Abstract
Rotavirus infection is the most common cause of viral diarrhea in infants and young children but uncommon and usually asymptomatic in adults. In the winter of 2017–2018, a large-scale outbreak of rotavirus in both children and adults was reported in Thailand. The current study focused on the prevalence, genotyping, and molecular characterization of rotavirus infections in Thai adults from July 2016 to December 2019. In 2,598 stool samples collected from adult residents of Bangkok (aged #x2265; 15 years) with acute gastroenteritis, rotavirus was detected via real-time RT-PCR analysis of the VP6 gene. G, P and I genotypes were determined by direct sequencing of VP7, VP4, and VP6 genes, respectively. Our results showed 8.7% (226/2,598) of stool samples were positive for rotavirus. The incidence of rotavirus was high during the winter season of 2017–2018 (17.7%) compared to another studied periods (4.5% between July 2016- October 2017 and 2.8% between March 2018- December 2019). Nucleotide sequencing of VP7 and VP4 revealed G3P[8] as the predominant strain (33.2%,75/226), followed by G9P[8] (17.3%,39/226), and G2P[4] (15.0%,34/226). Uncommon G and P combinations were additionally detected at low frequencies. VP6 sequencing was conducted to discriminate I genotype between the Wa and DS-1 genogroup. The unusual DS-1-like G3P[8] strain was most prevalent amomg rotavirus strains detected in this study (29.6%, 67/226), and the corresponding VP7 sequences showed high nucleotide identity with unusual DS-1-like globally circulating strains. Our study demonstrates that rotavirus outbreaks in adults are attributable not only to high prevalence of RV infection but also the unusual DS-like genogroup. The collective findings reinforce the importance of investigating rotavirus diagnosis in adults suffering from acute gastroenteritis and taking appropriate preventive measures.
Introduction
Rotavirus (RV) is a common pathogen associated with acute viral gastroenteritis in humans worldwide, especially in developing countries [1]. Although significantly more prevalent in young children, RV infection has been reported in parents and caretakers of children [2]. RV can transmit within families and between adults caring for children with RV infection, as evidenced by serological tests [3, 4]. Adults with RV infection may either be asymptomatic or present with mild to severe symptoms [5]. The incidence of rotavirus infections in adults varied between 3–18% with a high prevalence during the winter to spring months (November to March in the northern hemisphere) [6, 7]. Individual may have acquired immunity from previous natural exposures during childhood, leading to a lower incidence of infection in adulthood.
RV is a double-stranded RNA genome virus. RV belongs to the Reoviridae family and is classified into 10 species (A-J) based on antigenic and genetic variants of viral protein 6 (VP6). Among the species, Rotavirus group A (RVA) is commonly found in children worldwide [8]. The triple-layered viral particle composed of 11 gene segments encoding six structural proteins (VP1 to VP4, VP6, and VP7) and five or six non-structural proteins (NSP1 to NSP5/NSP6) [9]. Genetic characterization of the VP6 gene is used to differentiate between RV groups. VP7 and VP4 comprise the outer layer of the virion and thus form the basis of a binary classification system defining G (glycoprotein) and P (protease) genotypes, respectively. In the genotyping system, the genotype constellation is described by the nomenclature Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx, which defines the genotypes of VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5, respectively, with x indicating the number of corresponding genotypes [10]. At present, 36 G, 51 P, 26 I, 22 R, 20 C, 20 M, 31 A, 22 N, 22 T, 27 E and 22 H genotypes among the RVA have been identified in human and animal species worldwide [11]. To date, 6 G genotypes (G1-G4, G9, and G12) and 3 P genotypes (P[4], P[6], and P[8]) have been commonly identified within the G-P combinations of RVA. In humans, the predominant RV genotypes are G1P[8], G2P[4], G3P[8], G4P[8], G9P[8], and G12P[8], which often vary by region and time and are particularly prevalent in developing countries [8]. In humans, the major RV strains consist of the Wa-like genogroup (G1, G3, G4, G9, or G12-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1), DS-1-like genogroup (G2-P[4]-I2-R2-C2-M2-A2-N2-T2-E2-H2) and the less common AU-1-like genogroup (G3-P[9]-I3-R3-C3-M3-A3-N3-T3-E3-H3) which have origins in distinct animal species. The segmented nature of the genome enables both intra- and inter-genogroup reassortment between/within animal and human strains, which can lead to the wide spread among humans. Interspecies reassortment derived from zoonotic transmission increase the genetic diversity among circulating RV and the incidence of acute gastroenterisits in human [12]. Recently, the unusual DS-1-like inter-genogroup reassortment strains possessing genotype constellations G1/3/8/9-P[8]-I2-R2-C2-M2-A2-N2-T2-E2-H2 have emerged and spread among human populations in at least five continents (Asia, Australia, Europe, North America, and South America) [11, 12].
