Abstract
Brain injury caused by ischemic insult due to significant reduction or interruption in cerebral blood flow leads to disruption of practically all cellular metabolic pathways. This triggers a complex stress response followed by overstimulation of downstream enzymatic pathways due to massive activation of post-translational modifications (PTM). Mitochondria are one of the most sensitive organelle to ischemic conditions. They become dysfunctional due to extensive fragmentation, inhibition of acetyl-CoA production, and increased activity of NAD+ consuming enzymes. These pathologic conditions ultimately lead to inhibition of oxidative phosphorylation and mitochondrial ATP production. Both acetyl-CoA and NAD+ are essential intermediates in cellular bioenergetics metabolism and also serve as substrates for post-translational modifications such as acetylation and ADP-ribosylation. In this review we discuss ischemia/reperfusion-induced changes in NAD+ and acetyl-CoA metabolism, how these affect relevant PTMs, and therapeutic approaches that restore the physiological levels of these metabolites leading to promising neuroprotection.
Keywords: Ischemia, Brain, Mitochondria, NAD+, Acetyl-CoA
1. Introduction
At the onset, brain ischemia triggered by severely limited or absent blood flow, can be considered an acute mitochondrial disease. This is because the essential mitochondrial function, oxidative phosphorylation that generates ATP, is the first one ceased due to the depletion of tissue oxygen. Although the brain represents only 2% of body weight it is particularly sensitive to ischemic conditions since it utilizes about 20% of the total body oxygen consumption under resting conditions. Due to the ATP requirement for the majority of cellular functions, ischemia impacts practically every aspect of cellular metabolism in all cell types. Therefore, ischemia-induced brain damage is one of the most complex and devastating neurological condition.
Mitochondria are one of the major targets for development of effective treatment strategies in both acute brain injury and neurodegenerative diseases. They are essential in the maintenance of normal cellular functions, mainly through regulation of energy production [1–5]. The cells’ energy demand is affecting not only the activity of mitochondrial oxidative phosphorylation but also the mitochondrial structure and movement. Mitochondria respond to energetic stress by re-organizing their sub-cellular distribution and also by structural and morphological alterations [4,6–8]. This unique ability of mitochondria to spatially and morphologically adapt to changing intracellular environments is termed “mitochondrial dynamics”.
Bioenergetic stress and downstream effects caused by ischemic insult depletes NAD+ levels and compromises several metabolic pathways and mitochondrial functions [9–13]. Mitochondrial protein acetylation is dramatically reduced suggesting a deficiency in production of acetyl-CoA (AcCoA), a substrate for acetyltransferases. Additionally, mitochondria are extensively fragmented leading to failure in oxidative phosphorylation [14–16]. All these events lead to bioenergetics failure and ultimately cell death. In this review we will address the metabolic links between NAD+, AcCoA, and mitochondrial dynamics that are altered by ischemia/reperfusion triggered conditions.
2. Role of mitochondrial dynamics in mechanisms of pathophysiology
The state of mitochondrial dynamics is determined by the balance between activities of fission and fusion processes. There are several physiological functions of mitochondrial fission. Fragmented mitochondria can move more efficiently within the cell to reach areas where there is a higher demand for local ATP generation. Furthermore, by fragmentation the damaged and healthy mitochondrial proteins and DNA can be segregated into separate smaller organelles allowing the damaged, dysfunctional subpopulation to be eliminated by mitophagy [17,18]. Finally, during cell division mitochondrial fragmentation facilitates proper redistribution of mitochondrial mass into daughter cells [19]. However, an extensive and prolonged fission due to pathologic stress can lead to transformation of the whole mitochondrial population into submicron size organelles [14]. These individual organelles are too small to harbor the required amount of all essential metabolites and proteins for proper and effective function. Thus, the cellular demand for ATP generation cannot be met and ultimately will lead to bioenergetics failure and cell death. To reverse this process, the fragmented mitochondria need to fuse back so the contents of the small organelles can combine and stabilize protein and DNA levels for normal mitochondrial function. Therefore, fusion, by combining the contents of functionally compromised small organelles into functioning mitochondria, mitigates the effects of cellular stress. For example, CA1 neurons in the hippocampus are the most vulnerable to ischemic attack and mitochondria in these neurons are extensively fragmented following ischemic insult [14,15]. This highly fragmented state lasts for several days and CA1 neurons ultimately die. On the other hand the CA3 and dentate gyrus neurons of the hippocampus are resistant to ischemic conditions [20–22]. Although, mitochondria in these cells are fragmented directly after the ischemic insult, later at 24 h of reperfusion the highly fragmented population is significantly reduced, and the number of longer mitochondria increases when compared to immediate post-insult state [14]. This suggests that at later recovery time, factors stimulating the fission process are diminished or the fusion activity is sufficiently increased to reverse the fission process. Interestingly, similar temporal profile of mitochondrial fragmentation is observed also in astrocytes following acute brain injury [14,23].
Mitochondrial fission and fusion is a highly controlled process by several cytosolic and mitochondrial proteins belonging to the GTPase family (for review see [24–27]). There are a separate set of fusion proteins that control the outer and the inner membrane fusion. Mitofusin1 and mitofusin2 (Mfn1 and Mfn2) mediate the mitochondrial outer membrane fusion, while the inner membrane fusion is regulated by dynamin-like GTPase encoded by optic atrophy 1 gene (Opa1) [18,28]. Fission is facilitated by the dynamin-related protein1 (Drp1) which needs to be recruited from the cytosol to the outer mitochondrial membrane. Several proteins on the outer membrane serve as recruitment factors for Drp1, mitochondrial fission factor (MFF), Fis1 protein, and mitochondrial dynamic proteins 49/51 (MiD49/51) [29,30]. Overall modulation of the fission and fusion process is rather complex, involving several post-translational modifications [4,24,26,27]. Thus, the activity of these proteins is tightly regulated by phosphorylation, acetylation, ADP-ribosylation, S-nitrosylation, SUMOylation, ubiquitination, o-linked-N-acetyl-glucosamine glycosylation, and proteolytic cleavage [27,31–33]. Maintenance of the proper balance between mitochondrial fission and fusion by post-translational modifications is essential not only for facilitating normal mitochondrial bioenergetic function but also for dynamic cellular stress response to pathological conditions. In next paragraphs we discuss the impact of ischemia on NAD+ and AcCoA metabolism that modulates acetylation of cellular and mitochondrial proteins.
3. NAD+ metabolism and the cellular and mitochondrial acetylome
3.1. NAD+ metabolism and pathophysiology of brain injury
NAD+ is one of the most abundant metabolic intermediate that is required for about 500 enzymatic reactions. As a cofactor it is essential for activity of pyruvate dehydrogenase complex (PDHC) and several mitochondrial enzymes in the TCA cycle that reduce NAD+ to NADH. The matrix localized NADH serves as an electron donor to complex I of the respiratory chain where it is reoxidized back to NAD+. Similarly, NADH (and its phosphorylated form NADPH) participates as a cofactor in the glycolytic pathway, pentophosphate pathway, ketone body, fatty acid, and amino acid metabolism.
