More than 700,000 persons in the United States suffer with kidney failure but the vast majority will not have an opportunity to receive a kidney transplant, even though transplantation provides superior quality of life and survival compared with chronic dialysis. In 2019, there were 16,534 deceased donor and 6867 living donor kidney transplants, whereas nearly 95,000 patients remain on the kidney transplant waiting list. Remarkably, two important transplant strategies, compatible living donor kidney transplantation (LDKT) and transplantation with high-quality deceased donor organs, not only provide clinical benefit but are also cost saving to the health care system (1). Higher-risk kidney transplant options (i.e., with lower-quality deceased donor organs or blood type-incompatible LDKT) are more expensive but remain cost-effective, providing superior cost per quality-adjusted life year ratios than dialysis (1). Although a minority of patients with kidney failure have comorbidities that preclude safe transplantation, many others that are not currently wait-listed would likely pursue and benefit from transplantation if it were more accessible, without the prolonged waiting that often correlates with health deterioration on dialysis and may render a patient “too sick for transplant.”
The federal government is deeply invested in the care of patients with kidney disease, and the establishment of disease-specific Medicare entitlement in 1972 expanded dialysis access across age groups and comorbidity profiles to an extent not possible in some other countries. In concert, Medicare spending for kidney failure care has grown exponentially, to approximately $91,000 annually for the care of each hemodialysis patient and $76,000 for the care of each peritoneal dialysis patient, resulting in total spending of $36 billion in 2016 (2). By comparison, average annual spending for transplant recipients is approximately $38,000 (2). Moreover, current overall adjusted mortality per 1000 patient-years rates is 166 for hemodialysis patients and 154 for peritoneal dialysis patients, compared with only 29 for transplant recipients (2). Delivery of transplant education at dialysis centers correlates with listing rates (3), but the quality of transplant education provided by dialysis centers under the Centers for Medicare and Medicaid Services (CMS) education mandate is variable and inconsistent (4). Thus, current clinical practices favor the common use of the higher cost/lower benefit modality (in-center hemodialysis), which does not serve the best interests of patients and has tenuous economic sustainability.
To address these issues, the federal government, in July 2019, launched a historic initiative to shift practice toward increased use of home dialysis and transplantation through the “Advancing American Kidney Health” (AAKH) Executive Order (5). The AAKH blueprint is centered on three broad goals: (1) reducing the risk of kidney failure; (2) improving access to and the quality of person-centered treatment options; and (3) increasing access to kidney transplants, with the latter two directly tied to expanding transplantation. The goal of improving patient-centered care will be benchmarked against a target of 80% of patients who initiate RRT in 2025 and do so by receiving dialysis in the home or undergoing a transplant. Although ambitious, initiatives to foster reaching this metric were promptly drafted, including new reimbursement models designed for testing by the Center for Medicare and Medicaid Innovation. A nationally randomized, mandatory ESKD Treatment Choices Model includes payment adjustments favoring home versus in-center dialysis, whereas a voluntary model incorporates a Kidney Transplant Bonus of up to $15,000 when patient is transplanted and sustains at least 3 years of allograft function. The Kidney Transplant Bonus is designed to incentivize early consideration of transplant in treatment modality planning. The models will empirically assess the effect of payment restructuring over the next 3 years (with possible 1- to 2-year extensions), but in advance of these data, revising reimbursement incentives appears to be one rational strategy to help transform practice patterns.
Although early education and appropriate referrals are important strategies to mitigate disparities in transplant access, referral alone cannot increase transplantation without an expanded organ supply. Goal 3 of the AAKH Executive Order targets doubling the number of kidneys available for transplant by 2030 (5). Currently, approximately 20% of procured kidneys are not utilized for transplant, and recent studies have suggested substantial untapped potential for deceased donor kidney transplantation in the United States compared with countries such as France, primarily from broader use of organs from older donors with more comorbidity (6). To this end, in December 2019, the U.S. Department of Health and Human Services, through CMS and the Health Resources and Services Administration (HRSA), proposed new rules to support increasing both deceased donor kidney transplantation and LDKT. In the former, the focus is on the Organ Procurement Organizations (OPOs), the not-for-profit companies that are responsible for evaluation and procurement of deceased donor organs. Their performance will be evaluated on the basis of on new metrics that include defining donation and transplantation rates on the basis of national death records rather than self-reported data, and benchmarking performance against the donation and transplantation rates of the current top 25% of OPOs, with consequences for sustained underperformance including risks of decertification.