At present, two live oral RV vaccines, Rotarix (GlaxoSmithKline Biologicals S.A., wavre, Belgium; RV1, G1P[8]) and RotaTeq (Merck&Co., Inc., Whitehouse, NJ; RV5, G1-G4, and P[8]), with high efficacy, are licensed in more than 100 countries. However, vaccine efficacy clearly varies among different populations and drives the evolution of more dynamic and diverse wild-type strain populations in the post-vaccine era [13–15]. Moreover, introduction of RV vaccines may enforce additional selective pressure on currently circulating strains, resulting in the generation and spread of novel RV strains worldwide [16]. The above RV vaccines were licensed as optional vaccines in Thailand in 2006 and 2008, respectively [17]. However, RV outbreaks in Thailand still occurred among children after introduction of the vaccines, with different genotypes playing major roles in individual epidemics [18–20].
In Thailand, RV gastroenteritis is common in children but not as prevalent in adults. In the winter season of 2017–2018, an outbreak of acute RV-induced gastroenteritis in adults was reported in Thailand (mainly Bangkok). As mentioned earlier, immunity acquired following exposure during childhood may effectively contribute to protection against infection in individual adults. Therefore, the potential factors underlying the increased number of adults suffering from RV infections is worth investigation. The main objective of the current study was to determine the genotype distribution of RV in adults over an extended period from July 2016 to December 2019.
Materials and methods
This study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University (IRB 634/59; Bangkok, Thailand). The director of King Chulalongkorn Memorial Hospital authorized the use of stored samples. All clinical samples were investigated anonymously. The IRB waived the requirement for written informed consent.
Specimen collection
From July 2016 to December 2019, 4,472 stool specimens from routine testing service for acute gastroenteritis at King Chulalongkorn Memorial Hospital and Bangpakok 9 International Hospital (Bangkok, Thailand) were collected. Of these, 2,598 stool specimens from hospitalized and outpatient adults with acute gastroenteritis (≥15 years) were used for this study (1,058 males and 1,540 females). Enrolled patients presented with acute gastroenteritis, i.e. three or more loose liquid stools per day, along with moderate to severe dehydration with or without fever and vomiting. Available information of patients included sex, age, and collection date.
Rotavirus detection
Viral RNA was extracted from 10% (v/v) stool suspension in phosphate buffer saline. RNA was automatically extracted from 200 μL supernatant stool samples after centrifugation at 4,000 x g for 10 minutes using a magLEAD 12gC instrument (Precision System Science, Chiba, Japan) with a magLEAD Consumable Kit (Precision System Science, Chiba, Japan) according to the manufacturer’s instructions. Amplification of the partial VP6 gene was performed in a 25 μL reaction volume comprised 10 μM forward primer VP6-F (5’- GACGGVGCRACTACATGGT-3’) and reverse primer VP6-R (5’-GTCCAATTCATNCCTGGTGG -3’), based on the study of Kang G. et al. [21] and 2 μL RNA template using the QuantiTect SYBR Green 1-step real-time RT-qPCR Kit (Qiagen, Hilden, Germany). Reverse transcription was conducted at 50°C for 30 min, followed by heat denaturation at 95°C for 15 min. In total, 45 cycles of amplification were performed comprising denaturation at 94°C for 15 sec, annealing at 50°C for 30 sec, and extension at 72°C for 30 sec. Melting curve analysis was performed from 60°C to 95°C with 1°C increments. Amplified products were purified and sequenced for I genotype identification.
Co-infections of diarrhea-inducing viruses
To identify cases of co-infection, RV RNA was tested along with those of other gastroenteritis viruses, such as norovirus, astrovirus, sapovirus, bocavirus, enterovirus, adenovirus and parechovirus. Detection of other gastroenteritis viruses was performed by polymerase chain reation (PCR) or reverse-transcription PCR (RT-PCR) as previously described (norovirus [22], astrovirus, sapovirus, enterovirus [23], parechovirus, bocavirus [24] and adenovirus [25]).
Sequence determination of G, P, and I genotype of rotavirus and phylogenetic analysis
Samples initially tested positive based on VP6 screening were further subjected to amplification of genes encoding the structural proteins VP6, VP7, and VP4 using the SensiFAST 1-step RT-PCR kit (Bioline, London, UK). Consensus primer pairs specific for the VP6 gene from the study of Theamboonlers et al. [26] were used for amplification. The consensus primer pairs of BEG9/END9 and con2/con3 were used for amplification of VP7 [27] and VP4 [28], respectively. The 25 μL RT-PCR mixture contained 2X buffer, 10μM of consensus forward and reverse primers and 2 μL RNA. Reverse transcription steps included incubation at 45°C for 30 min, followed by 94°C for 5 min. PCR conditions were as follows: 40 cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 45 sec, and extension at 72°C for 2 min. Amplicons were agarose gel-purified and sequenced by FirstBASE Laboratories (SDN BHD, Salangor, Malaysia).
