Abstract
Maternal biological systems impact infant temperament as early as the prenatal period, though the mechanisms of this association are unknown. Using a prospective, longitudinal design, we found that maternal (N = 89) amplitudes of the late positive potential (LPP) in response to negative stimuli during the second, but not the third, trimester of pregnancy predicted observed and physiological indices of temperamental reactivity in infants at age 4 months. Maternal LPP was positively associated with observed infant fear and negatively associated with frontal EEG asymmetry and cortisol reactivity in infants at age 4 months. Results identify a putative mechanism, early in pregnancy, for the intergenerational transmission of emotional reactivity from mother to infant.
Keywords: mothers, emotional reactivity, pregnancy, infancy, LPP, temperament
Maternal emotionality is a known predictor of individual differences in infant temperament (Mangelsdorf, Gunnar, Kestenbaum, Lang, & Andreas, 1990), which is defined as infants’ own biologically-based propensities for experiencing and/or expressing the primary emotions (Goldsmith & Campos, 1982). Emotional reactivity is a central domain of temperament (Rothbart & Bates, 2006). In infants, high levels of negative emotional reactivity in early life predict increased emotional problems across childhood (Buss et al., 2013; Chronis-Tuscano et al., 2009; Dougherty, Klein, Durbin, Hayden, & Olino, 2010). Intuitively, heightened emotional reactivity in mothers, which can manifest in its extreme as symptoms of anxiety and/or depression, predicts greater emotional reactivity in offspring (Davis et al., 2004). This link between maternal emotional reactivity and offspring emotional reactivity suggests an intergenerational transmission of individual differences in reactivity that may translate to risk for disorder. Examinations of the processes by which emotional reactivity is transmitted from mothers to children are complicated by the fact that the emotions of mothers and children are under constant influence from the other member of the dyad, making directionality nearly impossible to surmise. This difficulty, then, is at least partially due to traditional designs that include cohorts of mothers and young infants assessed only during the postnatal period, making it impossible to identify processes of maternal reactivity that are true precursors to, and not products of, infant temperamental reactivity.
The second challenge for this work has been an absence of options for specific biological processes that serve as the mechanisms by which maternal emotional reactivity “gets under the skin” of developing infants in ways that may influence infant outcomes. Only a small body of work has examined infant temperament as an outcome of biological processes linked to maternal emotion observable during fetal development. Most such work assumes heterotypic stability, whereby different measures are used to test stability in the same underlying propensity, which manifests in different ways over development (Putnam, 2011). This idea initially seems problematic because it requires interpreting an assumed common factor across multiple systems, rather than simply tracking a single measure over time. However, it is well-suited to infancy, a period during which emotion systems increase in diversity and complexity (Izard & Malatesta, 1987; Sroufe, 1979). In fact, there is some evidence for homotypic stability in physiological measures of maternal emotionality during pregnancy and infant measures postpartum (Dipietro, Hilton, Hawkins, Costigan, & Pressman, 2002); however, these associations are small and somewhat inconsistent, as would be expected if the primary mode(s) by which reactivity symptoms manifest are changing as systems mature. A focus on homotypic continuity also necessitates the use of somewhat ubiquitous measures that make it difficult to identify specific mechanisms and/or targets for intervention.
Myriad studies have reported that maternal emotion behaviors affect displays (e.g., Cole, 1986; Luebbe, Kiel, & Buss, 2011) and developmental trajectories (e.g., Braungart-Rieker, Hill-Soderlund, & Karrass, 2010; Brooker et al., 2013) of emotion in offspring. A subset of this work links maternal emotion with infant temperament characteristics, reporting greater negative emotional reactivity in the offspring of mothers who are high in symptoms of anxiety and/or depression (Feldman et al., 2009; Weinberg & Tronick, 1998). Despite a focus on observable maternal behaviors with infants, there is evidence that individual differences in infant temperamental reactivity are more strongly predicted by prenatal than postnatal assessments of maternal emotion. For example, greater maternal symptoms of anxiety and depression during the third trimester of pregnancy, but not during the postpartum period, predict more crying and motor activity in response to novel stimuli in 4-month-old infants (Feldman et al., 2009; Weinberg & Tronick, 1998).
Identifying mechanisms of the intergenerational transmission of individual differences in reactivity is critical for understanding how and when maternal biological function impacts infant development in the formative years for systems of reactivity and regulation (Gunnar & Quevedo, 2007; Shirtcliff & Ruttle, 2010; Van den Bergh, Mulder, Mennes, & Glover, 2005). Alternatively, the ability to pinpoint mechanisms by which individual differences in maternal emotional reactivity may be transmitted intergenerationally would offer theoretical and empirical insights that comprise the foundation for enhanced normative outcomes across development (Nigg, 2006).
In the last two decades, researchers have identified an event-related potential (ERP), derived from time-locked electroencephalogram (EEG) recordings, that reflects a distinct and rapidly-occurring marker of neural reactivity to emotional stimuli - the Late-Positive Potential (LPP). The LPP is centro-parietally maximal and begins approximately 300 ms after the presentation of a visual stimulus, appearing larger for emotional compared to neutral stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Foti, Hajcak, & Dien, 2009; Hajcak, MacNamara, & Olvet, 2010). Greater LPP amplitudes are believed to denote greater neural engagement in emotion processing. Early LPP amplitudes, in particular, putatively reflect reactive stages of the unfolding emotion response (MacNamara, Foti, & Hajcak, 2009). The LPP is easily elicited from adults. However, because its quantification traditionally involves a relatively long passive viewing procedure, the LPP is not commonly elicited from infants. Nonetheless, the temperament field has a rich history of assessing both behavioral and physiological proxies of emotional reactivity during early life, allowing for a multimethod approach to testing conceptual overlap between maternal emotional reactivity and infants’ temperament-based emotional reactivity. Thus, our aim was to test, using a multimethod approach, whether a discrete neural process reflecting neural reactivity to emotional information in mothers (i.e., maternal LPP) predicted temperament-based indicators of negative emotional reactivity in infants. Our multimethod strategy was rooted in our assumption of heterotypic continuity. Thus, we selected four commonly-used indicators of infant temperament that tapped both behavioral (parent-report and observation) and physiological (neural and neuroendocrine activity) levels of function.
Most commonly, individual differences in temperament, including emotional reactivity in infants, is assessed via parent report or laboratory observations. Both approaches have distinct strengths. Parent reports are generally believed to assess more generalizable trait-level behaviors while observations are viewed as less prone to parental bias, thus more objectively indexing specific reactive responses in controlled conditions (Goldsmith & Gagne, 2012; Zentner & Bates, 2008). Correlations between parent-report and observational measures are typically modest, though both approaches have yielded positive associations between maternal and infant emotional reactivity (Brooker et al., 2013; Feldman et al., 2009). This is particularly true for paradigms assessing fearful reactivity, believed to specifically tap infants’ subsequent risk for anxiety problems (Chronis-Tuscano et al., 2009; Kagan, 1994; Kagan & Snidman, 1999).
