.362 | 0.103 |
| Satisfaction | ||||||
| Very good | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3.279 | 0.214 |
| Good | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Slight | 42 (46.7) | 14 (46.7) | 11 (36.7) | 21 (70.0) | ||
| None | 48 (53.3) | 16 (53.3) | 19 (63.3) | 9 (30.0) | ||
VSS Vancouver Scar Scale
Fig. 2.
Mean difference of the Vancouver Scar Scale between the MSC and placebo group
Fig. 3.
Representative hematoxylin-eosin (H&E) staining of cesarean section skin scars with the three groups. a Placebo group. b Low-dose group. c High-dose group
For all additional prespecified secondary outcomes, there were no significant differences among the three groups. Detailed research data and statistical results were provided in the attachment files. No obvious side effects or adverse effects, such as fever, chills, pain, inflammation, and wound infection, were reported in any of the three arms. Moreover, renal and liver functions and tumor markers, including CA125, CA153, CA199, and CEA, were all within the normal range (Table 3).
Table 3.
The value of the liver, kidney, and tumor markers before and at the sixth month [mean ± SD or median (Q25–Q75)]
| Baseline | Sixth month | |||||||
|---|---|---|---|---|---|---|---|---|
| All participants | Placebo group | Low-dose group | High-dose group | All participants | Placebo group | Low-dose group | High-dose group | |
| ALT | 9.72 ± 3.60 | 10.17 ± 4.04 | 9.63 ± 3.12 | 9.37 ± 3.65 | 16.82 ± 12.70 | 17.05 ± 9.97 | 17.09 ± 17.67 | 16.13 ± 6.54 |
| AST | 15.48 ± 3.40 | 15.93 ± 3.18 | 15.30 ± 3.04 | 15.20 ± 3.96 | 17.37 ± 6.34 | 16.62 ± 5.08 | 17.78 ± 7.94 | 17.75 ± 5.48 |
| UA | 345.58 ± 89.29 | 342.90 ± 83.96 | 328.40 ± 88.22 | 365.43 ± 94.42 | 333.10 ± 70.49 | 322.52 ± 62.01 | 343.48 ± 80.41 | 332.06 ± 67.91 |
| BUN | 3.77 ± 2.63 | 4.03 ± 4.42 | 3.61 ± 0.82 | 3.66 ± 0.93 | 4.65 ± 1.15 | 4.61 ± 1.01 | 4.74 ± 0.99 | 4.57 ± 1.55 |
| CA125 | 19.63 (15.21–26.75) | 19.62 (15.19–25.54) | 19.36 (14.65–30.49) | 20.75 (15.36–31.66) | 15.13 (9.87–19.72) | 15.85 (9.65–20.39) | 13.18 (9.07–18.53) | 15.13 (9.45–22.67) |
| CA153 | 13.21 (10.56–16.07) | 13.20 (10.70–16.87) | 12.81 (10.78–16.32) | 13.39 (9.68–15.76) | 8.62 (6.55–12.30) | 7.52 (6.27–11.86) | 8.89 (6.41–12.44) | 10.49 (7.33–12.44) |
| CA199 | 9.54 (6.91–14.48) | 9.29 (6.38–15.42) | 8.66 (7.03–19.38) | 10.27 (7.03–14.47) | 9.11 (6.48–14.45) | 10.58 (6.13–15.45) | 7.53 (6.53–15.76) | 10.78 (6.42–14.13) |
| CEA | 0.62 (0.31–0.87) | 0.70 (0.30–0.85) | 0.60 (0.38–0.90) | 0.61 (0.21–0.83) | 0.84 (0.53–1.48) | 1.21 (0.54–1.59) | 0.66 (0.51–1.39) | 0.79 (0.60–1.38) |
ALT alanine aminotransferase, AST aspartate aminotransferase, BUN blood urea nitrogen, CA125 cancer antigen 125, CA153 carbohydrate antigen 153, CA199 carbohydrate antigen 199, CEA carcinoembryonic antigen, UA uric acid
By the end of the trial period, 51.00% (46) of the women described their experience overall as “slight” and 49.00% (44) described as “none.” None of them described as “moderate,” “good,” or “very good.” However, the majority of women (90% (81)) stated that they would use the intervention again if there are any changes and would recommend this treatment method to their friends (Table 2).
