Abstract
The canine hookworms Ancylostoma braziliense, Ancylostoma ceylanicum, Ancylostoma caninum and Uncinaria stenocephala are not only capable of producing morbidity and mortality in dogs but are also neglected tropical zoonoses. Each hookworm species differs considerably in its geographical distribution, life cycle, biology, pathogenic impacts on both canine and human hosts, zoonotic potential, and response to treatment with anthelminthics. Here we describe the development and validation of two Taq-Man based multiplex PCR assays capable of detecting and differentiating all four canine hookworm species in faeces of naturally infected dogs. The analytical sensitivity of both assays was assessed using 10-fold serial dilutions of synthetic gene block fragments containing individual sequence targets of each hookworm species. The sensitivity of the assays and ability to detect mixed species infections were compared to a conventional PCR-Restriction Fragment Length Polymorphism based-approach when applied to laboratory and field samples from endemic areas.
The qPCRs detected at least one species of hookworms in 82.4% of PCR-RFLP-negative but microscopy-positive samples. The qPCRs detected an additional 68% mixed infections with different species of canine hookworms, and additional single species infection with A. caninum (47%), U. stenocephala (33%) and A. ceylanicum (0.02%) that were missed by PCR-RFLP. These multiplex qPCR assays will assist field based epidemiological surveillance studies towards an accurate and sensitive monitoring of canine hookworm infections in dogs, to inform their species-specific zoonotic risks to populations living in endemic areas, globally.
Author summary
There are four species of blood-sucking hookworms that infect dogs, namely Ancylostoma braziliense, Ancylostoma ceylanicum, Ancylostoma caninum and Uncinaria stenocephala. Each hookworm species differs in its geographical distribution, impact on canine health and more importantly its ability to infect and produce disease in humans. For example, dogs are the primary reservoir for A. ceylanicum, the second most common hookworm species infecting humans in the Asia Pacific. Ancylostoma braziliense is more geographically confined and responsible for chronic skin eruptions in humans. Accurate diagnosis of hookworm species in dogs is therefore essential for understanding the relative risk each species pose to human populations living in close association with dogs. As microscopic methods are unable to distinguish between species of hookworms in stool, highly efficient real time PCR assays capable of detecting and differentiating all four canine hookworm species in canine faeces were developed. These new assays are superior to previously published gel-based PCR assay displaying higher throughput, and ability to correctly identify single and mixed hookworm species infections with higher accuracy.
Introduction
The canine hookworms Ancylostoma braziliense, Ancylostoma ceylanicum, Ancylostoma caninum and Uncinaria stenocephala are widely distributed soil-transmitted helminths causing morbidity in dogs and are agents of zoonoses [1–8]. Each hookworm species differs considerably in its geographical distribution, life cycle, biology, pathogenic impact on both canine and human hosts, zoonotic potential and response to anthelminthic treatment [1,9–15]. Dogs and humans become infected percutaneously or orally via the ingestion of the ensheathed larvae in soil or via contaminated food or water [15]. For A. caninum, A. braziliense and U. stenocephala transmission by dogs preying on paratenic hosts is also possible [6–8,16]. Trans-mammary transmission in dogs also occurs for A. caninum [15,17,18]. Infection, especially with A. caninum, is a common cause of haemorrhagic diarrhoea and death in pups and chronic iron deficiency anaemia in adult animals [18,19]. All canine hookworm species cause cutaneous larva migrans (CLM) [20]. In human patients, A. braziliense is by far the most frequently implicated, as it is the only species capable of causing classical ‘creeping eruptions’, a prolonged highly pruritic and serpiginous eruption within the dermis that may persist for over 100 days, if untreated [9–11]. In humans, A. caninum is a well-recognized agent of eosinophilic enteritis and aphthous ileitis. Although most infections are asymptomatic, a single immature adult worm residing in the small intestine is capable of eliciting abdominal pain, intestinal bleeding, diarrhoea and weigh loss [13,21,22]. Recent findings of A. caninum eggs in the faeces of human patients highlights its potential to complete its life cycle in humans [23].
A. ceylanicum on the other hand, is the only canine (and feline) hookworm species known to produce patent, long-lived infection within the intestinal tract of humans both in both natural and experimental infections [24–30]. In the Asia Pacific A. ceylanicum is the second most common hookworm infecting humans after Necator americanus and is responsible for between 5.5–51.6% of human infections in Laos, Malaysia, Thailand, Cambodia and the Solomon Islands [30–35]. Ancylostoma spp. are mostly found in subtropical and tropical regions and may co-exist as sympatric species and/or be featured by specific ecological requirements with implications for their distribution. For instance, A. caninum is endemic in wet and dry regions of the tropics and subtropics globally, while A. ceylanicum thrives in the wet tropics and subtropics of Africa, Asia and Oceania. Ancylostoma braziliense appears limited in distribution to a narrow latitudinal areas spanning approximately 5°N to 10°S of Brazil, Kenya, Tanzania, northern Australia, Indonesia and Malaysia [1–5]. Conversely, U. stenocephala, inhabits predominantly temperate and colder climate such as those of Europe, Canada, southern Australia and the United States of America [14,36].
In semi-domesticated dogs residing in urban and rural areas of Asia, Africa and South America, between 40–100% of dogs may be infected with hookworms contributing to a high environmental dispersal of potentially zoonotic infectious larvae [21, 29–32]. The high proportion of Ancylostoma-infected dogs in these tropical climates is exacerbated by the co-existence of several risk factors that allow the parasite to thrive in dogs and significantly increase their risk of infecting humans i.e. free ranging community dogs, a lack of veterinary care, irregular deworming, roaming behaviour and poor environmental hygiene [35,37–39].
