SUMMARY
High temperatures (e.g. fever) and gut microbiota can both influence host resistance to infection. However, effects of temperature-driven changes in gut microbiota on resistance to parasites remain unexplored. We examined the temperature dependence of infection and gut bacterial communities in parasite-infected bumble bees. Infection intensity decreased by over 80% between 21 and 37 °C. Temperatures of peak infection were lower than predicted based on parasite growth in vitro, consistent with mismatches in the thermal performance curves of hosts, parasites, and gut symbionts. Gut bacterial community size and composition exhibited slight but significant, non-linear, and taxon-specific responses to temperature. Abundance of total gut bacteria and of Orbaceae, both negatively correlated with infection in previous studies, were positively correlated with infection here. Prevalence of the bee pathogen-containing family Enterobacteriaceae declined with temperature, suggesting that high temperature may confer protection against diverse gut pathogens.
Our results indicate that resistance to infection reflects not only the temperature dependence of host and parasite performance, but also the temperature-dependent activity of gut bacteria. The thermal ecology of gut parasite-symbiont interactions may be broadly relevant to infectious disease, both in ectothermic organisms that inhabit changing climates, and in endotherms that exhibit fever- based immunity.
Keywords: thermal performance asymmetry, temperature-mediated competition, gut microbiome, Bombus, Crithidia, Lactobacillus, Gilliamella
INTRODUCTION
Temperature has strong effects on the growth and metabolism of individual species, with subsequent consequences for ecological communities (Gillooly et al., 2001). The effects of temperature on growth, metabolism, and performance are described by the metabolic theory of ecology, which considers organismal metabolism as the combined output of numerous metabolic enzymes. Consequently, metabolic theory models predict the temperature dependence of organismal performance using equations based on enzyme kinetics (Molnár et al., 2017). The temperature-related changes in performance of any given species are described by its thermal performance curve, with performance generally increasing up to an optimal temperature, then declining sharply at supraoptimal temperatures (Molnár et al., 2017). Because species differ in their responses to temperature and thermal ranges of peak performance— a difference referred to as a ‘mismatch’ between two species’ thermal performance curves (Cohen et al., 2017)—temperature can also influence the outcome of species interactions including predation, parasitism, and competition (Dell et al., 2014; Bestion et al., 2018).
One context in which the effect of temperature on species interactions has been repeatedly demonstrated to reflect the predictions of metabolic theory is that of host-parasite interactions (Raffel et al., 2013; Cohen et al., 2019). Metabolic theory predicts that the success of parasites at any given temperature reflects the relative performance of hosts and parasites at that temperature, rather than the absolute performance of either in isolation (Cohen et al., 2017). As a result, the temperature dependence of parasite growth may differ for parasites grown in cell cultures vs. in live hosts (James, 2005; Cohen et al., 2017; Kirk et al., 2018).
Increases in body temperature—also known as fever—have been shown to ameliorate infection in plants, fish, mammals, amphibians, and insects (Kluger et al., 1998; Thomas and Blanford, 2003; Boltaña et al., 2013; Heinrich, 2013; Stahlschmidt and Adamo, 2013). Both endo- and ectothermic animals may use metabolic and behavioral strategies to raise their body temperatures when infected (Starks et al., 2000; Campbell et al., 2010; Stahlschmidt and Adamo, 2013). These febrile behaviors can allow hosts to achieve body temperatures in which the host immune system has a relative advantage over parasites (Casadevall, 2016). Consequently, fever can improve the health of individual hosts, with consequences that may be reflected at the landscape scale (Daskin et al., 2011; Cohen et al., 2019). In bees, particularly social bees that thermoregulate their nests, relatively high temperatures have been shown to ameliorate infections with microsporidia, other fungi, and viruses in both adults and larvae (Martín-Hernández et al., 2009; Di Prisco et al., 2011; Xu and James, 2012; Dalmon et al., 2019; Li et al., 2019).
Besides temperature, host microbiota—particularly gut symbionts—play an increasingly appreciated role in resistance to infection. Microbial symbionts can compete with parasites for space and resources, stimulate host immunity, and produce allelopathic chemicals that inhibit parasite growth (Maslowski and Mackay, 2010; Spor et al., 2011). In insects, including bees, in vitro culturing studies, sterile rearing experiments, and manipulations of the microbiota with fecal transplants have repeatedly demonstrated the ability of bacterial symbionts to suppress the growth and effects of infectious organisms within and outside of hosts (Sabaté et al., 2009; Koch and Schmid-Hempel, 2012; Onchuru et al., 2018; Praet et al., 2018).
Despite the recognized importance of temperature and microbial symbionts in resistance to infection, few studies have explored the effects of temperature on the microbiome, and the consequences of these effects for disease resistance have seldom been considered at all. Landscape surveys and experimental manipulations have shown that temperature can be an important driver of microbial community composition and function in soils and aquatic environments (Steinauer et al., 2015; Chiriac et al., 2017; Rubin et al., 2018). Exposure of rhizosphere communities to high temperatures can lead to loss of taxa and alterations in the ability of soils to support plant growth and suppress disease (Voort et al.; Rubin et al., 2018). In insects, experimental temperature elevations reduced populations of endo- and ectosymbionts (Parkinson et al., 2014; Kikuchi et al., 2016), suggesting that fever and high temperature could be costly for host-symbiont mutualisms and their role in resistance to infection. On the other hand, in cultures of amphibian skin symbionts, high temperatures can elevate production of compounds that inhibit parasite growth (Daskin et al., 2014). In bees, many core gut symbionts have relatively high (34-37 °C) preferred growth temperatures in vitro (Engel et al., 2013), suggesting that high temperatures could favor core gut symbionts over parasites, and thereby reinforce host-bacterial mutualisms.
The bumble bee (Bombus spp.)/Crithidla host-trypanosomatid study system is well suited for investigating how temperature affects infection resistance and microbiota. First, bumble bees are facultative endotherms, capable of producing large amounts of metabolic heat to optimize nest and body temperature during foraging and incubation (Heinrich, 1972). As a result, their ranges of body temperatures and thermal strategies overlap those of both poikilo- and homeothermic animals (Heinrich, 1972), suggesting that bumble bees could be a model for study of infection-microbiota interactions in both types of host. Second, trypanosomatid infection is widespread, common, and costly in Bombus (Schmid-Hempel, 2001; Brown et al., 2003; Schmid-Hempel and Tognazzo, 2010). As a result, responses of trypanosomatid infection to environmental variables is relevant for bee conservation, and may also be relevant for the insect-vectored stage of trypanosomatids that afflict crops, livestock, and humans (Maslov et al., 2013). Third, the well-characterized host microbiota of Bombus have a demonstrated role in resistance to infection (Koch and Schmid-Hempel, 2011, 2012) and higher temperatures of peak performance in vitro than trypanosomatid parasites (Engel et al., 2013; Palmer-Young, Raffel, et al., 2018). In vitro experiments have suggested that the effects of core bacterial symbionts – namely, their ability to acidify the gut lumen to levels that inhibit parasite growth—are temperature-dependent (Palmer-Young, Raffel, et al., 2018; Palmer-Young et al., 2019). These prior findings suggest that high temperatures could lead to symbiont-mediated increase in resistance to infection. However, no study has evaluated the effects of environmental temperature on bumble bee gut microbiota or trypanosomatid infection, nor the effects of temperature on microbiota-mediated protection against disease.
To determine the effects of environmental temperature on bumble bee gut microbiota and resistance to infection with trypanosomatid parasites, we measured gut bacterial community size and composition and infection intensity in bumble bees (Bombus Impatiens) inoculated with the parasite Crithidia bombi, then incubated for 7 d at temperatures between 21 and 37°C. Because bumble and honey bee muscle performance (Gilmour and Ellington, 1993; Harrison and Fewell, 2002), bumble bee respiration rate (Kammer and Heinrich, 1974), and bacterial gut symbiont performance all have higher temperatures of peak performance than does the parasite C. bombi (Palmer-Young, Raffel, et al., 2018), we predicted that infection would decrease across the temperature range previously recorded in wild bees (Heinrich, 1972). Based on metabolic theory and the concept of thermal mismatches (Cohen et al., 2019)—which predict that high temperatures improve performance of the host immune system and antiparasitic bacterial symbionts relative to performance of parasites—we also predicted that the temperature of peak infection in bees would be lower than the temperature of peak growth rate for parasite cell cultures. Finally, we predicted that higher temperatures would lead to lower absolute quantities of gut bacteria, due to elevation of per capita metabolic rates and consequent reduction of the gut ecosystem's carrying capacity at higher temperatures (Lemoine, 2019).
RESULTS
Crithidia bombi infection.
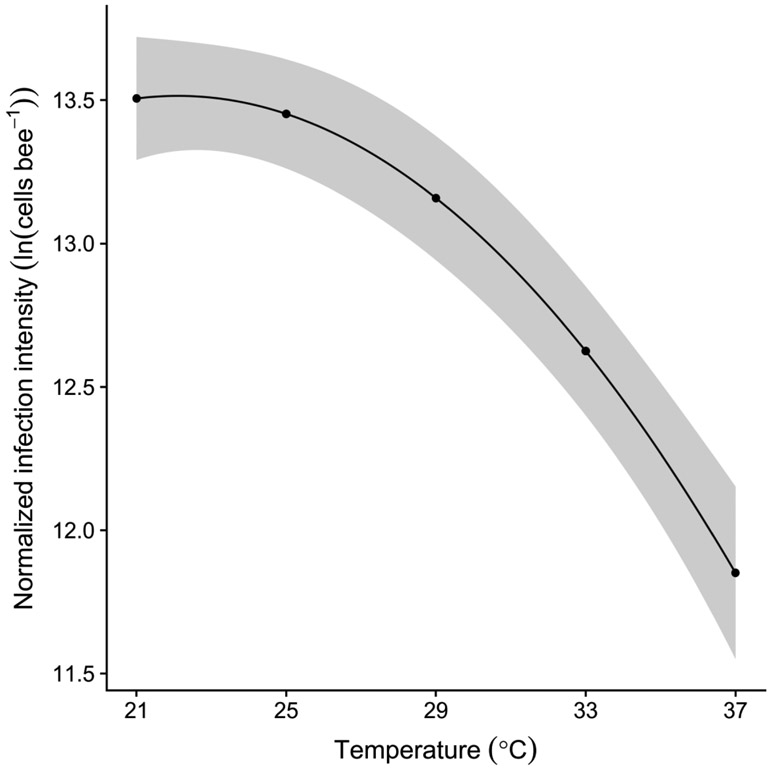
Infection intensity declined quadratically with temperature, as shown by the significance of models terms for both temperature and temperature2, i.e., the square of the mean-centered temperature (temperature: β = −0.10 ± 0.017 SE, χ21 = 36.02, P < 0.001; temperature2: β = −0.0075 ± 0.0034 SE, χ21 = 4.89, P = 0.027). After exponentiation from the log scale, intensity declined by 81% over the range of incubation temperatures, from 7.34 * 105 cells bee−1 at 21 °C to 1.40 * 105 cells bee−1 at 37 °C (Figure 1). Infection intensity varied significantly among bees from different colonies (χ24 = 47.84, P < 0.001) and was positively correlated with ln(bacterial abundance) (β = 0.22 ± 0.094 SE, χ21 = 5.31, P = 0.021).