Discussion
Based on the findings of the present work, UC-MSCs did not demonstrate the effects of improvement of cesarean section skin scars. No significant difference was found between groups in terms of the VSS score, secondary and adverse-effect outcomes at the first-, third-, and sixth-month follow-ups.
In a case-control prospective study by Abo-Elkheir et al., the authors proved that MSCs can effectively enhance wound regeneration in deep burns, representing a new modality providing great hope for burn injury patients [31]. A randomized phase 1/2 clinical trial focusing on skin expansion showed that it was safe and effective to intradermally transplant autologous stem cells to promote mechanical stretch-induced skin regeneration in vivo and was a feasible clinical application to provide amounts of tissue for complex skin defect [32]. Unfortunately, we could not observe significant cesarean section skin scar improvement based on VSS rating and other outcomes, such as wound healing at the second week, pigmentation and erythema around the incision, and skin area and volume. We considered that the insufficient effective dose may be a key important cause. Most of the previous effective clinical studies used the doses of 108 or 109 cells [33, 34].
The number of cells, 106, seems inadequate in this trial. What was more regrettable was that there were a number of cells, about 10%, which performed inactively. The reason that we focused on 106 UC-MSCs was the full consideration of the safety for those sensitive population, namely pregnant women. Although there was no statistical difference, the present study found that the VSS rating was lower with the dose increased. Understanding this appearance is of great significance for future clinical trials that target higher doses of UC-MSCs to intervene cesarean section skin scar.
The biological function of UC-MSC hydrogel was complex, and the greater potential may be related to paracrine, immunomodulation, and the vascular differentiation capacity of these cells. Several studies have shown that MSCs can secrete multiple cytokines and growth factors, including stromal cell-derived factor 1, basic fibroblast growth factor (bFGF), TGF-β, IGF-1, VEGF, and HGF, and increase angiogenesis and collagen accumulation in the wound tissue [35–37]. It has been reported that MSC hydrogel can stimulate wound tissue to generate granulation tissues [25, 38]. Similarly, in this study, the histologic evaluation showed that the capillary density of skin scar was increased in UC-MSC groups. In addition, in vivo evidence revealed that MSCs markedly expressed the anti-inflammatory factor IL-10 and suppressed the production of IL-6, IL-8, IL-1, IL-4, TNF-α, interferon-γ, and ICAM-1 [35, 39, 40]. This indicated that MSCs can induce high immunomodulation activity and reduce inflammation during wound healing.
Another reason for negative findings may be due to insufficient follow-up period. Some studies have suggested that it usually took 18–24 months for a typical scar to mature [11]. Considering a low rate of lost to follow-up in the special group of pregnant women, we only followed for 6 months at this design. Satisfactorily, all the participants completed the trial, with a primary outcome according to the protocol at the sixth month. In the next step, we will further follow up to observe the long-term efficacy in these participants.
Women with keloid formation may benefit from this therapy. However, in order to control the confounding deviation as much as possible, we excluded these women in the trial. These women should be the focus of the next study. In addition, our used stem cells were derived from allogeneic cells supplied by the company manufacturing. If possible, trials using autologous stem cells may yield better effectiveness.
A major concern with MSC therapy is the safety potential. In the present study, many eligible pregnant women who declined to participate in the trial are concerned about the short- and long-term safety of MSCs, especially the concern regarding their future breastfeeding. To date, most of the published preclinical and clinical trials have reported that MSCs are safe when being used for the treatment of injuries to the central nervous system, biliary lesions, musculoskeletal disease, digestive system disease, and cardiovascular disease [17, 18, 33, 34, 41].
Meanwhile, a meta-analysis that included 36 articles, involving a total of 1012 participants, evaluated the safety of MSC-based therapy in all prospective clinical trials [42]. This study did not detect any risk like malignancy, infection, organ system complications, and acute infusion toxicity in treated participants. On the other hand, this study proved that intravascular infusions of MSC therapy appeared to be safe. Our findings were consistent with the evidence from the above studies that the use of MSCs in pregnant women is safe.