Diagnosis of canine hookworm infections has traditionally relied on the identification of eggs isolated in faecal floatation and species-based identification by examining adult worms following necropsies or anthelmintic treatment [14,40]. For research purposes, these traditional techniques were replaced with molecular-based diagnostic assays nearly two decades ago, including PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) [41,42] targeting the internal transcribed spacer (ITS)-2 region as genetic marker for the detection and discrimination of hookworms from canine faeces. The PCR-RFLP, however, is relatively labour-intensive limiting large-scale epidemiological studies. Here we present novel Taq-Man based multiplex qPCRs to screen for canine hookworm species in mixed natural infections from canine faeces. Diagnostic parameters of the multiplex qPCR assays were validated and compared to the PCR-RFLP by using genomic DNA sourced from previously published epidemiological studies for canine hookworms conducted in Vietnam [43] and Southeast Queensland, Australia [44]
Materials and methods
Parasite material
A total of 191 faecal samples previously demonstrated positive for A. ceylanicum, A. caninum and U. stenocephala by PCR-RFLP were selected [42]. Of these 191 samples, a subset of 111 samples were also subjected to sodium chloride floatation and identified positive for hookworm eggs. Faecal samples were sourced from an existing collection donated by Dr. Dinh Ng-Nguyen from a previous investigation in 2014 in Vietnam [43] and from Dr. Lara Harriot from an investigation performed from 2012 to 2015 in Queensland (Australia) [44]. Of these 191 faecal samples 54 were confirmed positive for A. caninum, 60 A. ceylanicum and 4 U. stenocephala by PCR-RFLP [42]. Twelve samples harboured mixed infections with two or more species of hookworm. Hookworm-negative faeces were spiked with DNA of A. braziliense. This negative faecal sample was collected from a fully-dewormed pet dog residing in Melbourne, Victoria, and demonstrated free of hookworm eggs using sodium nitrate faecal floatation (SG 1.20). The hookworm-free faecal sample was divided into equal aliquots and spiked with serial dilution of genomic A. braziliense DNA belonging to an existing collection of hookworm genomic DNA of Prof. Rebecca Traub (University of Melbourne). DNA concentrations were quantified using a Qubit 4 Fluorometer (Life Technologies, Thermo Fisher Scientific Inc.) as per manufacturer’s instructions. Ten-fold serial dilutions of 0.219 ng/μl genomic DNA were prepared and spiked into seven tubes containing 150 mg of faeces each and 150 mg faeces with nuclease free water was used as negative control.
DNA was extracted from faeces using the ISOLATE Fecal DNA Kit (Bioline Sydney, Australia) according to the manufacturer’s instructions. Final DNA elution was made in 100 μl of elution buffer.
Multiplex qPCRs
The multiplex qPCR assays were designed to detect the ITS-1 rRNA region of A. braziliense, A. ceylanicum, A. caninum and U. stenocephala. Reference nucleotide sequences for the ITS-1 region of A. caninum (Reference GenBank accession number JQ812694; KP844730; DQ438071), A. ceylanicum (Reference GenBank accession number DQ780009; DQ831518), U. stenocephala (Reference GenBank accession number HQ262054; AF194145) and A. braziliense (Reference GenBank accession numbers JQ812692; DQ359149; DQ438056) were obtained from NCBI and aligned with GenBank sequences sourced from the available range of geographical isolates for each hookworm species, as well as other closely related canine helminths using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (S1 Text). Primers and probes targeting highly conserved region of the ITS-1 were designed manually and analysed for suitability within each multiplex assay using Oligo Analyzer (Integrated DNA technologies, https://sg.idtdna.com/calc/analyzer) and their genus or species-based specificity assessed using NCBI Blast (http://www.ncbi.nlm.nih.gov/blast). The primers were manufactured by Integrated DNA Technologies, USA. The probes were designed and manufactured using LNA (locked nucleic acid) technology (Integrated DNA Technologies, USA) to increase probe specificity. A four-channel Magnetic Induction Cycler (BioMolecular Systems, Sydney, Australia) was used for the amplification, detection, and data analysis (micPCR software).
Two qPCR multiplex assays were developed. The first reaction used a common primer pair designed (AcanceyF and AcanceyR) to amplify a 103 bp region of A. caninum and A. ceylanicum ITS-1 rRNA. The second qPCR used a common primer pair UncbrazF and UncbrazR to amplify a 119 bp and 118bp region of the ITS-1 rRNA of A. brazilienze and U. stenocephala, respectively. Equine herpes virus (EHV4) primers (EHVF and EHVR), probe (EHV probe) and genomic DNA were used as reaction internal control, and mammalian primers (Mam F and Mam R) and probe (MAM PROBE) designed to target a 92 bp region of the 16S mitochondrial gene of mammals was used as a DNA extraction control for both qPCR assays.
Optimisation of each singleplex and multiplex assays were carried out using synthetic double stranded DNA fragments (gBlocks Gene Fragments, IDT Technologies, Skokie, Illinois, USA) containing individual sequence targets of each hookworm species. Progressive concentrations of primer pairs and probes were tested to select the concentrations that guaranteed the highest efficiency and the greatest sensitivity.
The qPCR assays were conducted in 20 μL reactions containing 10 μL of GoTaq Probe qPCR Mastermix (Promega, Madison, WI), 1 μL of known quantity of EHV4 gDNA, and 2 μL of template DNA. Nuclease free-water was added to reach the final reaction volume. The cycling threshold was set at 0.2 units for A. caninum and A. ceylanicum and 0.1 unit for A. braziliense and U. stenocephala. The fluorescent threshold was set to 5% for all targets. The cycling conditions were identical for both reactions: 2 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. gBlocks Gene Fragments were used as positive controls and nuclease-free water was used as negative control in all runs. The efficiency and the intra-assay reproducibility of multiplex reactions were directly compared to each singleplex reaction by plotting Ct versus 10-fold serial dilutions of target gBlock Gene Fragments of known DNA concentration. The correlation coefficients (R2) of the standard curves were produced automatically by micPCR software (BioMolecular Systems, Sydney, Australia).
