Abstract
Previous studies suggest that marathon running induces lower extremity muscle damage. This study aimed to examine inter- and intramuscular differences in hamstring muscle damage after a marathon using transverse relaxation time (T2)–weighted magnetic resonance images (MRI). 20 healthy collegiate marathon runners (15 males) were recruited for this study. T2-MRI was performed before (PRE) and at 1 (D1), 3 (D3), and 8 days (D8) after marathon, and the T2 values of each hamstring muscle at the distal, middle, and proximal sites were calculated. Results indicated that no significant intermuscular differences in T2 changes were observed and that, regardless of muscle, the T2 values of the distal and middle sites increased significantly at D1 and D3 and recovered at D8, although those values of the proximal site remained constant. T2 significantly increased at distal and middle sites of the biceps femoris long head on D1 (p = 0.030 and p = 0.004, respectively) and D3 (p = 0.007 and p = 0.041, respectively), distal biceps femoris short head on D1 (p = 0.036), distal semitendinosus on D1 (p = 0.047) and D3 (p = 0.010), middle semitendinosus on D1 (p = 0.005), and distal and middle sites of the semimembranosus on D1 (p = 0.008 and p = 0.040, respectively) and D3 (p = 0.002 and p = 0.018, respectively). These results suggest that the distal and middle sites of the hamstring muscles are more susceptible to damage induced by running a full marathon. Conditioning that focuses on the distal and middle sites of the hamstring muscles may be more useful in improving recovery strategies after prolonged running.
Introduction
Prolonged running, such as in a marathon, has become a popular sports activity for health promotion. However, marathon races are often reported to induce muscle damage in the lower extremity muscles, manifesting as decreased muscle strength [1–4], occurrence of delayed onset muscle soreness [4, 5], and increased plasma creatine kinase (CK) levels [1, 2, 4], which usually peak at 1–3 days after the race and take several days to recover. To better understand the location of muscle damage in the leg muscles induced by a marathon, previous studies using ultrasound elastography have demonstrated that the thigh and lower leg muscles became harder after a marathon [6, 7], that the amount of change in muscle mechanical properties (e.g., muscle hardness) does not occur uniformly within the lower extremity muscles, and that the recovery time for each muscle varies. Muscle hardness in the vastus lateralis, biceps femoris, and soleus muscles returned to baseline at 8 days after the race, whereas the rectus femoris and gastrocnemius medial muscle hardness did not recover even after 8 days [6]. A study which determines each muscle damage after prolonged running can provide evidence for recreational and competitive runners about the time required for the recovery of each lower extremity muscle between training sessions and/or races.
Muscle functional magnetic resonance imaging (mfMRI) allows for the examination of differences in the intensity and/or pattern of exercise-induced muscle damage. This method relies on an exercise-induced increase in the proton transverse relaxation time (T2) of the muscle on MRI, which could provide information about the water content of muscle tissues. A delayed T2 increase, which occurs at 1–5 days after exercise, is a consequence of muscle damage (inflammatory oedema) induced by eccentric contractions [8]; a high correlation has been reported between changes in T2 and plasma CK levels measured within several days after eccentric exercise [9]. In addition, since T2 values are mapped out across cross-sectional images of muscles, mfMRI can determine muscle damage even in deep muscles; this high spatial resolution overcomes the limitations associated with ultrasound imaging. By using these advantages, a previous study examined the location of muscle damage within the quadriceps femoris muscles induced by downhill running and suggested that muscle damage is specifically located at the proximal and middle sites of the vastus intermedius muscle compared to other knee extensors [10]. Considering these findings, it may be possible that different patterns of running-induced muscle damage may also exist in each section of the hamstring muscles.
The hamstring muscles are composed of three muscles: the semimembranosus (SM), semitendinosus (ST), and biceps femoris (BF); the BF has a short (BFsh) and a long head (BFlh). The BFsh arises from the femur and shares a common distal tendon with BFlh, making it a monoarticular muscle that spans the knee joint only. By contrast, the SM, ST, and BFlh all arise from the ischial tuberosity on the pelvis and thus are biarticular muscles that span the hip and knee joints. During running, the hamstring muscles play an important role in decelerating the flexing hip and rapidly extending the knee [11]; biarticular hamstring muscles contract eccentrically in the terminal swing phase of running [12, 13]. In addition, hamstring muscles are known to be susceptible to muscle strain. Repetitive eccentric contractions associated with running may lead to the accumulation of eccentrically induced muscle damage [14, 15]. Such accumulation could, in turn, leave the hamstring muscles more at risk of strain injury [14]. Considering the fact that hamstring muscles are susceptible to muscle strain, identification of the sites in the hamstring muscles that are significantly damaged by prolonged running would contribute to an effective strategy for recovery focusing on injury prevention. In addition, this will provide evidence about the time required for the recovery of each site in the hamstring muscle between training sessions and/or competitions. Nevertheless, to our knowledge, there have been no studies on the location of muscle damage in the hamstring muscles induced by repetitive eccentric contractions after marathon running.
