Abstract
Indian rhesus macaques infected with the Rev-independent live-attenuated SIVmac239 strains control viremia to undetectable levels, have persistent but low cellular and humoral anti-SIV responses, and show no signs of immune deficiency. To analyze the immune mechanisms responsible for viral control, five macaques infected at day 1 after birth were subjected to CD8+ cell depletion at 6.7 y postinfection. This resulted in viremia increases to 3.7–5.5 log10 RNA copies, supporting a role of CD8-mediated responses in the control of viral replication. The rebounding viremia was rapidly controlled to levels below the threshold of detection, and occurred in the absence of SIV-specific CD8+ T cells and significant CD8+ T cell recovery in four of the five animals, suggesting that other mechanisms are involved in the immunological control of viremia. Monitoring immune responses at the time of viral control demonstrated a burst of circulating SIV-specific CD4+ T cells characterized as CD45RA−CD28+CD95+CCR7− and also granzyme B+, suggesting cytotoxic ability. Control of viremia was also concomitant with increases in humoral responses to Gag and Env, including a transient increase in neutralizing Abs against the neutralization-resistant SIVmac239 in four of five animals. These data demonstrate that a combination of cellular responses mediated by CD4+ T cells and humoral responses was associated with the rapid control of the rebounding viremia in macaques infected by the Rev-independent live-attenuated SIV, even in the absence of measurable SIV-specific CD8+ T cells in the blood, emphasizing the importance of different components of the immune response for full control of SIV infection.
Infection with live-attenuated SIV (LASIV) provides the best-known protection against challenge with pathogenic SIVmac strains (for reviews, see Refs. 1 and 2 and references therein). Attenuation of SIVmac239 has been achieved by different strategies, including deletion of nonessential genes, mutation of viral sequences, or replacing essential gene functions (3–14). Challenge of LASIV-vaccinated rhesus macaques with wild-type SIV showed that they were able to successfully control subsequent SIVmac239 or SIVmac251 challenge (for reviews, see Refs. 1 and 2 and references therein). The immune mechanisms responsible for this protection are not well defined. The study of this model is thought to provide critical information to identify immune mechanisms responsible for prevention of AIDS development. This information could be helpful for the design of vaccines against HIV infection in humans.
We previously reported the generation of nonpathogenic LASIVmac239 derivatives, in which the essential genomic regions encoding rev and the Rev binding site on the viral RNA (Rev-responsive element [RRE]) were replaced with the constitutive transport element (CTE) identified in the Mason-Pfizer monkey virus/simian retrovirus type 1 (SRV-1) (11–18). Rev is an essential viral protein that binds to RRE RNA site in the nucleus and promotes transport of viral mRNAs from the nucleus to the cytoplasm and efficient translation (19–21). The Rev-independent LASIV uses an alternative posttranscriptional control strategy for the expression of structural proteins, which is able to replace Rev/RRE. Infection of rhesus macaques with the Rev-independent LASIV manifests with lower peak viremia during the acute phase compared with infection by the wild-type SIVmac239, which was subsequently rapidly controlled to levels at or below the threshold of detection (12–14). The animals showed persistent low-level humoral and cellular immune responses, demonstrating persistent chronic infection (12–14). We reported that control of infection with the Rev-independent LASIV is long lasting without any signs of pathogenicity (12–14) in animals infected as juveniles (>7 y) as well as in macaques infected as neonates (5.9 y), which is the most sensitive model to evaluate the pathogenic potential of SIV-attenuated strains. Other LASIV, including SIVmac239Δnef, caused frequent pathogenic effects and AIDS to infected neonates (22, 23). Similar to infection with SIVmac239Δnef or Δ3 (24–27), animals infected with the Rev-independent LASIV showed protection against challenge with wild-type SIV (A. von Gegerfelt, manuscript in preparation), demonstrating great potency of these LASIV-induced immune responses.
The importance of CD8+ responses in the control of SIV infection has been elegantly demonstrated by systemic depletion of macaque CD8+ cells after injection of a cytotoxic anti-CD8 mAh (28–37). In these studies, transient depletion of CD8+ cells resulted in rapid rebound of viremia, which was attributed to the loss of viral control mediated by SIV-specific CD8+ CTL responses, a conclusion that was reinforced by the observation that rebounding virus control occurred simultaneously with the recovery of the CD8+ T cell population. CD8+ cell depletion in macaques infected with live-attenuated or avirulent strains of SIV has also been performed (34, 38–40). The outcomes of these depletion studies were different depending on the strain of LASIV used for infection. For instance, rapid viral rebound was reported in animals infected with SIVmac239Δnef (38), but not in macaques infected with SIVmac239Δ3 (34), suggesting lower chronic viremia in the latter, which could not be reactivated even in the absence of CD8+ cells. It was further found that depletion of CD8+ cells on the day of SIVmac251 challenge resulted in impaired control of the challenge virus as compared with non-CD8+-depleted LASIV-infected controls (34) and resulted in higher viremia than in nondepleted macaques, but lower (100-fold) viremia than in naive monkeys, suggesting that significant viral control is also mediated by mechanisms other than CD8+ cells.
The Rev-independent LASIV strains used in this study produced all the SIV proteins found in the parental SIVmac239 molecular clone, except Rev, or except Rev and Nef (11, 13). Animals, infected with these viruses at day 1 after birth, were able to fully suppress virus replication for many years (12, 13). Therefore, the Rev-independent LASIVmac239 is significantly different from other LASIV tested, such as the nef-deleted LASIV, which is pathogenic in neonatal macaques (22, 23). To explore the mechanisms leading to full virus suppression and lack of pathogenicity of Rev-independent LASIVmac239, we analyzed the virological and immunological outcome upon CD8+ lymphocyte depletion in five rhesus macaques chronically infected for 6.7 y. Previous studies involving CD8+ cell depletion in SIV-infected macaques did not address the type of cells associated with viral control (in most cases cell responses were measured by ELISPOT only). Therefore, we characterized the phenotype of the SIV-specific T cells responsible for the control of the rebounding viremia upon administration of the cM-T807 mAh, using detailed flow cytometric analysis. In agreement with previous studies, we found a rapid rebound in viral replication after CD8+ depletion. Viral rebound was controlled concomitantly with the increase of SIV-specific CD4+ T cell responses with a cytotoxic phenotype and increased Ab levels, including neutralizing Abs (Nabs) to SIVmac239, despite the lack of SIV-specific CD8+ T cell responses and poor total CD8+ T cell recovery in these animals. Our results show that SIV-specific CD4+ T cells and humoral immune responses mediate the potent control of the rebounding virus in the absence of SIV-specific CD8+ T cells.
Materials and Methods
Animals, infection, and in vivo CD8+ lymphocyte depletion
Indian rhesus macaques (Macaca mulatto) from the California National Primate Research Center were housed in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. The Rev-independent LASIVmac239 molecular clones contain multiple point mutations introduced in the rev gene and the RRE, and have either the CTE from the SRV-1 or the related CTE from an intracisternal A-particle (IAP) retroelement inserted after nt 9281 and are either nef− or nef+, as previously described (11, 12, 15, 17, 18), generating the pSIVmac239 molecular clone Rev−RRE−Nef+CTE+ and Rev−RRE−Nef−CTEIAP+. Virus stocks were generated in rhesus PBMC. The animals were infected via the i.v. route at day 1 after birth and monitored for 6.7 y. Transient depletion of peripheral CD8+ cells was performed by administering i.v. a single dose of the mAb cM-T807 (produced by Centocor, Malvern, PA; provided by K. Reimann, Harvard Medical School, Boston, MA) at 50 mg/kg body weight (35, 36, 40). Viral loads in plasma were determined by a RT-PCR assay with a threshold of detection of 30 copies/ml (41).
Lymphocyte phenotyping after CD8 depletion
Efficiency of CD8+ cell depletion was monitored in fresh peripheral blood samples by flow cytometry using two different anti-CD8 mAbs: the DK25 clone (DakoCytomation, Carpinteria, CA) recommended by the cM-T807 protocol and the SK1 clone from BD Pharmingen (San Jose, CA). Briefly, four-color flow cytometry was used, consisting of a single tube containing PerCP-conjugated anti-human CD8 (clone SK1; BD Pharmingen), FITC-conjugated anti-human CD3 (clone SP34; BD Pharmingen), PE-conjugated anti-human CD4 (clone M-T477; BD Pharmingen), and allophycocyanin-conjugated anti-human CD20 (clone L27; BD Pharmingen). RBCs were lysed, and the samples were fixed in paraformaldehyde by the Coulter Q-prep system (Coulter, Hialeah, FA). Flow cytometry was performed on a FACSCalibur flow cytometer (BD Pharmingen). Lymphocytes were gated by forward and side light scatter and were then analyzed with CellQuest software (BD Pharmingen). For the second staining, the anti-CD8 Ab was replaced by the DK25 clone conjugated to FITC (and combined with anti-CD3 PerCP, anti-CD4 PE, and anti-CD20 allophycocyanin).