Sequencing data were analyzed using Chromas 2.23 (Technelysium, Queensland, Australia). Nucleotide sequences identity was analyzed via BLAST. G (VP7), P (VP4), and I (VP6) genotypes were validated using the online RotaC rotavirus genotyping tool (RotaC version 2) [29].
Nucleotide sequences were aligned using Clustal Omega (www.ebi.ac.uk/Tools). Phylogenetic trees were constructed and molecular evolutionary analyses were conducted using MEGA 6.0 software. The Kimura-2 correction parameter model and Maximum likelihood method were applied to construct the phylograms of VP7, VP4, and VP6. The best-fit models were determined through testing the model parameter. Tree robustness was determined by bootstrapping (1,000 replicates), with bootstrap values >70% considered significant.
Nucleotide sequences were deposited in the GenBank database under the accession numbers MN836856-MN837068 and MN989603-MN989613 for VP7, MN989625-MN989850 for VP6, and MN837284-MN837475 and MN989614-MN989624 for VP4.
Statistical analyses
Statistical analyses were performed using IBM SPSS statistic for Windows, version 21 (IBM Corp., Armonk, NY). The analysis was conducted by dividing individuals into three age groups (15–29, 30–59 and > 59years old). Rate of RV infection was compared between three periods of time (July 2016-June 2017, July 2017-June 2018, July 2018-December 2019). A Chi-square table was used to compare the percentages of infected individuals between age groups and studied periods. All comparisons were two-sided and p-value < 0.05 was considered to be statistically significant.
Results
In this study, specimens from patients aged between 15–96 years old were investigated. The mean and median age of patients were 46.7 and 44.0 years, respectively. Among the 2,598 stool samples from adults (≥15 years) with acute gastroenteritis, 8.7% (226/2,598) were positive for RV based on the real-time RT-PCR VP6 primary screening assay (74 male and 152 female). The prevalence of RV infection was high during the winter season (November–March) as shown in Fig 1. Between July 2017 and June 2018, the prevalence of rotavirus infection in adults was highest among the three-year study period and accounted for 17.7% (179/1013). The rate of RV infection was highest in the 30–59 year age group (56.6%, 128/226), followed by 15–29 year age group (28.3%, 64/226), and ≥ 59 year age group (15.1%, 34/226) as shown in Fig 1 (p-value < 0.001).
Fig 1. Percentages of rotavirus infection classified by age group between July 2016 and December 2019.
The monthly number of samples from adults with acute gastroenteritis is shown in gray (left scale). Bar graphs show the percentage of RV-positive cases (right scale).
Among the total RV-positive samples, 85.0% (192/226) were solely infected with RV while 15.0% (34/226) of cases showed mixed infection with other diarrhea-inducing viruses. The major co-infecting virus was norovirus which accounted for 7.1% (16/226) followed by astrovirus 2.7% (6/226), bocavirus 2.7% (6/226), sapovirus 0.9% (2/226), and enterovirus 0.9% (2/226). Co-infection with adenovirus was not found. Moreover, triple infection with RV, astrovirus and bocavirus was detected in one patient. Bacterial pathogens and other causative agents not mentioned above were not investigated.
Based on the sequence of VP6, an unusual DS-1-like backbone (G1/3/8/9-P[4/6/8]-I2) was detected predominately in 48.2% (109/226) of RV-positive samples, followed by Wa-like strain (G1/3/8/9-P[6/8]-I1) (31.9%, 72/226), usual DS-1-like strain (G2-P[4]-I2) (19.4%, 44/226), and AU-1-like strain (G3-P[10]-I3) (0.4%, 1/226). When analyzed together with the distribution of G and P types, our results showed that G3P[8] (33.2%, 75/226) (unusual DS-1-like G3P[8] (29.6%, 67/226) and Wa-like G3P[8] (3.5%, 8/226)) was the most common genotype, followed by G9P[8] (17.3%, 39/226) (Wa-like G9P[8] (16.4%, 37/226) and unusual DS-1-like G9P[8] (0.9%, 2/226)), G2P[4] (15.0%, 34/226), and G1P[6] (8.4%, 19/226). Uncommon G and P types were additionally detected at low frequencies such as G3P[4] (3.5%, 8/226), G3P[10] (0.4%, 1/226), and G4P[8] (0.4%, 1/226) (Fig 2).
Fig 2. Distribution of rotavirus G and P genotypes between July 2016 and December 2019.
The monthly number of samples from adults with acute gastroenteritis is shown in gray (left scale). Bar graphs show the percentage of RV-positive cases (right scale).
