Abstract
Host-microbes interaction plays a crucial role in cardiovascular disease (CVD) pathogenesis, mechanistically via metaorganismal pathways. The trimethylamine N-oxide (TMAO) metaorganismal pathway is the most deeply investigated one, which comprises trimethylamine precursors, such as choline, trimethylamine lyase, trimethylamine, host liver FMO3, TMAO, and downstream effectors involving unfolded protein response (UPR), NF-κB and NLRP3 inflammasome. Accumulating data from clinical investigations of CVD patient cohorts and rodent models have supported the critical role of this metaorganismal pathway in the pathogenesis of CVD. We summarize an array of significant animal studies especially for arthrosclerosis with an emphasis on downstream molecular effectors of this metaorganismal pathway. We highlight clinical investigations of the prognostic value of plasma TMAO levels in predicting prospective risk for future major adverse cardiac events (MACE) indicated by composite end points of myocardial infarction (MI), stroke, heart failure (HF), other ischemic cardiovascular events, or death. Further, we discuss the latest advances of preclinical models targeting the gut microbiota trimethylamine lyase of the TMAO metaorganismal pathway for CVD intervention, as well as the catalog of gut microbiota TMA lyase genes and microbes in the human gut as the prerequisite for potential clinical intervention. In-depth characterization of TMAO metaorganismal pathway holds great promise for CVD clinical metagenomics, diagnostics and therapeutics.
Introduction
Trillions of microbes inhabit the human gut, which affect human health and disease. The 16S rRNA gene sequencing and next generation sequencing (NGS) allow culture free detection of bacterium community in human gut, which speed up the advancement of gut microbiome research (1–4). The association between gut microbiome and cardiovascular disease (CVD) received attention during the past several years. Patients with CVD shows different gut microbiota community structure from healthy controls, which are related to CVD phenotypes and cohorts (5–8).
Although there is no consistence in gut microbiota community with CVD among different groups in the world, a consensus has been reached that gut microbiota impacts cardiovascular health and disease via various metabolites, such as trimethylamine-N-oxide (TMAO), short-chain fatty acids and secondary bile acids (9–12). Host-microbes interaction plays crucial role in CVD pathophysiology, mechanistically via metaorganismal pathways. The TMAO metaorganismal pathway is the most deeply investigated one, which comprises trimethylamine precursors, such as choline, TMA lyase, TMA, host liver FMO3, TMAO, and downstream effectors involving unfolded protein response (UPR), NF-κB and NLRP3 inflammasome (13–20) (Figure 1).
Figure 1.
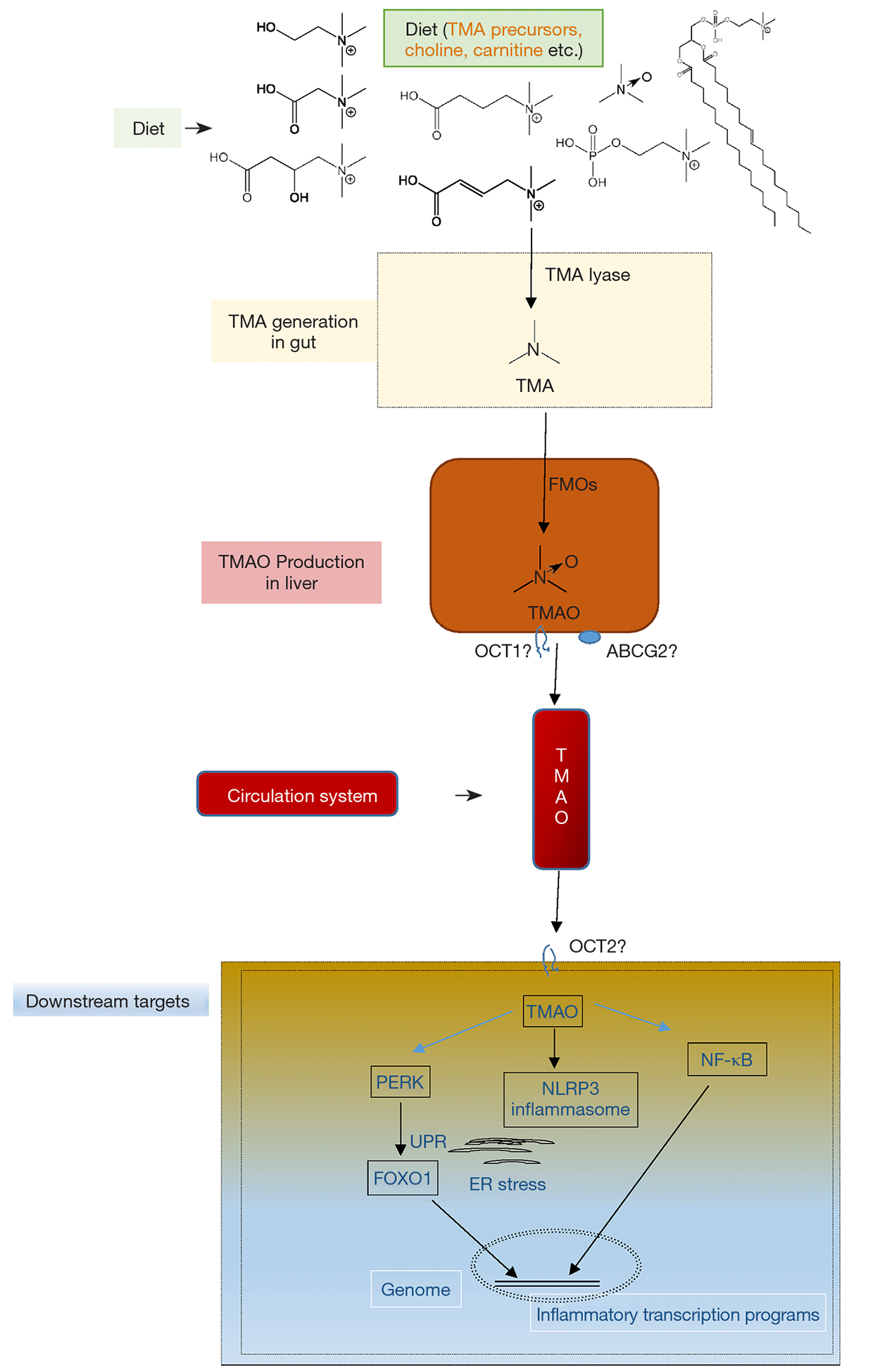
The trimethylamine N-oxide (TMAO) metaorganismal pathway. Key components of this metaorganismal pathway are illustrated, including trimethylamine precursors, such as choline, trimethylamine lyase, trimethylamine, host liver FMOs, TMAO, and downstream effectors involving UPR, NF-κB and NLRP3 inflammasome. TMA, trimethylamine; TMAO, trimethylamine N-oxide; FMOs, flavin monooxygenase; ER, endoplasmic reticulum; PERK, protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK); OCT1, organic cation transporter 1; OCT2, organic cation transporter 2; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; NF-κB, Nuclear factor kappa B; ABCG2, ATP Binding Cassette Subfamily G Member 2.