T cell responses and flow cytometry
The frequency of SIV-specific cytokine-producing T cells in rhesus macaques was determined by flow cytometric analysis, as previously described (42). Briefly, thawed cryopreserved PBMCs were incubated at 106 cells/ml in the presence of SIV Gag and Env peptide pools (15-aa peptides overlapping by 11 aa, at a final concentration of 1 μg/ml for each peptide; Infinity, Aston, PA). Some samples were also treated with the anti-CD28 (clone E293) and anti-CD49d (clone E25; BD Pharmingen) mAbs (1 μg/ml of each Ab) to provide additional costimulatory signals. Samples incubated in the absence of peptide stimulation were included in all the experiments and served as negative controls for each macaque analyzed. The cells were treated overnight with monensin to prevent protein secretion, and cell surface staining was performed using the following Ab mixture: CD3 allophycocyanin Cy7 (clone SP34-2), CD4 PerCP Cy5.5 (clone L200), CD45RA PE (clone 5H9), CD28 biotin (clone CD28.2; BD Pharmingen), and CD8 AF405 (clone 3B5; Caltag Laboratories, Carlsbad, CA). After washing the cells, the samples were incubated for 20 min at room temperature with streptavidin allophycocyanin Cy5.5 (Caltag Laboratories). The cells were washed twice, fixed, permeabilized with Cytofix/Cytoperm (BD Pharmingen), and stained for intracellular cytokine detection using the following Ab mixture: IFN-γ FITC (clone B27), IL-2 allophycocyanin (clone MQ1-17H12), and TNF-α PE Cy7 (clone MAb11; BD Pharmingen). Some samples were also analyzed using the following Ab mixture for cell surface staining: CD3 allophycocyanin Cy7, CD28 PerCP Cy5.5, CD95 FITC (cloneDX2; BD Pharmingen), CD45RA AF700 (clone F8-11-13; AbD Serotec, Oxford, U.K.), CCR7 PE (clone ISO503; R&D Systems, Minneapolis, MN), CD4 AmCyan (National Institutes of Health Nonhuman Primate Reagent Resource, Boston, MA), and CD8 AF405 (Caltag Laboratories), followed by permeabilization and intracellular staining with IFN PE Cy7 (BD Pharmingen) and granzyme B allophycocyanin (clone GB12; Invitrogen, Carlsbad, CA). Some PBMC samples were assayed for SIV-specific responses in the presence of anti-CD28 and CD49d mAbs. Data analysis was performed using the FlowJo platform (Tree Star, Ashland, OR), and all Ag-specific responses were determined within the CD3+ population after subtracting values obtained from samples incubated with medium alone.
Humoral immune responses and Nab measurements
Binding Abs against SIV Env and Gag were measured by ELISA using serial dilutions of the plasma samples (Advanced BioScience Laboratories, Kensington, MD). Samples with A450 absorbance value higher than twice the value obtained from naive animals were considered positive. The binding Ab titers are reported as the reciprocal value of the highest positive dilution. The Nabs against SIVmac251-TCLA (H9 cell grown) and SIVmac239CS.23 (293T pseudovirus) were measured in M7-luc and TZM-bl cells, respectively (43). The Nab titers are reported as the reciprocal serum dilution at which the relative luciferase units were reduced by 50% compared with virus control wells.
Results
Persistent long-term control of viremia in macaques infected by the Rev-independent SIV strains
The five animals enrolled in this study were infected with related strains of the Rev-independent LASIV at day 1 after birth (12, 13). These LASIV strains are derivatives of the pathogenic SIVmac239, do not produce the essential posttranscriptional regulator Rev, and lack the RRE located within the env open reading frame, but maintain the wild-type Env sequence. These strains have the viral posttranscriptional control replaced by the CTE of either SRV-1 or the related IAP (11, 12, 15–18), which use the cellular NXF1 export receptor to transport the full-length SIV transcript to the cytoplasm (44, 45). We previously showed that these SIV strains lack pathogenicity even in the presence of nef (12, 13). Animals 31469, 31470, and 31474 were infected with Rev−RRE−Nef+CTE+, and animals 31376 and 31388 were infected with Rev−RRE−Nef−CTEIAP+ (Fig. 1A), and plasma virus loads were determined over time (Fig. 1B). We previously reported (12, 13) that after the initial peak of primary viremia (ranging from 5.6 to 7.1 log10 of SIV RNA copies/ml plasma), the viral loads declined rapidly to levels below the threshold of the assay and remained at this level up to year 5.9 postinfection. In this study, we extend the observation period and show (Fig. 1B) that these five animals continue to control viremia to year 6.7; at the day of CD8+ cell depletion, the virus loads for four of the animals (31388, 31474, 31469, and 31470) were <30 RNA copies/ml plasma, whereas macaque 31376 showed 140 viral RNA copies/ml. Persistent low levels of both cellular and humoral immune responses demonstrate efficient control of the chronic LASIV infection, and none of these infected macaques displayed any signs of immunodeficiency or CD4+ T cell decline [(12, 13) and this report (Fig. 2B)], underscoring the non-pathogenicity of these LASIV strains.
FIGURE 1.
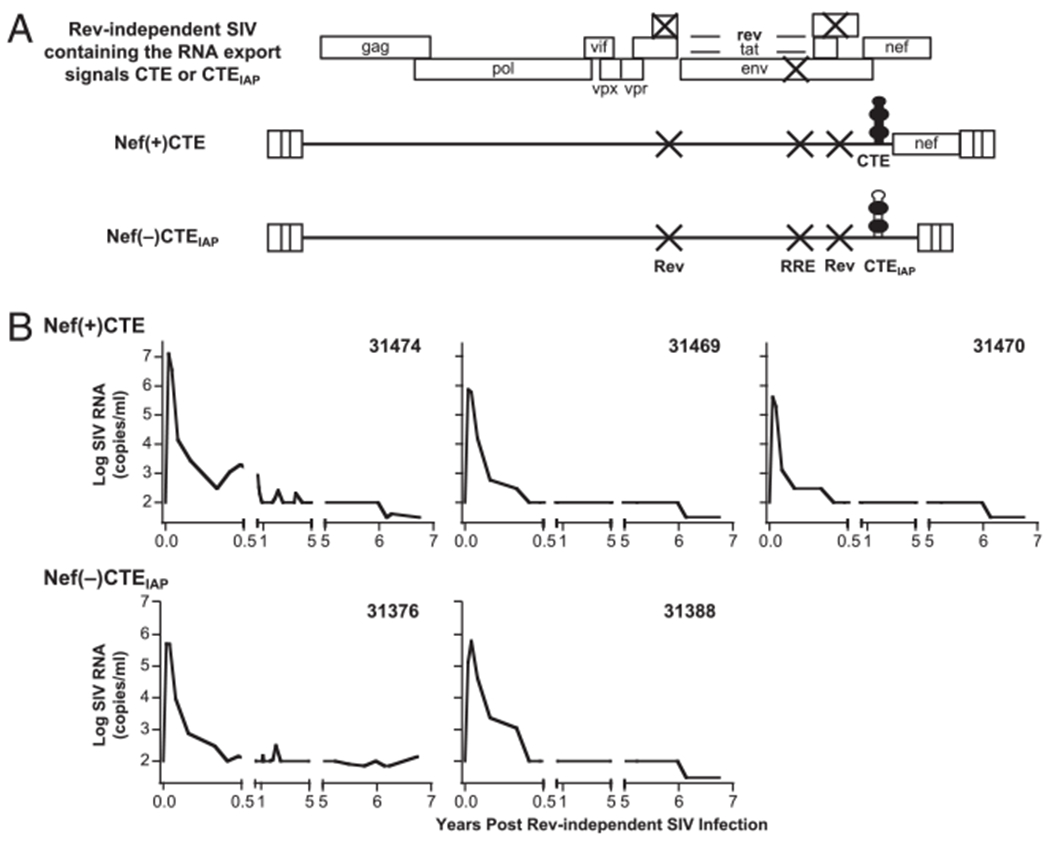
Rev-independent SIV strains with attenuated growth properties. A, Molecular clones of the Rev-independent SIVmac239. All viruses have multiple point mutations destroying rev and RRE, designed to preserve the coding potential of the overlapping tat and env coding regions (11–13). The clone Rev−RRE−Nef−CTEIAP+ contains the CTEIAP inserted 3′ to the terminator of env, which renders this clone nef-minus (13). The clone Rev−RRE−Nef+CTE+ contains the SRV-1 CTE and has the 5′ portion of nef(nt 9081–9280) inserted 3′ to the CTE, generating an intact nef open reading frame. B, The Rev-independent LASIV-infected animals were monitored over time. The plasma virus loads are shown over 6.7 y of infection up to the day of enrollment of the CD8 depletion study. Note that the threshold of the assays changed over the years of follow-up. The follow-up to 5.9 y postinfection has been reported previously (12). CTEIAP; CTE from the murine IAP retroelement.