Thus, this study aimed to identify the sites in the hamstring muscles that are significantly damaged by prolonged running (full marathon) using mfMRI. For this purpose, we measured running-induced changes in T2 values at the proximal, middle, and distal sites of each hamstring muscle. We hypothesized that the location of muscle damage following running would be greatest in the BFlh muscle, based on previous kinematic analyses of running, considering that the peak muscle–tendon stretch is greatest in the BFlh muscle among the biarticular hamstring muscles during the terminal swing phase of running [12, 13], which occurs simultaneously with high muscle activity [16]. In addition, given that the knee joint performs greater negative (eccentric) work than the hip joint during running [17], we hypothesized that muscle damage would be more pronounced in the distal site of the biarticular hamstring muscles.
Materials and methods
Subjects
Twenty collegiate runners (males, 15; females, 5) without lower extremity injuries were recruited for this study. Mean age, height, and body mass of participants were 20.5±1.4 years, 168.6±7.1 cm, and 59.4±7.1 kg, respectively. Participants were members of a track and field club who had registered for a marathon race (Fujisan Marathon in Japan, 2016–2017; 42.195 km) and had no history of lower limb injuries.
This study was approved by the local human research ethics committee of Waseda University (2014–246), complied with their requirements for human experimentation, and conformed to the principles of the Declaration of Helsinki. The purpose, procedures, and risks of the study were communicated to the participants, and written informed consent was obtained from each participant. Participants were asked not to perform exercise, such as running, and not to receive special care for recovery, such as a massage, during the observation period after marathon.
Procedures
Data collection
All participants completed the marathon. Maximal isometric knee flexion torque and T2-weighted magnetic resonance (MR) images of the thigh were obtained before (2 days before the race [PRE]) and at 1 (D1), 3 (D3), and 8 (D8) days after the marathon race. The course altitude was approximately 850 m above sea level from the start to approximately 22 km, increased to around 910 m, was almost flat from 23 km to 34 km, declined to 850 m from 34 km to 36 km, and was flat from 36 km to finish.
T2-MRI
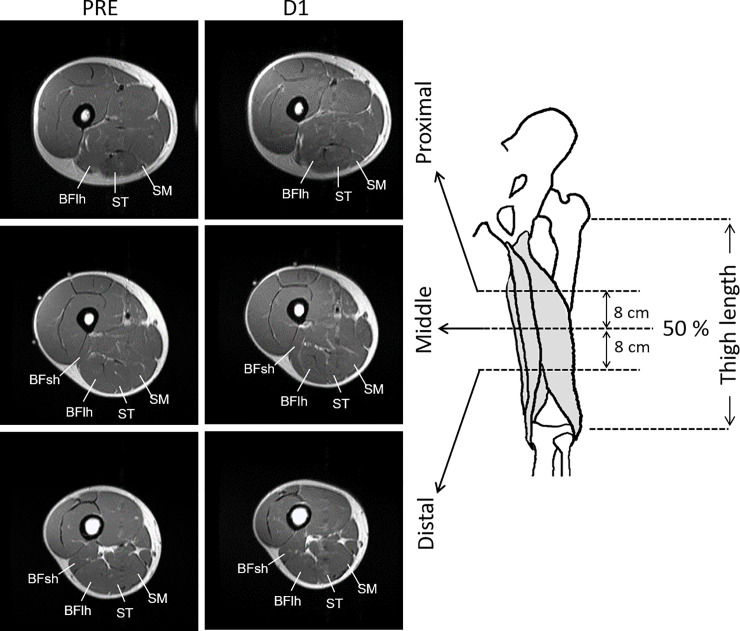
Thigh T2-MRI (Fig 1) was obtained with an MR scanner (Signa EXCITE 1.5T; GE Medical Systems, Waukesha, WI). Before scanning, participants lay quietly for 10 min. Ink lines were drawn transversely across the middle (50%) of the thigh (i.e., the distance from the great trochanter of the femur to the articular cleft between the femoral and tibial condyles) and oil capsules were put as markers on the skin surface at the lateral side. Participants lay supine with their legs fully extended and muscles relaxed in a magnet bore. The images were acquired with the following parameters: echo times, 25, 50, 75, and 100 ms; repetition time, 2000 ms; matrix, 256×160; field of view, 240 mm; slice thickness, 10 mm; gap, 10 mm. Images were analysed with ImageJ software (National Institute of Health, Bethesda, MD). Regions of interest were drawn in each slice by manually tracing the border of the anatomical cross-sectional area of each of the hamstring muscles at the middle (50%), proximal (8 cm proximal from the middle site), and distal (8 cm distal from the middle site) thigh sites (Fig 1). Care was taken to exclude noncontractile tissues, such as intramuscular fat and blood vessels. The T2 values for each pixel within the BFlh, BFsh, ST, and SM muscles were calculated, and the mean value was computed for each slice. As the BFsh is a monoarticular muscle with an origin at a more distal site than those of the other three muscles, only the middle and distal sites were analysed. T2 relaxation time was calculated by least-squares analysis, fitting the signal intensity at each of the four echo times (n×25 ms: 25, 50, 75, and 100 ms) to a monoexponential decay using the following equation:
where TE is the echo time, S0 is the signal intensity at 0 ms, and Sn is the signal intensity at TEn.