TMAO has been widely validated as a pro-atherogenic and pro-thrombotic microbe-host co-metabolite, causally linked to CVD (13–15,20–23), which affects one third of adults as the leading cause of death worldwide (24,25). TMAO is an oxidative product of TMA, catalyzed by hepatic flavin monooxygenases (FMOs) (26,27). Multiple nutrients with structural formula containing TMA group can be cleaved to produce free TMA by enzymes in microbes (28–30). Chronic red meat consumption results in an average of 3 times higher circulating TMAO than does non-meat or white meat (31). The two steps of TMAO production from nutrients constitute a metaorganismal pathway, linking to a number of complex diseases (19). Clinical investigations have highlighted the prognostic value of plasma TMAO levels in predicting prospective risk of complex diseases, including CVD, kidney diseases, diabetes and liver steatosis as well (32–35). Targeting this pathway holds great promise for CVD intervention as supported in preclinical models.
Gut microbiome and CVD
Initially gut microbiome was reported to contribute to metabolic diseases through the modulation of energy balance (increased energy harvest) and immunity (inflammation and autoimmunity) (36,37). Several years later our group reported that gut microbiota metabolism of dietary phosphatidylcholine (PC) promotes CVD (21) and the association between gut microbiome and CVD started to receive attention. The gut microbiome discrimination between patients with CVD and healthy controls were reported in several groups. Karlsson et al. used shotgun sequencing to analyze fecal microbiome from 12 patients with symptomatic atherosclerosis and 13 age and gender matched controls and found that the genus Collinsella was enriched in patients with symptomatic atherosclerosis, whereas Roseburia and Eubacterium were enriched in healthy controls (6). Yin et al. compared fecal microbiota community using 16S rRNA gene sequencing between 141 patients with stroke and transient ischemic attack and 94 asymptomatic controls and found more opportunistic pathogens, such as Enterobacter, Megasphaera, Oscillibacter, and Desulfovibrio, and fewer commensal or beneficial genera such as Bacteroides, Prevotella, and Faecalibacterium in patients (7). Emoto et al. compared fecal microbiota community by terminal restriction fragment length polymorphism (T-RFLP) of 16S rDNA amplicons in 39 patients with coronary artery disease (CAD), 30 age- and sex-matched no-CAD controls, and found that increased Frimicutes/Bacteroidetes ratio and Lactobacillus and decreased Bacteroides plus Preveotella in CAD (8). Jie et al. compared fecal microbiome in 218 patients with atherosclerotic CVD (ACVD) and 187 healthy controls and found that ACVD is rich in Enterobacteriaceae including Escherichia coli, Klebsiella spp., and Enterobacter aerogenes and Streptococcus spp., and Eubacterium eligens, Faecalibacterium prausnitzii, and Clostridiales sp and has diminished Bacteroides spp., Prevotella copri and Alistipes shahii (5). It seems that we cannot find a unique gut microbiome community structure that contributes to CVD, but a consensus was reached that gut microbiome contributes to CVD through gut microbiota derived metabolites including TMAO, aromatic acid metabolites, p-cresyl sulfate and indoxyl sulfate (19,38). Indoxyl sulfate contributes to CVD progression through stimulation of oxidative stress and inhibition of AMPK/ UCP2 signaling (39) and activation of multiple signaling pathways including mitogen activated protein kinase (MAPK) and nuclear factor-κB (NFκB) (40). P-cresyl sulfate mediates MCP-1 production in vascular smooth muscle cells through NF-kB pathway, contributing to the inflammatory response initiation involved in atherosclerosis lesion formation (41).