FIGURE 2.
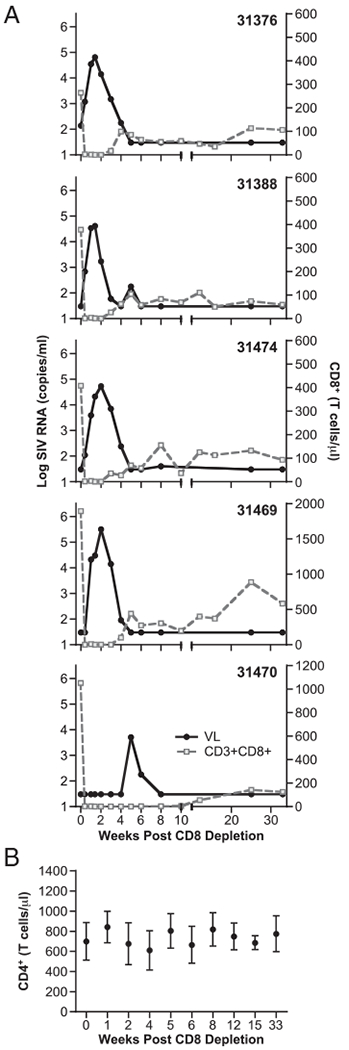
Changes in CD8+ T cells and viral loads after treatment with cM-T807 Ab. A, Macaques were subjected to CD8+ cell depletion 6.7 y postinfection with the Rev-independent SIVmac239 strains. A single treatment with the cM-T807 Ab led to temporal depletion of CD8+ lymphocytes and transient loss of control of viremia. The CD3+CD8+ T cell counts (measured with the anti-CD8 Ab DK25) per μl of blood (gray line) and plasma viral loads (black line) were monitored over time. B, Measurements of circulating CD4+ T cells of the five animals subjected to CD8+ cell depletion.
Effects of CD8+ lymphocyte depletion on replication of Rev-independent LASIV
To examine the role of CD8+ cells in the control of viremia of the Rev-independent LASIV-infected animals, the macaques were treated with a single high dose of cM-T807, an anti-CD8 mAb that had been shown to efficiently deplete all CD8+ cells, including CD8+ T lymphocytes and NK cells, from periphery and lymph nodes in rhesus macaques (35, 36, 40). At the time of CD8+ depletion, four of the animals had undetectable viral loads, whereas macaque 31376 had 140 copies of SIV RNA/ml plasma. After treatment, blood samples were taken at different time points to monitor changes in CD8+ and CD4+ lymphocytes and plasma viral loads (Fig. 2). After the anti-CD8 Ab administration, there was an immediate and complete loss of circulating CD8+ T lymphocytes in all the animals (Fig. 2A) without significant changes in the CD4+ T cell count (Fig. 2B). Concomitantly, a rapid increase of viremia occurred in four of the animals, reaching a peak at day 10–14 after CD8+ cell depletion, with viral loads ranging from 4.6 to 5.5 log10 of SIV RNA copies/ml plasma (Fig. 2A). The only exception to this pattern was animal 31470, which, despite a similar kinetic of CD8+ cell depletion, did not show virus rebound until 5 wk postdepletion (compared with ~2 wk for the other animals) and showed a peak of viremia of 3.7 log10. A possible explanation of this delayed viremia rebound is the lower chronic viremia in this animal. This is in agreement with the lower levels of humoral immune responses measured in 31470 (Table I), as also reported previously (12, 13). In all animals, virus replication was rapidly controlled, resulting in a steady decline of the viral loads to levels below the detection threshold of the assay (30 viral RNA copies/ml) by week 5 (week 8 for macaque 31470). Complete virus suppression was maintained in all animals for the entire follow-up period (33 wk).
Table I.
Humoral responses after CD8 cell depletion
| Animal | Weeks Postdepletion | Ab Titers |
|||
|---|---|---|---|---|---|
| Gaga | Enva | SIVmac239 Nabb | SIVmac251 Nabb | ||
| 31376 | 0 | 400 | 6,400 | <10 | 12,664 |
| 4 | 6,400 | 25,600 | 20 | 64,057 | |
| 8 | 1,600 | 25,600 | 16 | 45,169 | |
| 31388 | 0 | 400 | 6,400 | 27 | 5,321 |
| 4 | 25,600 | 25,600 | 185 | 60,538 | |
| 8 | 6,400 | 25,600 | 94 | 21,786 | |
| 31474 | 0 | 1,600 | 25,600 | 23 | 33,185 |
| 4 | 102,400 | 102,400 | 105 | 98,415 | |
| 8 | 25,600 | 102,400 | 47 | 78,614 | |
| 31469 | 0 | <100 | 100 | <10 | 537 |
| 4 | 6,400 | 25,600 | 26 | 55,125 | |
| 8 | 1,600 | 25,600 | 18 | 9,864 | |
| 31470 | 0 | <20 | <20 | 10 | <45 |
| 4 | <20 | <20 | <10 | <45 | |
| 8 | <100 | 6,400 | <10 | 3,030 | |
Reciprocal Ab titers against SIV Gag and Env were defined as the plasma dilution at or above two times the absorbance value obtained with plasma from naive rhesus macaques.
Nab titer assays were performed in TZM-bl cells using the SIVmac239CS.23 (293T pseudovirus) or in M7-Luc cells using SIVmac251 (H9 grown).
Comparison of the virus levels at peak of the primary viremia upon infection by the Rev-independent LASIV (12, 13) and after CD8 depletion showed remarkably lower viremia after cM-T807 treatment, with a reduction ranging from 0.4 to 2.4 log10 copies of SIV RNA/ml plasma (median, 1.8 log10). Animal 31474 showed the highest delta-peak level of 2.4 log10. It is possible that virus replication after CD8+ depletion activated potent recall immune responses in the chronically LASIV-infected macaques able to reduce the viremia (see below). Together, these data show that, after many years (6.7 y) of successful control of viremia, replication of the Rev-independent LASIV can be rapidly activated by removal of CD8+ cells, and that virus replication is potently controlled thereafter.
Lack of CD8 cell recovery after cM-T807 Ab treatment
We used flow cytometric analysis to monitor the changes of the CD8+ T cell subset within the T lymphocyte population. CD8+ T cells were monitored using two assays with two different anti-CD8 mAbs: clones DK25 and SK1 (DakoCytomation and BD Pharmingen, respectively; Fig. 2) were used with fresh whole blood samples, and clone 3B5 (Invitrogen; Fig. 3) was used with frozen PBMCs. Similar results were obtained with all three anti-CD8 mAbs. The CD8+ T cells represented between 15 and 40% (median 33%) of the circulating T lymphocytes at enrollment, and were efficiently depleted after Ab treatment. Interestingly, in four of the animals (31376, 31388, 31474, and 31470), control of viral replication occurred despite a very poor recovery of circulating CD8+ T cells. Reduction of viremia by >90% was achieved in animals 31376, 31388, and 31474 by week 4 post-CD8+ depletion, whereas CD8+ T cell count was ~10 to <1% (animal 31470 at week 8 postdepletion) of the predepletion levels. Poor recovery of CD8+ cells in periphery has been noted previously and was attributed to the age of the animals (age >5 y) (36). In accordance with this observation, the animals in our study were 6.7 y of age, which is most likely responsible for the observed long-term effect of CD8 depletion obtained despite a single Ab treatment. Only macaque 31469 showed a substantial recovery of the CD8+ T cell population in the blood, concomitant with control of viremia, similar to data reported by others (28–40). Importantly, despite the observed long-term CD8+ depletion, rapid control of viremia was observed in all the animals, suggesting that additional mechanisms, independent of CD8+ T cells, were responsible for this phenomenon.
FIGURE 3.