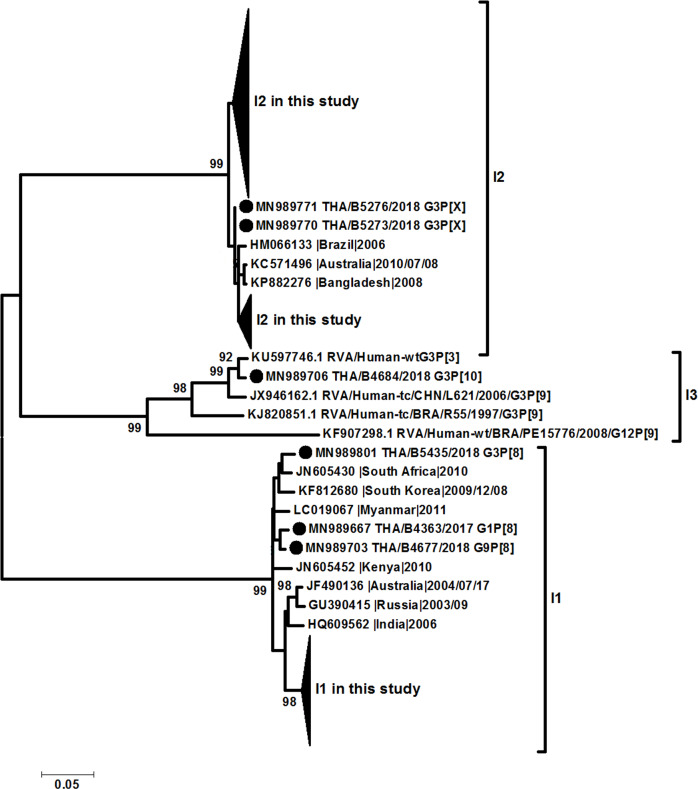
All samples positive for rotavirus VP6 screening can be sequenced for complete VP6 genome, whereas 99.1% (224/226) and 89% (201/226) of samples can be sequenced for complete VP7 and VP4 gene, respectively. The phylogenetic analysis of VP6 (I type) led to identification that sequences obtained in this study were clustered in 3 groups, Wa-like (I1) (31.9%, 72/226), DS-1-like (I2) (67.7%, 153/226), and AU-1-like (I3) (0.4%, 1/226) (Fig 3). On the other hand, the phylogenetic analysis of VP7 gene showed the different clustering of between Wa-like G3 and unusual DS-1-like G3 which clustered in different lineages (Fig 4). The percentage of nucleotide identity between Wa-like and unusual DS-1-like G3 was 81% while amino acid identity was 91%. Multiple amino acid variations were detected, especially in antigenic regions C (T212A and N213T) and F (N238D and N242A). The P[8] VP4 gene did not cluster in the different lineages and correlate with the phylogenetic tree of VP7 (Fig 5).
Fig 3. Phylogenetic tree of the VP6 gene.
Evolutionary history was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured by the number of substitutions per site. The analysis involved 252 nucleotide sequences. All positions with <95% site coverage were eliminated.
Fig 4. Phylogenetic tree of the VP7 gene.
Evolutionary history was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured by the number of substitutions per site. The analysis involved 252 nucleotide sequences. All positions with <95% site coverage were eliminated.
Fig 5. Phylogenetic tree of the VP4 gene.
Evolutionary history was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured by the number of substitutions per site. The analysis involved 185 nucleotide sequences. All positions with <95% site coverage were eliminated.
Discussion
In this study, the molecular characteristics of RV in adult patients with gastroenteritis in Bangkok, Thailand were investigated over a 3-year period (July 2016– December 2019) using a large number of stool specimens collected from various age groups. The RV genotype G3P[8]I2 (unusual DS-1-like G3P[8]) was the most dominant strain (29.6%), followed by G9P[8]I1 (Wa-like G9P[8]; 16.4%) and G2P[4]I2 (DS-1-like G2P[4]; 15.0%). The previous report from six provinces in Thailand showed that G3P[8]I2 has emerged in Thailand since 2015 [20]. The positive rate of rotavirus infection in adults presenting with acute gastroenteritis in this study was 8.7%. This rate was similar to some of the previous reports in Thailand [20, 30], but lower than a study in Bangkok which had a relatively small sample size [19]. Nevertheless, all of the previous studies agreed that the incidence of RV infection in adults increased during the winter season (November–March) indicating a similar seasonality pattern to that reported in children population [18, 31, 32]. Apart from being seasonal-specific, rotavirus gastroenteritis can be transmitted from children to adults [2–4]. Unfortunately, our study did not include the history of contact with sick children, therefore, the burden of RV gastroenteritis from children-to-adult transmission cannot be determined. The results of this study showed that rotavirus was an important agent for acute gastroenteritis in adults, and should not be underestimated.