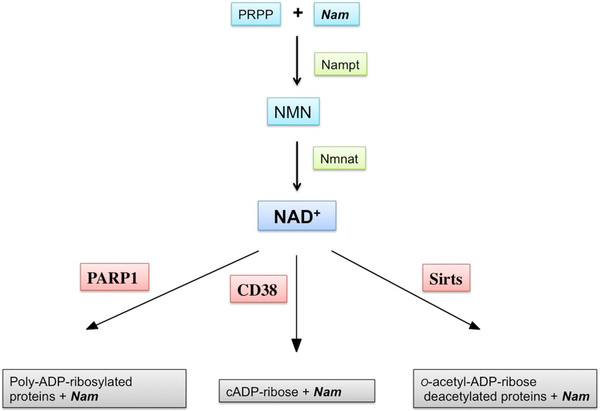
NAD+, apart being a cofactor, also serves as a substrate for NAD+-consuming enzymes that catalyze NAD+-dependent protein modifications including poly- and mono-ADP-ribosylation [34]. The CD38 enzyme that generates the second messenger cyclic-ADP-ribose (cADPR) also utilizes NAD+ as a substrate [35]. Finally, sirtuins, a class III NAD+ dependent de-acetylases remove the acetyl group from a target protein and transfer it on the ADP-ribose moiety after release of nicotinamide (Nam) [36–38] (Fig. 1).
Fig. 1.
NAD+ catabolism and the NAD+ salvage pathway. NAD+-consuming enzymes poly-ADP-ribose polymerase 1 (PARP1), CD38, and sirtuins (Sirts) cleave nicotinamide (Nam) from NAD+. PARP1 forms complex ADP-ribose polymers that are attached to the target protein. CD38 generates cADP ribose. Sirts conjugate ADP-ribose with an acetyl group removed from a lysine residue of an acetylated protein, generating o-acetyl-ADP-ribose. Released Nam is then recycled in the NAD+ salvage pathway by nicotinamide phosphotransferase (Nampt) that generates nicotinamide mononucleotide (NMN) from Nam and phosphoribose pyrophosphate (PRPP). NMN is then converted to NAD+ by nicotinamide mononucleotide adenylyl transferase (Nmnat).
The NAD+ pools have been shown to decrease due to pathologic conditions including ischemic insult or traumatic brain injury (TBI) [39–41]. This is caused by the generation of free radicals during reperfusion leading to DNA damage and activation of NAD+ consuming poly-ADP-ribose polymerase 1 (PARP1) [9,42]. PARP1 utilizes NAD+ as a substrate for poly-ADP-ribosylation of specific nuclear proteins, including histones, to facilitate DNA repair [43,44]. Uncontrolled activation of PARP1 can deplete cellular NAD+ leading to inhibition of ATP production and cell death [45,46]. The increase in poly-ADP-ribose (PAR) levels was reported already after the first 2 h of recovery following ischemic insult and was associated with NAD+ depletion [9,40,42,47]. An additional depletion of tissue NAD+ pools was observed at 24 h of recovery, which is linked to an increased activity of CD38 [47]. The NAD+ catabolism after acute brain injury was reduced by treating the animals with PARP1 inhibitors [9,39,42] or in PARP1 null animals [48].
All enzymes that utilize NAD+ as a substrate cleave the nicotinamide (Nam) moiety and generate ADP-ribose (for review see [13]). The released Nam can then be recycled via the NAD+ salvage pathway by nicotinamide phosphotransferase (Nampt). Nampt generates nicotinamide mononucleotide (NMN) from Nam and phosphoribose pyrophosphate (PRPP). The NMN is then used by nicotinamide mononucleotide adenylyl transferase (NMNAT) to synthetize NAD+ in the presence of ATP [13,49–51] (Fig. 1).
Another approach applied to prevent the depletion of post-ischemic NAD+ pools was to stimulate the NAD+ salvage pathway by administering precursors, Nam or NMN [40,52,53]. By feeding into the NAD+ salvage pathway one can facilitate NAD+ synthesis. However, Nam and NMN also inhibit PARP1 and CD38 [40,47,52,54,55] and therefore also inhibit the NAD+ depletion by reducing its degradation.
3.2. Brain injury and mitochondrial NAD+ metabolism
Although, it was established that acute brain injury is associated with brain tissue NAD+ depletion it is still elusive in which subcellular compartment the NAD+ levels are the most affected. In brain cells, depending on the cell type, 25% (in astrocytes) to 50% (in neurons) of NAD+ is localized to mitochondria [56]. The mechanisms that maintain mitochondrial NAD+ pools remain unclear.
Nampt, the rate-limiting enzyme of the NAD+ salvage pathway, has been detected in the nucleus and cytoplasm [57]. In the brain it is expressed mainly in the cytoplasmic fraction of neurons [58]. Therefore, to generate NAD+ in the mitochondria the product of Nampt, NMN, needs to be transported into the mitochondrial matrix. Mitochondrial NAD+ is then synthetized by mitochondrial isoform of NMNAT (NMNAT3) [59,60]. However, the mechanisms of NMN transport across the mitochondrial membrane are not known.
One could also replenish mitochondrial NAD+ via its translocation from the cytosolic compartment. In yeast and plants, a membrane carrier protein transporting NAD+ across the inner mitochondrial membrane from the cytoplasm into mitochondria has been identified [61,62]. Surprisingly, overexpression of plant and yeast mitochondrial NAD+ carrier in human cells caused a switch from oxidative phosphorylation to glycolytic metabolism, reduction of cellular ATP levels, and resulted in dramatic growth retardation [63]. Thus, these data suggest that direct NAD+ import is likely to be absent from mammalian mitochondria and the major mechanism that replenishes intra-mitochondrial NAD+ is the NMNAT3 enzyme driven synthesis [60,63]. However, recent findings challenge this conclusion [64] and also the presence of active NMNAT3 enzyme in human mitochondria was questioned [65]. Thus, until the NMN or NAD+ transporter in mammalian mitochondria will be unequivocally identified and characterized, the intra-mitochondrial NAD+ metabolism will remain a matter of debate.
There are two possible mechanisms that can lead to reduction of mitochondrial NAD+ levels. First, under high oxidative stress or mitochondrial calcium overload a large, high conductance pore can be formed in the inner mitochondria membrane, called the mitochondrial permeability transition (MPT) pore [66]. Since it allows solutes of molecular weight up to 1500 Da to diffuse across the inner membrane, the activation of MPT leads to mitochondrial depolarization, leakage of mitochondrial NAD+ into the cytosol, and osmotic swelling [67–69]. Even if pore opening is transient and does not lead to an extensive mitochondrial swelling, a potentially significant loss of matrix NAD+ will result in inhibition of all NAD+ dependent metabolic processes including oxidative phosphorylation [70].