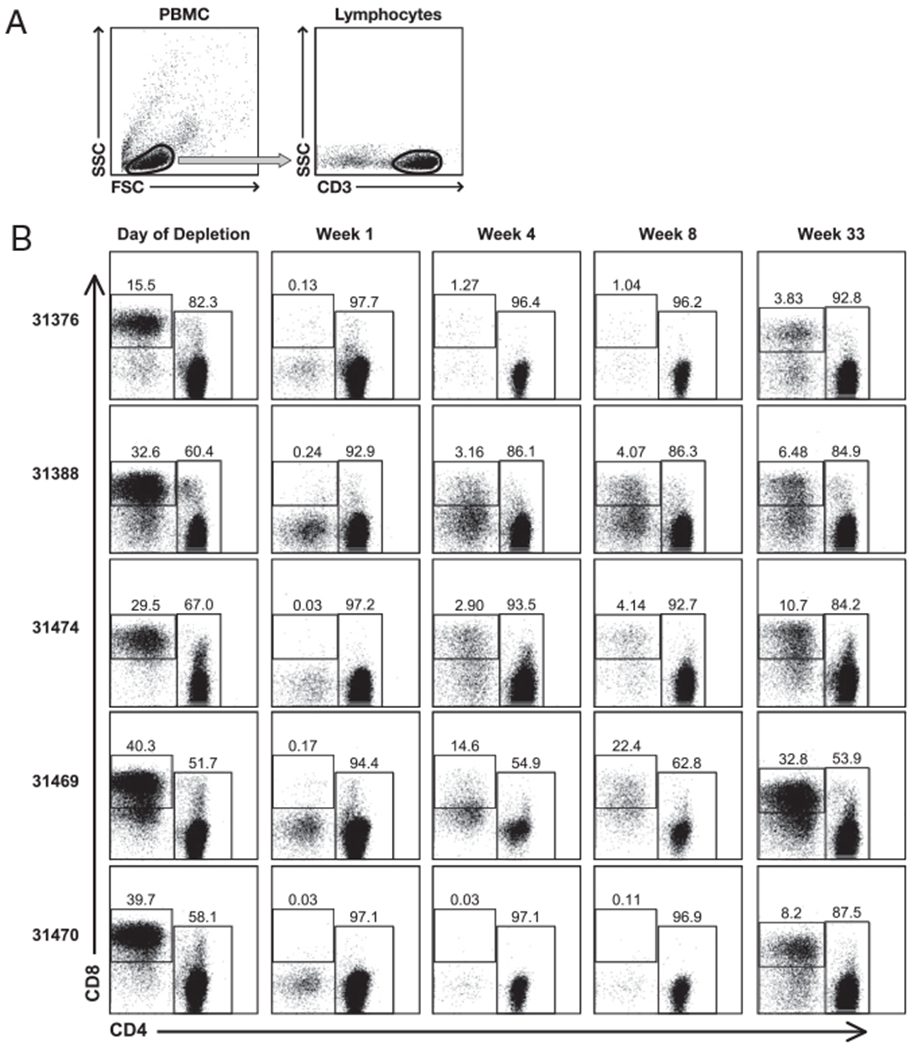
Frequency of CD8+ cells within the T lymphocyte population measured by flow cytometry. A, T lymphocytes were gated according to a scatter (left) and CD3 staining (right). B, Dot plots show the frequency of circulating CD8+ and CD4+ cells within gated CD3+ T cell population upon CD8+ lymphocyte depletion. The five macaques were monitored at the day of depletion and at weeks 1, 4, 8, and 33 postdepletion. Numbers represent the percentage of these two cell populations among the total CD3+ T lymphocytes.
Induction of SIV-specific CD4+ T cells, but lack of CD8+ T cell responses after CD8+ cell depletion
SIV-specific T cell responses were monitored in PBMC samples by intracellular staining and flow cytometric analysis. The lymphocyte population was gated based on forward and side scatter, and cytokine-positive cells were determined within the T cells (gated by CD3 staining). The phenotype of these Ag-specific T cells was further analyzed for CD4, CD8, and expression of different memory markers, as described in Materials and Methods. Because the infection by the nonpathogenic LASIV strains shows extremely low levels of virus replication, very low levels of Ag-specific cellular responses could be detected before the onset of the study (Fig. 4), which consisted of both SIV-specific CD4+ and CD8+ T cells in three of the animals (31376, 31388, and 31469) or only CD4+ cells in the other two animals (31474 and 31470).
FIGURE 4.
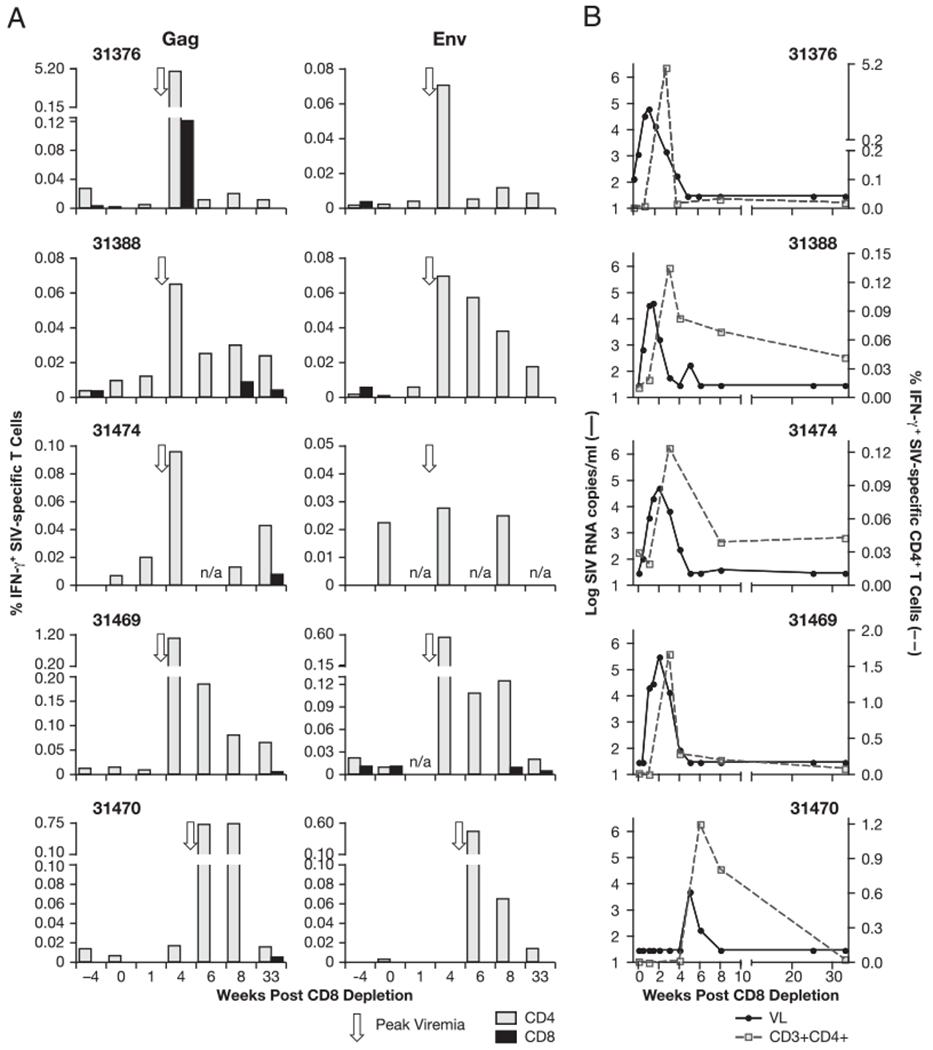
SIV-specific cellular immune responses induced upon CD8+ depletion. Analysis of blood samples collected 4 wk prior to depletion, at the day of depletion, and at weeks 1, 3, 4, 6 (animal 31470), 8, and 33 postdepletion is shown. A, The frequency of SIV-specific CD4+ (light gray bar) and CD8+ (black bar) T cells was measured in PBMCs by multicolor flow cytometry after in vitro stimulation with Gag (left panel) or Env (right panel) peptide pools, followed by intracellular staining for IFN-γ. Note the variable scales for the different animals. Arrow indicate the time of peak viremia. B, Viral loads and changes in the frequency of SIV-specific CD4+ T lymphocytes after depletion with the cM-T807 Ab. In all five animals, control of viremia coincides with a peak of Ag-specific CD4+ T cells.
After CD8+ cell depletion, we found a great increase in SIV-specific T cell responses (Fig. 4A) against Gag (left panel) and Env (right panel), demonstrating efficient induction of recall responses. The increase of SIV-specific cellular immune responses against both Gag and Env postdepletion coincided with the clearance of viremia (~1 wk postpeak viremia), and peaked at week 3 for animals 31376, 31388, 31469, and 31474 and at week 6 for animal 31470. These responses were generally greater against Gag, reflecting the greater Gag responses detected in these animals over the course of the long-term infection prior to CD8 depletion (12). The responses ranged from 0.065 to 5.3% and from 0.03 to 0.56% of the circulating T cells for Gag and Env, respectively. Macaque 31470, which had the delayed viral rebound, also showed high cellular immune responses against Gag and Env at the time of control of viremia. We noted that several animals maintained relatively high levels of cellular responses for a couple of weeks. Upon Gag and Env peptide stimulation, all responses led to the production of IFN-γ only, and neither IL-2 nor TNF-α was detected in any of the samples.
We found that the SIV-specific cellular responses were almost exclusively mediated by CD4+ T cells at the time of virus control (week 3 or 6). One exception was macaque 31376, which had a transient minor fraction of Gag-specific CD8+ T cells (2% of the SIV-specific T cells). Surprisingly, macaque 31469, the only animal that showed significant recovery of circulating CD8+ T cells, also had no detectable SIV-specific CD8+ T cell responses in the peripheral blood by the time of control of viremia. All animals, except 31376, developed extremely low levels of SIV-specific CD8+ T cells at much later time points, several weeks full control of viremia (week 8 or 33 post-CD8 depletion). Our data show that a burst of SIV-specific CD4+ T cell recall responses coincides with control of viremia (Fig. 4B), suggesting a potent role of this subset of T cells in virus control. Together, these findings indicate the contribution of mechanisms other than SIV-specific CD8+ cells mediating the rapid control of viremia.