TMAO, a metaorganismal product
The role of TMAO in CVD was initially discovered by Wang et al. through untargeted mass spectrometry (MS) based metabolomics approach (21) that was applied to identify metabolites in human plasma to discriminate healthy controls from CVD patients in two cohorts, randomly selected from GeneBank, a large data/sample bank containing more than 10,000 human plasma samples from patients undergone left selective coronary angiography with outcome data monitored after enrollment (42). The learning cohort was comprised of 50 cases subjected to risk for non-fatal myocardial infarction (MI), stroke or death within 3 years versus 50 controls without risk for non-fatal MI, stroke or death, and the validation cohort was comprised of 25 cases versus 25 controls (21). A metabolite with m/z =76 was found to have the smallest molecular weight while highly correlated to the other two analytes in the same metabolism pathway (21). The analyte with m/z =76 was eventually identified as TMAO via multiple MS and different liquid chromatography (LC) conditions by comparison with authentic compound (21). Because the structural formula of TMAO contains TMA group, it was initially hypothesized that TMAO is the metabolite of dietary PC. This hypothesis was examined by feeding mice and humans with isotope labeled PC where 1H atoms were replaced with deuterium (2H) at the TMA functional group. The corresponding isotope labeled TMAO product, alongside two metabolism related metabolites, choline and betaine, was detected for de novo biosynthesis (21,43). These data also showed that the broad spectrum antibiotics treatment to deprive gut microbiota suppresses the production of TMAO while the TMAO production recovers after the removal of antibiotics (21,43). In parallel, if d9-PC was intraperitoneally injected, no d9-TMAO was detectable in the conventional mice (21). Thus, gut microbiota plays an obligatory role in the biosynthesis of TMAO (21,43).
While in the above challenge experiments PC was replaced with free choline, it was further confirmed that choline contributes to the production of TMAO, depending on gut microbiota (43). PC is the main choline source in diet (44,45). In the small intestine, PC can be digested by pancreatic phospholipase D, to release free choline (46). Meanwhile, commensal gut bacterium contains phospholipase D with PC as substrate to release free choline (47). In human gut, the endogenously synthesized PC can be released from bile (48). Choline can be then cleaved to produce TMA, which is catalyzed by choline TMA lyase, a glycyl radical enzyme in microbes (28). Choline TMA lyase encoding gene was found via sequence alignment with the operon encoding enzymes of the ethanolamine metabolism (28). The choline TMA lyase gene clusters contain at least two genes, cutC and cutD, encoding the two subunits of TMA lyase, respectively. The cutD protein activates cutC protein by forming a glycyl radical, which abstracts hydrogen atom from the cysteine residue to form cysteine radical followed by the cysteine radical abstracting hydrogen from choline to release free TMA (28,49).
Choline TMA lyase encoding genes have been found in many bacteria. For instance, in the human microbiome project (HMP1), there are 19 strains with high abundance found in the oral cavity samples while 16 strains were found in the stool samples. Thus, it appears that the abundance of choline TMA lyase encoding bacterium is higher in oral cavity than stool (Figure 2). Choline TMA lyases are associated with other diseases besides CVD, e.g., choline TMA-lyase gene was overabundant in colorectal cancer (50).
Figure 2.