Figure 1.
Crithidia bombi infection intensity decreased with increasing temperature. Line and shaded band show fitted means and standard errors from generalized linear mixed model of normalized infection intensity after inverse log transformation from the scale of the linear predictor. Predictions are averaged over infection treatments and colonies and calculated for a bee of average gut bacterial abundance. Shaded bands indicate uncertainty from the fixed effects portion of the model only. Points show the five tested incubation temperatures.
Bacterial community composition.
Processing of 16s rRNA gene amplicon sequences resulted in a data set of 7,044,293 total sequences comprising 128 Exact Sequence Variants (ESVs). Samples were rarefied to a depth of 10,233 reads per sample (see rarefaction curves in Supplementary Figure 1). Temperature had slight but significant, bacterial family-specific effects on gut communities. Permutational MANOVA of proportional composition (based on weighted UniFrac distances between samples) indicated that temperature explained more than twice as much variation than any other experimental factor (F4, 411 = 12.46, R2 = 0.10, P < 0.001). There were also significant but smaller effects of colony (F4, 411 = 4.32, R2= 0.036, P < 0.001) and infection treatment (F1, 411 = 3.59, R2= 0.007, P = 0.027), although the latter explained less than 1% of variation across samples. There was no significant interaction between infection and temperature (F4, 411 = 1.08, P = 0.38).
Total and family-wise bacterial abundance.
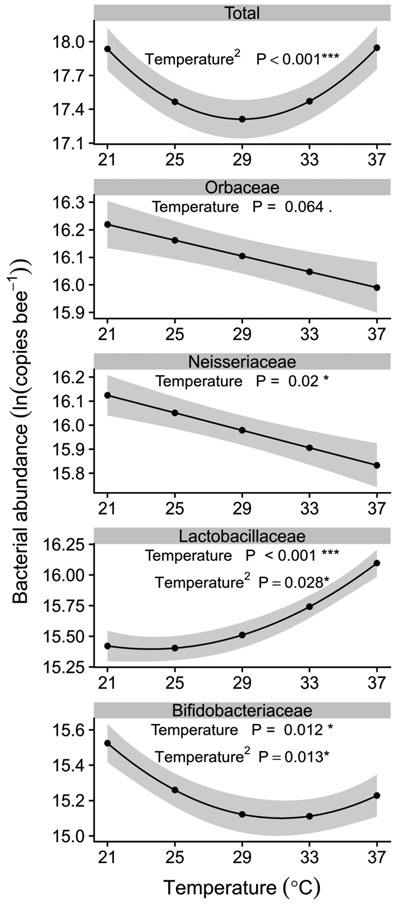
Total bacterial abundance (normalized 16s copy number) varied quadratically with temperature (temperature2: β = 0.0098 ± 0.0022 SE, χ21 = 20.49, P < 0.001, Figure 2). The lowest abundance occurred at intermediate temperatures (Figure 2). Averaged across colonies for a bee of average size, model-predicted abundance declined by 46% from 6.16 * 107 copies per bee at 21 °C to 3.30 * 107 copies per bee at 29 °C, then rose to 6.22 * 107 copies per bee at 37 °C (Figure 2). Bacterial abundance also differed significantly among bees from different colonies (χ24 = 15.37, P = 0.0040), and was negatively correlated with bee size (marginal cell length, β = −0.99 ± 0.18 SE, χ21 = 28.75, P < 0.001), but was not significantly affected by infection treatment (χ21 = 1.69, P = 0.19, Supplementary Table 2).
Figure 2. Differential effects of temperature on abundances of the four most abundant families of bumble bee gut bacteria.
Top panel depicts total bacteria abundance, as measured by qPCR of the 16s rRNA gene. Lower panels depict abundances of individual families, estimated for each sample by multiplying total abundance by the proportion of amplicon sequence reads associated with each family. Lines and shaded bands show fitted means and standard errors from negative binomial linear mixed model, averaged across colonies and plotted at the mean values for temperature and wing size. Annotations indicate significance of temperature and, where significant, temperature2 terms in models of abundance, as assessed by Wald χ2 tests. Effects of temperature and/or temperature2 were statistically significant for all families except Orbaceae (χ21 = 3.42, P = 0.064). Note that y-axis scales differ across panels. See Supplementary Table 2 for full model summaries.
Because we used the same primer set for qPCR and amplicon sequencing, we combined the total absolute abundance values from the 16s rRNA gene qPCR with the proportional abundance values from the amplicon sequencing to estimate absolute abundances of each bacterial family. The gut bacterial community was dominated bacteria from four families—Orbaceae, (32.7%), Neisseriaceae (31.6%), Lactobacillaceae (17.4%), and Bifidobacteriaceae (16.3%). Only one additional family, Enterobacteriaceae, accounted for >1% of reads (1.67%). Collectively, these five families accounted for 99.7% of detected bacteria.
Like total bacterial abundance, abundances of the main symbiont families were generally weakly but significantly affected by temperature, with changes of less than two-fold across the range of experimental temperatures. However, the effect of temperature was statistically significant for all except Orbaceae (Figure 2). Abundances of Orbaceae and Neisseriaceae, which were found in almost exactly equal copy numbers, both tended to decline with temperature (Orbaceae: β = −0.014 ± 0.0077 SE, χ21 = 3.43, P = 0.064; Neisseriaceae: β = −0.018 ± 0.0078 SE, χ21 = 5.41, P = 0.02). This effect was only significant for Neisseriaceae and of small magnitude, with fitted models predicting just a 26% drop in copy numbers from 21 to 37 °C (Figure 2).
Abundances of Lactobacillaceae and Bifidobacteriaceae varied quadratically with temperature (Figure 2). The strongest effects were seen for Lactobacillaceae, where model-fitted abundances roughly doubled (99% increase after exponentiation from the log scale) across the temperature range (temperature: β = 0.042 ± 0.0084 SE, χ21 = 25.15, P < 0.001; temperature2: β = 0.0039 ± 0.0018 SE, χ21 = 4.84, P = 0.028, Figure 2). Abundances of Bifidobacteriaceae most closely mirrored the patterns of total bacterial abundance, with an initial 34% decrease in abundance as temperatures approached 33 °C, followed by a 12% rise at the highest temperature (temperature: β = −0.019 ± 0.0074 SE, χ21 = 6.25, P = 0.012; temperature2: β = 0.0039 ± 0.0016 SE, χ21 = 6.19, P = 0.013, Figure 2). Together, the slight decreases in abundances of Orbaceae, Neisseriaceae, and Bifidobacteriaceae account for the initial fall in total bacterial abundance from 21 °C through 29 °C, whereas the relatively strong increase in abundance of Lactobacillaceae accounts for the rebound in total abundance above 29 °C.
In contrast to the significant effects of temperature, abundances of the four most abundant bacterial families were not significantly affected by infection treatment (Supplementary Table S2). Abundances of each family were negatively correlated with wing size, which was a significant predictor in every model, and varied across colonies for all families except Neisseriaceae (Supplementary Table S2).
Correlations between bacterial abundance and Crithidia bombi infection intensity.
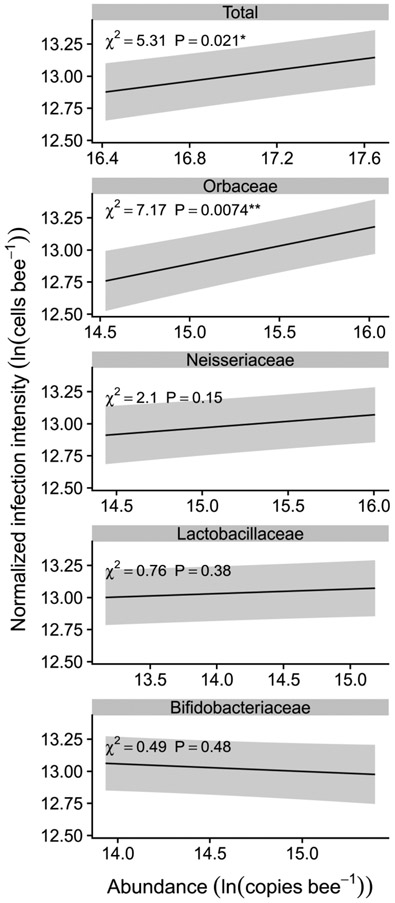
After accounting for effects of temperature and colony, infection intensity was positively correlated with total bacterial abundance (β = 0.22 ± 0.094 SE, χ21 = 5.31, P = 0.021, Figure 3; also noted in Results: Crithidia bombi infection) and with abundance of Orbaceae (β = 0.28 ± 0.11 SE, χ21 = 7.17, P = 0.0074, Figure 3), but not with abundance of Neisseriaceae, Lactobacillaceae, or Bifidobacteriaceae (Figure 3; see Supplementary Table 3 for full model summaries).
Figure 3. Total gut bacterial abundance and abundance of Orbaceae, but not other main families of gut bacteria, was positively correlated with C. bombi infection intensity.
Panels depict relationship between infection intensity and total gut bacteria (top panel) or family-wise abundance (lower panels). Lines and shaded bands show fitted means and standard errors from negative binomial linear mixed model, averaged across colonies and plotted at the mean value for temperature and, where significant, bee size. X-axes span the interquartile ranges of abundances of total bacteria and of each family. Shaded bands indicate uncertainty from the fixed effects portion of the model only. Annotations indicate significance of ln(abundance) term in negative binomial mixed model; degrees of freedom for χ2 statistic equals 1 for all panels. See Supplementary Table 3 for full model summaries.
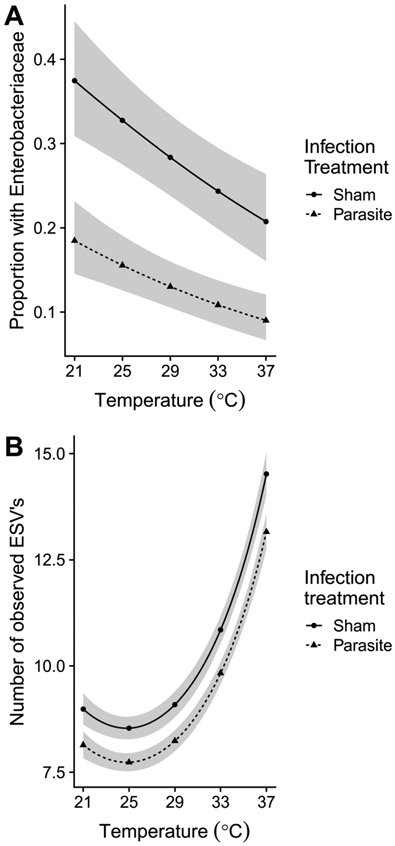
Occurrence of the next most abundant family, Enterobacteriaceae (1.7% of overall abundance), was also influenced by temperature. However, unlike abundance of the major symbiont families, Enterobacteriaceae was also affected by infection treatment. Prevalence declined by over 50% with increasing temperature (β = −0.052 ± 0.024 SE, χ21 = 4.74, P = 0.029), from an average of 27% at 21 °C to 13.1% at 37 °C (Figure 4). Presence of this family was overall less than half as likely for bees in the infection treatment (13.1 vs. 28.4% prevalence, Figure 4), an effect that was highly significant (β = 0.97 ± 0.27 SE, χ21 = 13.24, P < 0.001).