Sensitivity and specificity of the qPCRs
The analytical sensitivity (i.e. the ability of an essay to detect low concentrations of a given target substance or organism in a biological sample [45]) of the qPCRs was assessed using 10-fold serial dilutions of target gBlock Gene Fragments of known DNA concentration (i.e., 1.8 ng/ μl for A. caninum, 7,17 ng/μl for A. ceylanicum, 13.2 ng/μl for A. braziliense and 7.22 ng/ μl for U. braziliense). In addition, the analytical sensitivity of the qPCRs and PCR-RFLP was assessed and compared using 10-fold dilutions of known concentration of genomic DNA (i.e. 1.14 ng/ μl for A. ceylanicum, 1.65 ng/ μl for A. caninum, 2.94 ng/ μl for A. braziliense and 3.94 for U. stenocephala) sourced from an existing collection of hookworm genomic DNA of Prof. Rebecca Traub (University of Melbourne).
To investigate the analytical specificity of the assay (i.e. the ability of an assay to exclusively identify a particular target substance or organism rather than similar but different substances or organisms [45]) genomic DNA of the most common parasites infecting dogs, namely Echinococcus granulosus sensu lato, Dipylidium caninum, Taenia spp., Spirometra erinacei, Strongyloides stercoralis, Giardia duodenalis, Trichuris vulpis, Toxocara spp., and Neospora caninum were used. Additionally, we tested the newly developed qPCRs against the common human hookworm Necator americanus.
The diagnostic sensitivity (i.e. the ability of an assay to correctly classify an individual as positive if the individual is truly positive [45]) was assessed on a subset of 111 microscopy positive samples previously screened for hookworm eggs using sodium nitrate solution with S.G. 1.20 [43]. The 111 microscopy positive samples were subjected to the multiplex qPCRs and to the PCR-RFLP. As microscopy can be considered a gold standard to establish diagnosis (i.e. microscopy positive samples are truly positive for hookworm infection), the overall diagnostic sensitivity of the qPCRs was calculated as the number of hookworms correctly identified by the qPCRs (true positives) divided by the total number of microscopy positive samples (true positives and false negatives).
Assessment of the overall diagnostic specificity (i.e. the ability of an assay to correctly classify an individual as negative if the individual is truly negative) of the qPCRs was not possible due to truly negative samples being unavailable. Given that microscopy negative samples cannot be considered as truly negative for hookworm infection [46], microscopy cannot be considered a gold standard to rule out infection. Using an imperfect gold standard (i.e. assays with less than 100% diagnostic sensitivity and specificity) would lead to biased results [47–51].
Comparison of the PCR-RFLP and qPCRs was therefore performed relying on Kappa statistics [52] using Excel 2016 (Microsoft Corp., Redmond, WA). Agreement between both assays was considered poor if the coefficient (k) <0.00, slight if 0.00≤ k ≤0.20, fair if 0.21≤ k ≤0.40; moderate if 0.41≤ k ≤0.60, substantial if 0.61≤ k ≤0.80 and almost perfect if k >0.80. The 95% confidence intervals (95% CI) were calculated using the Wald method.
Results
Canine hookworm Multiplex qPCR optimization
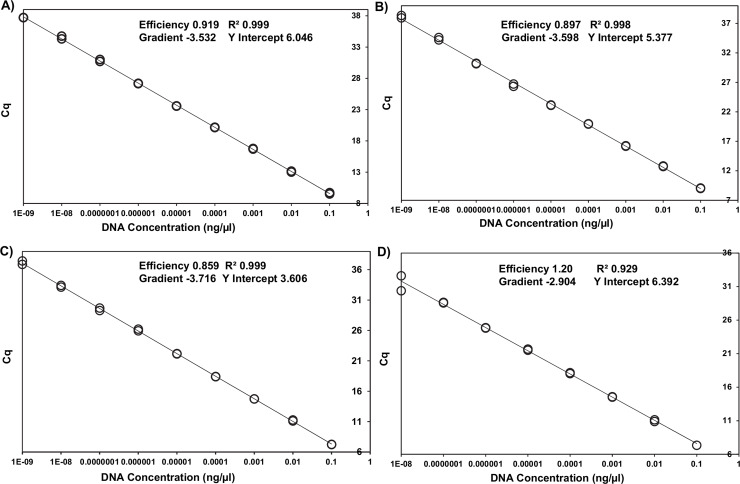
The optimized primer and probe concentrations are listed in Table 1. qPCRs efficiencies ranged from 86% to 120% with an R2 from 0.929 to 0.999 and Slope from -2.904 to -3.716 (Fig 1).
Table 1. Oligonucleotide primers and probes for qPCR assays for the detection of A. caninum, A. ceylanicum, A. braziliense, and U. stenocephala.
| Target | Primers and probes | Sequence (5’–3’) | Target | Size (bp) |
Conc. nm | Source |
|---|---|---|---|---|---|---|
| Hookworms | ACancey F | GGG AAG GTT GGG AGT ATC G | ITS-1 | 103 | 300 | This study |
| AcanceyR | CGA ACT TCG CAC AGC AAT C | 300 | This study | |||
| A. caninum | Acan probe | 5HEX/AG+T+CGT+T+A+C+TGG/3IABkFQ | 100 | This study | ||
| A. ceylanicum | AceyDOGprobe | 5Cy5/CCGTTC+CTGGGTGGC /3lAbRQSp | 100 | (Hii et al., 2018) | ||
| Hookworms | UncbrazF | GAG CTT TAG ACT TGA TGA GCA TTG | ITS-1 | 700 | This study | |
| UncbrazR | GCA GAT CAT TAA GGT TTC CTG AC | 700 | This study | |||
| A. braziliense | AbraProbe | 56FAM/TGA GCG CTA /ZEN/GGC TAA CGC CT/3IABkFQ/-3' | 119 | 200 | This study | |
| U. stenocephala | UncProbe | 5HEX/CAT TAG GCG /ZEN/GCA ACG TCT GGT G/3IABkFQ | 118 | 200 | This study | |
| Canine DNA | MAM F | CGACCTCGATGTTGGATCAG | 16S MtrRNA gene |
92 | 100 | (Hii et al. 2018) |
| MAM R | GAACTCAGATCACGTAGGACTTT | 100 | (Hii et al. 2018) | |||
| MAMPROBE | FAM/CCTAATGGT/ ZEN/ GCAGCAGCTATTAA/ LABKFQ | 200 | This study | |||
| Equine Herpes Virus | EHV FWD | GATGACACTAGCGACTTCGA | gB gene | 81 | 80 | (Bialasiewicz et al., 2009) |
| EHV-REV | TTTCGCGTGCCTCCTCCAG | 80 | (Bialasiewicz et al., 2009) | |||
| EHV PROBE | ROX/TTTCGCGTGCCTCCTCCAG/3IAbRQSp | 200 | (Bialasiewicz et al., 2009) |
+ Locked Nucleid Acid (LNA) basis
Fig 1.