In addition to the prevalence of adult RV infections, the molecular characteristics of RV-induced acute gastroenteritis in adults were investigated. The distribution of G and P types in Thailand have changed over time, but seasonality of RV infection remains unchanged [33]. The G1 genotype combination with P[8] strains has been predominantly identified in many parts of Thailand since 1993, followed by genotypes G2, G3, and G9 in different seasons and regions [34]. From 2000 to 2016 [20, 35], various G and P type combinations have been detected, such as G1P[8], G2P[4], G3P[8], and G9P[8]. On an annual basis, the dominant G and P types were identified as G9P[8] from 2000 to 2004, G1P[8] from 2005 to 2009, G3P[8] from 2009 to 2011, G1P[8] from 2012 to 2014, and G9P[8] from 2015 to 2016. Moreover, uncommon genotypes, such as G3P[10] and G12P[8], were detected. In the current study, G3P[8] was the predominant strain (33.2%, 75/226), consistent with previous reports from Indonesia (2015–2016) [36] and Brazil (2016–2017) [37, 38], followed by G9P[8] (17.3%, 39/226) and G2P[4] (15.0%, 34/226). In terms of genetic backbone, the DS-1-like strain (I2) was prevalent (55.3%, 125/226) with the highest being unusual DS-1-like G3P[8]I2 (53.6%, 67/125), followed by G2P[4]I2 (27.2%, 34/125).
The effects of vaccination on distribution patterns of co-circulating RV strains have been investigated. A previous study in Australia reported that the introduction of RV vaccines affected the ecology of RV by increasing diversity and differences in genotype predominance. Increased prevalence of G12P[8] across states was observed following the use of RotaTeq and G2P[4] and DS-1-like G3P[8] with the use of Rotarix [39]. Another study in Japan showed that the dominant genotype shifted from G3P[8] to G1P[8] and G2P[4] after vaccine introduction [40]. In the previous report in Thailand [20, 41], RV with DS-1-like backbone (G1,3,4,9P[8]I2) has been prevalent in children and adults since 2013, with a trend of continued significant increase until 2016 in distinct provinces. In the present study, unusual DS-1-like G3P[8] was identified as the major strain in Thailand. The sequences of VP7 and VP4 genes displayed high percentage nucleotide identity to the Australian unusual DS-1-like G3P[8] strains circulating between 2006 and 2011 [12]. This unusual DS-1-like G3P[8] strain has been identified in several countries with a range of vaccine coverage, with different rates of infection depending on geographic and temporal patterns, including Australia, Germany, Hungary, USA, Brazil, Japan, and Thailand [10–12, 20, 36, 38, 42–46], indicating that the DS-1-like backbone is derived from a globally circulating pool of RV. Studies on children infected with the unusual DS-1-like G1P[8] strain revealed no significant differences in clinical severity relative to disease caused by Wa-like G1P[8] [47, 48]. However, these strains contain several different amino acids, especially in the antigenic region, which may affect vaccine efficacy, as shown previously for unusual DS-1-like G1P[8] [49]. This reassortment has a considerable impact on communities, potentially conferring some advantage to viral propagation and survival, although severity of diarrhea may not be affected. While no data on effectiveness of vaccines against unusual DS-1-like G3P[8] are available at present, the efficacy of the RV vaccine against Wa-like G1P[8], unusual DS-1-like G1P[8], G2P[4] and Wa-like G9P[8] strains has been confirmed based on mean Vesikari score and distribution of Vesikari score [48–52].
The main limitation of this study is the lack of clinical severity and RV vaccination history of patients and their families. Although rotavirus vaccines were licensed in Thailand as optional vaccines since 2006, the vaccine coverage was low. In January 2020, Thailand implemented rotavirus vaccine to all infants. Vaccine introduction was not likely to be the factor contributing to the prevalence of the unusual DS-1-like genogroup in this study and the underlying reason remains to be established. Although our study subjects were mainly Bangkok resident which may not represent the overall national population, data were obtained from a large number of patients.
In conclusion, the increase in RV infections in adults during the winter season in 2017–2018 relative to that during the equivalent periods in the previous and subsequent years was of significant interest. Genotyping of the RV backbone in routine surveillance provided additional information to explain this finding. The unusual DS-1-like G3P[8] strain was most prevalent amomg rotavirus strains detected in this study (29.6%, 67/226). The results obtained may aid in further understanding of not only viral evolution but also symptomatic infection in adults. The effectiveness of the vaccine should be concerned for the unusual DS-1-like strain. Therefore, continuous and extensive surveillance, including genotyping of RV infection, is essential for preventive measures, vaccine efficacy, and control of viral gastroenteritis outbreaks.
Acknowledgments
We are grateful to the staff of the Center of Excellence in Clinical Virology for their technical and administrative assistance.
Data Availability
Sequences files are available from the GenBank database (Accession numbers MN836856-MN837068 and MN989603-MN989613 for VP7, MN989625-MN989850 for VP6, and MN837284-MN837475 and MN989614-MN989624 for VP4).