Second, mitochondrial NAD+ pools can also be reduced by activation of intra-mitochondrial enzymes that utilize NAD+ as substrate. The major NAD+ consuming enzyme following TBI or ischemic brain injury is PARP1. Although there are reports suggesting a presence of intra-mitochondrial PARP1 activity causing increase in poly-ADP-ribosylation of mitochondrial proteins following TBI, so far there is no consensus whether it can lead to pathologic depletion of intra-mitochondrial NAD+ pools [13]. Furthermore, to our knowledge there are no systematic studies examining the effect of acute brain injury on brain mitochondrial NAD+ pools.
3.3. NAD+ and mitochondrial protein acetylation
Sirtuins (Sirts) serve as metabolic sensors due to their dependence on NAD+ as a substrate [36]. The Sirt family of proteins is comprised from seven members that show a discrete pattern of subcellular localization. Sirt1, Sirt6, and Sirt7 are localized in the nucleus but also reports show a presence of Sirt1 in the cytosol, suggesting that Sirt1 can shuttle to the cytosol under specific circumstances [71]. Sirt2 is localized in the cytosol, and Sirt3, Sirt4, and Sirt5 were identified as mitochondrial proteins [72]. However, only Sirt3 is considered the major mitochondrial deacetylase [73,74]. Stimulation of Sirt3 by caloric restriction or following administration of NAD+ precursors leads to activation of TCA cycle enzyme glutamate dehydrogenase (GDH) [73] and isocitrate dehydrogenase 2 (IDH2) [75]. Furthermore, Sirt3 deacetylates components of the mitochondrial respiratory complexes, interacts with ATP synthase [76], and activates mitochondrial superoxide dismutase (SOD2). Deacetylation of SOD2 protects the cells against reactive oxygen species (ROS) [75,77]. Finally, Sirt3 plays an important role in protecting mitochondria against excitotoxic insult by deacetylating cyclophilin D (cypD), which leads to inhibition of its activity. CypD is a major regulator of the MPT pore [78]. As mentioned in the previous paragraph a prolonged opening of the MPT pore leads to dissipation of mitochondrial membrane potential, loss of matrix solutes including NAD+, inhibition of oxidative phosphorylation, cessation of mitochondrial ATP production, and swelling [68,79,80]. Thus, by deacetylating and inhibiting CypD, Sirt3 prevents MPT formation, and helps to maintain mitochondrial functions under stress conditions [77,81].
In summary, loss of mitochondrial NAD+ has multiple pathologic consequences that are associated with NAD+ roles as a cofactor for several key metabolic enzymes and also as a substrate for post-translational modifications. Therefore, depleted mitochondrial NAD+ pools lead to inhibition of oxidative phosphorylation and the TCA cycle enzyme activity. Furthermore, the activity of intra-mitochondrial enzymes that utilize NAD+ as a substrate for signaling reactions, most importantly deacetylation, are compromised. As a result, due to the reduced activity of Sirt3, increased acetylation of CypD and SOD2 will increase mitochondrial sensitivity to MPT inducing stress and mitochondria will be a more active source of free radicals.
4. Metabolic interplay of NAD+ and acetyl-CoA
4.1. Acetyl-CoA, protein acetylation, and ischemic injury
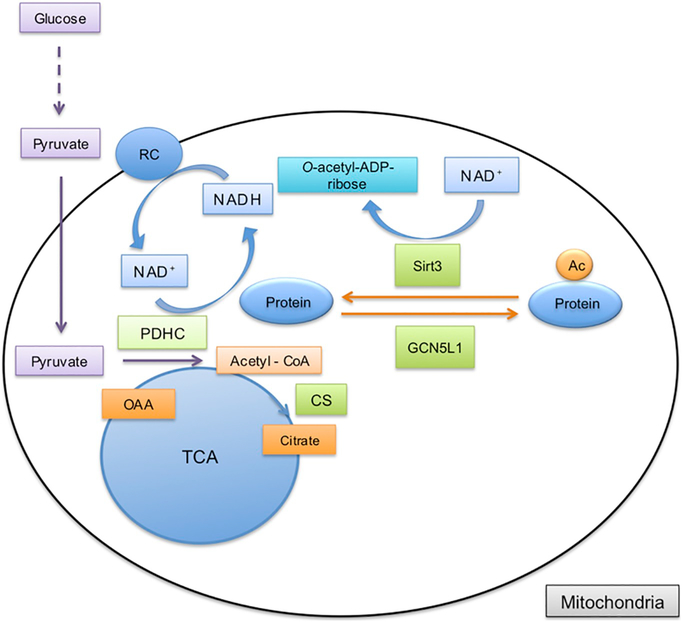
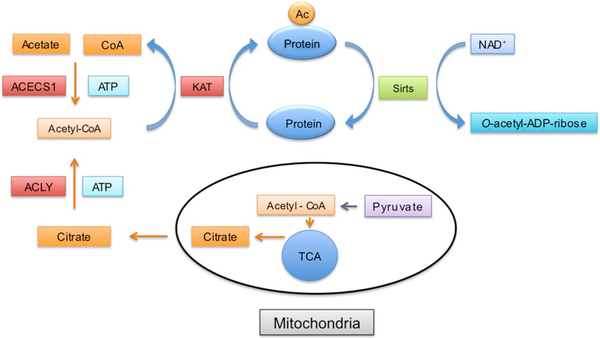
The acetylation status of proteins is determined by the dynamic interplay between deacetylases and acetyl‐transferases. The level of mitochondrial protein acetylation is controlled by mitochondria specific acetyl‐transferase, GCN5L1, and deacetylase, Sirt3 [82] (Fig. 2). Acetyl‐transferases use AcCoA as source of acetyl groups that are transferred onto lysine residues of the target protein. In brain cells the AcCoA is a metabolite mainly derived from glucose [83]. During glycolysis, glucose is converted to pyruvate, which is transported into mitochondria where the mitochondrial pyruvate dehydrogenase complex (PDHC) catalyzes the oxidative decarboxylation of pyruvate to generate AcCoA. As mentioned above PDHC requires NAD+ as a cofactor since during this process the NAD+ is reduced to NADH. In the next step of the TCA cycle mitochondrial citrate synthase (CS) forms citrate from AcCoA and oxaloacetate (Fig. 2). Citrate can either be oxidized by aconitase in the TCA cycle or it can be transported to the cytosol as a substrate for the ATP citrate lyase (ACLY). This enzyme generates cytosolic AcCoA from citrate in the presence of ATP (Fig. 3) [84]. Furthermore, in cytosol AcCoA can also be generated from acetate by acetyl-CoA synthetase (ACECS1) (for review see [85]). During this reaction ATP is used and pyrophosphate is also released. Two isoforms are known in mammalian cells [86].
Fig. 2.
Mitochondrial acetyl-CoA metabolism and protein acetylation. Pyruvate formed during glucose metabolism in the cytosol is transported into the mitochondria. Acetyl-CoA is then generated by pyruvate dehydrogenase complex (PDHC) from pyruvate. During this reaction NAD+ is reduced to NADH that donates electrons to complex I in the respiratory chain (RC). In the TCA cycle citrate is produced by citrate synthase (CS) from acetyl-CoA and oxaloacetate (OAA). Acetyl-CoA can also be used by mitochondrial acetyltransferase, GCN5L1, to acetylate mitochondrial proteins. The acetyl group is removed from the target protein by mitochondrial deacetylase, Sirt3, which uses NAD+ and releases the deacetylated protein and o-acetyl-ADP-ribose.