Fig 1. MRI measurement sites.
Examples of T2-MRI at the proximal, middle, and distal sites scanned before (PRE) and at 1 day (D1) after a full marathon race. BFlh, biceps femoris long head; BFsh, biceps femoris short head; MRI, magnetic resonance imaging; SM, semimembranosus; ST, semitendinosus.
The aforementioned analyses were performed three times for each slice and the average value was used for further analysis. The coefficient of variation of the three measurements for T2 values was 0.8±0.8%. The intraclass correlation coefficient of the measurements was 0.964. The absolute T2 values (ms) were used in between-time comparisons for each site. In addition, as the timing of T2 manifestations could vary among sites and individuals, peak T2 values (peak ΔT2, in ms) were calculated by subtracting the baseline (PRE) value from the peak value after marathon for each site, irrespective of the time point at which peak value occurred, and was used for between-site comparisons.
Maximal isometric knee flexion torque
After MR scanning, participants performed isometric right knee flexion with maximal effort while lying down in a prone position. The experimenter fixed the participant’s knee joint at 90° with a hand-held dynamometer (microFET2; Hoggan Scientific, Salt Lake, UT); the participants performed two isometric contractions against the experimenter’s force. They were asked to develop torque gradually over 5 s and reach the maximum, and they received verbal encouragement to sustain the maximum effort for 2 s from the examiner. Two peak torque values were averaged for the statistical analysis.
Statistical analyses
All statistical analyses were conducted using SPSS version 14.0 (IBM Corp., Armonk, NY). Changes in T2 values were compared by a three-way analysis of variance (ANOVA) with repeated measures (muscle × site × time point). When significant interaction effects were found, Bonferroni’s post hoc test was performed to compare changes from the PRE for each site. Absolute peak ΔT2 values were compared among the sites using one-way ANOVA (11 sites) followed by Bonferroni post hoc testing. Changes in maximal knee flxexion torque were compared by a one-way (4 time points) repeated measures ANOVA followed by Bonferroni post hoc testing. Partial η2 (for ANOVA) was calculated as indices of effect size (ES) for the ANOVA. To report the ES for post hoc comparisons, the mean change value from the PRE divided by the standard deviation of the change value was calculated [18]. Statistical significance was set at p<0.05.