Standard curves generated from 10-fold serial dilutions of target gBlock Gene Fragments of U. stenocephala (A), A. braziliense (B), A. ceylanicum (C), and A. caninum (D).
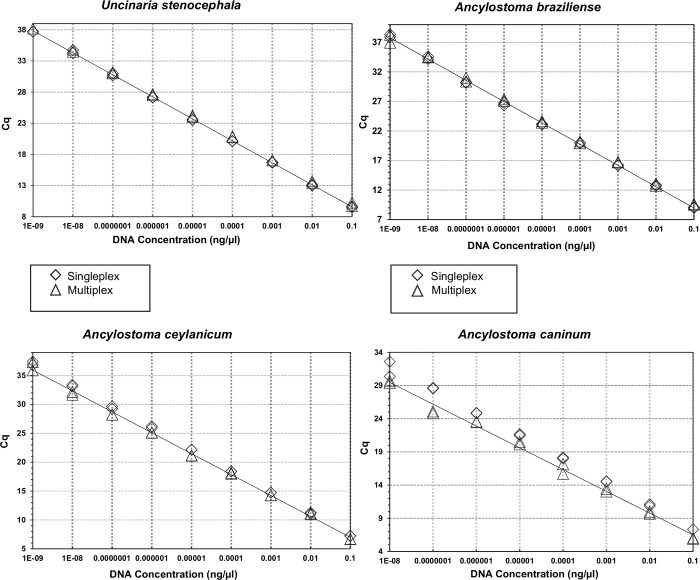
There were no significant differences between the efficiency, analytical sensitivity and intra-reaction reproducibility between singleplex and multiplex assays (Fig 2). No cross reactions between targeted hookworm species were observed for either singleplex or multiplex assays using gBlock Gene Fragments or genomic DNA controls.
Fig 2. Singleplex and multiplex qPCRs efficiencies.
Optimisation and comparison of the sensitivity and efficiency of each singleplex and multiplex qPCRs using gBlock Gene Fragments controls for U. stenocephala, A. braziliense, A. ceylanicum, A. caninum.
Sensitivity and specificity of the qPCRs
The analytical sensitivity of the assay was 0.0146 ng genomic DNA of A. braziliense per mg of spiked faeces. Mean Cq values associated corresponding to minimal detection limits were 37.66 (S.D.± 0.05) for U. stenocephala, 38.27 (S.D.± 0.06) for A. braziliense, 36.89 (S.D.± 0.368) for A. ceylanicum and 37.62 (S.D. 0.30) for A. caninum corresponding to 1.4 x 10−3 fg of target gBlock Gene DNA for A. ceylanicum, 2.6 x 10−3 fg of target gBlock Gene DNA for A. braziliense, 1.4 x 10−3 fg for U. stenocephala and 3.8 x 10−4 fg for A. caninum.
The limit of detection of the qPCRs and PCR-RFLP were respectively 0.00028 ng and 0.0028 ng for A. ceylanicum, 0.00033 ng and 0.033 ng for A. caninum, 0.00058 ng and 0.0058 ng for A. braziliense, and 0.0078 ng and 0.78 ng for U. stenocephala.
The multiplex qPCRs displayed a diagnostic sensitivity using microscopy as the gold standard of 97.3% (108/111; 95% CI 92.01–99.42). The diagnostic sensitivity of the PCR-RFLP against microscopy was 84.7% (94/111; 95% CI 76.74–90.31).
Neither multiplex qPCR assays cross reacted with genomic DNA of the tested parasites. No cross reactions with other target hookworm species was observed, thereby showing high analytical specificity.
Performance of the multiplex qPCRs for species of canine hookworm
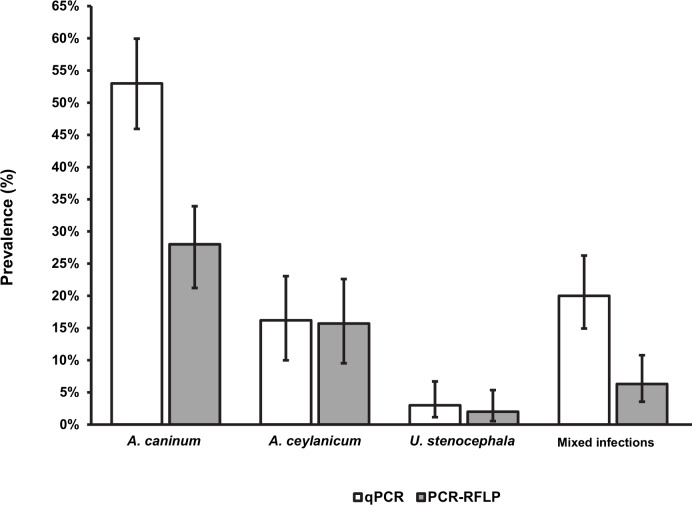
Performance of the canine hookworm multiplex qPCRs were determined using 191 DNA samples sourced from previous canine hookworm surveys in Vietnam and Queensland. Field samples, positive controls, EHV4 and mammalian DNA amplified within the expected Cq values. The detection of the signal derived from the amplification of mammalian DNA in all the samples confirmed the success of the extraction step. The multiplex qPCRs showed greater diagnostic sensitivity for the detection of hookworms in comparison to the PCR-RFLP (97% and 84%, respectively). The results for the multiplex quantitative qPCR and the PCR-RFLP are summarized in Table 2 and compared in Fig 3. Kappa statistics reported in Table 2 showed only fair agreement between the qPCRs and the PCR-RFLP for mixed infections (κ = 0.25, 95% CI 0.02–0.48) and A. caninum (κ = 0.43, 95% CI 0.31–0.56). Substantial agreement was shown for A. ceylanicum (κ = 0.79, 95% CI 0.7–0.89) and U. stenocephala (κ = 0.79, 95% CI 0.51–1).