Figure 4. Prevalence of Enterobacteriaceae (A) and bacterial alpha diversity (number of Exact Sequence Variants (ESV’s) per sample, B) exhibited opposite responses to temperature, but both metrics were lower in parasite-inoculated bees.
Lines and shaded bands show fitted means and standard errors from binomial (A) or Poisson (B) linear mixed models, back-transformed to probabilities (from the logit scale) or counts (from the log scale). Circles and solid line: parasite infection treatment; triangles and dotted line: sham infection treatment. Predictions in (B) are averaged over colonies. Shaded bands indicate uncertainty from the fixed effects portion of the model only. Points show the five tested incubation temperatures.
Temperature had a quadratic effect on bacterial alpha diversity, measured as the number of observed ESV's per sample (Figure 4). Both temperature (β = 0.030 ± 0.0027 SE, χ21 = 124.0, P < 0.001) and temperature2 (β = 0.0036 ± 0.00058 SE, χ21 = 38.28, P < 0.001) terms were highly significant predictors of richness. Model-fitted ESV richness declined slightly between 21 and 25 °C, then increased by 70% at the highest temperature (Figure 4). ESV richness was slightly (9.3%) lower in parasite-inoculated bees (infection treatment: β = 0.098 ± 0.032 SE, χ21 = 9.61, P = 0.002, Figure 4). Richness also varied significantly among bees from different colonies (χ24 = 25.41, P < 0.001).
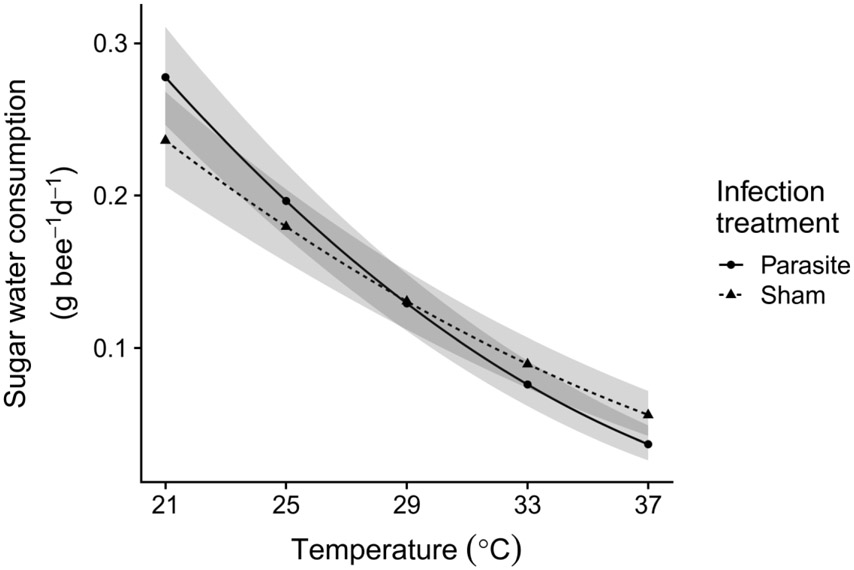
Sugar water consumption.
Rates of sugar water consumption declined strongly and significantly with temperature (β = −0.021 ± 0.0019 SE, χ21 = 171.52, P < 0.001, Figure 5). Covariate-adjusted model predictions, averaged across colonies and infection treatments, showed an 82% decrease in rate of sugar water consumption over the range of incubation temperatures, from 0.256 g bee−1 d−1 at 21 °C to 0.046 bee−1 d−1 at 37 °C (Figure 5). Although infected bees tended to have higher consumption rates at low temperatures but lower consumption rates at high temperatures, the effects of infection (χ21 = 0.05, P = 0.82) and the temperature x infection interaction were non-significant (χ21 = 3.60, P = 0.058), as was the overall effect of infection treatment (χ21 = 0.050, P = 0.82). Consumption was positively correlated with bee size (marginal cell length, β = 0.11 ± 0.025 SE, χ21 = 20.07, P < 0.001) and varied significantly among bees of different colonies (χ24 = 16.34, P = 0.0026)
Figure 5. Sugar water consumption by bumble bees declined with temperature, with no significant effect of infection treatment.
(χ2 (1) = 0.050, P = 0.82) or the infection x temperature interaction (χ2 (1) = 3.60, P = 0.058). Lines and shaded bands show fitted means and standard errors from general linear mixed model on square root-transformed consumption, back-transformed to the original scale of the measurement. Circles and solid line: parasite infection treatment; triangles and dotted line: sham infection treatment. Predictions are averaged over colonies and calculated for a bee of average size. Shaded bands indicate uncertainty from the fixed effects portion of the model only. Points show the five tested incubation temperatures.
Mortality.
The probability of premature death ranged from 7.5% (3 deaths) in sham-infected bees at 29 °C to 26% in sham-infected bees at 33 °C (10 deaths). Flowever, survival analysis showed no effect of temperature treatment (χ24 = 5.93, P = 0.20), infection treatment (χ21 = 0.90, P =0.34), or their interaction (χ24 = 1.61, P =0.81) on rates of mortality, although rates differed significantly among colonies (χ24 = 21.96, P < 0.001).
DISCUSSION
Increases in temperature reduced infection intensity.
In agreement with predictions from in vitro experiments (Palmer-Young, Raffel, et al., 2018), we found reductions in trypanosomatid infection intensity as temperature increased over the typical range of bee body temperatures (Heinrich, 1972). This decrease in infection is consistent with previous observations on the temperature dependence of parasites, bees, and bacterial symbionts. Parasites in cell culture had optimal growth temperatures of 27-32 °C (Palmer-Young, Raffel, et al., 2018). Hence, reductions in infection above 32 °C could be due to direct inhibition of growth, but reductions in infection between 21 °C and 32 °C likely reflect relative, rather than absolute, disadvantage of parasites in comparison to the bee immune system and gut bacterial symbionts. Bee muscle and whole-body metabolism have temperatures of peak performances >37 °C (Kammer and Heinrich, 1974; Gilmour and Ellington, 1993; Harrison and Fewell, 2002). If the immune system has a similar temperature of peak performance, then metabolic theory would predict improvement in host immune function across the range of temperatures in our experiments. All of the key gut bacterial symbionts also have peak performance temperatures of 35-37 °C, as judged by the standard conditions used for in vitro cultivation (Engel et al., 2013). In particular, L bombicola growth and production of parasite-inhibiting acids increased exponentially across the 21-37 °C temperature range (Palmer-Young, Raffel, et al., 2018). Thus, the reduction in infection with increasing temperature may also reflect increases in metabolic rates of acid-producing, parasite-inhibiting bacteria. Experiments that compare the temperature dependence of infection between bees raised under normal vs. germ- free conditions could help to clarify the importance of the microbiota in this enhanced immunity. If the microbiota are indeed a key determinant of the temperature dependence of infection, then the relationship between temperature and infection should be different—and more closely resemble the thermal performance curve of isolated parasites—in germ-free bees. Alternatively, if the pattern primarily reflects the mismatch between host immune function and parasite performance, similar temperature dependence of immunity would be expected in symbiont-colonized and germ-free bees.
Temperature had smaller but significant effects on bacterial symbiont communities.
In comparison to C. bombi, the size and general composition of the gut bacterial community was relatively robust to changes in temperature, as well as to infection treatment, but some changes were nevertheless statistically significant. In contrast to C. bombi infection levels, which declined with temperature, total bacterial abundance was lowest at intermediate temperature (29 °C). This represents the center of the range of body temperatures recorded in workers (Heinrich, 1972), is close to the set point at which bumble bees incubate developing brood (30 °C (Vogt, 1986)), and matches the 28-30 °C temperature recommended for bumble bee rearing (Velthuis and van Doorn, 2006). We hypothesize that the bumble bee immune system has optimal control over the bacterial community in this temperature range, and is able to curtail overgrowth that might otherwise deplete host resources or facilitate establishment of opportunistic infections. Research with honey bees and heat shock proteins (McKinstry et al., 2017) has suggested a tradeoff between temperature tolerance and immune function. Direct measurements of bee immune function could clarify the effects of temperature on hosts, while manipulation of the immune response could clarify the importance of the host immune system relative to interbacterial community dynamics in the observed patterns of gut bacterial abundance. Another approach to clarify the relative importance of host- and microbiota-mediated immunity could be to compare the effects of temperature elevation on injected or systemic vs. enteric pathogens; the latter would be expected to be more directly affected by gut microbes, although gut symbiont-mediated alteration of systemic immunity shows that microbiota may mediate resistance to systemic as well as gut infection (Dillon and Dillon, 2004; Kwong et al., 2017).
Although temperature explained more variation in bacterial composition than did any other experimental factor, average abundance of total bacteria and of specific core symbiont families did not vary by more than two-fold across the experimental temperature range. Abundances of Neisseriaceae and Orbaceae were exceptionally stable, with abundances not varying by more than 30% (Figure 2). Populations of Lactobacillaceae were the most responsive to temperature, with mean abundances increasing two-fold between the lowest and highest temperatures. Experiments in vitro with the widespread symbiont L bombicola—the most abundant member of this family among our samples—indicate that growth rate of this species increases throughout the experimental temperature range (Palmer-Young, Raffel, et al., 2018). Whether the Lactobacillaceae are more thermophilic than other major symbionts, or their maximum population size is simply less constrained by host surface area than those of the biofilm-forming Neisseriaceae and Orbaceae (Martinson et al., 2012), remains to be determined. Regardless of the exact mechanism, the Lactobacillaceae appear to have greater abundance in the gut as temperature increases.
Bacterial abundance was positively correlated with infection.
Although the reduction in C. bombi infection at high temperature corresponded with higher populations of Lactobacillaceae (Figure 2), which produced parasite-inhibiting organic acids in vitro (Palmer-Young, Raffel, et al., 2018), our findings do not indicate that an abundance of acid-producing bacteria precludes establishment of parasites. In models controlling for temperature, there was no significant correlation between Lactobacillaceae abundance and C. bombi infection (Figure 3), whereas the effect of temperature itself remained significant (Supplementary Table S3). Moreover, abundances of total bacteria, and specifically of Orbaceae—the most abundant family among our samples (32.7% of total 16s rRNA gene copy numbers)—were positively correlated with infection intensity in our study (Figure 3). We hypothesize that proliferation of both bacteria and parasites may be driven be a common factor, possibly related to suboptimal host immune function. For example, in honey bees, the same Snodgrassella alvi pretreatment that resulted in high gut bacterial abundance and proliferation of Gilliamella apicola (Orbaceae) also led to higher levels of infection with the trypanosomatid L. passim (Schwarz et al., 2016). Although previous studies with bumble bees have implicated Gilliamella-rich microbiota in resistance to C. bombi infection (Koch and Schmid-Hempel, 2012; Mockler et al., 2018), the Orbaceae clade is phenotypically diverse, varying in traits such as carbohydrate metabolism and resistance to antimicrobial peptides despite conserved 16s rRNAgene sequences (Engel et al., 2014; Kwong et al., , and may harbor strains associated with both health and disease. For example, a cross-colony survey correlated high levels of Gilliamella ("Gamma-1") with honey bee colony collapse disorder (Cox- Foster et al., 2007), intensity of infection with the honey bee-infective microsporidian Nosema ceranae (Rubanov et al., 2019), and general 'dysbiosis' associated with low adult bee mass, higher mortality, the scab-forming bacterium Frischella perrara, and Nosema infection (Maes et al., 2016).