Proclivities for infant emotional reactivity also can be quantified at the level of neural activity as asymmetric neural activity in the hemispheres of the frontal cortex (Davidson, 1992). Greater baseline activity in the right, relative to left, frontal hemisphere (i.e., right-frontal asymmetry) is linked to a heightened propensity for negative affect and withdrawal related emotions (Davidson, 1992; Harmon-Jones & Allen, 1998). Though this approach lacks an association with a specific biological process, decades of work have demonstrated it as a valid and reliable measure of individual differences in negative reactivity from the first year of life (Schmidt, 2008) through adulthood (Davidson & Irwin, 1999). For example, during infancy, greater relative right-frontal EEG asymmetry at baseline predicts more distress during maternal separation (Davidson & Fox, 1989) or interaction with an unfamiliar adult (Fox & Davidson, 1987). Frontal asymmetry in infants is also associated with mothers’ self-reported emotion; infants of mothers who report higher levels of depressive symptoms show greater relative right frontal EEG asymmetry than infants of nondepressed mothers (Dawson et al., 1999; Jones, Field, Davalos, & Pickens, 1997). Of interest, substantial work has linked depressive symptoms to LPP amplitudes in adults, with greater depressive symptoms linked to reduced LPP amplitude (Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000; MacNamara, Kotov, & Hajcak, 2016). Infants of mothers reporting greater anxiety symptoms also show greater relative right frontal EEG asymmetry. Meta analytic work suggests that effect sizes linking infant right frontal EEG asymmetry to symptoms of maternal depression are similar to those linking right frontal EEG asymmetry to symptoms of maternal anxiety (Thibodeau, Jorgensen, & Kim, 2006), although findings linking anxiety symptoms to right frontal EEG asymmetry have been somewhat less consistent (e.g., Heller, Nitscke, Etienne, & Miller, 1997; LoBue, Coan, Thrasher, & DeLoache, 2011), particularly when comorbidity with depression was accounted for (Thibodeau et al., 2006).
Finally, emotional reactivity in infants has also been proxied using changes in the body’s production of cortisol, a hormone product of activation of the hypothalamic-pituitary-adrenal axis, in response to negative emotional evens. Cortisol reactivity, or increased cortisol production relative to baseline, are believed to index physiological responses that prepare individuals to deal with perceived threat. In infants, cortisol reactivity is not always visible at the group (or mean) level (Gunnar, Talge, & Herrera, 2009), though individual differences in cortisol levels show relatively consistent positive associations with observed negativity in laboratory paradigms (Kalin, Shelton, Rickman, & Davidson, 1998). Maternal negative emotionality during the prenatal period also predicts heightened cortisol reactivity in infants following negative emotion elicitation (Davis et al., 2007). There is evidence for positive associations between cortisol reactivity and LPP amplitudes in adults (Alomari, Fernandez, Banks, Acosta, & Tartar, 2015) and at least preliminary evidence for links between cortisol reactivity and LPP-like amplitudes in infants (Gunnar & Nelson, 1994). Thus, some convergence of reactivity assessed through these two measures is evident. Perhaps most importantly for the current work, maternal cortisol levels appear to impact the development and function of neural structures and systems in infants (Buss, Davis, Muftuler, Head, & Sandman, 2010; Buss et al., 2012), including putative generators of the LPP (Liu, Huang, McGinnis-Deweese, Keil, & Ding, 2012). Consequently, there is both empirical and theoretical support for investigating intergenerational transmission of reactivity across these systems.
While our hypothesized positive association between maternal LPP and observed fear in infants was straightforward, given an established directional link between fear-based assessments and anxiety risk (Clauss & Blackford, 2012), expectations for the direction of effects linking maternal LPP to infant frontal EEG asymmetry and cortisol reactivity were more complex. A problem arises because, although maternal depressive and anxiety symptoms both predict greater relative frontal EEG asymmetry, they are associated with opposing patterns of LPP amplitude. Specifically, greater anxiety symptoms are linked to enhanced LPP amplitudes and depressive symptoms are linked to reduced LPP amplitudes (MacNamara et al., 2016). Importantly, when symptoms of anxiety and depression are simultaneously tested as predictors of LPP amplitudes, only the negative association between depressive symptoms and LPP amplitudes is observed. That is, when depressive symptoms were not statistically partialed out, anxiety symptoms were unrelated to LPP amplitudes (MacNamara et al., 2016); however, when anxiety symptoms are not partialed out of a measure of depression, greater depressive symptoms remain significantly associated with reduced LPP. Thus, when depression-anxiety comorbidity is anticipated, it may be reasonable to base hypotheses on patterns of results expected for depressive symptoms. As a result, we hypothesized that reduced LPP amplitudes in mothers during pregnancy would predict greater relative right frontal EEG asymmetry and greater cortisol reactivity in infants at age 4 months.
In this work, we used a prospective design that tests LPP in mothers prior to the birth of infants, when infant temperament is not concurrently impacting maternal emotional reactivity. This offers a control for infant behavior that is not typically present in developmental investigations of links between maternal characteristics and infant temperament. The longitudinal design also offers an opportunity to test for timing differences in the association between maternal emotional reactivity during pregnancy and infant temperament. Though lesser studied, there is evidence suggesting that maternal emotional reactivity earlier, as opposed to later, in pregnancy may be more robust predictors of child emotion outcomes (Buss, Davis, Muftuler, Head, & Sandman, 2010; Buss et al., 2012). Such findings are consistent with evidence for cascading developmental effects beginning during a critical period of developing neural circuitry in the second trimester (Tau & Peterson, 2009) and with evidence that women may become less sensitive to environmental stressors across pregnancy (Buss et al., 2010; Davis, & Sandman, 2010). If true, emotional reactivity in mothers would have less variability later in gestation, resulting in smaller effects of the in-utero environment on fetal development late in pregnancy. Thus, we additionally hypothesized that effects would be stronger during the second than the third trimester.
In sum, the current study tested a marker of emotional reactivity at the neural level, the LPP, during pregnancy as a predictor of infant temperament-based emotional reactivity. Consistent with recommendations for best practices in the field, we assessed multiple domains of infant temperament in order to assess the generalizability of this hypothesis at the conceptual level. We hypothesized that greater LPP amplitudes would be associated with greater mother-reported and observed fear in infants while smaller LPP amplitudes would be associated with greater relative right frontal EEG asymmetry and greater cortisol reactivity. We also predicted that effects would be stronger for maternal LPP assessed during the second, relative to the third trimester.
Method
Participants
The present study was conducted according to guidelines laid down in the Declaration of Helsinki, with written informed consent obtained from a parent or guardian for each child before any assessment or data collection. All procedures involving human subjects in this study were approved by the human subjects committee of the Institutional Review Board at Montana State University (Project title: The Montana Minds of Mothers Study; IRB #RB011615-FC). Ninety-two pregnant women enrolled in a longitudinal examination of emotion in mothers and offspring from pregnancy to postpartum. The majority of women were recruited through informational brochures distributed at local doctors (26%), e-mail announcements through the local university (25%), and word-of-mouth referrals (27%). Participants also signed up to participate after seeing flyers posted at local preschools and businesses (13%), receiving referrals from the local Women, Infants, and Children office (7%), or hearing announcements in local mothers’ clubs (2%). Both primigravida and multigravida women were allowed to enroll. Participation included two prenatal laboratory visits: one each during the second (M = 21.15 weeks gestation; SD = 3.79) and third trimester (M = 35.92 weeks gestation; SD = 1.47), and a final postnatal visit at infant age 4 months (M = 4.27, SD = 0.62). Rolling recruitment procedures resulted in 81 participants at the second trimester visit, 85 participants at the third trimester visit (11 new, 1 skipped this phase because the baby was born early, 6 dropped out of the study), and 75 participants at the infant visit (7 dropped out of study, 3 participants whose infants were not yet 4 months old when the study was ended because the PI switched institutions).
Of those participants who reported race and ethnicity, most were White (89%, Asian = 8%, American Indian = 1%, African-American = 1%, mixed race = 1%) and Non-Hispanic (96%). The median and mode level of education was a college degree (36%). Most (86%) families reported gross annual income, which ranged from less than $15,000 to more than $91,000; with the largest groups of families reported earning $51,000-$60,000 (17%) or more than $91,000 (25%) per year. On average, mothers were in their early thirties at the time of the second trimester visit, though maternal age ranged from 21 to 41 years (M = 30.49, SD = 4.22).
Pregnancy experiences were reported by mothers at the postnatal visit using a questionnaire adapted from previous work (Marceau et al., 2013). Participants indicated the presence or absence of 28 different conditions (e.g., flu, lead exposure, diabetes, etc.), experiences (e.g., induction, c-section), and medications taken at any time during their pregnancy. Rates of pregnancy and labor and delivery conditions were generally low, with participants reporting an average total of three (M = 3.35, SD = 2.22). The most commonly-reported prenatal complication was a fever or chills (n = 7), and the most common delivery-related risk factor was labor induction (n = 21). Participants reported using very few medications during pregnancy (M = 1.02, SD = 1.13), most frequently reporting treatments for heartburn or nausea. On average, infants were full term (M = 39.64, SD = 1.75 weeks; range 34-44 weeks). Only six infants were born prior to 37 weeks gestation. Given the low-risk nature of the sample, pregnancy and delivery complications were not considered further.