To understand the nature of the induced T cell responses, we used additional memory markers in flow cytometry to distinguish the Ag-specific T cells into subsets with central memory markers, characterized as CD3+CD45RA−CD28+, and effector memory markers, characterized as CD3+CD28−. The analysis of the total SIV-specific (Gag and Env) T cell response is shown in Fig. 5A. We found that the increase in the SIV-specific CD4+ T cell population was mainly due to CD45RA−CD28+CD4+ T cells, which were greatly increased at week 3 (time of virus control) and maintained at week 8, with peak values ranging from 0.1 to 5% of the circulating T cells. Paralleling the delay in viral rebound, monkey 31470 showed a delay (week 6) in the increase of these SIV-specific CD4+ T cells. The majority of its responses showed an effector phenotype characterized by the lack of CD28 expression. In fact, only two of the animals, 31469 and 31470, showed a significant increase of SIV-specific CD4+CD28− effector memory cells (peak response of 0.04 and 0.75% of the blood T cells, respectively). Analysis of the very low levels of SIV-specific CD8+ responses (animal 31376 at week 3; animals 31388, 31474, 31469, and 31470 at weeks 8 and/or 33) showed that these were CD8+CD28+ memory T cells. In all animals, the total SIV-specific responses declined over time after control of the rebounding virus, probably reflecting the lack of antigenic stimulation in the absence of detectable viral replication.
FIGURE 5.
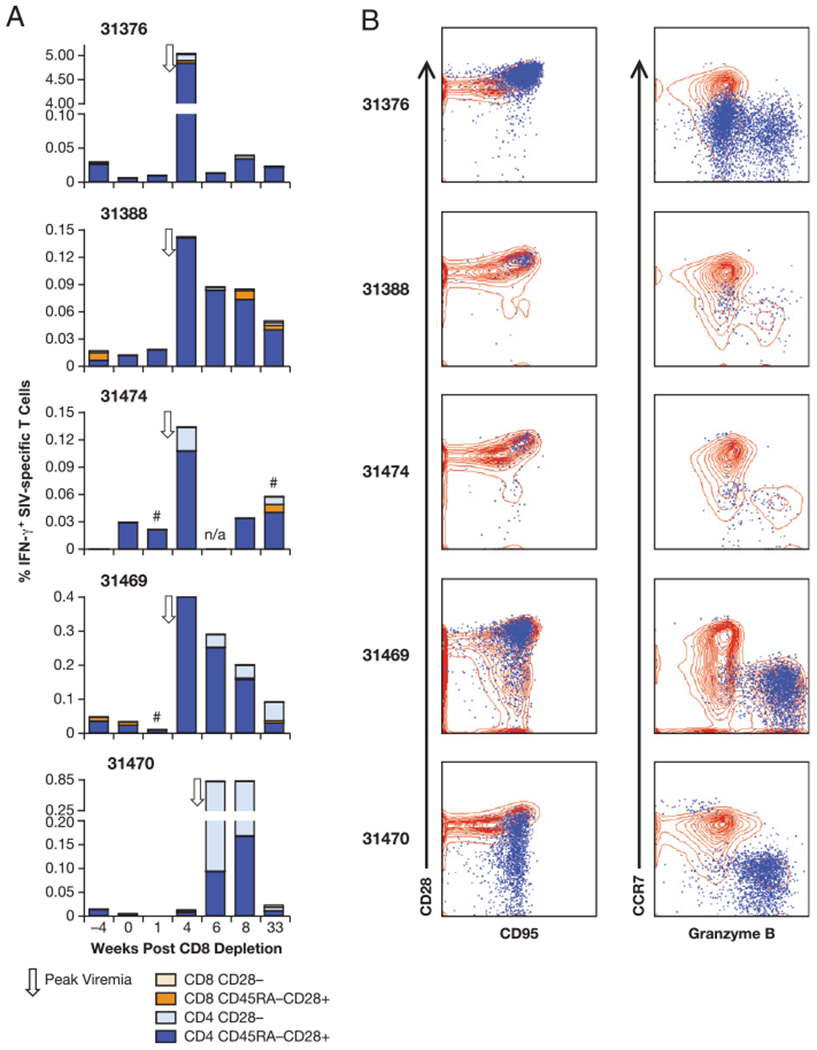
Phenotypic analysis of the SIV-specific T cells. A, Memory phenotype of the SIV-specific (Gag and Env) T cells upon CD8 depletion. The T cells were divided in CD3+CD28+CD45RA− and CD3+CD28−. The two subsets were further divided in CD4+ and CD8+ populations. B, Overlays showing the pattern of expression of the SIV-specific T cells (blue) and the general T cell population (red) for CD95 and CD28 (left panels) and CCR7 and granzyme B (right panels) at the time of control of rebounding virus (week 3 for four of the animals, and week 6 for macaque 31470). #, sample not analyzed for Env responses; n/a, sample not available.
The phenotype of the SIV-specific T cell responses was analyzed in more detail at three selected time points: prior to CD8 depletion, at the time of control of the rebounding virus (Figs. 5B, 6), and at week 33 postdepletion. In addition to CD28 and CD45RA, the analysis of these samples included CD95, CCR7, and granzyme B. Fig. 5B (left panel) shows that at the time of re-establishing viral control, the animals had a great increase of the SIV-specific T cells (over-layed in blue) that were CD95+ and mainly CD28+ in three animals (31376, 31388, and 31474). The other two macaques, 31469 and 31470, showed an additional significant population of cells lacking CD28, as described above (Fig. 5A). Interestingly, the majority of the CD95+CD28+/− T cells were CCR7−, and in all cases a significant fraction contained granzyme B (range 30–90% of the IFN-γ+ T cells; Fig. 5B, right panel), indicative of an effector phenotype with cytotoxic capabilities.
FIGURE 6.
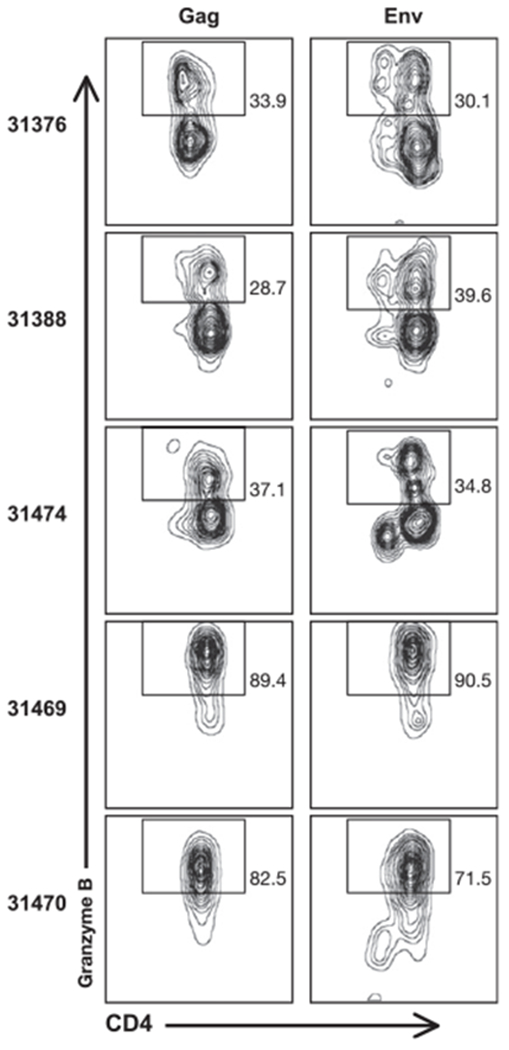
Frequency of granzyme B+ cells among the Gag-specific (left panels) or Env-specific (right panels) T lymphocytes at the time of control of rebounding viremia (week 6 for animal 31470, and week 3 for the other four macaques). Numbers in the plots denote the percentage of granzyme B+ included in the displayed gates.
The frequency of granzyme B+ cells among the Gag- and Env-specific CD4+ T cell responses was further analyzed at the same time points (Fig. 6). The frequency of these cells increased significantly compared with the time prior to depletion, when the T cell responses were very low (see also Fig. 5A). At the time of viral control, the frequency of granzyme B+ T cells within each of the five animals was similar for both Gag- and Env-specific cells (Fig. 6). In all animals, these responses were almost exclusively mediated by CD4+ T lymphocytes with an effector phenotype characterized by the lack of CCR7 expression. At week 33 after CD8 depletion, only animals 31469 and 31470 retained a subset of CCR7− granzyme B+ cells (70 and 50% of the SIV-specific T cells, respectively), indicating a contraction of cells with cytotoxic potential after the clearance of the rebounding virus (data not shown). In agreement with the lack of CD28 in their SIV-specific T cells, macaques 31469 and 31470 had the highest frequency of IFN-γ+ granzyme B+ T lymphocytes (90 and 70%, respectively; Fig. 6), whereas in macaque 31376, with the highest SIV-specific T cell responses, only 30% of the IFN-γ+ cells also expressed granzyme B (Fig. 6).