We hypothesize that rather than being a simple consequence of changes in bacterial abundance, temperature-driven resistance to infection may reflect changes in bacterial metabolic rates. With the exception of Neisseriaceae, all the major bee gut symbionts ferment carbohydrates to short- chain fatty acids (Kešnerová et al., 2017), lowering the pH of the gut to levels predicted to inhibit growth of trypanosomatids (Zheng et al., 2017; Palmer-Young et al., 2019). We expect that rates of acid production—a product of bacterial metabolism—increase exponentially with temperature below the temperature of peak growth rate, which appears to be close to 37 °C for most symbionts (Engel et al., 2013; Kwong and Moran, 2013; Palmer-Young, Raffel, et al., 2018). This temperature-driven increase in acid production would be expected to lower gut pH and inhibit parasite growth, a hypothesis that could be tested by measurement of gut chemistry at different temperatures (Zheng et al., 2017).
High temperature and trypanosomatid exposure conferred protection against potentially pathogenic bacteria.
Although the focus of this study was to determine how temperature affects resistance to C. bombi, we also found that high temperatures conferred resistance to colonization with presumably pathogenic members of the family Enterobacteriaceae. This family includes opportunistic pathogens of bees, such as Serratia marcescens (Raymann et al., 2018), as well as clinically and agriculturally important pathogens such Salmonella, Klebsiella, Yersinia, E. coli, and Erwinia spp. Previous studies with indoor-reared bumble bees showed that when colonies were moved to outdoor environments, core symbionts were displaced by members of Enterobacteriaceae of presumed environmental origin (Parmentier et al., 2016). Another study suggested the presence of two 'enterotypes' among wild bumble bees, one characterized by dominance of core symbionts and the other by Serratia and other Enterobacteriaceae (Li et al., 2015), again suggesting that presence of these taxa is suboptimal for bee health. Proliferation of enteric Enterobacteriaceae has also been linked to inflammatory bowel diseases in humans (Nagalingam and Lynch, 2012). Endothermic maintenance of high body temperatures has been proposed as a factor that limits the establishment of most environmental bacteria in mammals, both by direct inhibition of parasite growth and by augmentation of the host immune response (Casadevall, 2016). We propose that high nest and body temperatures, like those found among social bees, also provide a comparative advantage to thermophilic core gut symbionts, reinforcing mutualistic relationships with bacteria while limiting establishment of potential pathogens.
Whereas abundances of core symbiont families were unaffected by infection treatment, we found that inoculation with C. bombi resulted in lower prevalence of Enterobacteriaceae and lower gut bacterial alpha diversity. Both results suggest that the infection treatment provoked an immune response that enhanced resistance to colonization by non-core symbionts. Previous research in bumble bees has documented effects of the microbiota on trypanosomatids (Koch and Schmid-Hempel, 2011; Mockler et al., 2018), but not of exposure to C. bombi on either beneficial or pathogenic bacteria. However, C. bombi inoculation can cause an immune response that resembles the reaction to injection with heat-killed bacteria. This response includes upregulation of genes encoding antimicrobial peptides, other antibacterial effector proteins, and reactive oxygen species (Barribeau and Schmid-Hempel, 2013; Brunner et al., 2013), all of which likely enhance immunity to bacteria. In mosquitoes, exposure to Plasmodium (malarial) parasites likewise upregulated antibacterial immune pathways, including transcription of genes that appeared to be more effective against bacteria than against protozoans (Dong et al., 2006). Importantly for our study, where one-third of bees cleared the experimental infection, induction of antibacterial defenses did not require successful replication of parasites. Hence, mere transient exposure to trypanosomatids could act like a vaccination that elevates immunity to Enterobacteriaceae and other non-essential gut bacteria. However, detection of these effects at the landscape scale may be difficult, as different parasite strains can vary substantially in the extent to which they elicit immune responses, and responses to the same strain can vary across colonies (Barribeau and Schmid-Hempel, 2013; Barribeau et al., 2014).
Resource availability and pesticide exposure could alter thermoregulation in wild bees.
Given the potential benefits of high temperature for resistance to parasites, what environmental factors might constrain the thermoregulatory strategies that could optimize immunity? Like the immune response itself, endothermic elevation of nest or body temperature incurs a substantial metabolic cost, particularly at low ambient temperatures and in colonies of small size. Both conditions are present when queen bumble bees establish new colonies each spring. However, this is also the stage of the life cycle at which C. bombi can be most virulent (Brown et al., 2003; Fauser et al., 2017). The availability of adequate early-season floral resources may be a key determinant of the ability of queens to maintain body temperatures necessary for foraging, brood incubation, and parasite inhibition. However, in some regions, changing climates have resulted in higher frequency of spring frosts that damage early- blooming flowers, compromising key sources of nectar and pollen (Inouye, 2008). Honey bees, bumble bees, and other animals rapidly become torpid when sugar reserves are depleted (Esch, 1960; Heinrich, 1972; Angilletta et al., 2010). In bats, torpor exacerbates susceptibility to fungal infections, but infection is generally cleared when animals are fed sufficiently to allow maintenance of high body temperatures (Meteyer et al., 2011). The consequences of periods of low body temperature for the gut microbiota of facultative endotherms remain unknown, but could affect resistance to infection.
Another potential disruptor of thermoregulation is exposure to pesticides. Consumption of sublethal doses of neonicotinoids impaired thermogenesis in bumble bee workers and colonies, reducing individual metabolic rates by 15–25% and body temperatures by up to 3 °C (Crall et al., 2018; Potts et al., 2018). Both neonicotinoids and pyrethroids can lead to hypothermia in honey bees as well (Belzunces et al., 2012; Tosi et al., 2016; Meikle et al., 2018).Therefore, aside from directly affecting immune function (e.g., (Chaimanee et al., 2016)), pesticides may also exacerbate susceptibility to individual- and colony-level infection by impairment of thermoregulation. Intriguingly, low neonicotinoid concentrations stimulated heat production in honey bees (Tosi et al., 2016; Meikle et al., 2018); a similar hormetic effect may have contributed to nicotine’s enhancement of resistance to C. bombi in bumble bees (Baracchi et al., 2015; Richardson et al., 2015; Thorburn et al., 2015). As in insects, nicotine ingestion can produce hypothermia in mammals (Ruskin et al., 2007). It would be intriguing to investigate the consequences of nicotine-related alterations in body temperature for microbiota composition and resistance to infection in mammalian hosts.
Conclusions.
The effects of high body temperatures (fever) on resistance to infection have previously been considered to reflect changes in parasite performance relative to host immune function (Casadevall, 2016; Cohen et al., 2017). However, non-pathogenic gut microbiota can augment resistance to infection in many ecto- and endothermic organisms (Dillon and Dillon, 2004; Spor et al., 2011). As a result, the effects of temperature on infection might not be fully understood without considering how temperature affects the structure and function of the gut symbiont community. Our findings show that in bumble bees, high temperature can ameliorate infection with parasites without apparent harm to the major symbiont families. On the other hand, temperature elevation did harm key symbionts of other insects were harmed by (Parkinson et al., 2014; Kikuchi et al., 2016), suggesting that temperature elevation may be costly for microbiome-mediated benefits in some systems. In comparison to social bumble and honey bees, where the colony provides both gut microbial inoculum and thermoregulation, solitary insects experience a wide range of temperatures and gut microbiota (Corby-Harris et al., 2007; McFrederick et al., 2012; Moran et al., 2012). As a result, the effects of environmental temperature on microbiota may be considerably stronger in these purely ectothermic hosts, such as Plasmodiumvectoring mosquitoes, where microbiota also mediate resistance to infection (Cirimotich et al., 2011). As a topic with relevance to both endothermic animals that use fever as an immune strategy and ectothermic taxa that face novel infections in changing climates, interactions between temperature, infection, and gut symbionts warrant further investigation, and could be a key factor in diseases of clinical and conservation concern.
EXPERIMENTAL PROCEDURES
Experimental design
We tested the effects of experimental inoculation with parasites and rearing temperature on Crithidla bombi infection intensity, gut bacteria, sugar water consumption, and mortality in the common Eastern bumble bee, Bombus impatiens. Bumble bee workers were inoculated with 104 C. bombi parasite cells or a sham inoculum without parasite cells. Each inoculated bee was then reared individually for 7 d at one of five temperatures (21, 25, 29, 33, or 37 °C), chosen to capture the range of body temperatures previously recorded in bumble bees (Heinrich, 1972), and to include temperatures above and below the temperature of peak parasite growth rate (Palmer-Young, Raffel, et al., 2018). Seven days after inoculation, bees were frozen for dissection and analysis of microbiota and infection intensity. The experiment was conducted in 4 temporal blocks of inoculations, with 3 colonies used per block, and incubator temperatures reassigned after the first two blocks to avoid confounding the effects of incubator with those of temperature.
Bumble bees
Five colonies of B. Impatiens were obtained from a commercial rearing facility (Koppert Biological Supply, Howell, Michigan, USA). Colonies were reared at 27 °C in constant darkness, with red light illumination during periods of handling. Colonies were fed weekly with 50% (w/w) sugar water and every 2-3 d with pollen (Brushy Mountain Biological Supply, Moravian Falls, North Carolina, USA).
To facilitate collection of age-controlled bees, existing workers in each colony were marked on the thorax with white correction fluid (Wite-Out, BIC, Clichy France) 3 d prior to the beginning of the experiment. Thereafter, newly emerged workers (identified by their absence of thoracic marking) were collected twice weekly for experimental inoculations. Adult worker bees were isolated from the colony on the day prior to inoculation. Hence, they ranged in age from 1 to 5 d post-pupal eclosion at the time of inoculation. This protocol allowed us to pick bees that were old enough to have had time to acquire normal microbiota from the colony (Billiet et al., 2017), but were young enough to still have microbiota in the formative period (4-6 post-eclosion (Meeus et al., 2013; Powell et al., 2014)).
Parasites
Three strains of C. bombi were obtained from infected wild B. impatiens and B. terrestris by single cell sorting: Strains ‘12.6’ (from B. Impatiens in Lufkin, TX in 2014 by Hauke Koch, ‘IL13.2’ (from B. impatiens in Normal, IL in 2013 by Ben Sadd), and ‘C1.l’ (from B. terrestris in Corsica in 2012 by Ben Sadd) (Palmer-Young et al., 2016). Parasites were grown at 27 °C in vented culture flasks with modified Mattei growth medium as previously described (Salathe et al., 2012).