Table 2. Multiplex quantitative PCR and PCR-RFLP agreement statistics.
| PCR-RFLP | |||||
|---|---|---|---|---|---|
| qPCRs | POS | NEG | Total agreement % | Kappa (95% CI) | |
| A. caninum | POS | 50 | 52 | 70% | 0.43 (0.31, 0.56) |
| NEG | 4 | 85 | |||
| A. ceylanicum | POS | 52 | 9 | 91% | 0.79 (0.7, 0.89) |
| NEG | 8 | 122 | |||
| U. stenocephala | POS | 4 | 2 | 99% | 0.79 (0.51, 1) |
| NEG | 0 | 185 | |||
| Mixed infections | POS | 8 | 30 | 82% | 0.25 (0.02, 0.48) |
| NEG | 4 | 149 | |||
POS = positive; NEG = negative; 95% CI = 95% confidence interval
K agreement level: k) <0.00 poor; 0.00≤ k ≤0.20, slight; 0.21≤ k ≤0.40, fair; 0.41≤ k ≤0.60, moderate; 0.61≤ k ≤0.80; substantial; k >0.80 almost perfect.
Fig 3. Comparison between multiplex PCR-RFLP and multiplex qPCRs.
Conventional PCR results in grey, qPCRs results in white.
Discussion
Here we propose and validate two Taq-Man probe-based multiplex qPCRs for the detection of zoonotic species of canine hookworms. These diagnostic tools will assist large epidemiological field studies toward a timely surveillance and monitoring of canine hookworm infections in dogs and provide information on species-specific zoonotic risks to populations living in endemic areas, globally.
While several qPCR assays have been developed for high-throughput screening of human hookworm species [46,53–55], there is a considerable lack of diagnostic tools to understand species distribution of canine hookworms. During the last decade, the development of qPCRs targeting human hookworms has aided monitoring of the distribution, efficacy and impact of mass deworming programs targeting individual genera or species of both anthroponotic and zoonotic hookworms [46,53,54]. The zoonotic species of canine hookworms are emerging pathogens with A. ceylanicum alone estimated to infect up to 73 million people in the Asia Pacific [30–35]. Similarly, the development and field application of a multiplex qPCR assay for canine hookworm species can be applied to assess their distribution, inform zoonotic risk, facilitate field-based anthelmintic efficacy trials and monitor the impacts of One Health intervention strategies for their control.
The qPCRs herein described demonstrated higher diagnostic sensitivity (97%) in comparison to previously developed PCR-RFLP (84%) by Ng-Nguyen et al. (2015). For instance, our qPCRs successfully detected at least one species of hookworms in the 82.4% of PCR-RFLP-negative but microscopy-positive samples. The absolute sensitivity of the qPCRs was 10-fold more sensitive than the PCR-RFLP for A. ceylaniucm and A. braziliense and 100-fold more sensitive for A. caninum and U. stenocephala.
One of the main advantages of our qPCRs was the capability of detecting additional mixed hookworm species infections (68%) compared to PCR-RFLP. In addition, the qPCRs detected an additional 48 A. caninum single infections (47%), two U. stenocephala (33%) and one A. ceylanicum (0.02%) missed by PCR-RFLP. A small number of samples were qPCR-negative and PCR-RFLP positive however, it is likely that these samples were PCR-RFLP false-positives associated with the subjective and potentially inaccurate interpretation of gel electrophoresis results. These assays have proven specific as it detected all the four canine hookworms tested without cross reacting when tested against a range of 10 different intestinal parasites of dogs and the human hookworm N. americanus.
The primary disadvantage of the qPCR is its inability to differentiate A. caninum from A. duodenale DNA, given the high degree of nucleotide identity between DNA sequences of these two closely related hookworm species. The ability to differentiate canine from human hookworm species becomes apparent in resource-poor communities that practice outdoor defaecation, as dogs are known to be coprophagic [56]. Moreover, A. duodenale has been demonstrated to produce self-limiting infections in experimentally infected puppies in a non-controlled study [57], a phenomenon that is likely to be rare in natural A. caninum-endemic settings as the transmammary route of infection in pups is likely to provide cross-protective immunity against its closely related human-hookworm counterpart. Despite this limitation, the likelihood of false positive A. caninum infection in dogs as a result of the coprophagy or patent infections with A. duodenale appears negligible. DNA of Ancylostoma duodenale was detected in a single canine faecal sample in Turkana, Kenya at an overall prevalence of <0.062%, even in the presence of widespread canine coprophagic behaviour [58].
Kappa statistics showed only fair agreement (0.41 ≤|κ|≤ 0.6) between the qPCRs and the PCR-RFLP for mixed and single A. caninum infections. The primary reason for this was the qPCRs assays’ increased sensitivity for the detection of A. caninum and mixed hookworm-species infections. Good agreement (0.61 ≤|κ|≤ 0.80) was instead shown for A. ceylanicum and U. stenocephala.
The primary limitation of the study is that only microscopy positive samples were subjected to the PCR-RFLP assays and therefore diagnostic specificity was not assessed. Further studies testing the relationship between Cq values/copy numbers to egg numbers are needed before these qPCRs can be used as quantitative tools to assess infection intensity levels. The deployment of more sensitive techniques such as the qPCR assays herein validated, acquires pivotal importance especially in areas where preventive chemotherapy has been employed for many years and where conventional techniques may fail to detect light-intensity infections (i.e. shedding low number of eggs) [59,60]. The advantages of these high-throughput qPCRs over the previously published assays traditionally used for the diagnosis of canine hookworms is not only a greater sensitivity and specificity but also its reduced processing time [46]. Even though qPCR may be slightly more expensive than PCR-RFLP, this disadvantage is overcome by significantly reduced labour costs associated with shortened qPCR set-up times and real-time analysis of results.