Results
Marathon time
The average marathon completion time was 4 h, 7 min, 55 s ± 47 min, 39 s.
T2-MRI
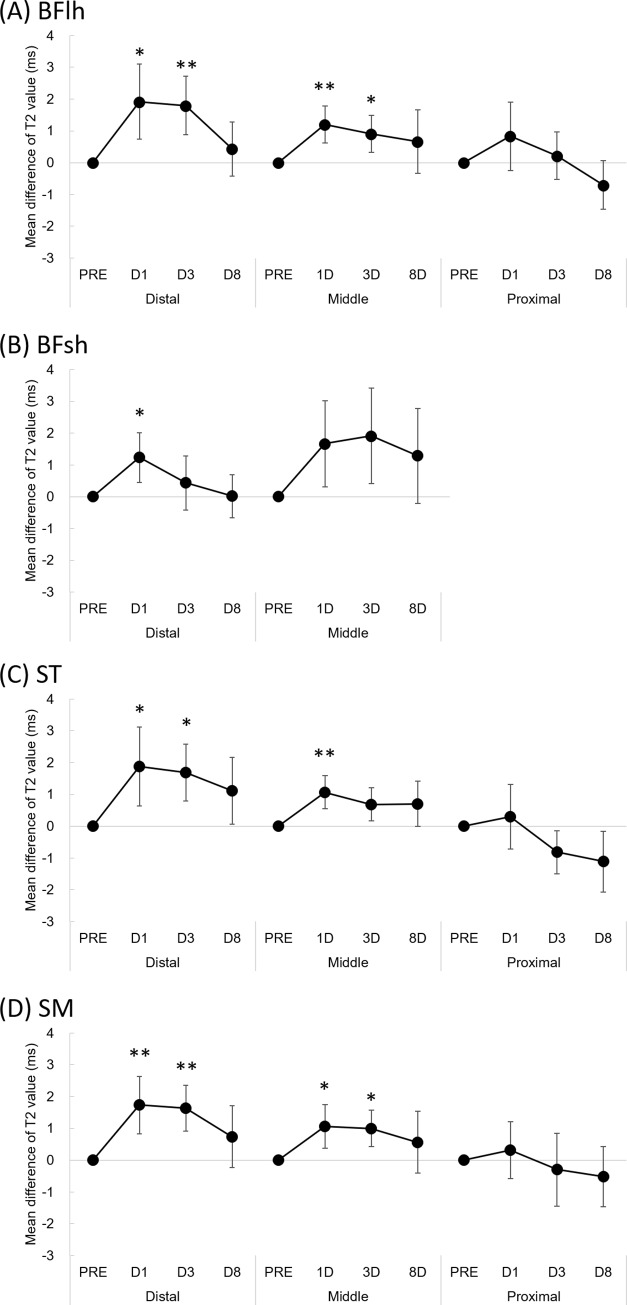
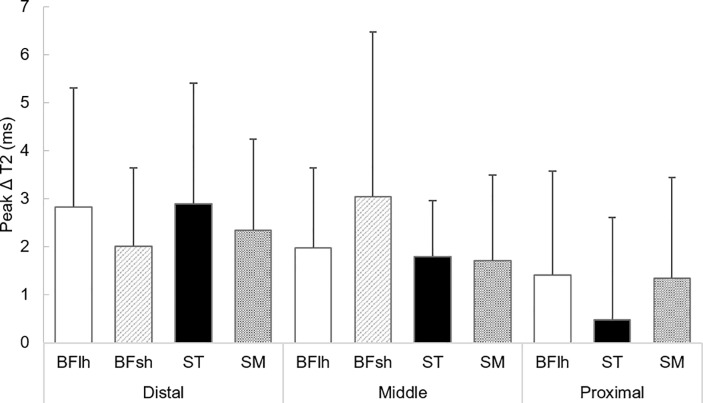
Three-way ANOVA analysis for T2 values indicated some interaction effects: muscle × site (F = 3.393, p = 0.006) and site × time point (F = 5.729, p<0.001) (Table 1). The absolute T2 values for each site are shown in the Table 2. Fig 2 shows the mean difference and 95% confidence intervals in T2 value from the baseline (PRE) for each muscle. Bonferroni’s post hoc analysis indicated that, regardless of muscle, the T2 values of the distal and middle sites increased significantly at D1 and D3 and recovered at D8 (no significant difference on D8 when compared with PRE), although those values of the proximal site remained constant. T2 significantly increased at distal and middle sites of the biceps femoris long head on D1 (p = 0.030 and p = 0.004, respectively) and D3 (p = 0.007 and p = 0.041, respectively), distal biceps femoris short head on D1 (p = 0.036), distal semitendinosus on D1 (p = 0.047) and D3 (p = 0.010), middle semitendinosus on D1 (p = 0.005), and distal and middle sites of the semimembranosus on D1 (p = 0.008 and p = 0.040, respectively) and D3 (p = 0.002 and p = 0.018, respectively). There was no significant difference in the T2 value of the middle BFsh muscle and the proximal site of the BFlh, ST, and SM at any time point compared with that of PRE. Fig 3 shows the peak ΔT2 value for each site. No significant differences in the peak ΔT2 values after the full marathon were observed among sites.
Table 1. Results of statistical analyses for the T2 values.
| Source | Three-way ANOVA | ||||
|---|---|---|---|---|---|
| df | F-value | p-value | η2 | ||
| Site | 2 | 14.950 | 0.000 | 0.125 | ** |
| Muscle | 3 | 21.158 | 0.000 | 0.233 | ** |
| Time point | 3 | 27.508 | 0.000 | 0.116 | ** |
| Site × muscle | 5 | 3.393 | 0.006 | 0.075 | * |
| Site × time point | 6 | 5.729 | 0.000 | 0.052 | ** |
| Muscle × time point | 9 | 0.368 | 0.950 | 0.005 | n.s. |
| Site × muscle × time point | 15 | 0.861 | 0.609 | 0.020 | n.s. |
* p < 0.01
** p < 0.001; n.s. not significantly different
Table 2. Time-dependent change in the absolute value of T2 values (Mean ± SD) measured before (PRE) and 1 day (D1), 3 days (D3) and 8 days (D8) after the marathon race.