Inoculation, rearing, and consumption measures
Experimental bees were removed from their colonies on the day prior to inoculation and housed overnight with access to 1:1 sugar water in 500 mL plastic cups containing 20-30 newly emerged workers each. Prior to inoculation, bees were transferred to individual 30 mL plastic vials and deprived of food for ~5 h.
Infection success of individual parasite strains can vary widely across colonies (Sadd and Barribeau, 2013). To improve the chances of successful infection in bees from a variety of colonies, the inoculum consisted of a 'cocktail' that included equal cell number of each of the three parasite strains (total 104 cells in 10 μL (Näpflin and Schmid-Hempel, 2018)). The inoculum consisted of a 1:1 mixture of 16 mM sucralose (as 1 g Splenda (Heartland Food Products, UK) in 8 mL water + 16 μL red #40 food dye) and parasite cells in growth medium. Sucralose (rather than sugar water) was used to reduce osmotic shock to parasites during inoculation, and thereby improve probability of successful infection. High concentrations of sugar are lethal to C. bombi (Cisarovsky and Schmid-Hempel, 2014), and we observed that cells rapidly became deformed and immotile in sugar water. To control for effects of the inoculation procedure, bees in the sham infection treatment were inoculated with the same 1:1 mixture of 16 mM sucralose with dye and growth medium, but without C. bombi cells.
In all, 525 bees were used (N = 90-129 per colony, N = 63-67 per temperature in the C. bombi infection treatment and 39-43 per temperature in the sham infection treatment, Supplementary Table 1). Final sample sizes are unequal for the parasite and sham-infection treatments because of uncertainty in rates of compliance during the inoculation. We conservatively expected only ~50% compliance during the inoculation among the bees fed the parasite inoculum. Consequently, we attempted to inoculate 2 bees with parasites for every 1 bee in the sham-infection treatment. Because rates of compliance generally exceeded 50%, final sample sizes are higher in the parasite-infection treatment.
After inoculation, bees were transferred to individual, inverted 60 mL translucent polystyrene deli cups, lined with a disk of filter paper to absorb excess moisture. Each bee was provided with a ~50 mg ball of pollen paste, and provisioned ad libitum with 50% sugar water from a 1.7 mL microcentrifuge tube. Sugar water tubes were checked daily and replaced with fresh sugar water as needed. Mortality was recorded daily at time of feeding (ca. 1200 local time). Bees that escaped during the experiment were scored as not having died; they were given an end time corresponding to the first date at which they were observed missing (N = 6).
Sugar water consumption was recorded during at least two 24 h intervals, normally 3-4 d and 4-5 d post-inoculation, for one bee per unique combination of infection treatment, temperature treatment, colony, and inoculation block. Consumption was calculated as the change in mass of the feeder tube from the beginning to the end of the trial. Net consumption, corrected for mass loss due to evaporation and handling, was determined by subtracting the mass loss of tubes in identical rearing setups, but without bees, at the corresponding temperature. At 7 d post-inoculation, bees were frozen in 2 mL microcentrifuge tubes on dry ice, then stored at −80 °C until dissection.
Dissection and DNA extraction
Bees were dissected to remove the mid- and hindgut using standard methods described in the BeeBook (Engel et al., 2013). The body was thawed on ice and surface-sterilized by rinsing for 3 min in 1% household bleach (0.05% sodium hypochlorite (NaOCl)) and 3 x 1 min in doubly deionized water. The gut was removed by pulling on the distal segment of the abdomen with sterile forceps. The mid- and hindgut of the alimentary tract were drawn out onto a UV-sterilized piece of aluminum foil, then transferred to a 96-well plate for DNA extraction. Length of the marginal cell on the right forewing (in mm) was measured as an index of bee size (Wilfert et al., 2007).
DNA was extracted using the Qiagen DNEasy blood and tissue kit (Qiagen, Hilden, Germany). Samples were treated with 180 μL lysis buffer (Qiagen buffer "ATL") and 20 μL proteinase K solution, then homogenized for 6 min at 30 Hz in a TissueLyser (Qiagen) with a 3.2 mm diameter steel ball and 50 μL of 0.1 mm glass beads. Homogenized samples were incubated overnight at 56 °C in a convection oven. Subsequent DNA extraction was performed according to the manufacturer's instructions. Extracted DNA was stored at −80 °C until use in PCR-based assays.
Quantification of infection intensity and bacterial abundance by qPCR
Bees in both infection treatments (parasite and sham control) were first screened for presence or absence of C. bombi by PCR using the primers CB-SSUrRNA-F2 (CTTTTGACGAACAACTGCCCTATC) and CB-SSUrRNA-B4 (AACCGAACGCACTAAACCCC) (Schmid-Hempel and Tognazzo, 2010). The product was visualized on a 1.5% agarose gel. This initial screen confirmed the absence of infection among bees in the sham infection treatment.
Infection intensity.
For bees in the parasite infection treatment, infection intensity was quantified by qPCR of C. bombi DNA; C. bombi quantities were then normalized to quantities of bumble bee actin in the corresponding sample to correct for extraction efficiency (Palmer-Young, Calhoun, et al., 2018). Quantification of C. bombi was made for each sample in triplicate with primers for the C. bombi 18s rRNA gene (‘CriRTF2’ (GGCCACCCACGGGAATAT) and ‘CriRTR2’ (CAAAGCTTTCGCGTGAAGAAA) (Ulrich et al., 2011). The assay used 20 μL reaction volume consisting of 2 μL DNA extract and 300 nM of each primer in 1x Power SYBR Green Mastermix (Applied Biosystems, Foster City, CA). Thermocycle conditions included 10 min initial denaturation at 95 °C followed by 40 cycles of denaturation (15 s at 95 °C) and annealing-extension (60 s at 60 °C). Absolute quantifications (number of parasite cell equivalents) were made relative to a standard curve consisting of 8 dilutions of C. bombi DNA (equivalent of 3.9 * 103 to 2.5 * 105 cells) extracted from cell cultures of known concentration. The standards were run in triplicate on each assay plate. Analysis of standard curves showed typical amplification efficiency of 90-100% with R2 > 0.98.
Bacterial abundance.
Quantification of gut bacteria was made using universal primers for the bacterial 16s rRNA gene (forward: 799F-mod3 (CMGGATTAGATACCCKGG) (Hanshew et al., 2013), reverse: 1115R (AGGGTTGCGCTCGTTG) (Kembel et al., 2014)), chosen to minimize amplification of DNA from plastids in pollen (McFrederick and Rehan, 2016; Rothman et al., 2018). The assay used 15 μL reaction volume with 1.5 μL of 10x diluted DNA extract. (An initial round of qPCR with undiluted extracts resulted in failure of amplification in >60% of samples.) Thermocycle conditions consisted of 3 min denaturation at 95 °C, followed by 40 amplification cycles of 10 s denaturation at 95 °C and 30 s simultaneous annealing and extension at 59 °C.
Absolute quantifications of bacteria (16s copies bee−1) were made relative to an 8-concentration standard curve (102 to 105 copies μL−1), run in triplicate on each plate. Analysis of standard curves showed typical amplification efficiency of 90-100% with R2 > 0.99. The standards were generated by cloning an amplicon of the 799-1115 V5-V6 region of the 16s rRNA gene from a stock culture of Lactobacillus micheneri (courtesy Floang Vuong, (McFrederick et al., 2018)). The amplicon was cloned into E. coli using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA); the reaction was confirmed by sequencing the insert of plasmids that were purified from transformed cultures (Purelink Plasmid Purification Kit, Invitrogen). The plasmid was linearized with the Pst1 restriction enzyme (New England Biolabs, Ipswich, MA). Concentration of 16s gene copies in the stock solution of linearized plasmid were estimated by fluorescence-based quantification of DNA concentration using a Qubit (Invitrogen).
Normalization.
For normalization, the amount of host DNA was determined by a separate qPCR assay, also run in triplicate, for the B. impatiens actin 5C gene (primers Forward: CAAACGCTCGCTCAAACG, Reverse: GTGTACGTGAATGGTCTTGCAC (Palmer-Young, Calhoun, et al., 2018)). The assay used 20 μL reaction volume consisting of 2 μL DNA extract and 300 nM of each primer in 1x Power SYBR Green Mastermix. Thermocycle conditions consisted of 10 min denaturation at 95 °C, followed by 40 amplification cycles of 15 s denaturation at 95 °C and 31 s simultaneous annealing and extension at 60 °C. Specificity was confirmed by melt-curve analysis. Quantifications were made in units of proportion of host DNA relative to a pooled DNA extract from 10 randomly selected experimental bees (Palmer-Young, Calhoun, et al., 2018). A standard curve, consisting of 8 dilutions of the pooled DNA extract, was run in triplicate on each plate.
Characterization of gut bacterial communities
Library preparation.
Gut bacterial community composition was determined by amplicon sequencing of the V5-V6 region of the 16s rRN A gene (McFrederick and Rehan, 2016; Rothman et al., 2018) on an Illumina (San Diego, CA) MiSeq using standard methods (Engel et al., 2013). Libraries were prepared using the same bacterial 16s rRNA primers (799F-mod3 and 1115R) used for bacterial qPCR. Use of the same primers for qPCR and amplicon sequencing enabled us to estimate absolute, as well as relative, abundances of individual taxa from sequence data. Libraries were prepared using two rounds of PCR as previously described (McFrederick and Rehan, 2016; Rothman et al., 2018). The first round of PCR amplified the target region and barcoded each sample's amplicons with unique 8-nucleotide sequences appended to the forward and reverse primers; the second round added the forward or reverse Illumina sequencing primer.
The first round of PCR (20 μL reaction volume) used 2 μL of 10x diluted DNA extract, 1 μL each of 10 μM barcoded forward (799F-mod3) and reverse (1115R) primers (final concentration: 500 nM), 10 μL of 2x Pfusion High-Fidelity DNA polymerase master mix (New England Biolabs, Ipswich, MA), and 6 μL ultrapure water. PCR was performed in a C1000 Touch thermal cycler (BioRad, Hercules, CA) (3 min denaturation at 94 °C; 24 cycles of 45 s at 94 °C, 60 s at 52 °C, 90 s at 72 °C; 10 min at 72 °C). To remove residual primers and dNTPs, each sample’s product was treated with 10 μL of a solution containing 0.05 units μL−1 exonuclease I (New England Biolabs) and 0.025 units μL−1 alkaline phosphatase (Sigma-Aldrich, St. Louis, MO). Samples were incubated for 30 min at 37 °C, followed by 5 min at 95 °C to inactivate the enzymes. The second round of PCR used 1 μL of the treated PCR product as template, 1 μL each of 10 uM forward and reverse primers (PCR2F: CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGC) and PCR2R: AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACG) to generate the forward and reverse Illumina adapter sequences; 10 μL of 2x Phusion MasterMix, and 13 μL ultrapure water. Thermocycle conditions consisted of 3 min at 95 °C; 14 cycles of 45 s at 95 °C, 60 s at 58 °C, 90 s at 72 °C; and 10' at 72 °C.