These newly developed qPCRs will facilitate large-scale detection and proper identification of canine hookworm species, globally, by researcher dealing with the assessment of STHs in endemic areas. Further, this tool will aid in promptly diagnosing and treating affected animals by veterinary clinicians whom can refer cases to diagnostic laboratories. Further, faecal samples can be easily stored and transported at room temperatures to laboratories with qPCR capabilities without the need of trained technicians or special equipment, making it an ideal tool for large survey especially when sampling animals living in remote areas. These new tools provide an alternative to the labour-intensive microscopic examination of faecal samples and to low-throughput gel-based PCR-RFLP for the diagnosis of canine hookworm infections.
These qPCRs will enable a comprehensive investigation of the epidemiology of canine hookworm infections in dogs and their risk posed to humans especially in areas where A. ceylanicum, A. caninum and A. braziliense are sympatric. Its greater accuracy in comparison with PRC-RFLP may be explained owing to outputs being based on an objective automated quantitation of fluorescent signals instead of a visual-based size discrimination of the bands in an electrophoresis gel.
Distinguishing between the four species of canine hookworm is also important to inform One Health based chemotherapeutic intervention strategies in dogs, as species-specific differences in anthelmintic efficacy have been reported [1,9–15]. For example, certain geographical isolates of A. caninum, the well-known cause of eosinophilic enteritis in humans, have demonstrated high-level of pyrantel resistance [61]. Poor efficacy of milbemycin oxime against U. stenocephala has also been demonstrated [62].
Furthermore, although hookworm eggs are morphologically indistinguishable, the zoonotic impact of each species differs considerably. For example, A. ceylanicum is the only canine hookworm known to produce patent infection in humans in both natural and experimental infections and is the second most common hookworm infecting humans in the Asia Pacific [16,19,53]. Conversely, A. caninum and A. braziliense cause accidental infections in humans although preliminary evidence on the ability of the former to produce patent infections in human patients have recently been reported [23].
WHO recommends different targeted treatments in endemic populations based on the prevalence detected in people from these geographical areas [54]. However, human treatment alone is insufficient for the control of STH in the presence of zoonotic parasites such as A. ceylanicum [30,33,55]. As we approach the goal of eliminating morbidity associated with human STHs, the need to monitor both canines and humans for emerging zoonoses such as A. ceylanicum and the subsequent requirement for accurate high throughput diagnostic techniques, will become increasingly important [56].
These novel multiplex qPCRs for the four species of canine hookworms showed higher diagnostic sensitivity compared to the PCR-RFLP and have greater ability to detect mixed infections compared to previously published assays. These qPCRs will assist large epidemiological surveillance investigations, the assessment of the role of dogs in the transmission of hookworms to humans and the efficacy of anthelminthic treatments in canine populations.
Supporting information
In grey primer pairs designed and in red probes.
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding for this work was provided by Boehringer Ingelheim Animal Health. Luca Massetti and Patsy A. Zendejas are recipients of Melbourne Research Scholarship from the University of Melbourne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yoshida Y, Okamoto K, Matsuo K, Kwo EH, Retnasabapathy A. The occurrence of Ancylostoma braziliense (de Faria, 1910) and Ancylostoma ceylanicum (Looss, 1911) in Malaysia. Southeast Asian J Trop Med Public Heal. 1973. [PubMed] [Google Scholar]
- 2.Setsuban P, Waddel HAH. Hookworms in cats and dogs in Queensland. Aust Vet J. 1973;49: 110–110. 10.1111/j.1751-0813.1973.tb09339.x [DOI] [PubMed] [Google Scholar]
- 3.Margono SS, Koesharjono C, Kosin E. Hookworm in dogs and cats in the area of Jakarta. Trop Geogr Med. 1979;31: 257–61. Available: http://www.ncbi.nlm.nih.gov/pubmed/505556 [PubMed] [Google Scholar]
- 4.Labarthe N, Serrão ML, Ferreira AMR, Almeida NKO, Guerrero J. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Vet Parasitol. 2004. 10.1016/j.vetpar.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Nyambura Njuguna A, Kagira JM, Muturi Karanja S, Ngotho M, Mutharia L, Wangari Maina N. Prevalence of Toxoplasma gondii and Other Gastrointestinal Parasites in Domestic Cats from Households in Thika Region, Kenya. Biomed Res Int. 2017;2017: 1–6. 10.1155/2017/7615810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsukaki G. Studies on the life history of the hookworm. Part VII: On the development of Ancylostoma caninum in the abnormal host. Yokohama Med Bull. 1951;2: 154–160. Available: https://www-cabdirect-org.ezp.lib.unimelb.edu.au/cabdirect/abstract/19510800600 [Google Scholar]
- 7.Norris DE. The Migratory Behavior of the Infective-Stage Larvae of Ancylostoma braziliense and Ancylostoma tubaeforme in Rodent Paratenic Hosts. J Parasitol. 1971;57: 998 10.2307/3277855 [DOI] [PubMed] [Google Scholar]
- 8.Lee KT, Little MD, Beaver PC. Intracellular (Muscle-Fiber) Habitat of Ancylostoma caninum in Some Mammalian Hosts. J Parasitol. 1975;61: 589 10.2307/3279448 [DOI] [PubMed] [Google Scholar]
- 9.Dove WE. Further studies on Ancylostoma braziliense and the etiology of creeping eruption. Am J Epidemiol. 1932;15: 664–711. 10.1093/oxfordjournals.aje.a117837 [DOI] [Google Scholar]