Procedure
Prenatal visits.
Procedures were identical for the two prenatal visits. Participants scheduled prenatal visits either over the phone with laboratory staff or via an online booking website. Two weeks prior to the laboratory visit, participants were mailed a packet of questionnaires to complete and bring with them to the laboratory. Questionnaires included assessments of symptoms of anxiety and depression and general demographic information. Upon arrival at the laboratory, mothers completed an informed consent procedure followed by a computerized passive viewing task1 while EEG data were collected.
The target gestational age for each visit was selected based on the goals of the study and included a second trimester (~20 weeks), third trimester (~36 weeks)2, and postpartum (~4 months) assessment. These time points were selected in order to (a) collect three data points, consistent with gold standards of the field and allowing us to isolate specific pre- (e.g., early vs. late) to postpartum changes, (b) include an assessment during a critical period of prenatal development with regard to the neural bases of infant emotional development (Davis et al., 2004; Davis & Sandman, 2010; Qiu et al., 2015), and (c) enable us to include, within the funding period, postnatal assessments for the outcome(s) of interest at an age where validity has been established.
Postnatal visit.
Because the postnatal visit targeted child age 4 months, laboratory staff confirmed the child’s birth date roughly one month after the anticipated due date. Questionnaires were identical to the prenatal assessments, but additionally assessed complications experienced across pregnancy and during childbirth, parenting-related stress, and a survey of infant temperament. Upon arrival at the laboratory, mothers completed an informed consent procedure. EEG data were then collected from infants during a 5-minute resting period (described in more detail below). EEG electrodes were then removed and infants completed a laboratory episode designed to elicit fear in infants3. Infants remained with a familiar experimenter while EEG data were collected from mothers during the same computerized task used for the prenatal visits.
Measures
Maternal LPP.
LPPs were derived from EEG recorded at each of the prenatal visits using a BioSemi Active 2 system (Cortech Solutions, LLC; Wilmington, NC) during a passive viewing task (Greg Hajcak & Nieuwenhuis, 2006). Mothers were fitted with a 32-channel Bio-Semi elastic head cap. Electroconductive gel was placed into plastic electrode holders embedded into the cap. Ag-AgCl-tipped active electrodes were then secured into electrode holders, arranged in according to the 10-20 labeling system. Data were sampled at a rate of 2048 Hz and referenced to the Common Mode Sense and Driven Right Leg electrodes during recording. Horizontal and vertical eye-movements were recorded via flat electrodes placed at the outer canthi of the left and right eye, and supra and infra orbital sites of the left eye, respectively. Additional flat electrodes were placed on each mastoid for offline re-referencing.
During recording, mothers completed two trial blocks, passive view and reappraisal, separated by a short break. Given our interest in reactivity rather than regulation, analyses focus on the passive view condition. For this, mothers were instructed to focus their attention on the screen while viewing 40 positive, 40 negative, and 40 neutral images from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). Pictures were presented for 1000 ms each in the center of a 23” computer monitor using Presentation® software (Neurobehavioral Systems, Inc.; Berkeley, CA). A blank screen was presented between images for 1500 ms. The order of images was randomized within block.
EEG data were processed offline using Brain Vision Analyzer (Brain Products GmbH). Continuous EEG was re-referenced to the average of the left and right mastoids, high-pass filtered at 0.1 Hz, low-pass filtered at 30 Hz, and corrected for eye movements and blinks (Gratton, Coles, & Donchin, 1983). Data were segmented (−400 to 2000 ms), and baseline corrected for 400 ms prior to stimulus presentation. Artifacts were identified, using a semiautomatic procedure, when one of the following criteria were met: a voltage step of more than 75 µV between data points, a difference of 150 µV per within 200 ms, amplitudes below 0.5 µV within a 50 ms period, and activity that exceeded +100µV or −100 µV. Remaining segments were visually inspected for artifacts.
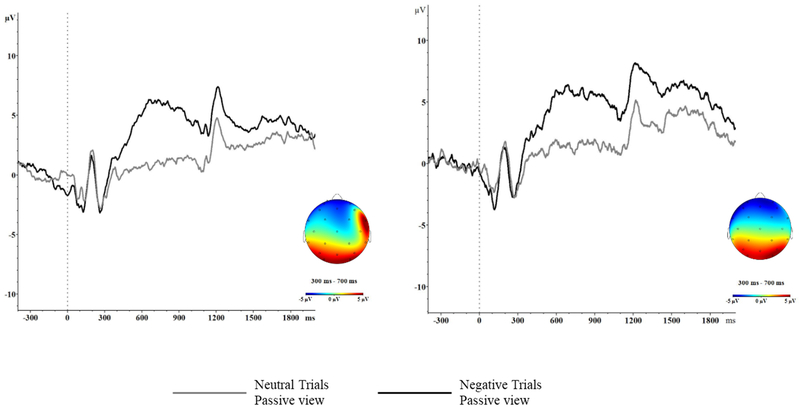
To focus on emotional reactivity (Dan Foti & Hajcak, 2008; Gross, 2002), LPP was defined as the mean EEG amplitude at electrode Pz between 300 and 700 ms post-stimulus. Averages were created only for negative and neutral trials. Negative trials were selected given our focus on infant temperamental reactivity in the negative emotion domain and neutral trials were included in order to control for neural processing in response to non-emotional images. Averages were not created if fewer than 8 trials were available (Moran, Jendrusina, & Moser, 2013). On average, there were roughly 30 usable trials for each LPP average during the first trimester, and 27 usable trials for each LPP average at the second trimester visit. The presence of an LPP effect in this sample has been previously established (Nyman, Mistry, Kiel, Schmall, & Brooker, under review), and is also visible in Figure 1.
Figure 1.
LPP Waveforms for the second trimester (left) and third trimester (right) assessments at Pz.
Infant reactivity (Mother-reported).
Infant negative emotional reactivity was reported by mothers using the negative emotionality scale of the Infant Behavior – Questionnaire – Revised version (IBQ-R; Gartstein & Rothbart, 2003), a 191-item parent report measure that asked mothers to describe whether various behaviors were typical of their child over the past 1-2 weeks (1 = never, 7 = always). The scale included 59 items and showed acceptable reliability (∝ = 0.83). The negative emotionality scale includes 4 subscales: sadness, distress, fear, and falling reactivity. Notably, sadness items comprise only 14 items (~23%) of the total scale and results are unchanged when sadness items are excluded.
Observed infant reactivity.
Observed negative reactivity was assessed using a modified Masks paradigm from the Prelocomotor version of the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith & Rothbart, 1999). For this episode, the infant was seated in a car seat in front of an enclosed gray booth. The experimenter captured the infant’s attention by knocking on the wall of booth, then lifted a mask up and displayed it for 10 seconds. After a 5 second pause, a second mask was displayed. This sequence was repeated in order to display a total of 4 masks in increasing order of putative threat: an evil queen, an old man, a glow-in the dark vampire, and a gas mask.
Similar to the previous modifications (Crockenberg & Leerkes, 2004, 2006), the masks procedure was conducted twice. During the first masks episode, intended to assess infant temperamental reactivity, mothers were instructed to stand behind and to the right of their infant, remaining uninvolved throughout the procedure (mother uninvolved). The second masks episode, intended to capture infant affect during maternal intervention, took place immediately following the first administration. For this, mothers were told that they could “talk to and interact with (child) as you normally would,” but were asked not to intervene directly with the mask (i.e., don’t touch the masks’; mother involved). All mothers completed the same order of administration in order to maintain the novelty of the masks during assessments of temperament. Because the goals of the current study center around temperamental reactivity, we focused on the mother uninvolved episode.