To equalize concentrations of DNA from each sample in the final pooled amplicon library, 18 μL of the product from the second round of qPCR was bound to, then eluted from a SequalPrep (Thermo Fisher, Waltham, MA) normalization plate according to the manufacturer's instructions. The normalized products were pooled (5 μL sample−1), then purified with the Purelink PCR product purification kit (Invitrogen). Amplicon size and abundance were checked on a 2100 Bioanalyzer (Agilent, Santa Clara, CA). Libraries were sequenced at the UC Riverside Genomics Core Facility on an Illumina MiSeq Sequencer (Illumina, San Diego, CA) using a MiSeq V3 Reagent Kit. The sequencing run consisted of 2 x 300 PCR cycles. Raw sequence data are available on the NCBI Sequence Read Archive (SRA) under Accession number PRJNA532469.
Bioinformatics.
Sequences of 16s rRNA amplicons were processed in macQIIME and QIIME2 (Caporaso et al., 2010; Bolyen et al., 2018). Reads were trimmed to removed low-quality regions, then binned to exact sequence variants (‘ESV’s’, .i.e., bacteria with identical 16s amplicon sequences) with DADA2 (Callahan et al., 2016). Taxonomic classification of each ESV was inferred using the SILVA database (Quast et al., 2013). Proportional composition and ESV richness were estimated for each sample after removal of ESV’s found in only one sample or in blanks (i.e., reagent controls) and rarefaction to a read depth of 10,233 reads. A phylogeny of the observed ESV’s was built using maximum likelihood in FastTree2 (Price et al., 2010) and used to estimate unifrac distances between samples (Lozupone and Knight, 2005).
Statistical analyses
Statistical analyses were conducted in R v3.5 for Windows (R Core Team, 2014). Models were fitted with packages Ime4 (for general linear models) (Bates et al., 2015) and glmmTMB (for negative binomial models) (Magnusson et al., 2017). Significance of individual predictor terms was tested with Wald χ2 tests, implemented with the Anova function in package car (Fox and Weisberg, 2011). Predictions from models of each response variable were estimated with package emmeans (Lenth, 2019). Plots were created with packages ggplot2 and cowplot (Wickham, 2009; Wilke, 2016). Temperature was centered at the mean value (29 °C) to permit estimation of a quadratic term for the effect of temperature (i.e., temperature2).
Infection intensity.
For bees in the parasite-infection treatment, effects of temperature on infection intensity were tested with a negative binomial family linear mixed-effects model in R package glmmTMB (Magnusson et al., 2017). The negative binomial model is suited to overdispersed, non-negative count data (Bliss and Fisher, 1953), while the zero inflation term allows us to simultaneously account for two separate processes that might generate zeroes— in this case, whether or not the infection established (a binomial process), and the resulting infection intensity in bees when the infection did establish (Martin et al., 2005).
Infection intensity was normalized to amount of host actin to control for gut size and DNA extraction efficiency. Normalized infection intensity was computed for each sample as the qPCR estimate of number of C. bombi parasite cells per gut divided by the same sample's quantity of bumble bee actin. Actin quantities were expressed as the proportion of actin found in a reference extraction. The reference extraction consisted of a pooled DNA extract from 10 randomly selected experimental bees (see Methods: Quantification of infection intensity and (Palmer-Young, Calhoun, et al., 2018). Samples with less than 10% of the actin found in the reference extraction were excluded a priori. The model used normalized infection intensity as the response variable; temperature (centered at the mean temperature, 29 °C), temperature2, and bee colony as fixed predictor variables. We chose to use bee colony as a fixed effect because we had fewer than 7 levels of this factor (Bolker et al., 2009). Models also used ln(normalized bacterial abundance) as a covariate that was previously correlated with C. bombi infection (Mockler et al., 2018) and inoculation block as a random effect. Size of the forewing marginal cell (an index of bee size (Wilfert et al., 2007)) was initially included a covariate, but excluded from the final model because it did not explain significant variation in infection (χ21 = 2.57, P = 0.11).
Microbiome composition.
Predictors of microbiome community structure were assessed with permutational MANOVA (Oksanen et al., 2017). The weighted UniFrac distance matrix of between-sample dissimilarity was used as the multivariate response variable. Infection treatment, temperature treatment (coded as a factor in this analysis only), their interaction, and bee colony were tested as predictor variables. Significance of individual terms was assessed with F tests. Proportion of variation explained by each term (R2) was determined as the ratio of the sum of squared variation associated with the predictor relative to that of that of the full model.
Total bacterial abundance.
Effects of temperature and infection treatments on gut bacterial abundance were tested with a negative binomial family linear mixed-effects model in R package glmmTMB (Magnusson et al., 2017). Normalized 16s copy number (raw copy number divided by proportion of bumble bee actin in a reference extraction, as in the analysis of infection intensity) was the response variable. As in the model for infection, samples with less than 10% of the actin found in the reference extraction were excluded a priori; samples with no measurable bacteria in the qPCR assay were also excluded. Infection treatment, temperature (centered at the mean temperature treatment, 29 °C) and temperature2, and bee colony were used as fixed predictor variables; size of the forewing marginal cell was used as a covariate, and inoculation block was included as a random effect. The infection x temperature interaction was included in the initial model, but excluded from the final model because it did not explain significant variation in bacterial abundance (χ21 = 0.36, P = 0.55).
Family-wise bacterial abundance.
Responses of family-wise abundances to temperature and infection treatments was assessed for each of the four bacterial families (Orbaceae, Neisseriaceae, Lactobacillaceae, and Bifidobacteriaceae, all found in >98% of samples) that accounted for >10% of the total number of reads in the 16s amplicon sequencing. To calculate absolute abundances of each family in each sample, we multiplied total normalized abundance by the proportion of reads corresponding to each family. Changes in abundances were assessed with negative binomial family generalized linear mixed models of the same structure used to evaluate temperature-dependent changes in total bacterial abundance. Infection treatment; temperature and, where significant, temperature2; and bee colony were used as fixed predictor variables. Size of the forewing marginal cell was used as a covariate, and inoculation block was included as a random effect. The infection x temperature interaction term was not a significant predictor in any of the models (P > 0.20 for all) and was excluded from the final analyses.
The fifth most abundant family, Enterobacteriaceae, was present in only 20% of samples. Its abundance was analyzed with a standard binomial model, rather than the negative binomial used for the four more abundant families. Terms for the infection x temperature interaction (χ21 = 0.57, P = 0.45), temperature2 (χ21 = 2.11, P = 0.15),, bee colony (χ2(4) = 5.93, P = 0.20), and wing size (χ21 = 0.45, P = 0.50) were excluded from the final model because they did not explain significant variation in prevalence.
Correlations between family-wise abundance and C. bombi infection intensity.
Correlations between abundances of each of the four most abundant bacterial families and C. bombi infection were assessed with negative binomial family generalized linear mixed models of the same structure used to evaluate temperature-dependent changes in infection intensity, except that ln(abundance +1) of the individual family was substituted for ln(total bacterial abundance) covariate. Temperature, temperature2, and bee colony were used as fixed predictor variables. Inoculation block was included as a random effect. Size of the forewing marginal cell was included as a covariate in the model with Neisseriaceae abundance, but was removed from the other models because it did not explain significant variation in infection (P > 0.05 for Wald chi-squared tests).
Alpha diversity (ESV richness).
Number of unique ESV's per sample was analyzed with a Poisson family linear mixed model that used infection treatment, temperature and temperature2, and bee colony as fixed predictor variables. Inoculation block was included as a random effect. The infection x temperature interaction (χ21 = 0.81, P = 0.37) and wing marginal cell size (χ21 = 0.05, P = 0.82) were excluded from the final model because they did not explain significant variation in ESV richness.
Sugar water consumption.
Effects of temperature and infection treatments on sugar water consumption (g bee−1 day−1) were tested with a linear mixed-effects model in R package Ime4 (Pinheiro & Bates). Net consumption values that were less than 0 after correction for evaporation were assigned a trivial positive mass of 1 mg. Square root-transformed net mass of sugar water consumed was the response variable; infection treatment, temperature treatment, their interaction, and bee colony were used as predictor variables. Size of the forewing marginal cell was used as a covariate. Inoculation block was included as a random effect, as was individual bee identity to account for non-independence of repeated measures on the same individual. A temperature2 term was initially included in the model, but excluded from the final model because it did not explain significant variation in consumption (χ21 = 0.79, P = 0.37).
Mortality.
Effects of temperature and infection treatment on mortality were tested with a Cox proportional hazards mixed-effects model (Therneau, 2015). Death hazard rate was the response variable; infection treatment, temperature treatment, their interaction, and bee colony were used as predictor variables; and inoculation block was included as a random effect. Exploratory plots and models showed no linear trends of mortality by temperature (χ21 = 0.20, P = 0.65), and models that included a temperature2 term failed to converge; therefore, this analysis treated temperature as a factor rather than as a continuous variable. Marginal cell size was initially included in the model, but excluded from the final model because it did not explain significant variation in the response (χ21 = 1.26, P = 0.26).
Supplementary Material
ORIGINALITY-SIGNIFICANCE STATEMENT.
Both gut microbiota and host body temperature, including fever, can influence resistance to infection. However, the effects of temperature on gut microbiota, and the consequences of these effects for resistance to infection, remain unexplored. Our experiments with bumble bees and an intestinal parasite show dramatic reductions in infection intensity at high temperatures, along with subtle changes in the size and community composition of the gut bacterial microbiota that contribute to host resistance. These results indicate that high body temperatures may reduce parasitic infection by clearing pathogens while sparing or encouraging growth of beneficial bacteria, highlighting an unexplored mechanism by which febrile temperatures could ameliorate disease. Our findings have implications for both bumble bee conservation and, more generally, our understanding of how febrile temperatures and changing climates affect host-parasite interactions in humans and wildlife.
ACKNOWLEDGMENTS
The authors thank Hauke Koch and Ben Sadd for donation of C. bombi strains and advice on qPCR; Koppert Biological Supply for donation of bumble bee colonies; Hoang Vuong and Kaleigh Russell for laboratory assistance; and anonymous reviewers for comments on the initial submission.
FUNDING
This project was funded by a National Science Foundation Postdoctoral Research Fellowship to EPY (NSF-DBI-1708945); USDA NIFA Hatch funds (CA-R-ENT-5109-H), NIH (5R01GM122060-02), and NSF MSB-ECA (1638728) to QSM; a USDA NIFA Predoctoral Fellowship to JAR (2018-67011-28123); and an NSF-CAREER grant (IOS 1651888) to TRR. Funders had no role in study design, data collection and interpretation, or publication.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
DATA AVAILABILITY
Raw sequence data are available on the NCBI Sequence Read Archive under Accession number PRJNA532469. All other data are supplied in the Supplementary Information, Data S1.