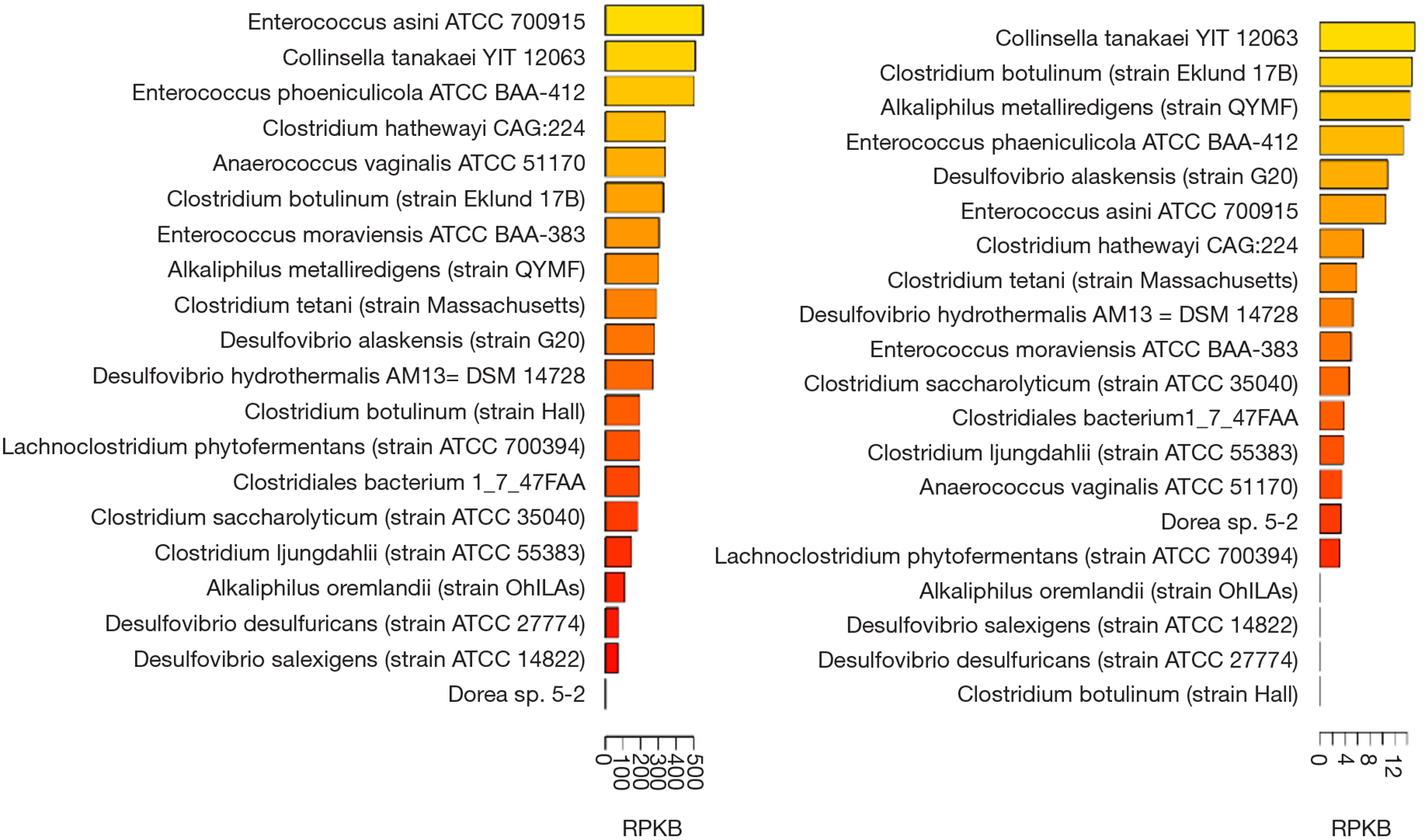
The distribution and abundance of cutC encoding bacteria in the samples of the human microbiome project 1(HMP1) (https://www.hmpdacc.org/resources/). The cutC abundance in oral cavity (A) is much more than that of the stool cutC (B) as shown in bar plot of the maximum abundance (RPKB, strain numbers per billion reads) among all samples in the cohort. The cutC gene abundances were computed following the method described in Thomas et al. (50).
Besides choline TMA lyase CutC/D, other TMA lyases using different preferential substrates to produce TMA have been reported, including cntA/B (the same to yeaW/ X), betaine reductase and TMAO reductase (29,30,51,52). Multiple FMOs, including FMO1, FMO2 and FMO3, have the capacity to catalyze the oxidation of TMA to TMAO as shown in the HEK293Ad cell line transfected with human FMO1–5 constructs and incubated with d9-TMA with the readout of d9-TMAO monitored by LC/MS/MS (27). After normalization to the corresponding expressing FMO protein level, FMO3 has the highest specific activity (27). In humans, FMO3 genetic deficiency due to germline mutations cause fish odor syndrome characterized by the accumulation in TMA and excretion in the breath, urine, sweat, saliva and vaginal secretions (53–55).
Dissecting the TMAO metaorganismal pathway in rodent models
The potential causal relationship between plasm TMAO and CVD was examined extensively in mice models (32,33). We summarized functional studies of the TMAO metaorganismal pathway in rodent models in Table 1, with an emphasis on the association of TMAO metaorganismal pathway with CVD. Moreover, we described key experimental results as follows, highlighting the complexity of TMAO effectors.
Table 1.
Dissecting TMAO metaorganismal pathway in mouse models.