Funding Statement
Yong Poovorawan; P-15-50004, The Research Chair Grant from the National Science and Technology Development Agency Yong Poovorawan; GCE 59-009-30-005, The Center of Excellence in Clinical Virology, Chulalongkorn University Jira Chansaenroj; Rachadapisek Sompote Fund for Postdoctoral Fellowship, Chulalongkorn University The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–8. 10.1056/NEJM199610033351404 . [DOI] [PubMed] [Google Scholar]
- 2.Kim HW, Brandt CD, Kapikian AZ, Wyatt RG, Arrobio JO, Rodriguez WJ, et al. Human reovirus-like agent infection. Occurrence in adult contacts of pediatric patients with gastroenteritis. JAMA. 1977;238(5):404–7. 10.1001/jama.238.5.404 . [DOI] [PubMed] [Google Scholar]
- 3.Grimwood K, Abbott GD, Fergusson DM, Jennings LC, Allan JM. Spread of rotavirus within families: a community based study. Br Med J (Clin Res Ed). 1983;287(6392):575–7. 10.1136/bmj.287.6392.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenman WM, Hinde D, Feltham S, Gurwith M. Rotavirus infection in adults. Results of a prospective family study. N Engl J Med. 1979;301(6):303–6. 10.1056/NEJM197908093010604 . [DOI] [PubMed] [Google Scholar]
- 5.Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis. 2004;4(2):91–9. 10.1016/S1473-3099(04)00928-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson EJ, Katz BZ, Polin JA, Reddy S, Weinrobe MH, Noskin GA. Rotavirus in adults requiring hospitalization. J Infect. 2012;64(1):89–95. 10.1016/j.jinf.2011.09.003 . [DOI] [PubMed] [Google Scholar]
- 7.Anderson EJ, Shippee DB, Weinrobe MH, Davila MD, Katz BZ, Reddy S, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis. 2013;56(6):755–60. 10.1093/cid/cis1010 . [DOI] [PubMed] [Google Scholar]
- 8.Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083 Epub 2017/11/10. 10.1038/nrdp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153(8):1621–9. Epub 2008/07/08. 10.1007/s00705-008-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol. 2011;156(8):1397–413. 10.1007/s00705-011-1006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz EM, Esona MD, Betrapally NS, De La Cruz De Leon LA, Neira YR, Rey GJ, et al. Whole-gene analysis of inter-genogroup reassortant rotaviruses from the Dominican Republic: Emergence of equine-like G3 strains and evidence of their reassortment with locally-circulating strains. Virology. 2019;534:114–31. 10.1016/j.virol.2019.06.007 . [DOI] [PubMed] [Google Scholar]
- 12.Cowley D, Donato CM, Roczo-Farkas S, Kirkwood CD. Emergence of a novel equine-like G3P[8] inter-genogroup reassortant rotavirus strain associated with gastroenteritis in Australian children. J Gen Virol. 2016;97(2):403–10. 10.1099/jgv.0.000352 . [DOI] [PubMed] [Google Scholar]
- 13.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. 10.1016/S0140-6736(10)60889-6 . [DOI] [PubMed] [Google Scholar]
- 14.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–23. 10.1016/S0140-6736(10)60755-6 . [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix(R) and RotaTeq(R), into the National Immunization Program of Australia. Pediatr Infect Dis J. 2011;30(1 Suppl):S48–53. 10.1097/INF.0b013e3181fefd90 . [DOI] [PubMed] [Google Scholar]
- 16.Matthijnssens J, Heylen E, Zeller M, Rahman M, Lemey P, Van Ranst M. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol Biol Evol. 2010;27(10):2431–6. 10.1093/molbev/msq137 . [DOI] [PubMed] [Google Scholar]
- 17.Muangchana C, Riewpaiboon A, Jiamsiri S, Thamapornpilas P, Warinsatian P. Economic analysis for evidence-based policy-making on a national immunization program: a case of rotavirus vaccine in Thailand. Vaccine. 2012;30(18):2839–47. 10.1016/j.vaccine.2012.02.047 . [DOI] [PubMed] [Google Scholar]
- 18.Chieochansin T, Vutithanachot V, Phumpholsup T, Posuwan N, Theamboonlers A, Poovorawan Y. The prevalence and genotype diversity of Human Rotavirus A circulating in Thailand, 2011–2014. Infect Genet Evol. 2016;37:129–36. 10.1016/j.meegid.2015.11.011 . [DOI] [PubMed] [Google Scholar]
- 19.Kittigul L, Swangsri T, Pombubpa K, Howteerakul N, Diraphat P, Hirunpetcharat C. Rotavirus infection in children and adults with acute gastroenteritis in Thailand. Southeast Asian J Trop Med Public Health. 2014;45(4):816–24. . [PubMed] [Google Scholar]