Importantly, OPOs do not act in isolation, and efforts to successfully expand organ procurement and placement require well coordinated partnership of OPOs with donor hospitals and transplant centers. Organs from Public Health Services infection risk donors, donors with known viral infections such as hepatitis C and lower predicted survival (as reflected in higher Kidney Donor Profile Indices), can provide survival benefit over dialysis, although at higher risk than transplant from “ideal” donors (1,7). However, transplant centers face significant performance scrutiny, commonly resulting in a risk-averse culture, with less high-risk organ utilization. Thus, although CMS recently removed the strict performance metrics for 1-year allograft and patient survival from the Conditions of Participation for transplant program reapproval (8), transplant centers must continue to publicly report their post-transplant outcomes, and are ranked and assessed for Centers of Excellence designations for private insurance contracting. Furthermore, there are other financial disincentives to expanded organ acceptance, because transplantation of clinically higher-risk organs increases the cost of transplantation without compensatory increases in Medicare reimbursement (9). Importantly, from the patient outcomes perspective, clinical utility of higher-risk organs can vary with recipient characteristics, and expanded organ use requires nuanced understanding of which patients may benefit from these organs and efforts to promptly direct them to appropriate recipients. Finally, because organ allocation policies are being actively revised to distribute organ offers over broader geographic boundaries, it will be critical to monitor how broader geographic sharing effects utilization and outcomes of higher-risk organs, which may be particularly vulnerable to injury from longer cold ischemia times during transport.
Expanding living donation, the other key strategy in expanding donor supply, has significant barriers, including financial disincentives to donors. Although donation-related medical expenses are paid by the recipient’s insurance or Medicare, living donors incur both direct and indirect costs in the donation process that are not reimbursed, such as travel, lost time from work, and dependent care (10,11). In one U.S. study, 92% of living kidney donors incurred direct costs (median $433, range $6–$10,240) and more than one third reported lost wages in the first year after donation (median $2712, range $546–$19,728) (10). A single-center, retrospective survey of living donors who were working for compensation at the time of donation found that increased length of time to return to work was a significant predictor of financial burden (12). Living kidney donation declined after 2005 and remained below 2005 levels until 2019, with the onset of the decline correlating with the economic recession, and most dramatic declines in men among all but the highest income quartile (13). Financial barriers may also contribute to racial disparities in LDKT access, which have increased over time (14). As a result, in December 2019, the HRSA issued a proposed rule to support LKDT by removing financial disincentives to living donation; specifically, expanding the scope of reimbursable expenses for living donors to include lost wages, and childcare and eldercare expenses, for those donors who lack other forms of financial support, administered through the National Living Donor Assistance Center (NLDAC).
Although the HRSA-proposed rule is an important step to improve financial neutrality for living donors, even more can be done (15). On the basis of provisions of the Organ Donation and Recovery Improvement Act of 2004 authorizing federal reimbursement of nonmedical expenses as a payer of last resort, awarding of travel and lodging grants to living donors requires income testing of the intended recipient. Means testing of the recipient is problematic in the case of nondirected donors, who offer their gift without an identified recipient, and is also likely a barrier for nonrelated directed donors without a close established relationship with their recipient (e.g., donors who learn of a need through social networking and may wish to be evaluated anonymously). Revision of recipient means testing in the NLDAC program to help mitigate financial barriers for all potential donors, regardless of their relationship to the intended recipient, could be a key step forward to increasing LDKT.
Another barrier to living donation is the medical leave time required for recovery, which has reportedly caused some donors to lose their jobs. Proposed legislation such as the “Living Donor Protection Act” is designed to prevent insurance discrimination on the basis of serving as an organ donor and to protect the right to use the Family Medical Leave Act to recover from donation surgery, and provides another opportunity to advance LDKT. With slow progress on a federal bill in recent years, a number of states have introduced state-level Living Donor Protection Acts. CMS could also consider provision of lifetime Medicare insurance as a financial and clinical safeguard.
The 2019 AAKH Executive Order brings great promise for expanding access to transplantation by dissolving silos created by current patient education and referral practices, incentivizing optimal procurement and placement of deceased donor organs, and removing barriers to LKDT. However, there are caveats that warrant ongoing attention (Figure 1). We hope AAKH is a starting point to unify collaborative efforts across policy makers, transplant and general nephrology providers, and OPOs in a shared goal of making access to kidney transplantation—the clinically and economically superior treatment for kidney failure—a near-term reality for all patients in need.