| Study | mouse | Genetic model | Diet | intervention | Disease model (trait) | Metaorganismal pathway components | Reference |
|---|---|---|---|---|---|---|---|
| Wang_2011 | C57BLV6J | Apoe−/− | Choline supplemented chow diet | Mice were supplied with drinking water containing broad spectrum antibiotics (vancomycin, neomycin, ampicillin, metronidazole) | Atherosclerosis | Choline, Fmo3, TMAO | Wang et al., 2011) |
| Koeth_2013 | C57BLV6J | Apoe-−/− | Choline or carnitine supplemented chow diet | Mice were supplied with drinking water containing broad spectrum antibiotics (vancomycin, neomycin, ampicillin, metronidazole) | Atherosclerosis | Choline, carnitine, TMA and TMAO, CD36, key bile acid synthetic enzymes Cyp7a1 and Cyp27a1 | Koeth et al., 2013 |
| Zhu_2016 | C57BL/6J, NZW/LacJ | C57BL/6J versus NZW/LacJ | Chemically defined chow (0.08% total choline) versus chow diet supplemented with 1% choline for 3 weeks before the evaluation of plasma TMAO and platelet function. | FeCl3 carotid artery injury thrombosis model, rose Bengal photochemical injury thrombosis model, cecal microbial transplant study | Platelet hyperresponsiveness and atherothrombotic heart disease | Choline, TMA lyase, FM03, TMAO, Ca2+ | Zhu et al.,2016 |
| Chen_2019 | C57BL/6 | C57BL/6 insulin receptor liverKO, C57BL/6 FoxOI liver KO, C57BL/6J FmoS−/−, ob/ob, PERK Flox, C57BL/6 XBP1 Flox | Standard chow diet I (Control for 0.12% TMAO diet, 1% Choline diet, 0.25% DIM diet), 0.12% TMAO diet, 1 % Choline diet, 0.25% DIM diet, Standard chow diet II (Control for 0.25% I3C diet), 0.25% I3C diet | Germ free female C57BL/6J mice colonized with CutC+ orCutC− E. coli strains | Metabolic syndrome | Choline, TMA, cutC, Fmo3, TMAO, PERK, FOXOI | Chen et al.,2019 |
TMA precursors, such as choline, carnitine and -butyrobetaine, promote atherosclerosis via TMAO metaorganismal pathway. Apoe−/− mice are similar to humans in atherosclerosis progression (56). When Apoe−/− mice were fed choline or TMAO supplemented chow diet for 16 weeks to quantify aortic lesion with oil red O-hematoxylin staining, results showed that both choline and TMAO promote atherosclerosis. In the female mice, the aortic lesion is highly correlated to plasma TMAO (21). In order to test whether choline promoting atherosclerosis is mediated by TMAO, Apoe−/− mice were fed choline supplemented chow diet alongside drinking water containing broad spectrum antibiotics (vancomycin, neomycin, ampicillin, metronidazole), to suppress the gut microbiota production of TMAO. The quantification of aortic lesion suggests that gut microbiota plays an important role in choline promoting atherosclerosis and the effect of choline might be mediated through TMAO (21). Further, TMAO inhibits expressions of the key bile acid synthetic enzymes Cyp7a1 and Cyp27a1 in liver, resulting in decreased bile acid pool and cholesterol reverse transport (57). Besides choline promoting atherosclerosis, other TMA containing nutrients such as carnitine and -butyrobetaine, can also promote atherosclerosis via the TMAO metaorganismal pathway (30,57).
TMAO promotes platelet aggregation and thrombosis by inducing Ca2+ release. The Ldlr−/− mice show decreased plasma level of TMAO after Fmo3-knock down by intraperitoneal injection of Fmo3 antisense oligonucleotide. Further, atherosclerotic lesion area was decreased with the decreased plasma TMAO (58), suggesting the TMAO metaorganismal pathway is linked to atherosclerosis. TMAO increases platelet hyper-reactivity thereby promoting thrombosis (22). In platelet, TMAO induces Ca2+ release from intracellular calcium stores, leading to platelet aggregation and thrombosis (22).
Given that TMAO is linked to inflammatory diseases, it is pivotal to understand the underlying molecular mechanisms of TMAO activating inflammasome, a group of protein complexes built around several proteins, including NLRP3, NLRC4, AIM2 and NLRP6, et al. (59–62). TMAO activates the NLRP3 inflammasomes, leading to endothelial dysfunction activation, putatively via pathways of mitogen-activated protein kinase (MAPK) and nuclear factor-B (14,20). Accordingly, TMAO pre-incubated aortic endothelial cells show increased leukocyte adhesion (20). Monocyte adhesion to the vascular endothelial cell constitutes an important step of atherosclerosis progression (63). Importantly, TMAO is linked to UPR during endoplasmic reticulum stress; TMAO binds to PERK at physiologically relevant concentrations; selectively activates the PERK branch of the UPR; and induces the transcription factor FoxO1, a key driver of metabolic disease (18).
The molecular functions and associations with complex disease traits of TMAO metaorganismal pathway have been strengthened by using gnotobiotic models. Gut microbiota transplantation to germ free mice showed that choline diet-induced TMAO production capability and atherosclerosis and thrombosis susceptibility are transmissible. Distinct plasma TMAO levels have been observed in the strains of the hybrid mouse diversity panel (HMDP) (64–66), in which the aortic lesion is positively correlated to plasma TMAO (67). The NZW/LacJ Apoe−/− mouse has the lowest plasma TMAO and the lowest aortic lesion, while the C57BL/6J Apoe−/− mouse has the highest plasma TMAO and the largest aortic lesion (67).