Analysis of anti-SIV humoral responses
The chronically LASIV-infected animals showed low levels of humoral immune responses prior to enrollment in the CD8 depletion protocol, indicative of the persistent, but low-level infection by the attenuated SIV (12, 13). At the day of CD8+ lymphocyte depletion, all the animals except 31470 had detectable Abs against SIV Gag and Env (Fig. 7), with the Ab titers against Env being higher than those against Gag (range 2–4.4 log10 for Env versus Gag range 1–3.2 log10). Macaque 31469 had low anti-Env, but no anti-Gag responses, whereas macaque 31470 (which showed the delayed viremia upon CD8+ depletion) was the only animal in the group that did not have detectable Gag or Env Abs at enrollment into the study, suggesting very low levels of ongoing antigenic stimulation. The plasma samples were also analyzed for neutralizing activity against SIVmac251 and the neutralization-resistant SIVmac239. At the day of treatment, Nabs to SIVmac251 were detected in all the animals except monkey 31470, and low titers of Nabs to SIVmac239 were detected in two macaques (31388 and 31474; Table I).
FIGURE 7.
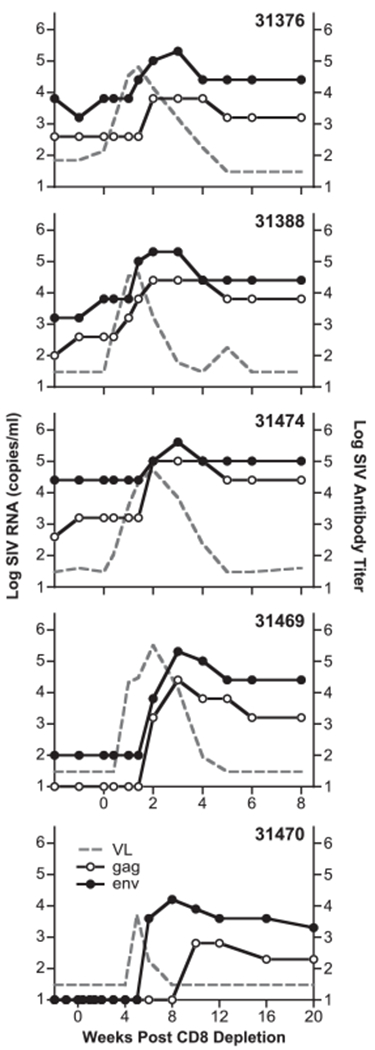
Increase in SIV-specific humoral immune responses after CD8+ lymphocyte depletion. Reciprocal log Ab titers to SIV Gag (open circles) and Env (black circles), respectively, were defined as the plasma dilution at or above two times the absorbance values of the naive controls. Plasma samples below the cutoff value of dilution 1/20 are shown in the graph as log 1. Ab titers for Gag and Env for the five animals and the SIV viral loads (from Fig. 1, gray lane) are shown. Note the different scale of the x-axis for animal 31470.
After CD8+ lymphocyte depletion and subsequent viral rebound, we found rapid and great increases in SIV-specific humoral recall responses against both Env and Gag in all the animals (Fig. 7, Table I). Peak titers developed between weeks 2 and 3 after CD8 depletion (~1 wk postviremia peak) in four of the animals, with Env titers ranging from 5.3 to 5.6 log10 and Gag titers ranging from 3.8 to 5.0 log10. In animal 31470, which had a delay in virus rebound, the humoral responses appeared later (weeks 8–10), as expected, from the kinetics of virus rebound. In summary, as a result of the rebounding viremia, the animals showed increases for both Gag and Env Ab titers by ~2 logs.
Like the binding Abs to Env, the levels of the Nabs against the SIVmac251 increased (Table I). Upon CD8+ depletion, we also found increases in the neutralizing activity against the neutralization-resistant SIVmac239 (Table I) in all animals, except animal 31470, which also had the lowest level of Env Ab. Animals 31376 and 31469, which did not show detectable levels of Nabs to SIVmac239 at enrollment, also showed increases upon CD8+ depletion, suggesting the presence of Nabs to SIVmac239 at levels below the threshold of the assay prior to depletion. This is similar to the previously reported finding by Metzner et al. (39), which noted detectable Nabs to SIVmac239 in macaques infected by SIVmac239Δnef, which increased transiently after CD8 depletion. In summary, the transiently increased viremia of the Rev-independent LASIV induced both binding and Nabs that were maintained during the observation period.
Discussion
Depleting CD8+ cells in vivo, which efficiently removes the CD8+ cells both from blood and lymph nodes (35, 36), established an important role of these cells in the control of SIV replication. Although this procedure removes SIV-specific CTL responses mediated by CD8+ T cells, multiple mechanisms may be responsible for viral rebound upon depletion of CD8+ cells. CD8+ depletion by the cM-T807 Ab removes not only T cells, but also NK cells that are important sources of the β-chemokines MIP-1 and RANTES, which inhibit CCR5 usage and contribute to viral control (46, 47). NKs are also effector cells of Ab-dependent cellular cytotoxicity. CD8+ T cells are also thought to produce a yet-unidentified soluble factors) that inhibits viral expression in the absence of cytotoxic activity (48, 49). It has been reported that vaccination with LASIV strains induces the production by CD8+ T cells of soluble factors, other than β-chemokines, able to inhibit SIV replication in a MHC-unrestricted manner (50). Thus, it is possible that removal of CD8+ cells results in increased viral expression by the combination of two mechanisms, as follows: 1) loss of viral control by MHC-restricted cytotoxic responses and 2) reduction of systemic levels of anti-SIV soluble factors produced or induced by CD8+ cells, either T lymphocytes or NK cells. The important homeostatic changes taking place after systemic depletion of CD8+ cells have been recently highlighted by Okoye et al. (51), in which CD8+ depletion resulted in increased proliferation of CD4+CCR5+ effector memory T cells secondary to endogenous IL-15 production.
In the cohort reported in this work, efficient control of viral rebound occurred in the presence of only marginal levels of CD8+ T cell recovery in four of the five CD8-depleted macaques, and in the absence of SIV-specific CD8+ T lymphocytes in the blood. Impaired recovery of circulating CD8+ T cells after depletion has been reported previously and was attributed to the age of the macaques, because it was observed that younger macaques recovered CD8+ T cells faster than older animals (>5 y) (36, 52). It is possible that the anti-CD8 Ab affects the pool of T cell precursors, which could be limited in some animals. The animals in the present work were 6.7 y old at enrollment, and therefore, their age may contribute to inefficient recovery of the CD8+ cells. In contrast, the study of such animals enables us to further dissect the role of CD8+ cells and other mechanisms responsible for suppression of viral replication.
Another study (34) showed that SIVmac251 challenge of CD8+ cell-depleted SIVmac239Δ3-vaccinated macaques resulted in higher viral loads compared with the nondepleted vaccinated animals, but 2 logs lower viremia than in unvaccinated macaques, indicating the contribution of CD8-independent mechanisms leading to reduced viremia. Viral control in the absence of significant CD8+ responses and the presence of very low cellular responses and low Nabs has been described (53) in animals vaccinated with the attenuated SIVmac239 that has a deletion of Env V1-V2, which were strongly protected against challenge with SIVmac239. In fact, protection in the absence of significant Nab titers appears to be a common feature of macaques infected with LASIV (24, 27, 54, 55). The contribution of cytotoxic mechanisms mediated by CD8+ T cells in the control of plasma viremia during the chronic phase of SIV infection has been recently addressed in studies in which CD8-depleted animals received antiretroviral treatment (56, 57). In these studies, it was found that the life span of the infected cells and the viral load decay were not affected by the presence or absence of CD8+ T lymphocytes, indicating that either CD8+ T cells contribute to viral control via noncytotoxic mechanisms or cytotoxicity is mediated by other cells.