Trained coders recorded the latency to the first observation of fear across eight 5-second coding epochs (2 per mask). Latencies were then reversed to reflect speed such that higher scores reflected earlier fear expressions. Facial fear was rated on a four-point scale where zero reflected no codable fear across three regions of the face (i.e., brows, eyes, mouth), and three reflected visible fear in all three facial regions or an intense expression of fear in two areas. Bodily fear was similarly rated. All episodes were double coded by two independent scorers who had been trained by a master coder (R.J.B.). Each coder was required to achieve a minimum reliability (kappa = 0.70) with the master coder before coding independently. A fear composite was created using regression-based factor scores. Because these scores were based on standardized variables, the infant negativity has a mean of zero and standard deviation of 1.00. To illustrate the range of each behavior present in the episode, descriptive statistics for the individual scales used to form the composite are provided in Supplementary Table 1. Each infant received a reactivity single score; greater scores reflected greater reactivity.
Infant frontal EEG asymmetry.
Propensities for negative reactivity were assessed via frontal asymmetry scores derived from infant EEG recorded during a resting baseline. Consistent with contemporary practices (Bell & Cuevas, 2012), to keep infants awake and calm, an experimenter presented a series of three low-intensity toys throughout the recording. EEG data were continuously recorded using a BioSemi Active 2 recording system and Ag-AgCl-tipped electrodes arranged in a 32-channel cap according to the 10-20 labeling system. Electrodes were also placed at the outer canthus of the left eye to collect horizontal eye movements and at the infra orbital site of the left eye to collect vertical eye movement. One electrode was placed on each mastoid for later re-referencing. Data were sampled at a rate of 2048 Hz. During recording, data were referenced to the Common Mode Sense and Driven Right Leg electrodes. Offline, the continuous EEG was re-referenced to the average of the two mastoids, high-pass filtered at 0.1 Hz, low-pass filtered at 30 Hz, and corrected for eye movement or blinks (Gratton et al., 1983). Continuous data were divided into 1-second segments with 50% overlap and baseline corrected for the entire segment length. Artifacts were marked in segmented data when one of the following criteria were met: a voltage step of more than 75 µV between data points, a difference of 150 µV per within 200ms, amplitudes below 0.5 µV within a 50ms period, and activity that exceeded +100µV or −100 µV. Segments were submitted to a Fast-Fourier Transform using a Hamming window and clean segments were averaged together. Power in the 6-9 Hz band was exported for each infant and log10 transformed to correct for positive skew in the data. Asymmetry scores were calculated as the difference in alpha power at homologous frontal electrodes (F4-F3) frequently used in previous work for calculating asymmetry in infants (Schmidt, 2008). Note that, because alpha reflects the inverse of cortical activity, positive scores reflect greater activity in the left (relative to right) hemisphere, while negative scores reflect greater activity in the right (relative to left) hemisphere (Davidson, 1992).
Infant cortisol reactivity.
To assess neuroendocrine reactivity to emotion-eliciting contexts, experimenters collected two saliva samples from infants during the laboratory visit. The first sample was collected upon arrival at the laboratory. The second sample was collected at the end of the visit, approximately 30 minutes after the infant had completed a series of positive and negative affect-eliciting tasks. Saliva samples were collected by the primary experimenter using and infant swab (Salimetrics, LLC part 5501.20). Experimenters were instructed to hold the swab under the infant’s tongue for a minimum of 90 seconds. If it was not possible to place the swab under the tongue, experimenters were instructed to place the swab in the corner of the mouth for a minimum of 90 seconds.
All samples were stored at −80°C until they were thawed and centrifuged. All samples were assayed in duplicate using a highly sensitive enzyme immunoassay (Salimetrics, Suffolk, UK) with a detection range from 0.012μg/dL-3μg/dL. Samples were included in analyses only if the coefficient of variation was less than 20%. Mean Coefficient of Variation (CV) values for included data were 7.18 (SD = 5.15) for pre-visit samples and 6.92 (SD = 4.82) for post-visit samples. Average intra- and inter-assay coefficients of variation were <7%. A reactivity score was created by subtracting pre-visit cortisol levels from post-visit cortisol levels. Scored in this way, higher values reflect greater neuroendocrine reactivity to the emotion-eliciting episodes (Gunnar, 1992). Given the anticipated timing of peak cortisol responses in infants (M. R. Gunnar, 1992) , cortisol levels would most closely reflect reactivity in the Masks episode. Cortisol reactivity scores were unrelated to sample times (mean r = −0.13), morning wake time (r = 0.06), time since waking (mean r = −0.14), or hours of sleep the previous night (r = −0.13). Cortisol reactivity scores were also unrelated to maternal reports of infants having napped on the day of the visit (t(54) = 0.90, p = 0.37), being on antibiotics (t(54) = 0.26, p = 0.79) or other medications (t(51) = −0.02, p = 0.99), or having had an “out of the ordinary, potentially stressful” even recently occur (t(53) = 0.36, p = 0.72). These variables are generally statistically controlled given an expectation for significant associations. However, because no associations were significant in our data, neither time of day nor medication use was considered further.
Maternal symptoms.
Severe emotional reactivity may manifest as symptoms of maternal anxiety and depression. Symptoms of anxiety and depression have been linked to LPP in pregnant women (Nyman et al., under review) and have been associated with infant temperament (Davis et al., 2004; Degnan, Almas, & Fox, 2010). Thus, in order to isolate the effects of the LPP on infant temperament characteristics in a way that is nonredundant with the effects of maternal symptoms, we used levels of concurrent maternal symptoms of depression and anxiety as covariates in all of our analyses.
Maternal depressive symptoms were assessed at all three visits using the Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden, & Sagovsky, 1987). The EPDS is a 10-item parent report measure that asked mothers to rate, on a 4-point Likert scale (0 = absence of the symptoms, 3 = most frequent experience of the symptoms) the degree to which they experienced a variety of symptoms over the last 7 days (e.g. feeling anxious for no good reason). The EPDS showed acceptable internal consistency at each visit (second trimester α = 0.82, third trimester α = 0.88) and at the postnatal visit (α = 0.82). Though the EPDS is treated as a first-stage screener rather than a diagnostic instrument, EPDS scores of 10 or higher are thought to indicate possible depression. The EPDS has been shown to be valid and during pregnancy and the postpartum period (Ji et al., 2011).
Maternal anxiety symptoms were assessed at all three visits using the Penn State Worry Questionnaire PSWQ; (Meyer, Miller, Metzger, & Borkovec, 1990). The PSWQ is a 16-item self-report measure that asked mothers to describe, using a 5-point Likert scale (1 = not at all typical, 3 = somewhat typical, 5 = very typical), the degree to which items were characteristic of them. The PSWQ showed acceptable internal consistency at both prenatal visits (second trimester α = 0.91 and third trimester α = 0.93, respectively) and at the postnatal visit (α = 0.93). The PSWQ is not a diagnostic instrument, though differences between normative and clinically anxious samples are apparent in PSWQ scores. For examples, mean PSWQ scores of 41 have been reported for community samples (Gillis, Haaga, & Ford, 1995) while mean scores of 64 are reported for individuals with Generalized Anxiety Disorder diagnoses (Meyer et al., 1990). The PSWQ has been shown to be valid and during pregnancy and the postpartum period (O’Connor et al., 2013; Wenzel et al., 2005).
Missing Data
LPPs were available from all 81 mothers at the second trimester visit, though LPP from one mother could not be scored at all electrode sites due to variable noise in the data. LPPs were available from 78 mothers at the third trimester visit, with missing data resulting from excessive artifact, experimenter error, or equipment malfunction. Symptoms of anxiety and depression were missing for 1 mother who did not return any questionnaire data. IBQ data were available for 67 infants; missing data resulted from missing or incomplete questionnaires at the postnatal visit. Scores of frontal EEG asymmetry were available for 47 infants, with missing data resulting from an absence of EEG data (task refusal, excessive distress) or data with excessive artifact.