In a gnotobiotic model, feces from NZW/LacJ Apoe−/− mice and from C57BL/6J Apoe−/− mice were used as donors to transplant to germ free mice, acquired by subjecting conventional mice on drinking water with broad spectrum antibiotics for 3 weeks, initially orally gavaging every other day for 2 weeks, followed by weekly for 11 more weeks and the mice were fed choline supplemented chow diet. At an age of 20 weeks, mice were sacrificed and the aortic plaque was quantified. The germ free mice which received feces from NZW/LacJ Apoe−/− mice have a relatively lower plasma TMAO and lower aortic lesion than C57BL/6J Apoe−/− mice (67). Thus, this gnotobiotic model of gut microbial transplantation demonstrated that atherosclerosis susceptibility is transferrable via transmission of the choline diet-induced TMAO production.
Wild type Clostridium sporogenes in the mice gut contributes to the production of TMAO (68). Intriguingly, disruption of the cutC gene in Clostridium sporogenes caused the loss of commensal bacterium transmitting thrombotic property (69). Thus, human gut commensals containing cutC are sufficient to transmit enhanced platelet reactivity and thrombosis potential.
The TMAO metaorganismal pathway has a molecular mechanism link to inflammation, thereby associated with a variety of inflammatory diseases, including atherosclerosis.
Targeting choline TMA lyase to attenuate atherosclerosis and thrombosis
Multiple choline TMA lyase inhibitors have the capacity of decreasing plasma TMAO levels, thereby ameliorating atherosclerosis and thrombosis. The natural compound, 3,3-dimethyl-1-butanol (DMB), which can be detected in some balsamic vinegars, red wines, and in some cold-pressed extra virgin olive oils and grape seed oils, has been shown to decrease plasma TMAO in mice fed choline or carnitine supplemented chow diet (70). Alongside decreasing plasma TMAO level, DMB can significantly ameliorate aortic lesions in Apoe−/− mice fed choline-supplemented chow diet via inhibition of enzymatic activities of multiple TMA lyases (70). In addition, DMB, a microbial choline TMA lyase inhibitor, attenuates choline diet-enhanced platelet responsiveness and in vivo rate of thrombus formation (71). In the FeCl3-induced carotid artery injury mice model, the time to cessation of flow appears to be significantly longer in the mice with DMB added to the drinking water when compared with regular water, which is in agreement with the decrease in plasma TMAO (71).
Mice fed western diet developed impaired cardiac systolic and diastolic function by echocardiography accompanied by increased circulating TMAO, the addition of DMB to mice drinking water can decrease plasma TMAO, therefore prevent cardiac systolic and diastolic dysfunction (72). Series choline analogues, including fluromethylcholine (FMC), chloromethylcholine, bromomethylcholine, iodomethylcholine (IMC), were tested as more potent inhibitors to choline TMA lyase. These lead compounds can effectively decrease plasma TMAO to a nearly non-detectable level either fed chow diet or choline supplemented chow diet. FMC and IMC were further functionally tested and both decreased platelet responsiveness from mice fed choline-supplemented chow diet compared with chow diet and increased the time of cessation of flow (71). Inhibitory effects of bacterium growth or changed liver and renal functions have not been observed in the mice administrated with DMB, FMC, or IMC, indicating little potential side effects for these lead compounds (70,71). In addition, some nutraceutical, such as resveratrol, appeared to decrease circulatory TMAO, putatively due to gut microbiota remodeling (73).
TMAO metaorganismal pathway and CVD
Following Wang et al. in 2011, measurement of plasma TMAO from subsequent human plasma samples in the GeneBank cohort (4,007 patients) showed that increased plasma TMAO was correlated with increased prospective risk for major adverse cardiac events (MACE, MI, stroke and death) within 3 years (43). In addition, it was reported that high levels of plasma TMAO are independently correlated with plaque rupture in patients with ST-segment—elevation MI (74).
In human gut, there are trillions of microbes, which play important roles in human health, including production of vitamins, such as vitamin K and biotin, serotonin, and fermentation of dietary fiber to produce short chain fatty acids and modulation of immunity as well (75–78). The LifeLines-DEEP population cohort data showed the variance of microbiota can explain 4.5% of the variance in body mass index, 6% in triglycerides, and 4% in high-density lipoproteins, after adjusting for age, sex and genetic risk factors (79). These findings support the concept that gut microbiota contributes to CVD.
The prognostic values of multiple TMA nutrients are dependent on gut microbial metabolite, TMAO
Circulating choline and betaine are significantly correlated to the prevalence of CVD, and also predict future risk for MACE (80). However, if adjusted with TMAO, the significant differences were not observed (80). The plasma TMAO levels can be stratified into two ranges by the median value, i.e., below median value (LOW) and above median value (HIGH), and the same method was applied to choline or betaine. In the GeneBank cohort, the LOW TMAO, HIGH choline or betaine plasma levels did not show significantly higher prognostic value in predicting risk for future MACE when compared with LOW choline or betaine, while the HIGH TMAO, HIGH choline or betaine levels showed significantly higher prognostic value in predicting risk for future MACE compared with LOW choline or betaine (80). Similarly, for carnitine, another TMAO precursor, the LOW TMAO, HIGH carnitine levels did not show significantly higher prognostic value than LOW carnitine, while the HIGH TMAO, HIGH carnitine levels showed significantly higher prognostic value than LOW carnitine (57). Thus, the prognostic values of multiple TMA nutrients are dependent on gut microbial metabolite, TMAO. Intriguingly, trimethyllysine (TML), a TMA containing compound in structural formula, can be cleaved to produce TMA and further contribute to the production of TMAO. However, the prognostic incident CVD risks of TML is independent of TMAO (81).