Our study using the CD8 depletion approach in LASIV-infected rhesus macaques is the first report that extensively characterizes the phenotype of SIV-specific T cells associated with control of the rebounding virus using flow cytometry. We found that CD8+ cell depletion was accompanied by the rapid emergence of SIV-specific CD4+ T cells in the blood. These CD4+ cells were characterized mainly as CD45RA−CD95+CD28+CCR7− and reached peak levels at the time of control of the rebounding viremia. The SIV-specific CD4+ T cells produced IFN-γ, but not IL-2, a cytokine associated with helper function, and a high frequency of these effector cells expressed granzyme B, supporting their killing capability. IL-2 production by blood CD4+ T cells upon antigenic stimulation is mainly restricted to CD28+CCR7+ memory cells. In the study reported in this work, the vast majority of CD4+ SIV-specific T lymphocytes were CCR7−, which could explain the lack of IL-2 secretion by these cells and, together with granzyme B expression, emphasize their cytotoxic potential. Veazey et al. (37) found that CD8+ cell depletion of naive macaques prior to challenge with SIVmac251 resulted in high levels of viremia, with extensive killing of CD4+CCR5+ memory T cells also in mucosal tissues. This massive killing resulted in the inability of the infected animals to mount an effective immune response with impairment of both cell-mediated and humoral responses. In contrast, in our study, CD8+ cell depletion was performed in macaques chronically infected with an attenuated SIV strain in the presence of efficient pre-existing immunity. These preserved SIV-specific CD4+ T cells were able to expand, display a cytotoxic phenotype, and most likely contribute efficiently to the containment of viral rebound. Although the cytotoxic capability of CD4+ T cells is well known, originally it was thought that their mechanism of killing was mediated via apoptotic signals delivered by Fas–Fas ligand interaction or by TNF-α secretion, whereas cytotoxicity mediated by the release of cytolytic granules was exclusively mediated by CD8+ T lymphocytes and NK cells (58). It is now apparent that a subset of CD4+ T cells expresses both perforin and granzymes (59–61), and that these cells are capable of killing both in vitro and in vivo. Direct killing in vivo by CD4+ T cells has been shown in several viral infections, including lymphocytic choriomeningitis virus, EBV, and gamma-herpesvirus (62–64), and the presence of CD4+ T cells with a cytotoxic phenotype has been described in HIV-1–infected individuals (65). In fact, studies with CD4+ T cells obtained from HIV-1–infected individuals and healthy controls demonstrated that such CD4+ T cells not only harbor granules containing perforin and granzyme B, but were also able to release them upon specific stimulation with CMV peptides, resulting in lysis of the target cells ex vivo (66).
In our CD8-depleted animals, in addition to the increase in Ag-specific CD4+ T cell responses, we also found an increase in SIV-specific humoral responses in all animals. In fact, the increase in both cellular and humoral responses coincided with viral rebound and clearance of viremia; animal 31470 had a delay in both peak of viremia and increase of immune responses, which suggests that the CD4 and Ab responses were stimulated specifically by the viral replication and not by any other mechanisms triggered by CD8 depletion. The Ab responses were characterized by transient increased levels of Nabs to SIVmac251 (all animals) and to the neutralization-resistant SIVmac239 (in four of the five macaques). Whereas these Abs contribute to the control of the rebounding virus, macaque 31470, which did not show detectable neutralizing activity to SIVmac239, had very high CD4+ responses against Gag and Env and successfully controlled viremia. In fact, the generation of SIV-specific CD4+ T cell responses, in the absence of SIV-specific CD8+ T cells, was the common feature in all the CD8-depleted animals. It is possible that the increased Ab responses could also contribute to viral control via additional mechanisms, such as Ab-dependent cellular cytotoxicity, which highlights the importance of a well-balanced immune response for control of the infection. The specific contribution of either CD4+ T cells or humoral responses in the control of the rebounding virus could be addressed by depletion of B cells, in addition to CD8. The small number of long-term LASIV-infected animals available did not allow addressing this question. Using a different model, Zahn et al. (67) recently reported that double depletion of CD8+ cells and B lymphocytes performed during primary infection of African Green monkeys failed to show significant contribution of humoral responses in viral containment.
Because infection of rhesus macaques with LASIV is characterized by control of viremia and lack of disease progression, the macaques had a preserved CD4+ T cell population with normal CD4 counts in the peripheral blood. The healthy CD4 levels present in these animals are probably critical for mounting an effective CD4+ T cell response upon CD8 depletion and to provide help to the B lymphocytes for the production of high titers of anti-SIV Abs, including anti-Env Nabs. Taken together, our results indicate important roles of different components of the immune responses, including CD8-independent responses in the control of viremia in SIV-infected rhesus macaques. Therefore, strategies for vaccines against HIV should include the development of a broad, well-balanced type of immunity, including CD8+ and CD4+ cells, and humoral responses.
Acknowledgments
We thank I. Kalisz (Advanced BioScience Laboratories) for assistance, J. Schmitz for discussions, T. Jones for editorial assistance, and Keith Reimann (Harvard Medical School) for provision of cM-T807.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Part of this work was supported by Grant RR-00169 from the National Center for Research Resources, National Institutes of Health to the California National Primate Research Center.
Abbreviations used in this paper:
- #
sample not analyzed for Env responses
- CTE
constitutive transport element
- CTEIAP
CTE from the murine IAP retroelement
- IAP
intracisternal A-particle
- LASIV
live-attenuated SIV
- n/a
sample not available
- Nab
neutralizing Ab
- RRE
Rev-responsive element
- SRV-1
simian retrovirus type 1
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, and Desrosiers RC. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol 7: 19–23. [DOI] [PubMed] [Google Scholar]
- 2.Whitney JB, and Ruprecht RM. 2004. Live attenuated HIV vaccines: pitfalls and prospects. Curr. Opin. Infect. Dis 17: 17–26. [DOI] [PubMed] [Google Scholar]
- 3.Blancou P, Chenciner N, Ho Tsong Fang R, Monceaux V, Cumont MC, Guétard D, Hurtrel B, and Wain-Hobson S. 2004. Simian immunodeficiency virus promoter exchange results in a highly attenuated strain that protects against uncloned challenge virus. J. Virol 78: 1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desrosiers RC, Lifson JD, Gibbs JS, Czajak SC, Howe AY, Arthur LO, and Johnson RP. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol 72: 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fultz PN,Vance PJ, Endres MJ,Tao B, Dvorin JD, Davis IC, Lifson JD, Montefiori DC, Marsh M, Malim ΜH, and Hoxie JA. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol 75: 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs JS, Regier DA, and Desrosiers RC. 1994. Construction and in vitro properties of SIVmac mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retroviruses 10: 607–616. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Whitney JB, Detorio M, and Wainberg MA. 2001. Construction and in vitro properties of a series of attenuated simian immunodeficiency viruses with all accessory genes deleted. J. Virol 75: 4056–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y, Whitney JB, Liang C, and Wainberg MA. 2001. Novel, live attenuated simian immunodeficiency virus constructs containing major deletions in leader RNA sequences. J. Virol 75: 2776–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kestler HW, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, and Desrosiers RC. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65: 651–662. [DOI] [PubMed] [Google Scholar]
- 10.Shacklett BL, Shaw KE, Adamson LA, Wilkens DT, Cox CA, Montefiori DC, Gardner ΜB, Sonigo P, and Luciw PA. 2002. Live, attenuated simian immunodeficiency virus SIVmac-M4, with point mutations in the Env transmembrane protein intracytoplasmic domain, provides partial protection from mucosal challenge with pathogenic SIVmac251. J. Virol 76: 11365–11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Gegerfelt A, and Felber BK. 1997. Replacement of posttranscriptional regulation in SIVmac239 generated a Rev-independent infectious virus able to propagate in rhesus peripheral blood mononuclear cells. Virology 232: 291–299. [DOI] [PubMed] [Google Scholar]
- 12.von Gegerfelt AS, Alicea C, Valentin A, Morrow M, van Rompay KK, Ayash-Rashkovsky M, Markham P, Else JG, Marthas ML, Pavlakis GN, et al. 2006. Long lasting control and lack of pathogenicity of the attenuated Rev-independent SIV in rhesus macaques. AIDS Res. Hum. Retroviruses 22: 516–528. [DOI] [PubMed] [Google Scholar]
- 13.von Gegerfelt AS, Liska V, Li PL, McClure HM, Horie K, Nappi F, Montefiori DC, Pavlakis GN, Marthas ML, Ruprecht RM, and Felber BK. 2002. Rev-independent simian immunodeficiency virus strains are nonpathogenic in neonatal macaques. J. Virol 76: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Gegerfelt AS, Liska V, Ray NB, McClure HM, Ruprecht RM, and Felber BK. 1999. Persistent infection of rhesus macaques by the rev-independent Nef simian immunodeficiency virus SIVmac239: replication kinetics and genomic stability. J. Virol 73: 6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolotukhin AS, Valentin A, Pavlakis GN, and Felber BK. 1994. Continuous propagation of RRE and Rev RRE human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J. Virol 68: 7944–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray M, Prasad S, Dubay JW, Hunter E, Jeang KT, Rekosh D, and Hammarskjbld ML. 1994. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA 91: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabemero C, Zolotukhin AS, Bear J, Schneider R, Karsenty G, and Felber BK. 1997. Identification of an RNA sequence within an intracisternal-A particle element able to replace Rev-mediated posttranscriptional regulation of human immunodeficiency virus type 1. J. Virol 71: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabemero C, Zolotukhin AS, Valentin A, Pavlakis GN, and Felber BK. 1996. The posttranscriptional control element of the simian retrovirus type 1 forms an extensive RNA secondary structure necessary for its function. J. Virol 70: 5998–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felber BK, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, and Pavlakis GN. 1989. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc. Natl. Acad. Sci. USA 86: 1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadzopoulou-Cladaras Μ, Felber BK, Cladaras C, Athanassopoulos A, Tse A, and Pavlakis GN. 1989. The rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. J. Virol 63: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino DM,Felber BK, Harrison JE, and Pavlakis GN. 1992. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol. Cell. Biol 12: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, and Ruprecht RM. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267: 1820–1825. [DOI] [PubMed] [Google Scholar]