- 20.Tacharoenmuang R, Komoto S, Guntapong R, Upachai S, Singchai P, Ide T, et al. High prevalence of equine-like G3P[8] rotavirus in children and adults with acute gastroenteritis in Thailand. J Med Virol. 2020;92(2):174–86. Epub 2019/09/10. 10.1002/jmv.25591 . [DOI] [PubMed] [Google Scholar]
- 21.Kang G, Iturriza-Gomara M, Wheeler JG, Crystal P, Monica B, Ramani S, et al. Quantitation of group A rotavirus by real-time reverse-transcription-polymerase chain reaction: correlation with clinical severity in children in South India. J Med Virol. 2004;73(1):118–22. 10.1002/jmv.20053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phumpholsup T, Chieochansin T, Vongpunsawad S, Vuthitanachot V, Payungporn S, Poovorawan Y. Human norovirus genogroup II recombinants in Thailand, 2009–2014. Arch Virol. 2015;160(10):2603–9. 10.1007/s00705-015-2545-5 . [DOI] [PubMed] [Google Scholar]
- 23.Chansaenroj J, Tuanthap S, Thanusuwannasak T, Duang-In A, Klinfueng S, Thaneskongtong N, et al. Human enteroviruses associated with and without diarrhea in Thailand between 2010 and 2016. PLoS One. 2017;12(7):e0182078 10.1371/journal.pone.0182078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chieochansin T, Thongmee C, Vimolket L, Theamboonlers A, Poovorawan Y. Human bocavirus infection in children with acute gastroenteritis and healthy controls. Jpn J Infect Dis. 2008;61(6):479–81. . [PubMed] [Google Scholar]
- 25.Sriwanna P, Chieochansin T, Vuthitanachot C, Vuthitanachot V, Theamboonlers A, Poovorawan Y. Molecular characterization of human adenovirus infection in Thailand, 2009–2012. Virol J. 2013;10:193 10.1186/1743-422X-10-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theamboonlers A, Maiklang O, Thongmee T, Chieochansin T, Vuthitanachot V, Poovorawan Y. Complete genotype constellation of human rotavirus group A circulating in Thailand, 2008–2011. Infect Genet Evol. 2014;21:295–302. 10.1016/j.meegid.2013.11.020 . [DOI] [PubMed] [Google Scholar]
- 27.Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28(2):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30(6):1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009;9:238 10.1186/1471-2180-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echeverria P, Blacklow NR, Cukor GG, Vibulbandhitkit S, Changchawalit S, Boonthai P. Rotavirus as a cause of severe gastroenteritis in adults. J Clin Microbiol. 1983;18(3):663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M, Parashar U, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192 Suppl 1:S114–9. 10.1086/431497 [DOI] [PubMed] [Google Scholar]
- 32.Satter SM, Gastanaduy PA, Islam K, Rahman M, Rahman M, Luby SP, et al. Hospital-based Surveillance for Rotavirus Gastroenteritis Among Young Children in Bangladesh: Defining the Potential Impact of a Rotavirus Vaccine Program. Pediatr Infect Dis J. 2017;36(2):168–72. Epub 2016/11/01. 10.1097/INF.0000000000001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lestari FB, Vongpunsawad S, Wanlapakorn N, Poovorawan Y. Rotavirus infection in children in Southeast Asia 2008–2018: disease burden, genotype distribution, seasonality, and vaccination. J Biomed Sci. 2020;27(1):66 Epub 2020/05/23. 10.1186/s12929-020-00649-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongsuwannna Y, Guntapong R, Tacharoenmuang R, Prapanpoj M, Kameoka M, Taniguchi K. A long-term survey on the distribution of the human rotavirus G type in Thailand. J Med Virol. 2010;82(1):157–63. 10.1002/jmv.21596 . [DOI] [PubMed] [Google Scholar]
- 35.Chan-It W, Chanta C. Emergence of G9P[8] rotaviruses in children with acute gastroenteritis in Thailand, 2015–2016. J Med Virol. 2018;90(3):477–84. 10.1002/jmv.24985 . [DOI] [PubMed] [Google Scholar]
- 36.Utsumi T, Wahyuni RM, Doan YH, Dinana Z, Soegijanto S, Fujii Y, et al. Equine-like G3 rotavirus strains as predominant strains among children in Indonesia in 2015–2016. Infect Genet Evol. 2018;61:224–8. 10.1016/j.meegid.2018.03.027 . [DOI] [PubMed] [Google Scholar]
- 37.Guerra SF, Soares LS, Lobo PS, Penha Junior ET, Sousa Junior EC, Bezerra DA, et al. Detection of a novel equine-like G3 rotavirus associated with acute gastroenteritis in Brazil. J Gen Virol. 2016;97(12):3131–8. 10.1099/jgv.0.000626 . [DOI] [PubMed] [Google Scholar]