Effect sizes are shown in parentheses.
| PRE | D1 | D3 | D8 | ||
|---|---|---|---|---|---|
| BFlh | |||||
| Distal | 31.7 ± 1.2 | 33.6 ± 2.7* [0.71] | 33.5 ± 2.0** [0.86] | 32.1 ± 1.9 [0.22] | |
| Middle | 32.2 ± 1.1 | 33.4 ± 1.5** [0.90] | 33.1 ±1.4* [0.68] | 32.8 ± 2.3 [0.29] | |
| Proximal | 35.5 ±1.8 | 36.3 ±2.1 [0.34] | 35.7 ± 1.7 [0.13] | 34.8 ± 1.3 [0.41] | |
| BFsh | |||||
| Distal | 32.2 ± 1.6 | 33.9 ± 2.9* [0.69] | 34.1 ± 3.2 [0.22] | 33.5 ± 3.4 [0.01] | |
| Middle | 32.8 ± 1.9 | 34.0 ± 2.5 [0.54] | 33.2 ± 2.3 [0.56] | 32.8 ± 2.2 [0.38] | |
| ST | |||||
| Distal | 30.4 ± 1.6 | 32.3 ± 2.7* [0.66] | 32.1 ± 2.0* [0.82] | 31.5 ± 2.4 [0.47] | |
| Middle | 30.9 ± 1.1 | 32.0 ± 1.5** [0.89] | 31.6 ± 1.7 [0.58] | 31.6 ± 2.0 [0.43] | |
| Proximal | 32.2 ± 2.0 | 32.5 ± 1.4 [0.3] | 31.3 ± 1.5 [0.53] | 31.0 ±1.2 [0.51] | |
| SM | |||||
| Distal | 31.5 ± 1.5 | 33.2 ± 2.5** [0.84] | 33.1 ± 1.6** [1.00] | 32.2 ± 2.2 [0.33] | |
| Middle | 31.7 ± 1.1 | 32.7 ± 1.4* [0.68] | 32.7 ± 1.3* [0.76] | 32.3 ± 1.9 [0.26] | |
| Proximal | 33.8 ± 2.9 | 34.1 ± 3.1 [0.15] | 33.5 ± 3.2 [0.11] | 33.3 ± 2.2 [0.24] | |
*Significantly different from PRE at p < 0.05.
**Significantly different from PRE at p < 0.01. BFlh, biceps femoris long head; BFsh, biceps femoris short head; ST, semitendinosus; SM, semimembranosus.
Fig 2.
Mean difference in T2 value from the baseline value (PRE) of the biceps femoris long head (A), biceps femoris short head (B), semitendinosus (C), and semimembranosus (D) muscles. Error bars indicate 95% confidence intervals. *Significantly different from PRE at p < 0.05. **Significantly different from PRE at p < 0.01. D1, 1 day after marathon; D3, 3 days after marathon; D8, 8 days after marathon. BFlh, biceps femoris long head; BFsh, biceps femoris short head; ST, semitendinosus; SM, semimembranosus.
Fig 3. Peak T2 changes from PRE (ΔT2) within 8 days after a full marathon.
Values are mean±standard deviation. BFlh, biceps femoris long head; BFsh, biceps femoris short head; ST, semitendinosus; SM, semimembranosus.
Maximal isometric knee flexion torque
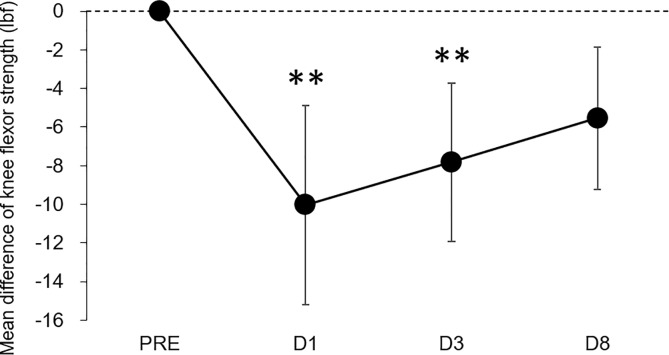
One-way ANOVA revealed a significant main effect of time on maximal knee flexion torque (F = 8.491, p<0.001, partial η2 = 0.309). Bonferroni post-hoc testing showed that the knee flexion torque significantly decreased compared with the baseline (PRE) value on D1 (−22.7%, p = 0.007, ES = 0.85) and D3 (−17.7%, p = 0.009, ES = 0.83) after marathon; however, there was no significant difference on D8 (−5.5%, p = 0.050, ES = 0.65) compared with PRE (Fig 4).
Fig 4. Mean difference in maximal isometric knee flexion torque from the baseline (PRE).
Error bars indicate 95% confidence intervals. D1, 1 day after marathon; D3, 3 days after marathon; D8, 8 days after marathon.