- 10.Beaver PC. Larva migrans. Experimental Parasitology. 1956. 10.1016/0014-4894(56)90032-7 [DOI] [PubMed] [Google Scholar]
- 11.Marc Brenner, A.; Mital BP. Cutaneous Larva Migrans: The Creeping Eruption. Cutan Larva Migrans. 2003;72: 111–115. Available: http://www.emedicine.com. [PubMed] [Google Scholar]
- 12.Prociv P, Croese J. Human enteric infection with Ancylostoma caninum: Hookworms reappraised in the light of a “new” zoonosis. Acta Trop. 1996. 10.1016/S0001-706X(96)00016-2 [DOI] [PubMed] [Google Scholar]
- 13.Walker NI, Croese J, Clouston AD, Parry M, Loukas A, Prociv P. Eosinophilic enteritis in Northeastern Australia: Pathology, association with Ancylostoma caninum, and implications. Am J Surg Pathol. 1995. 10.1097/00000478-199503000-00011 [DOI] [PubMed] [Google Scholar]
- 14.TroCCAP. 2017. Available: http://www.troccap.com/canine-guidelines/gastrointestinal-parasites/intestinal-threadworm/
- 15.Bowman DD, Lynn RC, Eberhard ML. Georgis’ Parasitology for Veterinarians. St Louis ES (USA)., editor. 2003. Available: https://mail.google.com/mail/u/0/#search/palmer+thesis?projector = 1
- 16.Murphy, MD, Spickler AR. Zoonotic Hookworms. 2013 [cited 10 May 2019]. Available: www.cfsph.iastate.edu [Google Scholar]
- 17.Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5: 91 10.1186/1756-3305-5-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller TA. Pathogenesis and immunity in hookworm infection. Trans R Soc Trop Med Hyg. 1968;62: 473–489. 10.1016/0035-9203(68)90130-2 [DOI] [PubMed] [Google Scholar]
- 19.Rep BH. Pathogenicity of Ancylostoma braziliense. IV. Blood loss caused by the worms in the prepatent period. Tropical and Geographical Medicine. Blackwell Science Ltd; 1996. Available: https://www-cabdirect-org.ezp.lib.unimelb.edu.au/cabdirect/abstract/19672901465 [PubMed] [Google Scholar]
- 20.Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26: 162–167. 10.1016/j.pt.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 21.Croese J, Loukas A, Opdebeeck J, Fairley S, Prociv P. Human Enteric Infection with Canine Hookworms. Ann Intern Med. 1994;120: 369 10.7326/0003-4819-120-5-199403010-00003 [DOI] [PubMed] [Google Scholar]
- 22.Prociv P, Croese J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet. 1990;335: 1299–1302. 10.1016/0140-6736(90)91186-e [DOI] [PubMed] [Google Scholar]
- 23.Ngcamphalala PI, Lamb J, Mukaratirwa S. Molecular identification of hookworm isolates from stray dogs, humans and selected wildlife from South Africa. J Helminthol. 2019; 1–9. 10.1017/S0022149X17001201 [DOI] [PubMed] [Google Scholar]
- 24.Anten JFG, Zuidema PJ. Hookworm infection in Dutch service-men returning from West New Guinea. Trop Geogr Med. 1964;16: 216–224. Available: https://www.cabdirect.org/cabdirect/abstract/19650802379 [PubMed] [Google Scholar]
- 25.Chiu J-K, Okamoto K, Yoshida Y. Ancylostoma Ceylanicum Infection in Dogs, Cats, and Man in Taiwan. Am J Trop Med Hyg. 1968;17: 378–381. 10.4269/ajtmh.1968.17.378 [DOI] [PubMed] [Google Scholar]
- 26.Areekul S, Radomyos P, Viravan C. Preliminary report of Ancylostoma Ceylanicum infection in Thai people. J Med Assoc Thail. 1970. [PubMed] [Google Scholar]
- 27.Carroll SM, Grove DI. Experimental infection of humans with Ancylostoma ceylanicum: clinical, parasitological, haematological and immunological findings. Trop Geogr Med. 1986;38: 38–45. Available: http://www.ncbi.nlm.nih.gov/pubmed/3961908 [PubMed] [Google Scholar]
- 28.Lim KN, Ismail WHW, Lim YAL, Mahmud R, Ngui R. Zoonotic Ancylostoma ceylanicum Infection Detected by Endoscopy. Am J Trop Med Hyg. 2014;91: 86–88. 10.4269/ajtmh.13-0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speare R, Bradbury RS, Croese J. A Case of Ancylostoma ceylanicum Infection Occurring in an Australian Soldier Returned from Solomon Islands. Korean J Parasitol. 2016;54: 533–6. 10.3347/kjp.2016.54.4.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradbury RS, Hii SF, Harrington H, Speare R, Traub R. Ancylostoma ceylanicum Hookworm in the Solomon Islands. Emerg Infect Dis. 2017;23: 252–257. 10.3201/eid2302.160822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RCA. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008. 10.1016/j.vetpar.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 32.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, et al. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011. 10.4269/ajtmh.2011.10-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngui R, Lim YALL, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. Geiger SM, editor. PLoS Negl Trop Dis. 2012;6: e1522 10.1371/journal.pntd.0001522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RCA, Conlan J V., Khamlome B, Pallant L, Fenwick S, Elliot A, et al. Soil-Transmitted Helminthiasis in Laos: A Community-Wide Cross-Sectional Study of Humans and Dogs in a Mass Drug Administration Environment. Am J Trop Med Hyg. 2012;86: 624–634. 10.4269/ajtmh.2012.11-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schar F, Inpankaew T, Traub RJJ, Khieu V, Dalsgaard A, Chimnoi W, et al. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol Int. 2014;63: 597–603. 10.1016/j.parint.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 36.Reinemeyer CR. Formulations and Clinical Uses of Pyrimidine Compounds in Domestic Animals. Pyrantel Parasit Ther Humans Domest Anim. 2016; 67–107. 10.1016/B978-0-12-801449-3.00015-6 [DOI] [Google Scholar]