Future perspective.
Since TMAO is mechanistically linked to CVD and can predict prospective risk for future MACE, the early monitoring of plasma TMAO is predictive to cardiac events. Furthermore, the administration of inibitors to block TMAO production as well as gut microbial transplantation has the potential to mitigate the progression of atherosclerosis and thrombosis. Taken together, TMAO takes the center stage in modulating the processes leading to the development of CVD. Several promising interventions targeting the TMAO metaorganismal pathway could be further investigated and ultimately be applied to patients with CVD.
Acknowledgments
Funding: Z Wang is supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL130819).
Keyword
- TMAO
Trimethylamine N-oxide
- CVD
cardiovascular disease
- MACE
major adverse cardiac events
Footnotes
Conflicts of Interest: Z Wang is named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, and have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab or Proctor & Gamble.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Matsuki T, Watanabe K, Fujimoto J, et al. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol 2002;68:5445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuki T, Watanabe K, Fujimoto J, et al. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 2004;70:7220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzon L, Militello V, Lavezzo E, et al. Human papillomavirus genotyping by 454 next generation sequencing technology. J Clin Virol 2011;52:93–7. [DOI] [PubMed] [Google Scholar]
- 4.Pospisilova S, Tichy B, Mayer J. [Human genome sequencing--next generation technology or will the routine sequencing of human genome be possible?]. Cas Lek Cesk 2009;148:296–302. [PubMed] [Google Scholar]
- 5.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson FH, Fak F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 2012;3:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin J, Liao SX, He Y, et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emoto T, Yamashita T, Sasaki N, et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb 2016;23:908–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 2019;16:137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang WHW, Backhed F, Landmesser U, et al. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:2089–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 2015;66:343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Jiao X, Ma Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 2016;481:63–70. [DOI] [PubMed] [Google Scholar]
- 14.Boini KM, Hussain T, Li PL, et al. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol Biochem 2017;44:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ML, Zhu XH, Ran L, et al. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Deeb OS, Atef MM, Hafez YM. The interplay between microbiota-dependent metabolite trimethylamine N-oxide, Transforming growth factor beta/SMAD signaling and inflammasome activation in chronic kidney disease patients: A new mechanistic perspective. J Cell Biochem 2019;120:14476–85. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Geng J, Zhao J, et al. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front Physiol 2019;10:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Henderson A, Petriello MC, et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab 2019. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018;9:416–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor- kappaB. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016;165:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Deeb OS, Atef MM, Hafez YM. The interplay between microbiota-dependent metabolite trimethylamine N-oxide, Transforming growth factor beta/SMAD signaling and inflammasome activation in chronic kidney disease patients: A new mechanistic perspective. J Cell Biochem 2019. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Wood D, Ralston J, et al. The World Heart Federation’s vision for worldwide cardiovascular disease prevention. Lancet 2015;386:399–402. [DOI] [PubMed] [Google Scholar]
- 25.Okwuosa IS, Lewsey SC, Adesiyun T, et al. Worldwide disparities in cardiovascular disease: Challenges and solutions. Int J Cardiol 2016;202:433–40. [DOI] [PubMed] [Google Scholar]
- 26.Lang DH, Yeung CK, Peter RM, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol 1998;56:1005–12. [DOI] [PubMed] [Google Scholar]
- 27.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A 2012;109:21307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A 2014;111:4268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019;40:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janeiro MH, Ramirez MJ, Milagro FI, et al. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisel SH, Warrier M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu Rev Nutr 2017;37:157–81. [DOI] [PubMed] [Google Scholar]
- 34.Tan X, Liu Y, Long J, et al. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol Nutr Food Res 2019;63:e1900257. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes Rev 2019;20:883–94. [DOI] [PubMed] [Google Scholar]
- 36.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 37.Sanz Y, Olivares M, Moya-Perez A, et al. Understanding the role of gut microbiome in metabolic disease risk. Pediatr Res 2015;77:236–44. [DOI] [PubMed] [Google Scholar]
- 38.Lin CJ, Chuang CK, Jayakumar T, et al. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch Med Sci 2013;9:662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang K, Xu X, Nie L, et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol Lett 2015;234:110–9. [DOI] [PubMed] [Google Scholar]