Fig. 3.
Cytosolic acetyl-CoA metabolism and protein acetylation. Citrate generated from acetyl-CoA is transported into the cytosol where it is a substrate for ATP citrate lyase (ACLY). This enzyme converts citrate back to acetyl-CoA in the presence of ATP. Acetyl-CoA can be also synthetized by cytosolic acetyl-CoA synthetase (ACECS1) from acetate, CoA and ATP. Similarly, as in mitochondria, acetyl-CoA is used for acetylation of proteins by acetyltransferases (KAT). The acetylated proteins are deacetylated by sirtuins where the deacetylation is coupled to NAD+ hydrolysis and o-acetyl-ADP-ribose is released.
Interestingly, mainly neurons are immunopositive for acetylated histones [87], probably due to their higher AcCoA levels. This is most likely because although neurons constitute only about 10% of brain cells they consume 70% of glucose and oxygen supplied to this organ. Thus, high glycolytic and TCA cycle metabolic flux leads to generation of higher AcCoA levels that drive the acetyl‐transferase activity. This is then reflected in increased acetylation of neuronal histone and non-histone proteins. To our knowledge there are no reports of either cell-type specific or subcellular distribution of AcCoA pools in the brain. Furthermore, studies examining changes in cellular or mitochondrial AcCoA levels following ischemic insult would be also required to shed more lights on mechanism that lead to post-ischemic pathophysiology.
During ischemia the glucose and oxygen delivery to the brain is abolished and the production of AcCoA is discontinued after glucose pools are depleted [88,89]. As a consequence of reduced cytosolic and nuclear AcCoA levels the histone acetylation is significantly reduced following ischemia [87,90]. This substantial reduction of histone acetylation affects chromatin folding, the control of DNA accessibility and transcriptional activation [91], leading to deficiency in proper response to stress conditions. The pathologic implications of such excessively low levels of histone acetylation are supported by the neuroprotective effect of class I, II and IV histone deacetylase (HDAC) inhibitors [87,92,93]. Treatment of animals subjected to ischemia with pan HDAC inhibitors such as Trichostatin A (TSA) or suberanilohydroxamic acid (SAHA), normalized the post-ischemic histone acetylation and resulted in significant neuroprotection [92], for review see [94].
Interestingly, to achieve neuroprotective effects against ischemic brain damage the increased activity of class III NAD+-dependent HDACs, Sirts, are required. This is probably because Sirt targets control expression of genes involved in neuroprotection pathways and also Sitr1, Sirt2, and Sirt3–5 modulate activity of non-histone proteins and transcription factors linked to cellular bioenergetic metabolism, inflammation, and autophagy. Since cellular NAD+ levels are significantly depleted following acute brain injury, the activity of these enzymes is compromised during the recovery period. Thus, by replenishing the NAD+ levels or administering Sirt activators (such as resveratrol) the acetylation of the target proteins can be restored. Although reports are not available, one would expect that the loss of mitochondrial NAD+ would lead to the inhibition of Sirt3 activity and increased acetylation of mitochondrial proteins. As mentioned above this could lead to further inhibition of oxidative phosphorylation and the TCA cycle with increased sensitivity of mitochondria to MPT inducing stress and higher ROS production rates. Replenishing the mitochondrial NAD+ levels could then reverse the negative effect of hyperacetylation due to activation of Sirt3.
4.2. N-acetyl-aspartate as indicator of neuronal damage and source of acetyl-CoA in non-neuronal brain cells
N-acetyl-aspartate (NAA) is the most abundant acetylated brain metabolite synthetized in neuronal mitochondria. Synthesis of NAA is catalyzed by L-aspartate N-acetyltransferase (Asp-NAT) via trans-acetylation of AcCoA and aspartate. Several studies demonstrated that in adult rat brain NAA synthesis takes place in neuronal mitochondria from AcCoA, generated from glucose and aspartate, a product of TCA cycle [95–97]. The NAA synthesis rate is one to two orders of magnitude slower when compared with the synthesis rate of other brain metabolites [98]. NAA is predominantly localized in neurons, oligodendrocytes-type-2, and myelin, whereas astrocytes and mature oligodendrocytes contain very low levels of NAA [99]. Following the synthesis, NAA is transported down the axons and used by oligodendrocytes for myelin synthesis, repair, and maintenance. NAA provides 30% of necessary AcCoA for myelin lipid synthesis. Specifically, NAA is taken by oligodendrocytes in axo-glial contact zones and converted into acetate and subsequently AcCoA (see [98]). Although NAA synthesis and turnover is very slow and relies on existing AcCoA and aspartate, following ischemic injury NAA levels decrease quickly and correlate with the fast decrease in ATP [100–102]. This was interpreted that following the acute brain injury like TBI or stroke, during the ‘metabolic crisis’ due to disrupted oxidative glucose metabolism, the NAA may serve as substrate and provide acetyl moieties to sustain oxidative metabolism and also help to maintain histone proteins acetylation levels.
4.3. Acetyl-carnitine and ketone bodies offer neuroprotection via acetyl-CoA metabolism
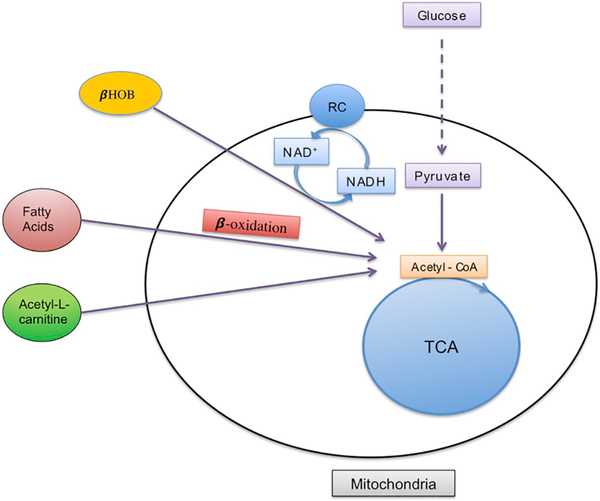
The brain is capable of replenishing the AcCoA pool via metabolism of alternative substrates, i.e. ketones, fatty acids, and acetyl-carnitine [103] (Fig. 4). This innate ability of brain to utilize alternative substrates for energy is highly important during pathological conditions such as stress, stroke, and brain trauma, which are characterized by impaired oxidative glucose metabolism and increased lactate production. The ability of the brain to use these substrates has been known for years, however, recent research re-examines these phenomena with specific attention to cell-, and compartment-specific points of view. From circulating ketone bodies represented by β-hydroxybutyrate (βOHB), acetoacetate, and acetone, βOHB is the most abundant ketone body. It is generated by the liver under starvation and is transported into brain cells by the monocarboxylate transporters via a sodium-independent and sodium-dependent manner (for review see [104]). It can enter directly into the mitochondria, however this pathway is yet to be understood. Once in mitochondria, βOHB is converted to acetoacetate via β-hydroxybutyrate dehydrogenase (BDH), which requires NAD+ as cofactor, thus this reaction results in production of acetoacetate and NADH [74]. It is interesting to note that BDH contains several sites for Sirt3 regulation, but whether the activity of BDH is affected by acetylation remains to be determined [105]. The ability to utilize βOHB for brain energy and metabolism is a subject of regional and developmental regulation. Specifically, βOHB is a preferred substrate for energy and metabolism in the developing brain. However, it is also present in the adult brain during caloric restriction, starvation, and after exogenous administration in high concentration [106]. All brain cells are capable of utilizing βOHB for respiration, however, neurons and oligodendrocytes use βOHB more efficiently than astrocytes [107]. Furthermore, βOHB has been shown to increase mitochondrial respiration, ATP production, and NAD+/NADH ratio in cortical neurons even in the presence of 1 mM of glucose [108]. Experiments using 13C NMR (nuclear magnetic resonance spectroscopy) showed that βOHB was oxidized to a greater extent in neurons when compared to cortical astrocytes [109].