Discussion
We determined whether there are inter- and intramuscular differences in damage to the hamstring muscles after a full marathon using T2-MRI. The main findings of the present study were that no significant intermuscular differences in the magnitude of T2 change after a marathon were found and that T2 significantly increased at the distal and middle sites of the biarticular hamstring muscles on D1 and D3 after the marathon race; however, no significant difference in the T2 value of the proximal site was observed at any time point when compared with PRE. These results suggest that running a full marathon induces muscle damage (inflammatory oedema) appearing as a T2 increase in the hamstring muscles and that the magnitude of muscle damage induced by prolonged running would be greater in the distal and middle sites than in the proximal site of the hamstring muscles.
Marathon running resulted in significant decreases in maximal knee flexion torque on D1 and D3 (Fig 4). This result is similar to that of a previous study, which showed a significant reduction in maximal voluntary knee flexion torque following a full marathon [4]. However, no significant differences in the magnitude of T2 change (peak ΔT2) after running were found among muscles (Fig 3). These results did not support our hypothesis that muscle damage would be greatest in the BFlh muscle. We drafted this hypothesis based on previous observations, which suggested that the magnitude of muscle–tendon strain during contraction is the more relevant parameter for muscle damage [19] and that peak muscle–tendon stretch is greatest in the BFlh during running compared to the ST and SM [12, 13]. Schache et al. [12] investigated the peak muscle–tendon stretch of the biarticular hamstring muscles at different running speeds and demonstrated that the peak stretch was greater for BFlh compared to ST and SM across a range of running speeds. They also found that there was no statistical difference in the magnitude of the maximum stretch between these muscles during slower speed below 18 km/h. The participants in the present study completed a full marathon at an average time of 4 h, 7 min, 55 s ± 47 min, 39 s (i.e., at an average speed of 11 km/h). Thus, the findings of Schache et al. [12] would support the result of the current study; however, the degree of muscle damage induced by prolonged running, such as after a full marathon, may not simply be explained based on the intermuscular difference in stretching magnitude: one possibility is the alternate muscle activity among the synergists due to the fatigue during prolonged running, while the previous study suggested the alterations in the neuromuscular activation patterns among the quadriceps muscles during the repetitive fatiguing exercise [20]. This phenomenon may occur in the hamstring muscles during marathon running. However, given the results of the mfMRI analysis indicating the intensity and/or pattern of exercise-induced muscle damage after exercise, whether the alternate muscle activity among the hamstring muscles occurs during the marathon race is uncertain. Even though it is a speculation, is worthy of further examination.
T2 values significantly increased at the distal and middle sites of the BFlh, ST, and SM muscles on D1 and D3 after running (Table 2, Fig 2), with a pronounced effect size found, whereas the T2 value of the proximal site at any time point did not significantly increase compared with PRE. This suggests that the distal and middle sites of the biarticular hamstring muscles are more susceptible to damage after a marathon. The amount of negative work performed by the muscle would be the best predictor of the magnitude of damage, as indicated by force deficits following active or passive muscle stretching [21]. Therefore, a possible explanation for the present results is that the location of muscle damage after prolonged running is primarily influenced by the intensity of repetitive negative work performed by the lower extremity. During running, the knee joint performs greater negative work than the hip joint (e.g., −0.41 J/kg for the knee and −0.10 J/kg for the hip at a running speed of 3.5 m/s) [17]. In addition, a previous study reported that during eccentric knee flexion, the greatest tissue motion in the BFlh muscle is observed along the distal musculotendinous junction [22]. Considering these findings and the fact that a greater degree of muscle tissue strain during eccentric contraction causes muscle damage [19], the magnitude of muscle damage induced by prolonged running would be greater in the distal than in the proximal portions of the hamstring muscles.
A previous study suggested that the accumulation of eccentrically-induced muscle damage associated with running may leave the hamstring muscles more vulnerable to strain injury [14]. The aetiology and risk of hamstring strain injury is closely related to high-speed running [23], and transiently elevated high-speed running exposure (<24 km/h) at 7–14 days prior to injury increases the likelihood of injury [24, 25]. However, sports in which a high rate of hamstring strain injury has been reported [26], such as Australian football, require prolonged and high-intensity intermittent performance, and players cover long distances during a game (12,939±1145 m for total distance) at various speeds; movement distance with a higher-speed running (>14.5 km/h) accounts only for 30% of the total distance [27]. The present results indicated that repetitive eccentric contractions associated with lower speed (at an average speed of 11 km/h) but prolonged running, such as in a marathon, produce inflammatory oedema at the distal and middle sites of biarticular hamstring muscles. In addition, considering that this microscopic damage in hamstring muscles takes several days to recover, accumulation of prior running exposure even at lower speeds may contribute to the susceptibility of strain injury for some time after. This hypothesis is yet to be explored. Moreover, the magnitude of muscle damage is largely reduced when the same or similar eccentric exercise is repeated within several weeks [28]. Therefore, differences in training and competition level may affect the magnitude of muscle damage induced by prolonged running. This should be regarded as a limitation; future research should ascertain whether similar findings are also seen in runners from different competitive levels or other competitive athletes.