Figure 1.
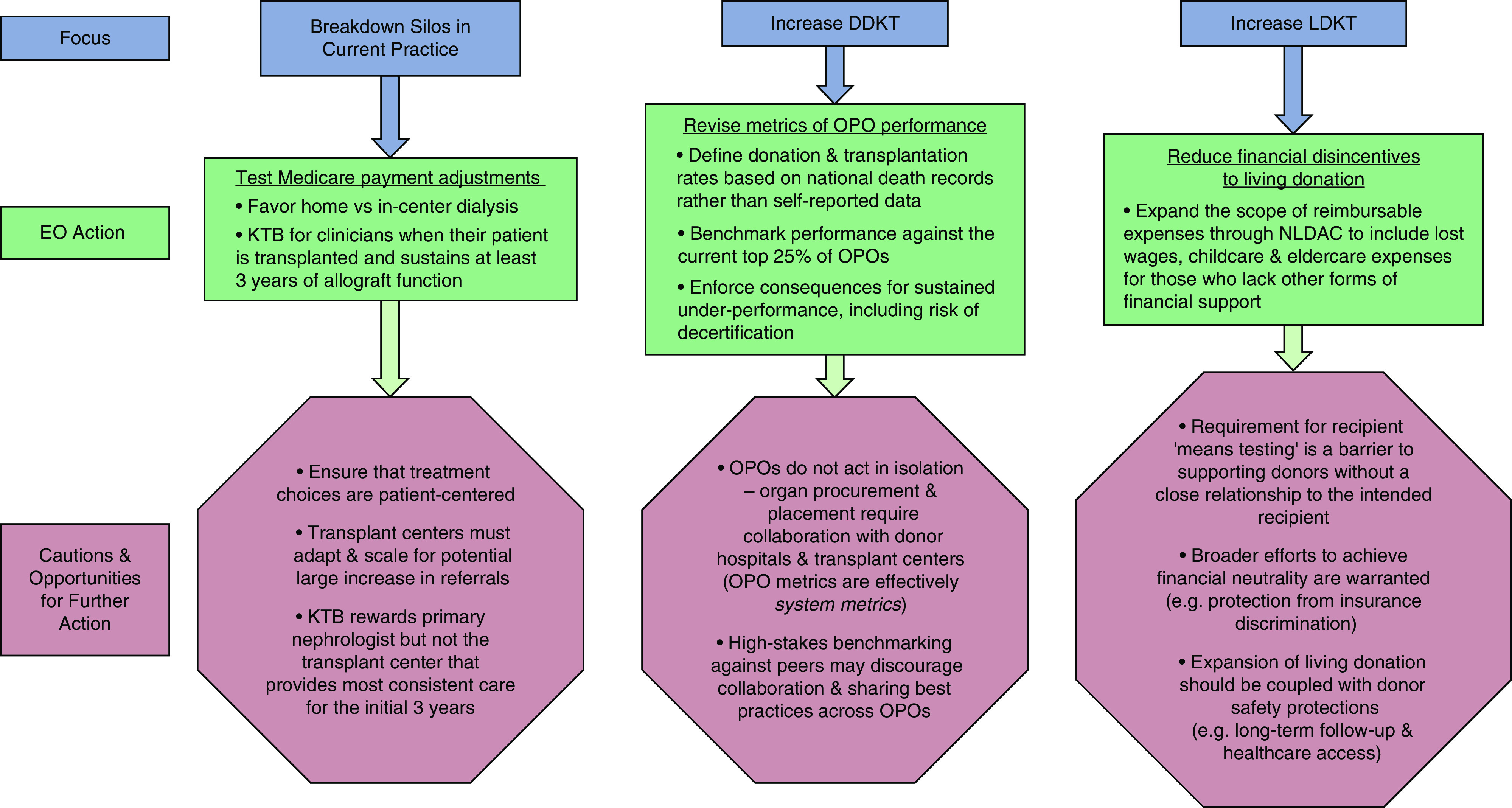
Opportunities and challenges for expanding kidney transplant access through the AAKH Executive Order. AAKH, Advancing American Kidney Health; DDKT, deceased donor kidney transplantation; EO, Executive Order; KTB, Kidney Transplant Bonus; LDKT, living donor kidney transplantation; NLDAC, National Living Donor Assistance Center; OPO, Organ Procurement Organization.