Fig. 4.
Acetyl-CoA synthesis supported by β-hydroxybutyrate (βOHB), fatty acids β-oxidation, and acetyl-l-carnitine metabolism. Acetyl-CoA can be generated from pyruvate, βOHB, β-oxidation of fatty acids (particularly in astrocytes), and from acetyl-l-carnitine. During these metabolic reactions NAD+ is reduced to NADH.
Increased ketone body utilization results in the significant rise of AcCoA and decrease in available Coenzyme A. Thus, βOHB is capable of supporting oxidative metabolism by increasing mitochondrial concentrations of AcCoA and increasing intra-mitochondrial concentrations of NADH (see review [110]).
Although a ketogenic diet has been used for treatment of refractory epilepsy for decades, the mechanisms of neuroprotection offered by ketones are just recently beginning to be understood. Hepatic generation of ketones following mobilization of endogenous triglycerides, fatty acids, and their subsequent metabolism via β-oxidation has been well studied under starvation, caloric restriction, and exogenous administration in both humans and animals. However, little is known about the brain’s endogenous ability to generate ketones. It was demonstrated that in vitro astrocytes are the only cells capable to use fatty acids for oxidative metabolism via β-oxidation, suggesting that astrocytes can generate ketones for neighboring neurons [111]. While Cahoy et al. [112] showed that genes responsible for fatty acid metabolism are present in all cells, however, the comparison of neuronal and astrocytic transcriptional profiles lead to conclusion that fatty acids oxidation is a constitutive metabolic pathway in astrocytes [112,113].
Hence, the astrocytic AcCoA pool can be replenished by fatty acids oxidation and is subjected to regional and developmental regulation [114]. Recent evidence demonstrates that in addition to astrocytes neural stem/progenitor cells are also dependent on fatty acid oxidation in their quiescent state [115]. Astrocytes are capable to upregulate fatty acid oxidation in response to injury and stimulation of this pathway by 3,3,5 triiodo-L-thyronine (T3) resulted in decreased lesion volume in stroke model [116].
Apart from feeding into mitochondrial respiration, ketone bodies also decease the production of ROS by complex I [117], induce BDNF gene expression via activation of the transcription factor NF-kB, and its interaction with the histone acetyltransferase p300/EP300 [108]. Furthermore, βOHB is an inhibitor of class I histone deacetylases (HDACs) [118]. Thus, the neuroprotective effect of βOHB is also exerted via mechanisms similar to pan-HDACs inhibitors TSA and SAHA.
Acetyl-carnitine, the shortest acylcarnitine generated via β-oxidation, doesn’t require transferases for intra-mitochondrial transport and provides directly acetyl moieties for the TCA cycle. Using 13C NMR, Scafidi et al., showed that astrocytes utilize acetyl-l-carnitine for energy and neurotransmitter synthesis [119]. Exogenous administration of acetyl-carnitine has been shown to be neuroprotective following ischemia, traumatic brain injury, multiple sclerosis, and peripheral nerve injury [120–123]. Thus, these alternative substrates, βOHB, fatty acids, and acetyl-l-carnitine, provide acetyl moieties for bioenergetics and lipids metabolism. Additionally, by altering AcCoA levels, they can affect histone and non-histone protein acetylation. Thus, they may serve as a therapeutic intervention following the acute brain injury or for chronic neurodegenerative diseases.
5. Conclusions
Both NAD+ and AcCoA are cellular metabolic intermediates that are essential for amino acids, fatty-acids, and bioenergetic metabolism. Furthermore, they influence gene expression by serving as cofactors for epigenetic modifiers mediating post-translational alterations of histone and non-histone proteins. Thus, the concentrations of AcCoA and NAD+ affect the acetylation levels of proteins controlling transcriptional regulation and metabolic status. As we discussed both NAD+ and AcCoA metabolism is disturbed following ischemic stress and there is a complex interplay between downstream effects due to imbalance in NAD+ and AcCoA homeostasis. The majority of AcCoA is generated via NAD+ dependent processes from pyruvate resulting in an intimate relationship between the mechanisms involved in NAD+, AcCoA metabolism, and mitochondrial function and dynamics. Due to the complexity of postischemic pathology that involves changes in almost every metabolic pathway, a successful treatment strategy will need to comprise of a multi-targeted approach, using compound that affects multiple pathways. Administration of intermediates that can modulate the post-insult NAD+ and AcCoA levels represents a promising way to manipulate several pathways since these metabolites are involved in many enzymatic reactions and also play a significant role in regulating enzymes activity and gene expression via post-translational modifications.
Acknowledgements
This work was supported by U.S. Department of Veterans Affairs Merit award BX000917 to TK.
Footnotes
Conflict of interests
The authors declare no conflict of interests.
Transparency document
The Transparency document associated with this article can be found in online version.
This article is part of a Special Issue entitled: Post-Translational Modifications In Brain Health And Disease edited by Paula Moreira, Susana Cardoso and Sónia Correia.