In this study, we demonstrate that the distal and middle sites of the biarticular hamstring muscles are more susceptible to damage induced after running a marathon. Based on our results, conditioning that focuses on the distal and middle sites of the hamstring muscles may be more useful in improving recovery strategies as well as for preventing injury after prolonged running. In addition, our results showed that inflammatory oedema appearing as T2 increase takes several days to recover. Therefore, accumulation of low-intensity running sessions may contribute to the susceptibility of strain injury afterwards. However, our results are inconsistent with the clinical observations that the proximal muscle–tendon junction of the BFlh muscles is more commonly affected in acute hamstring muscle injury [23, 29]. This discrepancy could be explained by the possible difference in the neuromechanical behaviour and joint kinetics between high-speed running, in which an injury occurs, and prolonged running. Therefore, further research is required to identify the regional damage patterns after repetitive high-speed running relative to the injury site. It is likely that such studies can provide further insights into the mechanisms of muscle strain injury during high-speed running.
In conclusion, the results indicated that marathon running induces a significant damage to hamstring muscles; however, the amount of change in inflammatory oedema appearing as T2 increase did not differ among hamstring muscles. Furthermore, site-dependent changes in T2 values after a full marathon were observed within muscles; that is, pronounced inflammatory oedema was observed in the distal and middle sites of the biarticular hamstring muscles. Our findings provide a better understanding of the site-specific muscle damage patterns of the hamstring muscle induced by prolonged running.
Data Availability
All relevant data are uploaded to Zenodo (DOI: 10.5281/zenodo.3734098).
Funding Statement
This work was supported by the Mizuno Sports Promotion Foundation.
References
- 1.Clifford T, Allerton DM, Brown MA, Harper L, Horsburgh S, Keane KM, et al. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metab. 2017;42(3):263–70. 10.1139/apnm-2016-0525 [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Howatson G, van Someren KA, Walshe I, Pedlar CR. Influence of compression garments on recovery after marathon running. J Strength Cond Res. 2014;28(8):2228–35. 10.1519/JSC.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 3.Petersen K, Hansen CB, Aagaard P, Madsen K. Muscle mechanical characteristics in fatigue and recovery from a marathon race in highly trained runners. Eur J Appl Physiol. 2007;101(3):385–96. 10.1007/s00421-007-0504-x [DOI] [PubMed] [Google Scholar]
- 4.Takayama F, Aoyagi A, Shimazu W, Nabekura Y. Effects of marathon running on aerobic fitness and performance in recreational runners one week after a race. J Sports Med (Hindawi Publ Corp). 2017;2017:9402386 10.1155/2017/9402386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. 2010;20(6):843–52. 10.1111/j.1600-0838.2009.01005.x [DOI] [PubMed] [Google Scholar]
- 6.Inami T, Nakagawa K, Yonezu T, Fukano M, Higashihara A, Iizuka S, et al. Tracking of time-dependent changes in muscle hardness after a full marathon. J Strength Cond Res. 2019;33(12):3431–7. 10.1519/JSC.0000000000002495 [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa K, Inami T, Yonezu T, Kenmotsu Y, Narita T, Kawakami Y, et al. Unstable rocker shoes promote recovery from marathon-induced muscle damage in novice runners. Scand J Med Sci Sports. 2018;28(2):621–9. 10.1111/sms.12911 [DOI] [PubMed] [Google Scholar]
- 8.Ochi E, Tsuchiya Y, Nosaka K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur J Appl Physiol. 2016;116(11–12):2145–54. 10.1007/s00421-016-3462-3 [DOI] [PubMed] [Google Scholar]
- 9.Larsen RG, Ringgaard S, Overgaard K. Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scand J Med Sci Sports. 2007;17(1):76–83. 10.1111/j.1600-0838.2006.00525.x [DOI] [PubMed] [Google Scholar]