An analysis of patterns of missing data from all measured variables suggested that data were missing completely at random (Little’s MCAR χ2(422) = 422, p = 0.167. Missing data were handled using a full-information maximum likelihood (FIML) procedure that uses all available data to estimate nonbiased parameter estimates, thus allowing one to take advantage of the full sample from which at least some data are available. FIML is appropriate with MCAR data when variables that can account for patterns of missingness are included in the analysis (Enders, 2010). However, because FIML relies on patterns of available data, mothers scores on all variables were not included. The final sample for analysis comprised 89 mothers.
Results
Descriptive statistics and correlations for all variables are presented in Tables 1 and 2, respectively. Paired samples t-tests suggested that levels of depression (t(67) = 1.642, p = 0.105) and anxiety t(66) = −0.471, p = 0.639) were stable from the second to third trimester of pregnancy. Similarly neural reactivity to both negative (t(65) = 0.468, p = 0.641) and neutral (t(64) = 0.200, p = 0.842) images was not significantly different for the second and third trimester visits and there was no change in the difference between negative and neural amplitudes over time (F(1, 63) = 0.001, p = 0.978).
Table 1.
Variable means and standard deviations
| Mean | SD | |
|---|---|---|
| Second Trimester | ||
| Depressive symptoms | 6.42 | 4.10 |
| Anxiety symptoms | 40.63 | 11.01 |
| Maternal neutral LPP | 0.16 | 4.36 |
| Maternal negative LPP | 4.44 | 5.42 |
| Third Trimester | ||
| Depressive symptoms | 5.46 | 4.74 |
| Anxiety symptoms | 40.70 | 12.04 |
| Maternal neutral LPP | 0.52 | 5.27 |
| Maternal negative LPP | 4.04 | 6.02 |
| Infant Temperament (4 mo.) | ||
| Mother-reported reactivity | 2.94 | 0.60 |
| Observed reactivity* | 0.00 | 1.00 |
| Frontal EEG asymmetry | 0.02 | 1.03 |
| Cortisol reactivity | −0.07 | 0.30 |
Notes:
standardized variable
Table 2.
Bivariate correlations for all variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Second trimester depressive symptoms | |||||||||||
| 2. Second trimester anxiety symptoms | 0.416 | ||||||||||
| 3. Third trimester depressive symptoms | 0.483 | 0.266 | |||||||||
| 4. Third trimester anxiety symptoms | 0.367 | 0.738 | 0.552 | ||||||||
| 5. Second trimester neutral LPP | −0.069 | 0.045 | −0.115 | −0.065 | |||||||
| 6. Second trimester negative LPP | −0.045 | 0.000 | −0.016 | −0.031 | 0.364 | ||||||
| 7. Third trimester neutral LPP | −0.160 | 0.012 | 0.036 | 0.038 | 0.342 | 0.568 | |||||
| 8. Third trimester negative LPP | −0.027 | 0.033 | −0.090 | 0.026 | 0.307 | 0.317 | 0.382 | ||||
| 9. Mother-reported reactivity | 0.103 | 0.060 | 0.128 | −0.014 | −0.081 | 0.004 | −0.027 | −0.117 | |||
| 10. Observed reactivity | −0.015 | 0.010 | 0.130 | 0.044 | 0.006 | 0.207 | 0.142 | 0.085 | −0.017 | ||
| 11. Frontal EEG asymmetry | 0.096 | −0.026 | −0.087 | 0.036 | −0.180 | 0.339 | 0.005 | 0.073 | 0.294 | 0.147 | |
| 12. Cortisol reactivity | −0.067 | 0.239+ | 0.178 | 0.219 | 0.059 | −0.209 | 0.045 | −0.239+ | −0.309 | −0.199 | −0.380 |
Note: Bivariate correlations prior to data imputation. Bolded correlations are significant at p ≤ 0.01. Italicized correlations are significant at p ≤ 0.05,
p ≤ 0.10.
Results from the second trimester visit are presented in Table 3. Second trimester LPP to negative emotional stimuli was not associated with maternal reports of temperamental reactivity in 4-month-old infants. Rather, second trimester maternal LPP to negative emotional stimuli predicted temperamental reactivity as assessed by observed emotion and physiological arousal in infants at age 4 months. Specifically, greater maternal LPP was associated with greater observed fear in infants, consistent with previous work on fearful-temperament-based anxiety risk. Also consistent with previous work, blunted maternal LPP was associated with blunted observed reactivity in the laboratory, greater relative right frontal EEG asymmetry in infants at 4 months of age, and greater cortisol reactivity in response to social novelty.
Table 3.
Regression results predicting infant temperament characteristics from maternal LPP
| Mother-reported reactivity | Observed reactivity | Frontal asymmetry | Cortisol reactivity | |||||
|---|---|---|---|---|---|---|---|---|
| ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI | |
| Second trimester | ||||||||
| Depressive symptoms | 0.085 | −0.245, 0.415 | −0.016 | −0.335, 0.304 | 0.067 | −0.273, 0.406 | −0.271+ | −0.554, 0.012 |
| Anxiety symptoms | 0.017 | −0.290, 0.324 | 0.037 | −0.246, 0.320 | −0.028 | −0.325, 0.269 | 0.355** | 0.093, 0.616 |
| Neutral LPP | −0.083 | −0.323, 0.157 | −0.084 | −0.331, 0.163 | −0.248* | −0.496, 0.000 | 0.113 | −0.128, 0.354 |
| Negative LPP | 0.039 | −0.212, 0.291 | 0.225+ | −0.028, 0.477 | 0.398** | 0.139, 0.658 | −0.221* | −0.446, 0.005 |
| Third trimester | ||||||||
| Depressive symptoms | 0.194 | −0.226, 0.614 | 0.161 | −0.227, 0.550 | −0.172 | −0.615, 0.272 | 0.101 | −0.304, 0.505 |
| Anxiety symptoms | −0.076 | −0.371, 0.220 | −0.007 | −0.299, 0.284 | 0.107 | −0.239, 0.453 | 0.175 | −0.121, 0.471 |
| Neutral LPP | 0.037 | −0.227, 0.302 | 0.206 | −0.130, 0.488 | 0.001 | −0.290, 0.291 | 0.121 | −0.190, 0.432 |
| Negative LPP | −0.122 | −0.391, 0.147 | −0.089 | −0.489, 0.311 | 0.062 | −0.284, 0.407 | −0.271+ | −0.591, 0.049 |
Note:
p ≤ 0.01,
p ≤ 0.05, p ≤ 0.10, N = 89 during second trimester, N = 88 during third trimester
Results from the third trimester are presented in Table 3. Neural reactivity to negative emotional stimuli was not associated with mother-reported temperamental reactivity, observed emotion, or right frontal EEG asymmetry. Blunted maternal neural reactivity to negative emotional stimuli marginally predicted greater cortisol reactivity in infants at 4 months of age.
Given the patterns of bivariate correlations shown in Table 2, we conducted two follow-up analyses to assess the specificity of our findings. First, we reran analyses using a single analysis at each trimester; such that the four measures of child temperament served as simultaneous outcomes predicted by maternal symptoms and LPP amplitudes. Because overlapping variance among predictors and outcomes were accounted for by the inclusion of all measures in the model, this tests the unique, nonoverlapping associations between neural reactivity to negative images and each outcome. Tested in this way, effect sizes remained similar to the original models for neural reactivity to negative images as a predictor of observed negativity (ß = 0.207, 95% CI [−0.049, 0.462], p = 0.113), frontal EEG asymmetry (ß = 0.415, 95% CI [0.168, 0.663], p = 0.001), and cortisol reactivity (ß =−0.210, 95% CI [−0.438, −0.018], p = 0.072), though probability values changed. The pattern of results for the third trimester was also unchanged.