References
- [1].Martin LJ, Mitochondrial and cell death mechanisms in neurodegenerative diseases, Pharmaceuticals (Basel) 3 (2010) 839–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schon EA, Przedborski S, Mitochondria: the next (neurode)generation, Neuron 70 (2011) 1033–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anne Stetler R, Leak RK, Gao Y, Chen J, The dynamics of the mitochondrial organelle as a potential therapeutic target, J. Cereb. Blood Flow Metab. 33 (2013) 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen H, Chan DC, Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases, Hum. Mol. Genet. 18 (2009) R169–R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Helley MP, Pinnell J, Sportelli C, Tieu K, Mitochondria: a common target for genetic mutations and environmental toxicants in Parkinson’s disease, Front. Genet. 8 (2017) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mannella CA, Structure and dynamics of the mitochondrial inner membrane cristae, Biochim. Biophys. Acta 1763 (2006) 542–548. [DOI] [PubMed] [Google Scholar]
- [7].Hackenbrock CR, Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria, J. Cell Biol. 37 (1968) 345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hackenbrock CR, Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria, J. Cell Biol. 30 (1966) 269–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Strosznajder RP, Gadamski R, Czapski GA, Jesko H, Strosznajder JB, Poly (ADP-ribose) polymerase during reperfusion after transient forebrain ischemia: its role in brain edema and cell death, J. Mol. Neurosci. 20 (2003) 61–72. [DOI] [PubMed] [Google Scholar]
- [10].Chiarugi A, Moskowitz MA, Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders, J. Neurochem. 85 (2003) 306–317. [DOI] [PubMed] [Google Scholar]
- [11].Chiarugi A, Intrinsic mechanisms of poly(ADP-ribose) neurotoxicity: three hypotheses, Neurotoxicology 26 (2005) 847–855. [DOI] [PubMed] [Google Scholar]
- [12].Strosznajder RP, Czubowicz K, Jesko H, Strosznajder JB, Poly(ADP-ribose) metabolism in brain and its role in ischemia pathology, Mol. Neurobiol. 41 (2010) 187–196. [DOI] [PubMed] [Google Scholar]
- [13].Owens K, Park JH, Schuh R, Kristian T, Mitochondrial dysfunction and NAD+ metabolism alterations in the pathophysiology of acute brain injury, Transl Stroke Res 4 (2013) 618–634. [DOI] [PubMed] [Google Scholar]
- [14].Owens K, Park JH, Gourley S, Jones H, Kristian T, Mitochondrial dynamics: cell-type and hippocampal region specific changes following global cerebral ischemia, J. Bioenerg. Biomembr. 47 (2015) 13–31. [DOI] [PubMed] [Google Scholar]
- [15].Kumar R, Bukowski MJ, Wider JM, Reynolds CA, Calo L, Lepore B, Tousignant R, Jones M, Przyklenk K, Sanderson TH, Mitochondrial dynamics following global cerebral ischemia, Mol. Cell. Neurosci. 76 (2016) 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao YX, Cui M, Chen SF, Dong Q, Liu XY, Amelioration of ischemic mitochondrial injury and Bax-dependent outer membrane permeabilization by Mdivi-1, CNS Neurosci. Ther. 20 (2014) 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lemasters JJ, Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging, Rejuvenation Res. 8 (2005) 3–5. [DOI] [PubMed] [Google Scholar]
- [18].Youle RJ, van der Bliek AM, Mitochondrial fission, fusion, and stress, Science 337 (2012) 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K, Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission, J. Biol. Chem. 282 (2007) 11521–11529. [DOI] [PubMed] [Google Scholar]
- [20].Smith ML, Auer RN, Siesjo BK, The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia, Acta Neuropathol. 64 (1984) 319–332. [DOI] [PubMed] [Google Scholar]
- [21].Sheng H, Laskowitz DT, Pearlstein RD, Warner DS, Characterization of a recovery global cerebral ischemia model in the mouse, J. Neurosci. Methods 88 (1999) 103–109. [DOI] [PubMed] [Google Scholar]
- [22].Onken M, Berger S, Kristian T, Simple model of forebrain ischemia in mouse, J.Neurosci. Methods 204 (2012) 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Gotz M, Winklhofer KF, Hrelia S, Bergami M, Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance, Cell Metab. 18 (2013) 844–859. [DOI] [PubMed] [Google Scholar]
- [24].Ni HM, Williams JA, Ding WX, Mitochondrial dynamics and mitochondrial quality control, Redox Biol. 4 (2015) 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Olichon A, Guillou E, Delettre C, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P, Mitochondrial dynamics and disease, OPA1, Biochim. Biophys. Acta 1763 (2006) 500–509. [DOI] [PubMed] [Google Scholar]
- [26].Suen DF, Norris KL, Youle RJ, Mitochondrial dynamics and apoptosis, Genes Dev. 22 (2008) 1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klimova N, Long A, Kristian T, Significance of mitochondrial protein post-translational modifications in pathophysiology of brain injury, Transl Stroke Res 9 (2018) 223–237. [DOI] [PubMed] [Google Scholar]
- [28].Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC, Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion, Mol. Biol. Cell 20 (2009) 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee H, Yoon Y, Mitochondrial fission and fusion, Biochem. Soc. Trans. 44 (2016) 1725–1735. [DOI] [PubMed] [Google Scholar]
- [30].Onoue K, Jofuku A, Ban-Ishihara R, Ishihara T, Maeda M, Koshiba T, Itoh T, Fukuda M, Otera H, Oka T, Takano H, Mizushima N, Mihara K, Ishihara N, Fis1 acts as a mitochondrial recruitment factor for TBC1D15 that is involved in regulation of mitochondrial morphology, J. Cell Sci. 126 (2013) 176–185. [DOI] [PubMed] [Google Scholar]
- [31].Wilson TJ, Slupe AM, Strack S, Cell signaling and mitochondrial dynamics: implications for neuronal function and neurodegenerative disease, Neurobiol. Dis. 51 (2013) 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR 3rd, Hoshijima M, Dillmann W, Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes, J. Biol. Chem. 287 (2012) 30024–30034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP, SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress, Mol. Cell. Biol. 34 (2014) 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koch-Nolte F, Fischer S, Haag F, Ziegler M, Compartmentation of NAD+-dependent signalling, FEBS Lett. 585 (2011) 1651–1656. [DOI] [PubMed] [Google Scholar]
- [35].States DJ, Walseth TF, Lee HC, Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38, Trends Biochem. Sci. 17 (1992) 495. [DOI] [PubMed] [Google Scholar]
- [36].Houtkooper RH, Pirinen E, Auwerx J, Sirtuins as regulators of metabolism and healthspan, Nat. Rev. Mol. Cell Biol. 13 (2012) 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kouzarides T, Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19 (2000) 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Verdone L, La Fortezza M, Ciccarone F, Caiafa P, Zampieri M, Caserta M, Poly (ADP-ribosyl)ation affects histone acetylation and transcription, PLoS One 10 (2015) e0144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kauppinen TM, Swanson RA, The role of poly(ADP-ribose) polymerase-1 in CNS disease, Neuroscience 145 (2007) 1267–1272. [DOI] [PubMed] [Google Scholar]
- [40].Park JH, Long A, Owens K, Kristian T, Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia, Neurobiol. Dis. 95 (2016) 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmezas CI, Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment, Brain 138 (2015) 992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA, Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase, J. Cereb. Blood Flow Metab. 17 (1997) 1143–1151. [DOI] [PubMed] [Google Scholar]
- [43].Chiarugi A, Poly(ADP-ribosyl)ation and stroke, Pharmacol. Res. 52 (2005) 15–24. [DOI] [PubMed] [Google Scholar]