- 40.Wakamatsu T, Yamamoto S, Ito T, et al. Indoxyl Sulfate Promotes Macrophage IL-1beta Production by Activating Aryl Hydrocarbon Receptor/NF-kappa/MAPK Cascades, but the NLRP3 inflammasome Was Not Activated. Toxins (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciel RA, Rempel LC, Bosquetti B, et al. p-cresol but not p-cresyl sulfate stimulate MCP-1 production via NF-kappaB p65 in human vascular smooth muscle cells. J Bras Nefrol 2016;38:153–60. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 2007;13:1176–84. [DOI] [PubMed] [Google Scholar]
- 43.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheatham CL, Goldman BD, Fischer LM, et al. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2012;96:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis ED, Zhao YY, Richard C, et al. Measurement of the abundance of choline and the distribution of choline-containing moieties in meat. Int J Food Sci Nutr 2015;66:743–8. [DOI] [PubMed] [Google Scholar]
- 46.Rydzewska G, Rossignol B, Morisset J. Involvement of phospholipase D in caerulein-induced phosphatidylcholine hydrolysis in rat pancreatic acini. Am J Physiol 1993;265:G725–34. [DOI] [PubMed] [Google Scholar]
- 47.Chittim CL, Martinez Del Campo A, Balskus EP. Gut bacterial phospholipase Ds support disease-associated metabolism by generating choline. Nat Microbiol 2019;4:155–63. [DOI] [PubMed] [Google Scholar]
- 48.Northfield TC, LaRusso NF, Hofmann AF, et al. Biliary lipid output during three meals and an overnight fast. II. Effect of chenodeoxycholic acid treatment in gallstone subjects. Gut 1975;16:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craciun S, Marks JA, Balskus EP. Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem Biol 2014;9:1408–13. [DOI] [PubMed] [Google Scholar]
- 50.Thomas AM, Manghi P, Asnicar F, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med 2019;25:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreesen JR. Glycine metabolism in anaerobes. Antonie Van Leeuwenhoek 1994;66:223–37. [DOI] [PubMed] [Google Scholar]
- 52.Pascal MC, Burini JF, Chippaux M. Regulation of the trimethylamine N-oxide (TMAO) reductase in Escherichia coli: analysis of tor::Mud1 operon fusion. Mol Gen Genet 1984;195:351–5. [DOI] [PubMed] [Google Scholar]
- 53.Dolphin CT, Riley JH, Smith RL, et al. Structural organization of the human flavin-containing monooxygenase 3 gene (FMO3), the favored candidate for fish-odor syndrome, determined directly from genomic DNA. Genomics 1997;46:260–7. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki H, Shimizu M. Genetic polymorphism of the flavin-containing monooxygenase 3 (FMO3) associated with trimethylaminuria (fish odor syndrome): observations from Japanese patients. Curr Drug Metab 2007;8:487–91. [DOI] [PubMed] [Google Scholar]
- 55.Rehman HU. Fish odor syndrome. Postgrad Med J 1999;75:451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Getz GS, Reardon CA. ApoE knockout and knockin mice: the history of their contribution to the understanding of atherogenesis. J Lipid Res 2016;57:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 2018;564:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666–71. [DOI] [PubMed] [Google Scholar]
- 61.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. [DOI] [PubMed] [Google Scholar]
- 62.Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature 2012;481:278–86. [DOI] [PubMed] [Google Scholar]
- 63.Moss JW, Ramji DP. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol 2016;13:513–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rau CD, Civelek M, Pan C, et al. A Suite of Tools for Biologists That Improve Accessibility and Visualization of Large Systems Genetics Datasets: Applications to the Hybrid Mouse Diversity Panel. Methods Mol Biol 2017;1488:153–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lusis AJ, Seldin MM, Allayee H, et al. The Hybrid Mouse Diversity Panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J Lipid Res 2016;57:925–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghazalpour A, Rau CD, Farber CR, et al. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm Genome 2012;23:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romano KA, Vivas EI, Amador-Noguez D, et al. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015;6:e02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skye SM, Zhu W, Romano KA, et al. Microbial Transplantation With Human Gut Commensals Containing CutC Is Sufficient to Transmit Enhanced Platelet Reactivity and Thrombosis Potential. Circ Res 2018;123:1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015;163:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 2018;24:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen K, Zheng X, Feng M, et al. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front Physiol 2017;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen ML, Yi L, Zhang Y, et al. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016;7:e02210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan Y, Sheng Z, Zhou P, et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Interv 2019;12:e007281. [DOI] [PubMed] [Google Scholar]
- 75.Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev 1997;6 Suppl 1:S43–5. [DOI] [PubMed] [Google Scholar]
- 76.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol 2000;95:2698–709. [DOI] [PubMed] [Google Scholar]
- 77.Fleming SE, Fitch MD, Chansler MW. High-fiber diets: influence on characteristics of cecal digesta including short-chain fatty acid concentrations and pH. Am J Clin Nutr 1989;50:93–9. [DOI] [PubMed] [Google Scholar]
- 78.Pratt VC, Tappenden KA, McBurney MI, et al. Short-chain fatty acid-supplemented total parenteral nutrition improves nonspecific immunity after intestinal resection in rats. JPEN J Parenter Enteral Nutr 1996;20:264–71. [DOI] [PubMed] [Google Scholar]
- 79.Fu J, Bonder MJ, Cenit MC, et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ Res 2015;117:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li XS, Wang Z, Cajka T, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018. doi: 10.1172/jci.insight.99096. [DOI] [PMC free article] [PubMed] [Google Scholar]