Finally, we reran the analyses predicting frontal EEG asymmetry replacing the frontal EEG asymmetry measure with individual measures of power at in the left (F3) and right (F4) hemispheres. This approach follows current recommendations (Allen, Coan, & Nazarian, 2004) for understanding whether significant findings might be driven by increases or decreases in power in one hemisphere. Maternal characteristics did not individually predict left or right hemisphere power, suggesting that the difference in power, rather than individual hemisphere power was driving the overall effect.
Discussion
Using a prospective study design beginning in pregnancy, we found that maternal neural reactivity to emotional stimuli during the second trimester predicted infants’ temperamental negative reactivity at four months of age. Effects were present across behavioral and biological levels of analysis but were greatest for physiological measures, which included right frontal EEG asymmetry and cortisol reactivity. All effects were moderate to large in size.
Greater LPP amplitudes in response to negative images during the second trimester of pregnancy were associated with greater observed fear in infants at age 4 months. This positive association likely reflects the focus of our observed measure on fear elicitation. Fear reactivity is an established predictor of anxiety symptoms (Buss, 2011; Chronis-Tuscano et al., 2009). Given that anxiety has been previously linked to enhanced LPP amplitudes (MacNamara & Hajcak, 2010), this positive association likely reflects common propensities for anxiety-related negative emotional reactivity in mothers and infants. Importantly, we controlled for the presence of maternal antenatal symptoms of anxiety and depression; thus, our findings do not simply reflect putative anxiety risk in infants of more anxious mothers. It is worth noting, however, that we controlled for anxiety in mothers assessed primarily as general worry rather than social anxiety, as would have been proxied by our infant paradigm.
Also consistent with previous work, smaller LPP amplitudes during the second trimester of pregnancy predicted greater relative right frontal EEG asymmetry and greater cortisol reactivity in four-month-old infants. In fact, the largest effect sizes in the data suggested that blunted LPP amplitudes to negative images was linked to biological function in infants roughly 9 months later (at 4 months of age). Both right frontal EEG asymmetry and heightened cortisol reactivity have been linked to increased negative emotional reactivity in infants. Given suggestions that associations with maternal depression are more robust than associations with maternal anxiety, we expected that these patterns of infant response would be predicted by depression-related patterns of maternal LPP. That is, we expected that blunted, rather than augmented, maternal LPP amplitudes would predict right frontal EEG asymmetry and enhanced cortisol reactivity in infants. Thus, while we would not rule out the possibility that augmented LPP amplitudes may predict infant temperament characteristics, it may be important to test this hypothesis in samples where comorbidity is low.
There is, then, at least suggestive evidence in our study for heterotypic continuity in emotional reactivity across generations (mother and child) from the pre- to early postnatal period. Several theories have been proposed to explain the ways that maternal biological functions that characterize the in utero environment strongly influence both baseline and reactive responses in infants that persist over time (Barker, 1998; Del Giudice, Ellis, & Shirtcliff, 2011;Gunnar & Quevedo, 2007; Sandman, Davis, Buss, & Glynn, 2011; Shirtcliff & Ruttle, 2010). In general, these developmental origin hypotheses posit that elevated levels of stress reactivity in mothers, sometimes measured in the domain of emotion, “programs” a propensity for greater infant reactivity or baseline function in infants that mirrors a reactive response. That is, when the in-utero environment is characterized by high levels of maternal reactivity, the fetus comes to anticipate the task of development under harsh or stressful circumstances; thus, baseline function in these infants may look more like reactive responses in infants with less reactive mothers. Similarly, reactive responses may be exacerbated. Appropriately, cortisol has been identified across theories as a primary target by which this process takes place (Del Giudice et al., 2011; Sandman et al., 2011; Shirtcliff & Ruttle, 2010). Cortisol is a hormone that crosses the placenta during pregnancy and appears to directly affect neural development in offspring. Similar to the current study, higher levels of maternal stress during the second, but not third, trimester predicted reduced gray matter density in the prefrontal cortex and temporal cortices (C. Buss et al., 2010). Activity in the prefrontal cortex was, of course, used to derive our infant measure of asymmetry and both the prefrontal cortex and temporal cortices have been implicated as part of a network that generates LPP in adults (Liu et al., 2012). Thus, the timing and form of heterotypic continuity in emotional reactivity observed in the current study is consistent with both theories about developmental programming and previous research on the functional significance of substrates for which findings were significant.
Specifically, infant temperament was predicted by maternal LPP during the second, but not third trimester. This effect was hypothesized based on previous work, although the precise mechanisms by which it operates have not been fully explicated. One possible reason may be that declines in stress reactivity appear late in gestation (Entringer et al., 2010), an effect that has also been suggested in relation to the LPP for this sample (Nyman et al., under review). Thus, maternal reactivity in the second trimester may be particularly important for child outcomes (Fan et al., 2016; O’Keane et al., 2011), including emotional development (Blair, Glynn, Sandman, & Davis, 2011). Indeed, the second trimester overlaps with an important sensitive critical period of neural development: the end of neurogenesis and beginning of synapse formation and pruning (Lenroot & Giedd, 2006). Of course, the degree to which second-trimester effects related to the LPP persist over time remains unknown. It should also be noted that elevated third trimester maternal cortisol does predict other domains of child functioning (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999) suggesting that there may be different critical periods for other infant outcomes.
Finally, we note that because of our design, none of the findings can reflect the possibility that greater negativity in infants simply make mothers more emotionally reactive, as this interpretation would necessitate an effect that operates backward in time. That is, the predictive nature of our findings relies on both the prospective longitudinal design and the fact that maternal LPP was measured before maternal reactivity could be influenced by interactions with infant temperament, assuming that the primary mechanism of child-to-parent effects is child behavior. This assumption is, of course, open to debate, but is strengthened in our sample by the lack of influence of pregnancy experiences and controls for prenatal maternal symptoms.
Despite its strengths, the current study is not without limitations. First, this work was conducted in a low-risk community sample who reported relatively low mean levels of depression and anxiety. Artificially restricted variability would, of course, more likely lead to null rather than significant findings, but this does create uncertainty about the extent to which the current results are generalizable to other populations. Similarly, null results may have resulted from a lack of representation of individuals who are high in symptoms of anxiety and depression. Limitations of the selected measures may also have contributed to the lack of consistency across domains of assessment or timing. For example, it is not necessarily the LPP is globally unrelated to maternal reported temperament, but the breadth of the scale selected for this work may have limited our ability to detect true underlying associations. Scales that more specifically tap flattened affect or “depressed-like” behavioral in infants may be more revealing. In addition, and as discussed above, measures that more specifically capture physiological processes linked to anxiety risk (e.g., heart rate variability or childhood ERN) may reveal more about the childhood consequences of elevated maternal LPP during pregnancy. Each of this should be considered in the interpretation of the current work and the design of future studies.
In addition, we note that although our analyses controlled for symptoms of depression and worry-related anxiety, the focus on general worry in maternal assessments and social fear in child assessments leaves intact the possibility that our findings reflect a residual association between maternal social anxiety symptoms and enhanced social fearfulness in infants. This possibility is underscored by the fact that maternal LPP was unrelated to maternal-reported negative reactivity, which included fear as only one aspect of overall reactivity. Effect sizes from past work suggest that maternal symptoms would not fully account for LPP amplitudes, making it unlikely that a subset of maternal symptoms would fully account for the association observed in our data. However, given our goal of identifying mechanisms for intergenerational transmission, future research should include efforts to control for other types of symptoms as well.