- [44].Schreiber V, Dantzer F, Ame JC, de Murcia G, Poly(ADP-ribose): novel functions for an old molecule, Nat. Rev. Mol. Cell Biol. 7 (2006) 517–528. [DOI] [PubMed] [Google Scholar]
- [45].Lo EH, Bosque-Hamilton P, Meng W, Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation, Stroke 29 (1998) 830–836. [DOI] [PubMed] [Google Scholar]
- [46].Dawson VL, Inhibition of poly(adenosine diphosphate-ribose) polymerase (PARP) in experimental models of neurologic diseases: cell death prevention, Retina 25 (2005) S31–S32. [DOI] [PubMed] [Google Scholar]
- [47].Long A, Park JH, Klimova N, Fowler C, Loane DJ, Kristian T, CD38 knockout mice show significant protection against ischemic brain damage despite high level poly-ADP-ribosylation, Neurochem. Res. 42 (2017) 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL, Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia, Nat. Med. 3 (1997) 1089–1095. [DOI] [PubMed] [Google Scholar]
- [49].Jayaram HN, Kusumanchi P, Yalowitz JA, NMNAT expression and its relation to NAD metabolism, Curr. Med. Chem. 18 (2011) 1962–1972. [DOI] [PubMed] [Google Scholar]
- [50].Coleman MP, Freeman MR, Wallerian degeneration, wld(s), and nmnat, Annu. Rev. Neurosci. 33 (2010) 245–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Belenky P, Bogan KL, Brenner C, NAD+ metabolism in health and disease, Trends Biochem. Sci. 32 (2007) 12–19. [DOI] [PubMed] [Google Scholar]
- [52].Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, Chan P, Adams JD, Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model, Pharmacol. Biochem. Behav. 73 (2002) 901–910. [DOI] [PubMed] [Google Scholar]
- [53].Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP, Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons, NeuroMolecular Med. 11 (2009) 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Klaidman LK, Mukherjee SK, Hutchin TP, Adams JD, Nicotinamide as a precursor for NAD+ prevents apoptosis in the mouse brain induced by tertiarybutylhydroperoxide, Neurosci. Lett. 206 (1996) 5–8. [DOI] [PubMed] [Google Scholar]
- [55].Chini CC, Tarrago MG, Chini EN, NAD and the aging process: role in life, death and everything in between, Mol. Cell. Endocrinol. 455 (2017) 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA, Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes, J. Neurosci. Res. 85 (2007) 3378–3385. [DOI] [PubMed] [Google Scholar]
- [57].Kitani T, Okuno S, Fujisawa H, Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor, FEBS Lett. 544 (2003) 74–78. [DOI] [PubMed] [Google Scholar]
- [58].Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S, Neuronal protective role of PBEF in a mouse model of cerebral ischemia, J. Cereb. Blood Flow Metab. 30 (2010) 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Orsomando G, Cialabrini L, Amici A, Mazzola F, Ruggieri S, Conforti L, Janeckova L, Coleman MP, Magni G, Simultaneous single-sample determination of NMNAT isozyme activities in mouse tissues, PLoS One 7 (2012) e53271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kitaoka Y, Munemasa Y, Kojima K, Hirano A, Ueno S, Takagi H, Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration, Cell Death Dis. 4 (2013) e860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Todisco S, Agrimi G, Castegna A, Palmieri F, Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae, J. Biol. Chem. 281 (2006) 1524–1531. [DOI] [PubMed] [Google Scholar]
- [62].Palmieri F, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, Trauth AU, Fiermonte G, Tjaden J, Agrimi G, Kirchberger S, Paradies E, Fernie AR, Neuhaus HE, Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins, J. Biol. Chem. 284 (2009) 31249–31259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].VanLinden MR, Dolle C, Pettersen IK, Kulikova VA, Niere M, Agrimi G, Dyrstad SE, Palmieri F, Nikiforov AA, Tronstad KJ, Ziegler M, Subcellular distribution of NAD+ between cytosol and mitochondria determines the metabolic profile of human cells, J. Biol. Chem. 290 (2015) 27644–27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Davila A, Liu L, Chellappa K, Redpath P, Nakamaru-Ogiso E, Paolella LM, Zhang Z, Migaud ME, Rabinowitz JD, Baur JA, Nicotinamide adenine dinucleotide is transported into mammalian mitochondria, elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Felici R, Lapucci A, Ramazzotti M, Chiarugi A, Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria, PLoS One 8 (2013) e76938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bernardi P, The permeability transition pore. Control points of a cyclosporin Asensitive mitochondrial channel involved in cell death, Biochim. Biophys. Acta 1275 (1996) 5–9. [DOI] [PubMed] [Google Scholar]
- [67].Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P, Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart, J. Biol. Chem. 276 (2001) 2571–2575. [DOI] [PubMed] [Google Scholar]
- [68].Kristian T, Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage, Cell Calcium 36 (2004) 221–233. [DOI] [PubMed] [Google Scholar]
- [69].Kristian T, Fiskum G, A fluorescence-based technique for screening compounds that protect against damage to brain mitochondria, Brain Res. Brain Res. Protoc. 13 (2004) 176–182. [DOI] [PubMed] [Google Scholar]
- [70].Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE, Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes, Ann. N. Y. Acad. Sci. 1147 (2008) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y, Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1, J. Biol. Chem. 282 (2007) 6823–6832. [DOI] [PubMed] [Google Scholar]
- [72].Verdin E, Hirschey MD, Finley LW, Haigis MC, Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling, Trends Biochem. Sci. 35 (2010) 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr., Weissman S, Verdin E, Schwer B, Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation, Mol. Cell. Biol. 27 (2007) 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Newman JC, He W, Verdin E, Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease, J. Biol. Chem. 287 (2012) 42436–42443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA, Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction, Cell 143 (2010) 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yang W, Nagasawa K, Munch C, Xu Y, Satterstrom K, Jeong S, Hayes SD, Jedrychowski MP, Vyas FS, Zaganjor E, Guarani V, Ringel AE, Gygi SP, Harper JW, Haigis MC, Mitochondrial sirtuin network reveals dynamic SIRT3dependent deacetylation in response to membrane depolarization, Cell 167 (2016) 985–1000 (e1021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP, Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges, Cell Metab. 23 (2016) 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P, Properties of the permeability transition pore in mitochondria devoid of cyclophilin D, J. Biol. Chem. 280 (2005) 18558–18561. [DOI] [PubMed] [Google Scholar]
- [79].Kristian T, Gertsch J, Bates TE, Siesjo BK, Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O, J. Neurochem. 74 (2000) 1999–2009. [DOI] [PubMed] [Google Scholar]
- [80].Bernardi P, Mitochondrial transport of cations: channels, exchangers, and permeability transition, Physiol. Rev. 79 (1999) 1127–1155. [DOI] [PubMed] [Google Scholar]
- [81].Elrod JW, Molkentin JD, Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore, Circ. J. 77 (2013) 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Scott I, Webster BR, Li JH, Sack MN, Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1, Biochem. J. 443 (2012) 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Szutowicz A, Bielarczyk H, Jankowska-Kulawy A, Pawelczyk T, Ronowska A, Acetyl-CoA the key factor for survival or death of cholinergic neurons in course of neurodegenerative diseases, Neurochem. Res. 38 (2013) 1523–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Srere PA, The citrate cleavage enzyme. I. Distribution and purification, J. Biol. Chem. 234 (1959) 2544–2547. [PubMed] [Google Scholar]