- 10.Maeo S, Ando Y, Kanehisa H, Kawakami Y. Localization of damage in the human leg muscles induced by downhill running. Sci Rep. 2017;7(1):5769 10.1038/s41598-017-06129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schache AG, Dorn TW, Blanch PD, Brown NA, Pandy MG. Mechanics of the human hamstring muscles during sprinting. Med Sci Sports Exerc. 2012;44(4):647–58. Epub 2011/09/14. 10.1249/MSS.0b013e318236a3d2 [DOI] [PubMed] [Google Scholar]
- 12.Schache AG, Dorn TW, Wrigley TV, Brown NA, Pandy MG. Stretch and activation of the human biarticular hamstrings across a range of running speeds. Eur J Appl Physiol. 2013;113(11):2813–28. Epub 2013/09/10. 10.1007/s00421-013-2713-9 [DOI] [PubMed] [Google Scholar]
- 13.Thelen DG, Chumanov ES, Hoerth DM, Best TM, Swanson SC, Li L, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108–14. 10.1249/01.mss.0000150078.79120.c8 [DOI] [PubMed] [Google Scholar]
- 14.Fyfe JJ, Opar DA, Williams MD, Shield AJ. The role of neuromuscular inhibition in hamstring strain injury recurrence. J Electromyogr Kinesiol. 2013;23(3):523–30. Epub 2013/02/14. 10.1016/j.jelekin.2012.12.006 . [DOI] [PubMed] [Google Scholar]
- 15.Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57(2):209–21. 10.1016/S0006-3495(90)82524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashihara A, Nagano Y, Ono T, Fukubayashi T. Relationship between the peak time of hamstring stretch and activation during sprinting. Eur J Sport Sci. 2016;16(1):36–41. Epub 2014/11/02. 10.1080/17461391.2014.973913 [DOI] [PubMed] [Google Scholar]
- 17.Schache AG, Blanch PD, Dorn TW, Brown NA, Rosemond D, Pandy MG. Effect of running speed on lower limb joint kinetics. Med Sci Sports Exerc. 2011;43(7):1260–71. Epub 2010/12/07. 10.1249/MSS.0b013e3182084929 [DOI] [PubMed] [Google Scholar]
- 18.Dankel SJ and Loenneke JP. Effect sizes for paired data should use the change score variability rather than the pre-test variability. J Strength Cond Res. 2018. 10.1519/jsc.0000000000002946 [DOI] [PubMed] [Google Scholar]
- 19.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74(2):520–6. Epub 1993/02/01. 10.1152/jappl.1993.74.2.520 [DOI] [PubMed] [Google Scholar]
- 20.Akima H, Foley JM, Prior BM, Dudley GA, Meyer RA. Vastus lateralis fatigue alters recruitment of musculus quadriceps femoris in humans. J Appl Physiol. 2002;92(2):679–84. Epub 2002/01/18. 10.1152/japplphysiol.00267.2001 [DOI] [PubMed] [Google Scholar]
- 21.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. The Journal of physiology. 1995;488 (Pt 2):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silder A, Reeder SB, Thelen DG. The influence of prior hamstring injury on lengthening muscle tissue mechanics. J Biomech. 2010;43(12):2254–60. Epub 2010/05/18. 10.1016/j.jbiomech.2010.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during slow-speed stretching: clinical, magnetic resonance imaging, and recovery characteristics. Am J Sports Med. 2007;35(10):1716–24. Epub 2007/06/15. 10.1177/0363546507303563 [DOI] [PubMed] [Google Scholar]
- 24.Duhig S, Shield AJ, Opar D, Gabbett TJ, Ferguson C, Williams M. Effect of high-speed running on hamstring strain injury risk. Br J Sports Med. 2016. Epub 2016/06/12. 10.1136/bjsports-2015-095679 [DOI] [PubMed] [Google Scholar]
- 25.Ruddy JD, Pollard CW, Timmins RG, Williams MD, Shield AJ, Opar DA. Running exposure is associated with the risk of hamstring strain injury in elite Australian footballers. Br J Sports Med. 2018;52(14):919–28. 10.1136/bjsports-2016-096777 . [DOI] [PubMed] [Google Scholar]
- 26.Orchard JW, Seward H, Orchard JJ. Results of 2 decades of injury surveillance and public release of data in the Australian Football League. Am J Sports Med. 2013;41(4):734–41. Epub 2013/03/06. 10.1177/0363546513476270 [DOI] [PubMed] [Google Scholar]
- 27.Coutts AJ, Quinn J, Hocking J, Castagna C, Rampinini E. Match running performance in elite Australian Rules Football. Journal of science and medicine in sport / Sports Medicine Australia. 2010;13(5):543–8. 10.1016/j.jsams.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 28.McHugh MP. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports. 2003;13(2):88–97. 10.1034/j.1600-0838.2003.02477.x [DOI] [PubMed] [Google Scholar]
- 29.Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: Association between anatomical location and extent of injury-A large single-center MRI report. J Sci Med Sport. 2016;19(4):317–22. Epub 2015/04/30. 10.1016/j.jsams.2015.04.005 [DOI] [PubMed] [Google Scholar]