Third, we cannot rule out the possibility that some level of habituation to our photo stimuli occurred resulted from the repeated measures design. We cannot directly test, in our data, whether differences in the association between maternal LPP and infant temperament over time result directly from habituation. However, there was no evidence for habituation overall, no differences in LPP amplitudes from the second to third trimester, and no change in the difference between negative and neutral amplitudes over time. Previous work reporting habituation of the LPP (Codispoti, Ferrari, & Bradley, 2006) found small amounts of habituation using only three different images per participant. Specifically, each picture was presented 60 times in a single experimental session (as opposed to our presentation of each picture twice with a 16 week lag between presentations). Even with this high and dense rate of repetition, habituation was only seen after 41-60 presentations of each picture, with amplitudes appearing to plateau after about 30 presentations. More importantly for our work, which covaries out neutral LPP amplitudes, the LPP effect – or modulation of LPP by picture content – showed no evidence of habituation. Given this and the consistency of our findings with previous work on the effects of maternal emotion during pregnancy on infant temperament, we have some degree of confidence that our results are not accounted for by habituation, though this can only be directly tested in future work.
In sum, this work offered a unique prospective test of the degree to which infant temperamental reactivity could be predicted by a biological marker of emotion reactivity in mothers during pregnancy. Our findings reveal a specific neural process that appears to contribute to early programming of biological and behavioral correlates of emotion in early life. Our work also reveals nuances about timing and context that can aid in the understanding of the intergenerational transmission of emotional reactivity from mothers to offspring.
Supplementary Material
Acknowledgments
Data collection for this project was supported by K01 MH100240 from the National Institute of Mental Health and P20 GM104417 from the National Institute of General Medical Sciences. Infrastructure support was provided by P20 GM103474 from the National Institute of General Medical Sciences. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest with regard to the funding source for this study.
Abbreviations:
- LPP
Late positive potential
Footnotes
Though not immediately relevant for the current report, mothers-to-be also completed a go/no-go task, which is noted to maintain transparency in research procedures. The current focus is on the passive-viewing paradigm given its efficacy for eliciting LPP.
Recruitment for this study was already underway prior to the redefinition of full-term pregnancy as 39 weeks by the National Institutes of Health.
Though not immediately relevant for the current report, infants completed a total of 5 emotion-eliciting episodes (2 fear, 1 anger, 2 positivity), which is noted to maintain transparency in research procedures. We focused on the fear episode given our interest in negative reactivity and some question regarding the effectiveness of the anger-elicitation paradigm for infants who were not yet reaching and grasping.
Contributor Information
Rebecca J. Brooker, Department of Psychological and Brain Sciences, Texas A&M University, 515 Coke Street, College Station, TX 77843, USA
Elizabeth J. Kiel, Department of Psychology, Miami University; 90 North Patterson Avenue, Oxford, OH 45056, USA
Annmarie MacNamara, Department of Psychological and Brain Sciences, Texas A&M University, 515 Coke Street, College Station, TX 77843, USA.
Tristin Nyman, Department of Psychological and Brain Sciences, Texas A&M University, 515 Coke Street, College Station, TX 77843, USA.
Neha A. John-Henderson, Department of Psychology, Montana State University, PO Box 173440, Bozeman, MT, 59717, USA
Ryan Van Lieshout, Department of Psychiatry and Behavioural Neurosciences, McMaster University, St. Joseph’s Healthcare Hamilton, West 5th Campus, 100 West 5th, Hamilton, ON L8N 3K7, Canada.
Louis A. Schmidt, Department of Psychology, Neuroscience, & Behaviour, McMaster University, 1280 Main Street West, Hamilton, Ontario L8S 4K1, Canada
References
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Frontal EEG Asymmetry, Emotion, and Psychopathology, 67(1–2), 183–218. doi: 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Alomari AR, Fernandez M, Banks BJ, Acosta J, & Tartar LJ (2015). Acute stress dysregulates the LPP ERP response to emotional pictures and impairs sustained attention: Time-sensitive effects. Brain Sciences, 5(2). doi: 10.3390/brainsci5020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D (1998). In utero programming of chronic disease. Clinical Sciences, 95, 115–128. [PubMed] [Google Scholar]
- Bell MA, & Cuevas K (2012). Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development, 13(3), 281–294. doi: 10.1080/15248372.2012.691143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. doi: 10.3109/10253890.2011.594121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, & Karrass J (2010). Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Developmental Psychology, 46(4), 791–804. h doi: 10.1037/a0019673 [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental Science, 16(6), 864–878. doi: 10.1111/desc.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, & Sandman CA (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology, 35(1), 141–153. doi: 10.1016/j.psyneuen.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, & Sandman CA (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences, 109(20), E1312. doi: 10.1073/pnas.1201295109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA (2011). Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology, 47(3), 804–819. doi: 10.1037/a0023227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Kiel EJ, Brooker RJ, Beekman C, & Early MC (2013). Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. Journal of Clinical Child & Adolescent Psychology, 42(5), 603–616. doi: 10.1080/15374416.2013.769170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, … Fox NA (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 928–935. doi: 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(10), 1066–1075.e1. doi: 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, & Bradley MM (2006). Repetitive picture processing: Autonomic and cortical correlates. Brain Research, 1068(1), 213–220. doi: 10.1016/j.brainres.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Cole PM (1986). Children’s spontaneous control of facial expression. Child Development, 57(6), 1309–1321. doi: 10.2307/1130411 [DOI] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150(6), 782. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, & Leerkes EM (2004). Infant and maternal behaviors regulate infant reactivity to novelty at 6 months. Developmental Psychology, 40(6), 1123–1132. doi: 10.1037/0012-1649.40.6.1123 [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, & Leerkes EM (2006). Infant and maternal behavior moderate reactivity to novelty to predict anxious behavior at 2.5 years. Development and Psychopathology, 18, 17–34. doi: 10.1017/S0954579406060020 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. doi: 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1992). Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition, 20(1), 125–151. doi: 10.1016/0278-2626(92)90065-T [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & Fox NA (1989). Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology, 98(2), 127–131. doi: 10.1037/0021-843X.98.2.127 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & Irwin W (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3(1), 11–21. doi: 10.1016/S1364-6613(98)01265-0 [DOI] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81(1), 131–148. doi: 10.1111/j.1467-8624.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel-Schetter C, Hobel C, Chicz-DeMet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. doi: 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, & Sandman CA (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy, 6(3), 319–331. doi: 10.1207/s15327078in0603_1 [DOI] [Google Scholar]
- Dawson G, Frey K, Self J, Panagiotides H, Hessl D, Yamada E, & Rinaldi J (1999). Frontal brain electrical activity in infants of depressed and nondepressed mothers: Relation to variations in infant behavior. Development and Psychopathology, 11(03), 589–605. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Almas AN, & Fox NA (2010). Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry, 51(4), 497–517. doi: 10.1111/j.1469-7610.2010.02228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Resilience and Adaptive Aspects of Stress in Neurobehavioural Development, 35(7), 1562–1592. doi: 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro JM, Hilton SC, Hawkins M, Costigan KA, & Pressman EK (2002). Maternal stress and affect influence fetal neurobehaivoral development. Developmental Psychology, 38(5), 659–668. doi: 10.1037/0012-1649.38.5.659 [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Durbin CE, Hayden EP, & Olino TM (2010). Temperamental Positive and Negative Emotionality and Children’s Depressive Symptoms: A Longitudinal Prospective Study from Age Three to Age Ten. Journal of Social and Clinical Psychology, 29(4), 462–488. doi: 10.1521/jscp.2010.29.4.462 [DOI] [Google Scholar]
- Enders C (2010). Applied missing data analysis. New York: The Guilford Press. [Google Scholar]
- Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, … Wadhwa PD (2010). Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress, 13(3), 258–268. doi: 10.3109/10253890903349501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Zou Y, Zhang Y, Zhang J, Ma X, Liu Y, … Dart AM (2016). Effects of maternal cortisol during pregnancy on children’s blood pressure responses. Neuroendocrinology, 103(3–4), 282–290. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, & Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 919–927. doi: 10.1097/CHI.0b013e3181b21651 [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46, 521–530. doi: 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- Foti Dan, & Hajcak G (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6), 977–988. doi: 10.1162/jocn.2008.20066 [DOI] [PubMed] [Google Scholar]
- Fox NA, & Davidson RJ (1987). Electroencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month-old infants. Developmental Psychology, 23(2), 233–240. doi: 10.1037/0012-1649.23.2.233 [DOI] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development, 26(1), 64–86. doi: 10.1016/S0163-6383(02)00169-8 [DOI] [Google Scholar]
- Goldsmith HH, & Gagne JR (2012). Behavioral assessment of temperament In Zentner M & Shiner RL (Eds.), Handbook of Temperament (pp. 209–228). New York, New York: The Guilford Press. [Google Scholar]
- Goldsmith HH, & Campos JJ (1982). Toward a theory of infant temperament In Emde RN & Harmon RJ (Eds.), The development of attachment and affiliative systems (pp. 161–193). New York, New York: Plenum. [Google Scholar]
- Goldsmith HH, & Rothbart MK (1999). The Laboratory Temperament Assessment Battery (Lab-TAB): Pre-locomotor Version 3.1. Department of Psychology, University of Oregon. [Google Scholar]
- Gratton G, Coles MGH, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. doi: 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Gross JJ (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. doi: 10.1017/S0048577201393198 [DOI] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58(1), 145–173. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR (1992). Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics, 90(3), 491–497. [PubMed] [Google Scholar]
- Gunnar MR, & Nelson CA (1994). Event-related Potentials in Year-Old Infants: Relations with Emotionality and Cortisol. Child Development, 65(1), 80–94. doi: 10.1111/j.1467-8624.1994.tb00736.x [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953–967. doi: 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An Integrative Review. Developmental Neuropsychology, 35, 129–155. doi: 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Nieuwenhuis S (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6(4), 291–297. doi: 10.3758/CABN.6.4.291 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJB (1998). Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology, 74(5), 1310–1316. doi: 10.1037/0022-3514.74.5.1310 [DOI] [PubMed] [Google Scholar]
- Heller W, Nitscke JB, Etienne MA, & Miller GA (1997). Patterns of regional brain activity differntiate types of anxiety. Journal of Abnormal Psychology, 106(3), 376–385. doi: 10.1037/0021-843X.106.3.376 [DOI] [PubMed] [Google Scholar]
- Izard C, & Malatesta C (1987). Perspectives on emotional development I: Differential emotions theory of early development In Osofsky JD (Ed.), Handbook of infant development (pp. 494–554). New York: Wiley. [Google Scholar]
- Jones NA, Field T, Davalos M, & Pickens J (1997). EEG stability in infants/children of depressed mothers. Child Psychiatry & Human Development, 28(2), 59–70. doi: 10.1023/A:1025197101496 [DOI] [PubMed] [Google Scholar]
- Kagan J (1994). Galen’s prophecy: Temperament in human nature. New York, New York: Basic Books. [Google Scholar]
- Kagan J, & Snidman N (1999). Early childhood predictors of adult anxiety disorders. Biological Psychiatry, 46(11), 1536–1541. h doi: 10.1016/S0006-3223(99)00137-7 [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, & Davidson RJ (1998). Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behavioral Neuroscience, 112(1), 251–254. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JE, & Quitkin FM (2000). Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology, 36(3), 211–236. doi: 10.1016/S0167-8760(00)00078-7 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual [Technical Report A-8]. Gainesville, FL: University of Florida. [Google Scholar]
- Lenroot RK, & Giedd JN (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Methodological and Conceptual Advances in the Study of Brain-Behavior Dynamics: A Multivariate Lifespan Perspective, 30(6), 718–729. doi: 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, & Ding M (2012). Neural substrate of the late positive potential in emotion processing. The Journal of Neuroscience, 32(42), 14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue V, Coan JA, Thrasher C, & DeLoache JS (2011). Prefrontal asymmetry and parent-rated temperament in infants. PLoS ONE, 6(7), e22694. doi: 10.1371/journal.pone.0022694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebbe AM, Kiel EJ, & Buss KA (2011). Toddlers’ context-varying emotions, maternal responses to emotions, and internalizing behaviors. Emotion, 11(3), 697–703. doi: 10.1037/a0022994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Foti D, & Hajcak G (2009). Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion, 9(4), 531–543. doi: 10.1037/a0016251 [DOI] [PubMed] [Google Scholar]
- MacNamara A, & Hajcak G (2010). Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety, 27(3), 234–243. doi: 10.1002/da.20679 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and symptom-based predictors of emotional processing in Generalized Anxiety Disorder and Major Depressive Disorder: An event-related potential study. Cognitive Therapy and Research, 40(3), 275–289. doi: 10.1007/s10608-015-9717-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf S, Gunnar M, Kestenbaum R, Lang S, & Andreas D (1990). Infant proneness-to-distress temperament, maternal personality, and mother-infant attachment: Associations and goodness of fit. Child Development, 61(3), 820–831. doi: 10.1111/j.1467-8624.1990.tb02824.x [DOI] [PubMed] [Google Scholar]
- Marceau K, Hajal N, Leve LD, Reiss D, Shaw DS, Ganiban JM, … Neiderhiser JM (2013). Measurement and associations of pregnancy risk factors with genetic influences, postnatal environmental influences, and toddler behavior. International Journal of Behavioral Development, 37(4), 366–375. doi: 10.1177/0165025413489378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the penn state worry questionnaire. Behaviour Research and Therapy, 28(6), 487–495. doi: 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, & Moser JS (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. doi: 10.1016/j.brainres.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3), 395–422. doi: 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- Nyman T, Mistry S, Kiel EJ, Schmall LJ, & Brooker RJ (under review). Perceived social support moderates neural reactivity to negative stimuli during pregnancy: Implications for maternal depression. [Google Scholar]
- O’Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, … Patrick K (2011). Increased pituitary–adrenal activation and shortened gestation in a sample of depressed pregnant women: A pilot study. Journal of Affective Disorders, 130(1), 300–305. doi: 10.1016/j.jad.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Putnam SP (2011). Stability and instability of childhood traits: Implications for personality development of animals. Developmental Psychobiology, 53(6), 510–520. doi: 10.1002/dev.20578 [DOI] [PubMed] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, … Meaney MJ (2015). Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry, 5, e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament in children’s development In Eisenberg N (Ed.), Handbook of child psychology: Vol 3. Social, emotional, and personality development (6th ed., pp. 99–166). New York: Wiley. [Google Scholar]
- Sandman CA, Davis EP, Buss C, & Glynn LM (2011). Prenatal programming of human neurological Function. International Journal of Peptides, 2011, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite TJ (1999). Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology, 34(3), 163–173. doi: [DOI] [PubMed] [Google Scholar]
- Schmidt LA (2008). Patterns of second-by-second resting frontal brain (EEG) asymmetry and their relation to heart rate and temperament in 9-month-old human infants. Personality and Individual Differences, 44(1), 216–225. doi: 10.1016/j.paid.2007.08.001 [DOI] [Google Scholar]
- Shirtcliff EA, & Ruttle P (2010). Immunological and neuroendocrine dysregulation following early deprivation and stress. Attachment and Early Disorders of Development, Klett-Cotta, Stuttgart, Munich. [Google Scholar]
- Sroufe LA (1979). Socioemotional development In Osofsky JD (Ed.), Handbook of infant development (pp. 462–516). New York: Wiley. [Google Scholar]
- Tau GZ, & Peterson BS (2009). Normal Development of Brain Circuits. Neuropsychopharmacology, 35, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S (2006). Depression, anxiety, and restring frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology, 115(4), 715–729. doi: 10.1037/0021-843X.115.4.715 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mulder EJH, Mennes M, & Glover V (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Prenatal Programming of Behavior, Physiology and Cognition, 29(2), 237–258. doi: 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Weinberg MK, & Tronick EZ (1998). Emotional characteristics of infants associated with maternal depression and anxiety. Pediatrics, 102(Supplement E1), 1298–1304. [PubMed] [Google Scholar]
- Zentner M, & Bates JE (2008). Child temperament: An integrative review of concepts, research programs, and measures. International Journal of Developmental Science, 2(1), 7–37. doi: 10.3233/DEV-2008-21203 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.