- [85].Rae C, Fekete AD, Kashem MA, Nasrallah FA, Broer S, Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice, Neurochem. Res. 37 (2012) 2541–2553. [DOI] [PubMed] [Google Scholar]
- [86].Starai VJ, Escalante-Semerena JC, Acetyl-coenzyme A synthetase (AMP forming), Cell. Mol. Life Sci. 61 (2004) 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, Moroni F, Chiarugi A, Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain, Mol. Pharmacol. 70 (2006) 1876–1884. [DOI] [PubMed] [Google Scholar]
- [88].Kato T, Ischemic effect on CoASH and acetyl-CoA concentration levels in cerebrum, cerebellum and liver of mice, J. Neurochem. 31 (1978) 1545–1548. [DOI] [PubMed] [Google Scholar]
- [89].Calvani M, Arrigoni-Martelli E, Attenuation by acetyl-l-carnitine of neurological damage and biochemical derangement following brain ischemia and reperfusion, Int. J. Tissue React. 21 (1999) 1–6. [PubMed] [Google Scholar]
- [90].Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, Endres M, Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury, Exp. Neurol. 210 (2008) 531–542. [DOI] [PubMed] [Google Scholar]
- [91].Eberharter A, Becker PB, Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics, EMBO Rep. 3 (2002) 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Murphy SP, Lee RJ, McClean ME, Pemberton HE, Uo T, Morrison RS, Bastian C, Baltan S, MS-275, a class I histone deacetylase inhibitor, protects the p53-deficient mouse against ischemic injury, J. Neurochem. 129 (2014) 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, Leeds P, Chuang DM, Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke, Stroke 43 (2012) 2430–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ziemka-Nalecz M, Zalewska T, Neuroprotective effects of histone deacetylase inhibitors in brain ischemia, Acta Neurobiol. Exp. (Wars) 74 (2014) 383–395. [DOI] [PubMed] [Google Scholar]
- [95].Arun P, Moffett JR, Namboodiri AM, Evidence for mitochondrial and cytoplasmic N-acetylaspartate synthesis in SH-SY5Y neuroblastoma cells, Neurochem. Int. 55 (2009) 219–225. [DOI] [PubMed] [Google Scholar]
- [96].Choi IY, Gruetter R, Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1–13C]glucose in the rat brain in vivo, J. Neurochem. 91 (2004) 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xu S, Yang J, Shen J, Measuring N-acetylaspartate synthesis in vivo using proton magnetic resonance spectroscopy, J. Neurosci. Methods 172 (2008) 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Moffett JR, Arun P, Ariyannur PS, Namboodiri AM, N-acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation, Front. Neuroenerg. 5 (2013) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Urenjak J, Williams SR, Gadian DG, Noble M, Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro, J. Neurochem. 59 (1992) 55–61. [DOI] [PubMed] [Google Scholar]
- [100].Casey PA, McKenna MC, Fiskum G, Saraswati M, Robertson CL, Early and sustained alterations in cerebral metabolism after traumatic brain injury in immature rats, J. Neurotrauma 25 (2008) 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vagnozzi R, Tavazzi B, Signoretti S, Amorini AM, Belli A, Cimatti M, Delfini R, Di Pietro V, Finocchiaro A, Lazzarino G, Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment—part I, Neurosurgery 61 (2007) 379–388 (discussion 388–379). [DOI] [PubMed] [Google Scholar]
- [102].Cvoro V, Wardlaw JM, Marshall I, Armitage PA, Rivers CS, Bastin ME, Carpenter TK, Wartolowska K, Farrall AJ, Dennis MS, Associations between diffusion and perfusion parameters, N-acetyl aspartate, and lactate in acute ischemic stroke, Stroke 40 (2009) 767–772. [DOI] [PubMed] [Google Scholar]
- [103].Brady S, Siegel G, Albers RW, Brady S , Basic Neurochemistry: Principles of Molecular, Cellular, and Medical Neurobiology, Elsevier Science & Technology, San Diego, United States, 2011. [Google Scholar]
- [104].Newman JC, Verdin E, Ketone bodies as signaling metabolites, Trends Endocrinol. Metab. 25 (2014) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW, Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 6601–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McKenna MC, Scafidi S, Robertson CL, Metabolic alterations in developing brain after injury: knowns and unknowns, Neurochem. Res. 40 (2015) 2527–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J, Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture, J. Neurosci. Res. 18 (1987) 551–561. [DOI] [PubMed] [Google Scholar]
- [108].Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP, 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons, J. Neurochem. 139 (2016) 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jiang L, Mason GF, Rothman DL, de Graaf RA, Behar KL, Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo, J. Cereb. Blood Flow Metab. 31 (2011) 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U, Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools, Biochem. Pharmacol. 71 (2006) 399–407. [DOI] [PubMed] [Google Scholar]
- [111].Auestad N, Korsak RA, Morrow JW, Edmond J, Fatty acid oxidation and ketogenesis by astrocytes in primary culture, J. Neurochem. 56 (1991) 1376–1386. [DOI] [PubMed] [Google Scholar]
- [112].Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA, A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function, J. Neurosci. 28 (2008) 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Eraso-Pichot A, Braso-Vives M, Golbano A, Menacho C, Claro E, Galea E, Masgrau R, GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes, Glia 66 (2018) 1724–1735. [DOI] [PubMed] [Google Scholar]
- [114].Jernberg JN, Bowman CE, Wolfgang MJ, Scafidi S, Developmental regulation and localization of carnitine palmitoyltransferases (CPTs) in rat brain, J. Neurochem. 142 (2017) 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Knobloch M, Pilz GA, Ghesquiere B, Kovacs WJ, Wegleiter T, Moore DL, Hruzova M, Zamboni N, Carmeliet P, Jessberger S, A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity, Cell Rep. 20 (2017) 2144–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sayre NL, Sifuentes M, Holstein D, Cheng SY, Zhu X, Lechleiter JD, Stimulation of astrocyte fatty acid oxidation by thyroid hormone is protective against ischemic stroke-induced damage, J. Cereb. Blood Flow Metab. 37 (2017) 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM, Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation, Neuroscience 145 (2007) 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ, Herson PS, Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury, J. Cereb. Blood Flow Metab. 33 (2013) 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC, Metabolism of acetyl-l-carnitine for energy and neurotransmitter synthesis in the immature rat brain, J. Neurochem. 114 (2010) 820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield DA, Calvani M, Pennisi G, Giuffrida Stella AM, Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine, Neurochem. Res. 28 (2003) 1321–1328. [DOI] [PubMed] [Google Scholar]
- [121].Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G, Neuroprotection by acetyl-l-carnitine after traumatic injury to the immature rat brain, Dev. Neurosci. 32 (2010) 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL 3rd, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS, Randomized double-blind placebocontrolled trial of acetyl-l-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy, J. Clin. Oncol. 31 (2013) 2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jalal FY, Bohlke M, Maher TJ, acetyl-l-carnitine reduces the infarct size and striatal glutamate outflow following focal cerebral ischemia in rats, Ann. N. Y. Acad. Sci. 1199 (2010) 95–104. [DOI] [PubMed] [Google Scholar]