- 23.Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, and Ruprecht RM. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med 5: 194–203. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RR, Lifson JD, Czajak SC, Cole KS, Manson KH, Glickman R, Yang J, Montefiori DC, Montelaro R, Wyand MS, and Desrosiers RC. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol 73: 4952–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata R, Siemon C, Czajak SC, Desrosiers RC, and Martin MA. 1997. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J. Virol 71: 8141–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenner-Racz K, Stahl Hennig C, Uberla K, Stoiber H, Ignatius R, Heeney J, Steinman RM, and Racz P. 2004. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proc. Natl. Acad. Sci. USA 101: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, and Desrosiers RC. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol 73: 8356–8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med 189: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EY, Veazey RS, Zahn R, McEvers KJ, Baumeister SH, Foster GJ, Rett MD, Newberg MH, Kuroda MJ, Rieber EP, et al. 2008. Contribution of CD8+ T cells to containment of viral replication and emergence of mutations in Mamu-A*01-restricted epitopes in simian immunodeficiency virus-infected rhesus monkeys. J. Virol 82: 5631–5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lifson JD, Rossio JL, Piatak M Jr., Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, et al. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol 75: 10187–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madden LJ, Zandonatti MA, Flynn CT, Taffe MA, Marcondes MC, Schmitz JE, Reimann KA, Henriksen SJ, and Fox HS. 2004. CD8+ cell depletion amplifies the acute retroviral syndrome. J. Neurovirol 10(Suppl. 1): 58–66. [DOI] [PubMed] [Google Scholar]
- 32.Matano T, Shibata R, Siemon C, Connors M, Lane HC, and Martin MA. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol 72: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller YM, Do DH, Boyer JD, Kader M, Mattapallil JJ, Lewis MG, Weiner DB, and Katsikis PD. 2009. CD8+ cell depletion of SHIV89.6P-infected macaques induces CD4+ T cell proliferation that contributes to increased viral loads. J. Immunol 183: 5006–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz JE, P Johnson R, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, et al. 2005. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239-delta3-vaccinated rhesus macaques. J. Virol 79: 8131–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–860. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, P Racz, Tenner-Racz K, Scallon BJ, Dalesandro M, et al. 1999. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am. J. Pathol 154: 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veazey RS, Aciemo PM, McEvers KJ, Baumeister SH, Foster GJ, Rett MD, Newberg MH, Kuroda MJ, Williams K, Kim EY, et al. 2008. Increased loss of CCR5+ CD45RA− CD4+ T cells in CD8+ lymphocyte-depleted simian immunodeficiency virus-infected rhesus monkeys. J. Virol 82: 5618–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, Perelson AS, Marx PA, Ho DD, Kostrikis LG, and Connor RI. 2000. Effects of in vivo CD8+ T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med 191: 1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzner KJ, Moretto WJ, Donahoe SM, Jin X, Gettie A, Montefiori DC, Marx PA, Binley JM, Nixon DF, and Connor RI. 2005. Evaluation of CD8+ T-cell and antibody responses following transient increased viraemia in rhesus macaques infected with live, attenuated simian immunodeficiency virus. J. Gen. Virol 86: 3375–3384. [DOI] [PubMed] [Google Scholar]
- 40.Van Rompay KK, Blackwood EJ, Landucci G, Forthal D, and Marthas ML. 2006. Role of CD8+ cells in controlling replication of non-pathogenic simian immunodeficiency virus SIVmac1A11. Virol. J 3: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suryanarayana K, Wiltrout TA, Vasquez GM, Hirsch VM, and Lifson JD. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retroviruses 14: 183–189. [DOI] [PubMed] [Google Scholar]
- 42.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, et al. 2008. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine 26: 5223–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori DC 2005. Evaluating neutralizing antibodies against HIV, SIV and SHIV in a luciferase reporter gene assay. Curr. Protoc. Immunol 64:12.11.1–12.11.17. [DOI] [PubMed] [Google Scholar]
- 44.Felber BK, Zolotukhin AS, and Pavlakis GN. 2007. Posttranscriptional control of HIV-1 and other retroviruses and its practical applications. Adv. Pharmacol 55: 161–197. [DOI] [PubMed] [Google Scholar]
- 45.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, and Izaurralde E. 1998. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1: 649–659. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein HB, Kinter AL, Jackson R, and Fauci AS. 2004. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res. Hum. Retroviruses 20: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 47.Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, et al. 1998. Natural killer cells from human immunodeficiency virus (HrV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Invest 102: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locher CP, Blackbourn DJ, and Levy JA. 1999. Suppression of human immunodeficiency virus type 1 replication by a soluble factor produced by CD8+ lymphocytes from HIV-2-infected baboons. Immunol. Lett 66: 151–157. [DOI] [PubMed] [Google Scholar]
- 49.Walker CM, Erickson AL, Hsueh FC, and Levy JA. 1991. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J. Virol 65: 5921–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauduin MC, Glickman RL, Means R, and Johnson RP. 1998. Inhibition of simian immunodeficiency virus (SIV) replication by CD8+ T lymphocytes from macaques immunized with live attenuated SIV. J. Virol 72: 6315–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M Jr., et al. 2009. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J. Exp. Med 206: 15751588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neville DM Jr., Scharff J, Hu HZ, Rigaut K, Shiloach J, Slingerland W, and Jonker M. 1996. A new reagent for the induction of T-cell depletion, anti-CD3-CRM9. J. Immunother. Emphasis Tumor Immunol 19: 85–92. [DOI] [PubMed] [Google Scholar]
- 53.Mansfield K, Lang SM, Gauduin MC, Sanford HB, Lifson JD, Johnson RP, and Desrosiers RC. 2008. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J. Virol 82: 4135–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almond N, Corcoran T, Hull R, Walker B, Rose J, Sangster R, Silvera K, Silvera P, Cranage M, Rud E, and Stott EJ. 1997. Mechanisms of protection induced by attenuated simian immunodeficiency virus. IV. Protection against challenge with virus grown in autologous simian cells. J. Med. Primatol 26: 34–43. [DOI] [PubMed] [Google Scholar]
- 55.Langlois AJ, Desrosiers RC, Lewis MG, KewalRamani VN, Littman DR, Zhou JY, Manson K, Wyand MS, Bolognesi DP, and Montefiori DC. 1998. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J. Virol 72: 6950–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, et al. 2010. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 6: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ, and Dandekar S. 2010. In vivo CD8+ T-cell suppression of SIV viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 6: e1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shresta S, Pham CT, Thomas DA, Graubert TA, and Ley TJ. 1998. How do cytotoxic lymphocytes kill their targets? Curr. Opin. Immunol 10: 581–587. [DOI] [PubMed] [Google Scholar]
- 59.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, et al. 2002. Characterization of CD4+ CTLs ex vivo. J. Immunol 168: 5954–5958. [DOI] [PubMed] [Google Scholar]
- 60.Williams NS, and Engelhard VH. 1996. Identification of a population of CD4+ CTL that utilizes a perforin-rather than a Fas ligand-dependent cytotoxic mechanism. J. Immunol 156: 153–159. [PubMed] [Google Scholar]
- 61.Williams NS, and Engelhard VH. 1997. Perforin-dependent cytotoxic activity and lymphokine secretion by CD4+ T cells are regulated by CD8+ T cells. J. Immunol 159: 2091–2099. [PubMed] [Google Scholar]
- 62.Jellison ER, Kim SK, and Welsh RM. 2005. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J. Immunol 174: 614–618. [DOI] [PubMed] [Google Scholar]
- 63.Landais E, Saulquin X, Scotet E, Trautmann L, Peyrat MA, Yates JL, Kwok WW, Bonneville M, and Houssaint E. 2004. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 103: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 64.Stuller KA, and Flafio E. 2009. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J. Virol 83: 4700–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, and Kelleher AD. 2004. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term non-progressor and in CMV infection. Blood 103: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 66.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, and Koup RA. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med 203: 2865–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zahn RC, Rett MD, Li M, Tang H, Korioth-Schmitz B, Balachandran H, White R, Pryputniewicz S, Letvin NL, Kaur A, et al. 2010. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood 115: 3070–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]