REFERENCES
- Angilletta MJ, Cooper BS, Schuler MS, and Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci Elite Ed 2: 861–881. [DOI] [PubMed] [Google Scholar]
- Baracchi D, Brown MJF, and Chittka L (2015) Behavioral evidence for self-medication in bumblebees? F1000Research 4: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barribeau SM, Sadd BM, du Plessis L, and Schmid-Hempel P (2014) Gene expression differences underlying genotype-by-genotype specificity in a host-parasite system. Proc Natl Acad Sci 111: 3496–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barribeau SM and Schmid-Hempel P (2013) Qualitatively different immune response of the bumblebee host, Bombus terrestris, to infection by different genotypes of the trypanosome gut parasite, Crithidia bombi. Infect Genet Evol 20: 249–256. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, and Walker S (2015) Fitting linear mixed-effects models using Ime4. J Stat Softw 67: 1–48. [Google Scholar]
- Belzunces LP, Tchamitchian S, and Brunet J-L (2012) Neural effects of insecticides in the honey bee. Apidologie 43: 348–370. [Google Scholar]
- Bestion E, García-Carreras B, Schaum C-E, Pawar S, Yvon-Durocher G, and Cameron D (2018) Metabolic traits predict the effects of warming on phytoplankton competition. Ecol Lett 21: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiet A, Meeus I, Van Nieuwerburgh F, Deforce D, Wackers F, and Smagghe G (2017) Colony contact contributes to the diversity of gut bacteria in bumblebees (Bombus terrestris). Insect Scl 24: 270–277. [DOI] [PubMed] [Google Scholar]
- Bliss C.l. and Fisher RA (1953) Fitting the negative binomial distribution to biological data. Biometrics 9: 176–200. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, and White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Boltaña S, Rey S, Roher N, Vargas R, Huerta M, Huntingford FA, et al. (2013) Behavioural fever is a synergic signal amplifying the innate immune response. Proc R Soc B 280: 20131381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA, et al. (2018) QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science, PeerJ Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJF, Schmid-Hempel R, and Schmid-Hempel P (2003) Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. J Anlm Ecol 72: 994–1002. [Google Scholar]
- Brunner FS, Schmid-Hempel P, and Barribeau SM (2013) Immune gene expression in Bombus terrestris : signatures of infection despite strong variation among populations, colonies, and sister workers. PLOS ONE 8: e68181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, and Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Kessler B, Mayack C, and Naug D (2010) Behavioural fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology 137: 1487–1491. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A (2016) Thermal Restriction as an Antimicrobial Function of Fever. PLoS Pathog 12:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimanee V, Evans JD, Chen Y, Jackson C, and Pettis JS (2016) Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J Insect Physiol 89: 1–8. [DOI] [PubMed] [Google Scholar]
- Chiriac CM, Szekeres E, Rudi K, Baricz A, Hegedus A, Drago N, and Coman C (2017) Differences in Temperature and Water Chemistry Shape Distinct Diversity Patterns in Thermophilic Microbial Communities. Appl Env Microbiol 83: e01363–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, and Dimopoulos G (2011) Natural Microbe-Mediated Refractoriness to Plasmodium Infection in Anopheles gambiae. Science 332: 855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisarovsky G and Schmid-Hempel P (2014) Combining laboratory and field approaches to investigate the importance of flower nectar in the horizontal transmission of a bumblebee parasite. Entomol Exp Appl 152: 209–215. [Google Scholar]
- Cohen JM, McMahon TA, Ramsay C, Roznik EA, Sauer EL, Bessler S, et al. (2019) Impacts of thermal mismatches on chytrid fungus Batrachochytrium dendrobatidis prevalence are moderated by life stage, body size, elevation and latitude. Ecol Lett 0: [DOI] [PubMed] [Google Scholar]
- Cohen JM, Venesky MD, Sauer EL, Civitello DJ, McMahon TA, Roznik EA, and Rohr JR (2017) The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol Lett 20: 184–193. [DOI] [PubMed] [Google Scholar]
- Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, and Promislow DEL (2007) Geographical Distribution and Diversity of Bacteria Associated with Natural Populations of Drosophila melanogaster. Appl Env Microbiol 73: 3470–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, et al. (2007) A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science 318: 283–287. [DOI] [PubMed] [Google Scholar]
- Crall JD, Switzer CM, Oppenheimer RL, Versypt ANF, Dey B, Brown A, et al. (2018) Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362: 683–686. [DOI] [PubMed] [Google Scholar]
- Dalmon A, Peruzzi M, Le Conte Y, Alaux C, and Pioz M (2019) Temperature-driven changes in viral loads in the honey bee Apis mellifera. J Invertebr Pathol 160: 87–94. [DOI] [PubMed] [Google Scholar]
- Daskin JH, Alford RA, and Puschendorf R (2011) Short-Term Exposure to Warm Microhabitats Could Explain Amphibian Persistence with Batrachochytrium dendrobatidis. PLOS ONE 6: e26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskin JH, Bell SC, Schwarzkopf L, and Alford RA (2014) Cool Temperatures Reduce Antifungal Activity of Symbiotic Bacteria of Threatened Amphibians – Implications for Disease Management and Patterns of Decline. PLOS ONE 9: el00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell AI, Pawar S, and Savage VM (2014) Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J Anim Ecol 83: 70–84. [DOI] [PubMed] [Google Scholar]
- Di Prisco G, Zhang X, Pennacchio F, Caprio E, Li J, Evans JD, et al. (2011) Dynamics of Persistent and Acute Deformed Wing Virus Infections in Honey Bees, Apis mellifera. Viruses 3: 2425–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RJ and Dillon VM (2004) The gut bacteria of insects: nonpathogenic Interactions. Annu Rev Entomol 49: 71–92. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, and Dimopoulos G (2006) Anopheles gamblae Immune Responses to Human and Rodent Plasmodium Parasite Species. PLOS Pathog 2: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, James RR, Koga R, Kwong WK, McFrederick QS, and Moran NA (2013) Standard methods for research on Apis mellifera gut symbionts. J Apic Res 52: 1–24. [Google Scholar]
- Engel P, Stepanauskas R, and Moran NA (2014) Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics. PLOS Genet 10: el004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch H (1960) Über die Körpertemperaturen und den WÔrmehaushalt von Apis melllflca. Z Für Vgl Physiol 43: 305–335. [Google Scholar]
- Fauser A, Sandrock C, Neumann P, and Sadd BM (2017) Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol Entomol 42: 306–314. [Google Scholar]
- Fox J and Weisberg S (2011) An R companion to applied regression, Second Ed. Thousand Oaks CA: Sage. [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, and Charnov EL (2001) Effects of Size and Temperature on Metabolic Rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- Gilmour KM and Ellington CP (1993) Power Output of Glycerinated Bumblebee Flight Muscle. J Exp Biol 183: 77–100. [Google Scholar]
- Hanshew AS, Mason CJ, Raffa KF, and Currie CR (2013) Minimization of chloroplast contamination in 16S rRNA gene pyrosequencing of insect herbivore bacterial communities. J Microbiol Methods 95: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JF and Fewell JH (2002) Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp Biochem Physiol A Mol Integr Physiol 133: 323–333. [DOI] [PubMed] [Google Scholar]
- Heinrich B (1972) Patterns of endothermy in bumblebee queens, drones and workers. J Comp Physiol 77: 65–79. [Google Scholar]
- Heinrich B (2013) The hot-blooded insects: strategies and mechanisms of thermoregulation, Springer Science & Business Media. [Google Scholar]
- Inouye DW (2008) Effects of Climate Change on Phenology, Frost Damage, and Floral Abundance of Montane Wildflowers. Ecology 89: 353–362. [DOI] [PubMed] [Google Scholar]
- James RR (2005) Temperature and chalkbrood development in the alfalfa leafcutting bee, Megachile rotundata. Apidologie 36: 15–23. [Google Scholar]
- Kammer AE and Heinrich B (1974) Metabolic Rates Related to Muscle Activity in Bumblebees. J Exp Biol 61: 219–227. [DOI] [PubMed] [Google Scholar]
- Kembel SW, O’Connor TK, Arnold HK, Hubbell SP, Wright SJ, and Green JL (2014) Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc Natl Acad Sci 111: 13715–13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kešnerová L, Mars RAT, Ellegaard KM, Troilo M, Sauer U, and Engel P (2017) Disentangling metabolic functions of bacteria in the honey bee gut. PLOS Biol 15: e2003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Tada A, Musolin DL, Hari N, Hosokawa T, Fujisaki K, and Fukatsu T (2016) Collapse of insect gut symbiosis under simulated climate change. mBio 7: e01578–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D, Jones N, Peacock S, Phillips J, Molnár PK, Krkošek M, and Luijckx P (2018) Empirical evidence that metabolic theory describes the temperature dependency of within-host parasite dynamics. PLOS Biol 16: e2004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, and Soszynski D (1998) Role of Fever in Disease. Ann N Y Acad Sci 856: 224–233. [DOI] [PubMed] [Google Scholar]
- Koch H and Schmid-Hempel P (2012) Gut microbiota instead of host genotype drive the specificity in the interaction of a natural host-parasite system. Ecol Lett 15: 1095–1103. [DOI] [PubMed] [Google Scholar]
- Koch H and Schmid-Hempel P (2011) Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci USA 108: 19288–19292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK, Mancenido AL, and Moran NA (2017) Immune system stimulation by the native gut microbiota of honey bees. Open Sci 4: 170003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WK and Moran NA (2013) Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst Evol Microbiol 63: 2008–2018. [DOI] [PubMed] [Google Scholar]
- Lemoine NP (2019) Considering the effects of temperature x nutrient interactions on the thermal response curve of carrying capacity. Ecology 100: e02599. [DOI] [PubMed] [Google Scholar]
- Lenth R (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means.