- 38.Luchs A, da Costa AC, Cilli A, Komninakis SCV, Carmona RCC, Boen L, et al. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J Gen Virol. 2019;100(1):7–25. 10.1099/jgv.0.001171 . [DOI] [PubMed] [Google Scholar]
- 39.Roczo-Farkas S, Kirkwood CD, Cowley D, Barnes GL, Bishop RF, Bogdanovic-Sakran N, et al. The Impact of Rotavirus Vaccines on Genotype Diversity: A Comprehensive Analysis of 2 Decades of Australian Surveillance Data. J Infect Dis. 2018;218(4):546–54. 10.1093/infdis/jiy197 . [DOI] [PubMed] [Google Scholar]
- 40.Tsugawa T, Tatsumi M, Tsutsumi H. Virulence-associated genome mutations of murine rotavirus identified by alternating serial passages in mice and cell cultures. J Virol. 2014;88(10):5543–58. 10.1128/JVI.00041-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda S, Tacharoenmuang R, Guntapong R, Upachai S, Singchai P, Ide T, et al. Full genome characterization of novel DS-1-like G9P[8] rotavirus strains that have emerged in Thailand. PLoS One. 2020;15(4):e0231099 Epub 2020/04/23. 10.1371/journal.pone.0231099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins C, Mijatovic-Rustempasic S, Ward ML, Cortese MM, Bowen MD. Genomic Characterization of the First Equine-Like G3P[8] Rotavirus Strain Detected in the United States. Genome Announc. 2017;5(47). 10.1128/genomeA.01341-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kikuchi W, Nakagomi T, Gauchan P, Agbemabiese CA, Noguchi A, Nakagomi O, et al. Detection in Japan of an equine-like G3P[8] reassortant rotavirus A strain that is highly homologous to European strains across all genome segments. Arch Virol. 2018;163(3):791–4. 10.1007/s00705-017-3668-7 . [DOI] [PubMed] [Google Scholar]
- 44.Guerra SFS, Soares LS, Lobo PS, Penha Junior ET, Sousa Junior EC, Bezerra DAM, et al. Detection of a novel equine-like G3 rotavirus associated with acute gastroenteritis in Brazil. J Gen Virol. 2016;97(12):3131–8. 10.1099/jgv.0.000626 . [DOI] [PubMed] [Google Scholar]
- 45.Doro R, Marton S, Bartokne AH, Lengyel G, Agocs Z, Jakab F, et al. Equine-like G3 rotavirus in Hungary, 2015—Is it a novel intergenogroup reassortant pandemic strain? Acta Microbiol Immunol Hung. 2016;63(2):243–55. 10.1556/030.63.2016.2.8 . [DOI] [PubMed] [Google Scholar]
- 46.Arana A, Montes M, Jere KC, Alkorta M, Iturriza-Gomara M, Cilla G. Emergence and spread of G3P[8] rotaviruses possessing an equine-like VP7 and a DS-1-like genetic backbone in the Basque Country (North of Spain), 2015. Infect Genet Evol. 2016;44:137–44. 10.1016/j.meegid.2016.06.048 . [DOI] [PubMed] [Google Scholar]
- 47.Nakagomi T, Nguyen MQ, Gauchan P, Agbemabiese CA, Kaneko M, Do LP, et al. Evolution of DS-1-like G1P[8] double-gene reassortant rotavirus A strains causing gastroenteritis in children in Vietnam in 2012/2013. Arch Virol. 2017;162(3):739–48. 10.1007/s00705-016-3155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono M, Tsugawa T, Nakata S, Kondo K, Tatsumi M, Tsutsumi H, et al. Rotavirus genotype and Vesikari score of outpatients in Japan in the vaccine era. Pediatr Int. 2020. 10.1111/ped.14150 . [DOI] [PubMed] [Google Scholar]
- 49.Jere KC, Bar-Zeev N, Chande A, Bennett A, Pollock L, Sanchez-Lopez PF, et al. Vaccine Effectiveness against DS-1-Like Rotavirus Strains in Infants with Acute Gastroenteritis, Malawi, 2013–2015. Emerg Infect Dis. 2019;25(9):1734–7. 10.3201/eid2509.190258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braeckman T, Van Herck K, Meyer N, Pircon JY, Soriano-Gabarro M, Heylen E, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ. 2012;345:e4752 10.1136/bmj.e4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201(3):363–9. 10.1086/649843 . [DOI] [PubMed] [Google Scholar]
- 52.Nakagomi T. Vaccine Effectiveness against DS-1-Like Rotavirus Strains. Emerg Infect Dis. 2020;26(1):184 10.3201/eid2601.191377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences files are available from the GenBank database (Accession numbers MN836856-MN837068 and MN989603-MN989613 for VP7, MN989625-MN989850 for VP6, and MN837284-MN837475 and MN989614-MN989624 for VP4).