Disclosures
K. Lentine is a member of the American Society of Nephrology Quality Committee, serves on Sanofi speaker’s bureau, and performs consulting for CareDx. R. Mannon is a member of the American Society of Nephrology Policy and Advocacy Committee.
Funding
None.
Acknowledgments
K. Lentine receives support for living donation research from National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK120551 and the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation. R. Mannon is supported by Nebraska Medicine. The authors thank D. L. White, Regulatory and Quality Officer for the ASN Alliance for Kidney Health, for his critical review of this manuscript.
The content of this manuscript is the sole responsibility of the authors and does not represent the official views of the authors’ institutions or the American Society of Nephrology.
Author Contributions
K. Lentine and R. Mannon conceptualized, visualized, reviewed and edited the manuscript, and K. Lentine was responsible for writing the original draft.
References
- 1.Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, Kasiske BL, Alhamad T, Lentine KL: An economic assessment of contemporary kidney transplant practice. Am J Transplant 18: 1168–1176, 2018 [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System : USRDS 2018 Annual Data Report. Available at: https://www.usrds.org/2016/view/v2_07.aspx. Accessed February 7, 2020
- 3.Waterman AD, Peipert JD, Xiao H, Goalby CJ, Kawakita S, Cui Y, Lentine KL: Education strategies in dialysis centers associated with increased transplant wait-listing rates. Transplantation 104: 335–342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterman AD, Peipert JD, Goalby CJ, Dinkel KM, Xiao H, Lentine KL: Assessing transplant education practices in dialysis centers: Comparing educator reported and Medicare data. Clin J Am Soc Nephrol 10: 1617–1625, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services : Advancing American kidney health. Available at: https://aspe.hhs.gov/system/files/pdf/262046/AdvancingAmericanKidneyHealth.pdf. Accessed March 3, 2020
- 6.Aubert O, Reese PP, Audry B, Bouatou Y, Raynaud M, Viglietti D, Legendre C, Glotz D, Empana JP, Jouven X, Lefaucheur C, Jacquelinet C, Loupy A: Disparities in acceptance of deceased donor kidneys between the United States and France and estimated effects of increased US acceptance. JAMA Intern Med 179: 1365, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL: Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 14: 2310–2316, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services (CMS) H. The Federal Register : Medicare and Medicaid programs; regulatory provisions to promote program efficiency, transparency, and burden reduction. 2019. Available at: https://www.federalregister.gov/documents/2019/09/30/2019-20736/medicare-and-medicaid-programs-regulatory-provisions-to-promote-program-efficiency-transparency-and. Accessed March 3, 2020 [PubMed]
- 9.Axelrod DA, Schnitzler MA, Xiao H, Naik AS, Segev DL, Dharnidharka VR, Brennan DC, Lentine KL: The changing financial landscape of renal transplant practice: A national cohort analysis. Am J Transplant 17: 377–389, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigue JR, Schold JD, Morrissey P, Whiting J, Vella J, Kayler LK, Katz D, Jones J, Kaplan B, Fleishman A, Pavlakis M, Mandelbrot DA; KDOC Study Group : Predonation direct and indirect costs incurred by adults who donated a kidney: Findings from the KDOC study. Am J Transplant 15: 2387–2393, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tietjen A, Hays R, McNatt G, Howey R, Lebron-Banks U, Thomas CP, Lentine KL: Billing for living kidney donor care: Balancing cost recovery, regulatory compliance, and minimized donor burden. Curr Transplant Rep 6: 155–166, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson DB, Wiseman JF, Vock DM, Berglund DM, Roman AM, Ibrahim HN, Matas AJ: Financial burden associated with time to return to work after living kidney donation. Am J Transplant 19: 204–207, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Gill J, Joffres Y, Rose C, Lesage J, Landsberg D, Kadatz M, Gill J: The change in living kidney donation in women and men in the United States (2005-2015): A population-based analysis. J Am Soc Nephrol 29: 1301–1308, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML, Gordon EJ, Crews DC, Boulware LE, Segev DL: Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA 319: 49–61, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, Bakr MA, Gallon L, Garvey CA, Guleria S, Li PK, Segev DL, Taler SJ, Tanabe K, Wright L, Zeier MG, Cheung M, Garg AX: KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 101[Suppl 1]: S1–S109, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