Interestingly, although participants in both the placebo and MSC groups were both not satisfied with the skin scar at the sixth month, most of them still want to try it again. It means that these younger mothers have gradually accepted MSC therapy after this clinical trial and looked forward to it.
Conclusions
In conclusion, in this three-arm RCT trial involving women with primiparous singleton pregnancies, the UC-MSC-treated group was not significantly different from the placebo group for the reduction of cesarean section skin scar and neither did the increasing recognition of participants’ satisfaction.
Supplementary information
Acknowledgements
We would like to thank all participants for their cooperation and all the supporting clinical staff at the Department of Obstetrics, Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University, for their assistance with carrying out this randomized controlled trial.
Abbreviations
- MSCs
Mesenchymal stem cells
- RCT
Randomized clinical trial
- VSS
Vancouver Scar Scale
- UC-MSCs
Umbilical cord mesenchymal stem cells
Authors’ contributions
DF, QX, ZL, ZH, and XG participated in the design and coordination of the trial. MZ, SW, SY, and ZL participated in the operation of the trial. JR, DL, HM, HZ, and DF collected the data. DF, JR, and DL analyzed the data. The authors read and approved the final manuscript.
Funding
The study was funded by the Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University (FSFY20160501).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The trial was approved by the Ethics Committee of the Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University (no. 2016-0501).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhongchao Han, Email: hanzhongchao@hotmail.com.
Xiaoling Guo, Email: fsguoxl@163.com.
Zhengping Liu, Email: liuzphlk81@outlook.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-020-01695-7.
References
- 1.Li HT, Luo S, Trasande L, Hellerstein S, Kang C, Li JX, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008-2014. JAMA. 2017;317:69–76. doi: 10.1001/jama.2016.18663. [DOI] [PubMed] [Google Scholar]
- 2.Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One. 2016;11:e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biccard BM, Madiba TE, Kluyts HL, Munlemvo DM, Madzimbamuto FD, Basenero A, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. Lancet. 2018;391:1589–1598. doi: 10.1016/S0140-6736(18)30001-1. [DOI] [PubMed] [Google Scholar]
- 4.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15:e1002494. doi: 10.1371/journal.pmed.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and hypertrophic scars: a spectrum of clinical challenges. Am J Clin Dermatol. 2016;17:201–223. doi: 10.1007/s40257-016-0175-7. [DOI] [PubMed] [Google Scholar]
- 6.Kwan PO, Tredget EE. Biological principles of scar and contracture. Hand Clin. 2017;33:277–292. doi: 10.1016/j.hcl.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 8.Carswell L, Borger J. Hypertrophic Scarring Keloids. StatPearls Publishing LLC, Treasure Island (FL). 2020. [PubMed]
- 9.Atiyeh BS, Amm CA, El Musa KA. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthet Plast Surg. 2003;27:411–417. doi: 10.1007/s00266-003-3049-3. [DOI] [PubMed] [Google Scholar]
- 10.Rasaii S, Sohrabian N, Gianfaldoni S, Hadibarhaghtalab M, Pazyar N, Bakhshaeekia A, et al. Intralesional triamcinolone alone or in combination with botulinium toxin A is ineffective for the treatment of formed keloid scar: A double blind controlled pilot study. Dermatol Ther. 2018;32:e12781. [DOI] [PubMed]
- 11.Li Q, Zhang C, Fu X. Will stem cells bring hope to pathological skin scar treatment? Cytotherapy. 2016;18:943–956. doi: 10.1016/j.jcyt.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Vilaca-Faria H, Salgado AJ, Teixeira FG. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson's Disease? Cells. 2019;8:118. [DOI] [PMC free article] [PubMed]
- 14.Li M, Qiu L, Hu W, Deng X, Xu H, Cao Y, et al. Genetically-modified bone mesenchymal stem cells with TGF-beta3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp Cell Res. 2018;367:24–29. doi: 10.1016/j.yexcr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134:2648–2657. doi: 10.1038/jid.2014.169. [DOI] [PubMed] [Google Scholar]