- Li J, Powell JE, Guo J, Evans JD, Wu J, Williams P, et al. (2015) Two gut community enterotypes recur in diverse bumblebee species. Curr Biol 25: R652–R653. [DOI] [PubMed] [Google Scholar]
- Li Jianghong, Wang T, Evans JD, Rose R, Zhao Y, Li Z, et al. (2019) The Phylogeny and Pathogenesis of Sacbrood Virus (SBV) Infection in European Honey Bees, Apis mellifera. Viruses 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C and Knight R (2005) UniFrac: a New Phylogenetic Method for Comparing Microbial Communities. Appl Env Microbiol 71: 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes PW, Rodrigues PAP, Oliver R, Mott BM, and Anderson KE (2016) Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol Ecol 25: 5439–5450. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Skaug H, Nielsen A, Berg C, Kristensen K, Maechler M, et al. (2017) glmmTMB: Generalized Linear Mixed Models using Template Model Builder. [Google Scholar]
- Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, et al. (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8: 1235–1246. [DOI] [PubMed] [Google Scholar]
- Martín-Hernández R, Meana A, García-Palencia P, Marín P, Botías C, Garrido-Bailón E, et al. (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol 75: 2554–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson VG, Moy J, and Moran NA (2012) Establishment of Characteristic Gut Bacteria during Development of the Honeybee Worker. Appl Env Microbiol 78: 2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov DA, Votýpka J, Yurchenko V, and Lukeš J (2013) Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasltol 29: 43–52. [DOI] [PubMed] [Google Scholar]
- Maslowski KM and Mackay CR (2010) Diet, gut microbiota and immune responses. Nat Immunol 12: 5–9. [DOI] [PubMed] [Google Scholar]
- McFrederick QS and Rehan SM (2016) Characterization of pollen and bacterial community composition in brood provisions of a small carpenter bee. Mol Ecol 25: 2302–2311. [DOI] [PubMed] [Google Scholar]
- McFrederick QS, Vuong HQ, and Rothman JA (2018) Lactobacillus micheneri sp. nov., Lactobacillus timberlakei sp. nov. and Lactobacillus quenuiae sp. nov., lactic acid bacteria isolated from wild bees and flowers. IntJ Syst Evol Microbiol 68: 1879–1884. [DOI] [PubMed] [Google Scholar]
- McFrederick QS, Wcislo WT, Taylor DR, Ishak HD, Dowd SE, and Mueller UG (2012) Environment or kin: whence do bees obtain acidophilic bacteria? Mol Ecol 21: 1754–1768. [DOI] [PubMed] [Google Scholar]
- McKinstry M, Chung C, Truong H, Johnston BA, and Snow JW (2017) The heat shock response and humoral immune response are mutually antagonistic in honey bees. Scl Rep 7: 8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeus I, Mommaerts V, Billiet A, Mosallanejad H, Van de Wiele T, Wäckers F, and Smagghe G (2013) Assessment of mutualism between Bombus terrestris and its microbiota by use of microcolonies. Apidologie 44: 708–719. [Google Scholar]
- Meikle WG, Adamczyk JJ, Weiss M, and Gregorc A (2018) Effects of bee density and sublethal imidacloprid exposure on cluster temperatures of caged honey bees. Apidologie 49: 581–593. [Google Scholar]
- Meteyer CU, Valent M, Kashmer J, Buckles EL, Lorch JM, Blehert DS, et al. (2011) Recovery of little brown bats (Myotls lucifugus) from natural infection with Geomyces destructans, white- nose syndrome. J Wildl Dis 47: 618–626. [DOI] [PubMed] [Google Scholar]
- Mockler BK, Kwong WK, Moran NA, and Koch H (2018) Microbiome Structure Influences Infection by the Parasite Crithidia bombi in Bumble Bees. Appl Environ Microbiol 84: e02335–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár PK, Sckrabulis JP, Altman KA, and Raffel TR (2017) Thermal Performance Curves and the Metabolic Theory of Ecology—A Practical Guide to Models and Experiments for Parasitologists. J Parasitol 103: 423–439. [DOI] [PubMed] [Google Scholar]
- Moran NA, Hansen AK, Powell JE, and Sabree ZL (2012) Distinctive Gut Microbiota of Honey Bees Assessed Using Deep Sampling from Individual Worker Bees. PLOS ONE 7: e36393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalingam NA and Lynch SV (2012) Role of the Microbiota in Inflammatory Bowel Diseases. Inflamm Bowel Dis 18: 968–984. [DOI] [PubMed] [Google Scholar]
- Näpflin K and Schmid-Hempel P (2018) High Gut Microbiota Diversity Provides Lower Resistance against Infection by an Intestinal Parasite in Bumblebees. Am Nat 000–000. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. (2017) vegan: Community Ecology Package. [Google Scholar]
- Onchuru TO, Martinez AJ, and Kaltenpoth M (2018) The cotton Stainer’s gut microbiota suppresses infection of a cotransmitted trypanosomatid parasite. Mol Ecol 27:3408–3419. [DOI] [PubMed] [Google Scholar]
- Palmer-Young EC, Calhoun AC, Mirzayeva A, and Sadd BM (2018) Effects of the floral phytochemical eugenol on parasite evolution and bumble bee infection and preference. Sci Rep 8: 2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young EC, Raffel TR, and McFrederick QS (2019) pH-mediated inhibition of a bumble bee parasite by an intestinal symbiont. Parasitology 146: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young EC, Raffel TR, and McFrederick Quinn S (2018) Temperature-mediated inhibition of a bumblebee parasite by an intestinal symbiont. Proc R Soc B Biol Scl 285: 20182041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young EC, Sadd BM, Stevenson PC, Irwin RE, and Adler LS (2016) Bumble bee parasite strains vary in resistance to phytochemicals. Scl Rep 6: 37087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JF, Gobin B, and Flughes WO. (2014) Short-term heat stress results in diminution of bacterial symbionts but has little effect on life history in adult female citrus mealybugs. Entomol Exp Appl 153: 1–9. [Google Scholar]
- Parmentier L, Meeus I, Mosallanejad H, de Graaf DC, and Smagghe G (2016) Plasticity in the gut microbial community and uptake of Enterobacteriaceae (Gammaproteobacteria) in Bombus terrestris bumblebees’ nests when reared indoors and moved to an outdoor environment. Apldologle 47: 237–250. [Google Scholar]
- Potts R, Clarke RM, Oldfield SE, Wood LK, Flempel de Ibarra N, and Cresswell JE (2018) The effect of dietary neonicotinoid pesticides on non-flight thermogenesis in worker bumble bees (Bombus terrestris). J Insect Physiol 104: 33–39. [DOI] [PubMed] [Google Scholar]
- Powell JE, Martinson VG, Urban-Mead K, and Moran NA (2014) Routes of Acquisition of the Gut Microbiota of the Floney Bee Apis mellifera. Appl Environ Microbiol 80: 7378–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praet J, Parmentier A, Schmid-Flempel R, Meeus I, Smagghe G, and Vandamme P (2018) Large-scale cultivation of the bumblebee gut microbiota reveals an underestimated bacterial species diversity capable of pathogen inhibition. Environ Microbiol 20: 214–227. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, and Arkin AP (2010) FastTree 2 – Approximately Maximum-Likelihood Trees for Large Alignments. PLOS ONE 5: e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014) R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, and Rohr JR (2013) Disease and thermal acclimation in a more variable and unpredictable climate. Nat dim Change 3: 146–151. [Google Scholar]
- Raymann K, Coon KL, Shaffer Z, Salisbury S, and Moran NA (2018) Pathogenicity of Serratia marcescens Strains in Honey Bees. mBio 9: e01649–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, et al. (2015) Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc R Soc Lond B Biol Scl 282: 20142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JA, Carroll MJ, Meikle WG, Anderson KE, and McFrederick QS (2018) Longitudinal Effects of Supplemental Forage on the Honey Bee (Apis mellifera) Microbiota and Inter- and Intra-Colony Variability. Microb Ecol 76: 814–824. [DOI] [PubMed] [Google Scholar]
- Rubanov A, Russell KA, Rothman JA, Nieh JC, and McFrederick QS (2019) Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Scl Rep 9: 3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RL, Koch GW, Martinez A, Mau RL, Bowker MA, and Hungate BA (2018) Developing climate-smart restoration: Can plant microbiomes be hardened against heat waves? Ecol Appl 28:1594–1605. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Anand R, and LaHoste GJ (2007) Menthol and nicotine oppositely modulate body temperature in the rat. Eur J Pharmacol 559: 161–164. [DOI] [PubMed] [Google Scholar]
- Sabaté DC, Carrillo L, and Carina Audisio M (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160: 193–199. [DOI] [PubMed] [Google Scholar]
- Sadd BM and Barribeau SM (2013) Heterogeneity in infection outcome: lessons from a bumblebee-trypanosome system. Parasite Immunol 35: 339–349. [DOI] [PubMed] [Google Scholar]
- Salathé R, Tognazzo M, Schmid-Hempel R, and Schmid-Hempel P (2012) Probing mixed-genotype infections I: Extraction and cloning of infections from hosts of the trypanosomatid Crlthldla bomb!. PLOS ONE 7: e49046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P (2001) On the evolutionary ecology of host–parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwissenschaften 88: 147–158. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel R and Tognazzo M (2010) Molecular divergence defines two distinct lineages of Crlthldla bombi (Trypanosomatidae), parasites of bumblebees. J Eukaryot Microbiol 57: 337–45. [DOI] [PubMed] [Google Scholar]
- Schwarz RS, Moran NA, and Evans JD (2016) Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proc Natl Acad Sci 113: 9345–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A, Koren O, and Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279–290. [DOI] [PubMed] [Google Scholar]
- Stahlschmidt ZR and Adamo SA (2013) Context dependency and generality of fever in insects. Naturwissenschaften 100: 691–696. [DOI] [PubMed] [Google Scholar]
- Starks PT, Blackie CA, and Seeley TD (2000) Fever in honeybee colonies. Naturwissenschaften 87: 229–231. [DOI] [PubMed] [Google Scholar]
- Steinauer K, Tilman D, Wragg PD, Cesarz S, Cowles JM, Pritsch K, et al. (2015) Plant diversity effects on soil microbial functions and enzymes are stronger than warming in a grassland experiment. Ecology 96: 99–112. [DOI] [PubMed] [Google Scholar]
- Therneau TM (2015) R package coxme: Mixed effects cox models. [Google Scholar]
- Thomas MB and Blanford S (2003) Thermal biology in insect-parasite interactions. Trends Ecol Evol 18:344–350. [Google Scholar]
- Thorburn LP, Adler LS, Irwin RE, and Palmer-Young EC (2015) Variable effects of nicotine, anabasine, and their interactions on parasitized bumble bees. F1000Research 4: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S, Démares FJ, Nicolson SW, Medrzycki P, Pirk CWW, and Human H (2016) Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). J Insect Physiol 93–94: 56–63. [DOI] [PubMed] [Google Scholar]
- Ulrich Y, Sadd BM, and Schmid-Hempel P (2011) Strain filtering and transmission of a mixed infection in a social insect. J Evol Biol 24: 354–62. [DOI] [PubMed] [Google Scholar]
- Velthuis HHW and van Doom HWV (2006) A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37: 421–451. [Google Scholar]
- Vogt FD (1986) Thermoregulation in Bumblebee Colonies. I. Thermoregulatory versus Brood-Maintenance Behaviors during Acute Changes in Ambient Temperature. Physiol Zool 59: 55–59. [Google Scholar]
- Van der Voort M, Kempenaar M, van Driel M, Raaijmakers JM, and Mendes R Impact of soil heat on reassembly of bacterial communities in the rhizosphere microbiome and plant disease suppression. Ecol Lett 19: 375–382. [DOI] [PubMed] [Google Scholar]
- Wickham H (2009) ggplot2: elegant graphics for data analysis, Springer; New York. [Google Scholar]
- Wilfert L, Gaudau BB, and Schmid-Hempel P (2007) Natural variation in the genetic architecture of a host-parasite interaction in the bumblebee Bombus terrestrls. Mol Ecol 16: 1327–1339. [DOI] [PubMed] [Google Scholar]
- Wilke CO (2016) cowplot: streamlined plot theme and plot annotations for “ggplot2.” CRAN Repos. [Google Scholar]
- Xu J and James RR (2012) Temperature stress affects the expression of immune response genes in the alfalfa leafcutting bee, Megachile rotundata. Insect Mol Biol 21: 269–280. [DOI] [PubMed] [Google Scholar]
- Zheng H, Powell JE, Steele MI, Dietrich C, and Moran NA (2017) Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci 114: 4775–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.