- 37.Mahdy MAK, Lim YAL, Ngui R, Fatimah MS, Choy SH, Yap NJ, et al. Prevalence and zoonotic potential of canine hookworms in Malaysia. Parasites and Vectors. 2012. 10.1186/1756-3305-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pumidonming W, Salman D, Gronsang D, Abdelbaset AEAEAE, Sangkaeo K, Kawazu S-IS-I, et al. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J Vet Med Sci. 2016;78: 1779–1784. 10.1292/jvms.16-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer CS, Thompson RCA, Traub RJ, Rees R, Robertson ID. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet Parasitol. 2008;151: 181–190. 10.1016/j.vetpar.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 40.Companion Animal Parasite Council. 2016. Available: https://capcvet.org/guidelines/trichuris-vulpis/
- 41.Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC. The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Heal. 2004;9: 688–701. 10.1111/j.1365-3156.2004.01252.x [DOI] [PubMed] [Google Scholar]
- 42.Palmer CS, Traub RJ, Robertson ID, Hobbs RP, Elliot A, While L, et al. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet Parasitol. 2007;145: 304–313. 10.1016/j.vetpar.2006.12.018 [DOI] [PubMed] [Google Scholar]
- 43.Ng-Nguyen D, Hii SF, Nguyen V-AT, Van Nguyen T, Van Nguyen D, Traub RJ. Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit Vectors. 2015;8: 401 10.1186/s13071-015-1015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harriott L, Gentle M, Traub RC, Soares Magalhães RJ, Cobbold R. Zoonotic and economically significant pathogens of peri-urban wild dogs across north-eastern New South Wales and south-eastern Queensland, Australia. Wildl Res. 2019;46: 212–221. 10.1071/WR18110 [DOI] [Google Scholar]
- 45.Saah AJ, Hoover DR. “Sensitivity” and “specificity” reconsidered: The meaning of these terms in analytical and diagnostic settings. Annals of Internal Medicine. American College of Physicians; 1997. pp. 91–94. 10.7326/0003-4819-126-1-199701010-00026 [DOI] [PubMed] [Google Scholar]
- 46.Hii SF, Senevirathna D, Llewellyn S, Inpankaew T, Odermatt P, Khieu V, et al. Development and evaluation of a multiplex quantitative real-time polymerase chain reaction for hookworm species in human stool. Am J Trop Med Hyg. 2018;99: 1186–1193. 10.4269/ajtmh.18-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyko EJ, Alderman BW, Baron AE. Reference test errors bias the evaluation of diagnostic tests for ischemic heart disease. Journal of General Internal Medicine. 1988. 10.1007/BF02595925 [DOI] [PubMed] [Google Scholar]
- 48.Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technology Assessment. 2007. 10.3310/hta11500 [DOI] [PubMed] [Google Scholar]
- 49.Whiting P, Rutjes AWS, Reitsma JB, Glas AS, Bossuyt PMM, Kleijnen J. Sources of Variation and Bias in Studies of Diagnostic Accuracy: A Systematic Review. Annals of Internal Medicine. 2004. 10.7326/0003-4819-140-3-200402030-00010 [DOI] [PubMed] [Google Scholar]
- 50.Valenstein PN. Evaluating diagnostic tests with imperfect standards. Am J Clin Pathol. 1990. 10.1093/ajcp/93.2.252 [DOI] [PubMed] [Google Scholar]
- 51.Begg CB. Biases in the assessment of diagnostic tests. Stat Med. 1987. 10.1002/sim.4780060402 [DOI] [PubMed] [Google Scholar]
- 52.Watson PF, Petrie A. Method agreement analysis: A review of correct methodology. Theriogenology. 2010. 10.1016/j.theriogenology.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 53.Verweij JJ, Brienen EAT, Ziem J, Polderman AM, Yelifari L, Van Lieshout L. Simultaneous Detection and Quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in Fecal Samples Using Multiplex Real-Time PCR. Am J Trop Med Hyg. 2007;77: 685–690. 10.4269/ajtmh.2007.77.685 [DOI] [PubMed] [Google Scholar]
- 54.Stracke K, Clarke N, Awburn C V., Vaz Nery S, Khieu V, Traub RJ, et al. Development and validation of a multiplexed-tandem qPCR tool for diagnostics of human soil-transmitted helminth infections. Carabin H, editor. PLoS Negl Trop Dis. 2019;13: e0007363 10.1371/journal.pntd.0007363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaz Nery S, Qi J, Llewellyn S, Clarke NE, Traub R, Gray DJ, et al. Use of quantitative PCR to assess the efficacy of albendazole against Necator americanus and Ascaris spp. in Manufahi District, Timor-Leste. Parasit Vectors. 2018;11: 373 10.1186/s13071-018-2838-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RCA. The role of dogs in transmission of gastrointestinal parasites in a remote tea-growing community in Northeastern India. Am J Trop Med Hyg. 2002. 10.4269/ajtmh.2002.67.539 [DOI] [PubMed] [Google Scholar]
- 57.Schad GA. Ancylostoma duodenale: Maintenance through six generations in helminth-naive pups. Exp Parasitol. 1979. 10.1016/0014-4894(79)90077-8 [DOI] [PubMed] [Google Scholar]
- 58.Mulinge E, Njenga SM, Odongo D, Magambo J, Zeyhle E, Mbae C, et al. Molecular identification of zoonotic hookworms in dogs from four counties of Kenya. J Helminthol. 2020. 10.1017/S0022149X1900018X [DOI] [PubMed] [Google Scholar]
- 59.Medley GF, Turner HC, Baggaley RF, Holland C, Hollingsworth TD. The Role of More Sensitive Helminth Diagnostics in Mass Drug Administration Campaigns: Elimination and Health Impacts. Adv Parasitol. 2016. 10.1016/bs.apar.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 60.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: A meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014. 10.1016/j.ijpara.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kopp SR, Kotze AC, McCarthy JS, Coleman GT. High-level pyrantel resistance in the hookworm Ancylostoma caninum. Vet Parasitol. 2007;143: 299–304. 10.1016/j.vetpar.2006.08.036 [DOI] [PubMed] [Google Scholar]
- 62.Niamatali S, Bhopale V, Schad GA. Efficacy of milbemycin oxime against experimentally induced Ancylostoma caninum and Uncinaria stenocephala infections in dogs. J Am Vet Med Assoc. 1992;201: 1385–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/1429184 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In grey primer pairs designed and in red probes.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.