- 16.Liu YL, Liu WH, Sun J, Hou TJ, Liu YM, Liu HR, et al. Mesenchymal stem cell-mediated suppression of hypertrophic scarring is p53 dependent in a rabbit ear model. Stem Cell Res Ther. 2014;5:136. doi: 10.1186/scrt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord ,mesenchymal stem cells on cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver. 2018;12:73–78. doi: 10.5009/gnl17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blau HM, Daley GQ. Stem cells in the treatment of disease. N Engl J Med. 2019;380:1748–1760. doi: 10.1056/NEJMra1716145. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176–186. doi: 10.1016/j.actbio.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Han ZB, Liu JF, Wang YW, Zhang JZ, Li CT, et al. Serum-free media and the immunoregulatory properties of mesenchymal stem cells in vivo and in vitro. Cell Physiol Biochem. 2014;33:569–580. doi: 10.1159/000358635. [DOI] [PubMed] [Google Scholar]
- 22.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 23.Sato C, Yamamoto Y, Funayama E, Furukawa H, Oyama A, Murao N, et al. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plast Reconstr Surg. 2018;141:390–398. doi: 10.1097/PRS.0000000000004068. [DOI] [PubMed] [Google Scholar]
- 24.Fang F, Huang RL, Zheng Y, Liu M, Huo R. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J Dermatol Sci. 2016;83:95–105. doi: 10.1016/j.jdermsci.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Zeng X, Tang Y, Hu K, Jiao W, Ying L, Zhu L, et al. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: a case report. Medicine (Baltimore) 2017;96:e9212. doi: 10.1097/MD.0000000000009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan D, Wu S, Ye S, Wang W, Wang L, Fu Y, et al. Random placenta margin incision for control hemorrhage during cesarean delivery complicated by complete placenta previa: a prospective cohort study. J Matern Fetal Neonatal Med. 2019;32:3054-61. [DOI] [PubMed]
- 27.Fan D, Xia Q, Wu S, Ye S, Liu L, Wang W, et al. Mesenchymal stem cells in the treatment of cesarean section skin scars: study protocol for a randomized, controlled trial. Trials. 2018;19:155. doi: 10.1186/s13063-018-2478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan D, Liu Z. Efficacy and safety of MSCs in treatment for caesarean section skin scars: a prospective randomized, double-blinded, placebo-controlled trial. In: 7th International Symposium Europe China Stem Cells and Regenerative Medicine Shangrao (China). Shangrao; 2017.
- 29.Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16:535–538. doi: 10.1097/00004630-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Chiang YY, Huang YC. Fractional CO2 Laser in the Treatment for Cesarian Scar. Taipei Medical University WanFang Hospital. 2012; https://clinicaltrials.gov/ct2/show/NCT01654406?term=%22caesarean+scar%22&rank=5. Accessed 2 Mar 2016.
- 31.Abo-Elkheir W, Hamza F, Elmofty AM, Emam A, Abdl-Moktader M, Elsherefy S, et al. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study. Am J Stem Cells. 2017;6:23–35. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou SB, Zhang GY, Xie Y, Zan T, Gan YK, Yao CA, et al. Autologous stem cell transplantation promotes mechanical stretch induced skin regeneration: a randomized phase I/II clinical trial. EBioMedicine. 2016;13:356–364. doi: 10.1016/j.ebiom.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalifeh Soltani S, Forogh B, Ahmadbeigi N, Hadizadeh Kharazi H, Fallahzadeh K, Kashani L, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21:54–63. doi: 10.1016/j.jcyt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19:194–199. doi: 10.1016/j.jcyt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438:410–419. doi: 10.1016/j.bbrc.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 36.Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7:163. doi: 10.1186/s13287-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Liu Y, Zhang X, Zhu D, Qi X, Cao X, et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics. 2018;8:5348–5361. doi: 10.7150/thno.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Chen L, Liu Y, Luo B, Xie N, Tan T, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res. 2016;8:4912–4921. [PMC free article] [PubMed] [Google Scholar]
- 40.Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8:e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang J, Zhang H, Zhao C, Wang D, Ma X, Zhao S, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int J Rheum Dis. 2017;20:1219–1226. doi: 10.1111/1756-185X.13015. [DOI] [PubMed] [Google Scholar]
- 42.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.