Abstract
The Rhesus macaque (Macaca mulatta) is one of the best studied species of Old World monkeys. DNA sequencing of the entire Rhesus macaque genome, completed in 2007, has demonstrated that humans and macaques share about 93% of their nucleotide sequence. Rhesus macaques have been widely used for medical research including drug testing, neurology, behavioral and cognitive science, reproduction, xenotransplantation and genetics. Because of the Rhesus macaque’s sensitivity to bacteria, parasites and viruses that cause similar disease in humans, these animals represent an excellent model to study infectious diseases. The recent pandemic of HIV and the discovery of SIV, a lentivirus genetically related to HIV Type 1 that causes AIDS in Rhesus macaques, have prompted the development of reagents that can be used to study innate and adaptive immune responses in macaques at the single cell level. This review will focus on the distribution of memory cells in the different immunologic compartments of Rhesus macaques. In addition, the strategies available to manipulate memory cells in Rhesus macaques to understand their trafficking and function will be discussed. Emphasis is placed on studies of memory cells in macaques infected with SIV because many studies are available. Lastly, we highlight the usefulness of the Rhesus macaque model in studies related to the aging of the immune system.
Introduction
The ability to maintain memory after encounter with an antigen is one of the central features of the immune system. The memory T-cell pool functions as a dynamic repository of antigen-experienced T-lymphocytes that accumulate over the lifetime of a host. While naïve T cells even with optimal T-cell receptor (TCR) stimulation and costimulation are largely incapable of immediate synthesis of certain effector cytokines such as interferons (IFNs), memory T cells acquire this capability upon receiving appropriate differentiation signals during the transition of T cell from naive to memory state.1,2
The most advanced discoveries about the induction, development, maintenance and function of the T-cell memory pool have been derived from studies in mice. However, because there are substantial differences in the life span and the immune system of mice and humans,3 studies in non-human primates (NHP) can provide information on the applicability to humans from concepts derived in murine models.
Most NHP used in the United States and European Union for research on HIV Type 1 and 2 are Old World monkeys that diverged from humans approximately 30 million years ago.4 These animals are not inbred and are naturally infected by viral pathogens highly related to human CMV, EBV, HTLVI and II, HHV8 and HPV.
Three macaque species account for 79% of all NHP used in research in the UK and 63% of all federally funded research grants for projects using primates in the US.5 The Rhesus macaque (RM) model is the best model for studying the pathogenesis of SIV and for evaluating vaccines for HIV Type 1. In fact, SIV inoculation in RM causes immunodeficiency disease that is similar to that observed in HIV infected individuals, both from a virological (tropism and kinetic of expansion of the virus, establishment of reservoir) as well an immunological prospective.6–9 Because of these studies, a large body of knowledge has been accumulated on the MHC-I and II diversity in these species. Also, several phenotypic markers that define innate and adaptive immune cells are highly conserved between humans and Old World NHP (see Table 1).10
Table 1.
Commercial antibodies that cross-react with rhesus macaques cells
| Naïve and Memory T Cells | Beckton Dickinson | Beckman Coulter | Invitrogen | Milteny | eBioscence |
|---|---|---|---|---|---|
| CD3 | SP34 | 10D12 | FN18,Cris-7 | ||
| CD4 | Leu-3A, L200 | 13B8 | S3.4 | OKT4 | |
| CD8 | RPA-T8, SKl | B9.11 | 3B5 | 2B5 | OKT8, RPA-T8 |
| 17d8, G42–8 | 143–44 | Bw135/80 | HIT8a | ||
| CD8β | 2ST8.5H7 | ||||
| CD11a | HI111 | 25.3 | HI100 | ||
| CD28 | CD28.2, L293 | CD28.2 | 15 E8 | 15 E8 | CD28.2 |
| CD45RA | 5H9, L48 | 2H4, ALB11 | MEM-56 | T6D11 | |
| CD45RO* | |||||
| CD62L | SK11 | ||||
| CD95 | DX2 | DX2 | DX2 | DX2 | |
| T Regulatory Cells | |||||
| CD25 | M-A251 | 1HT44H3 | 3G10 | 4.00E + 03 | BC96 |
| CTLA4 | BNI3.1 | BNI3 | 14D3 | ||
| FoxP3 | 3G3 | PCH101 | |||
| Cytokines | |||||
| IL-2 | MQ1–17H12 | N7.48A | MQ1–17H12 | N7.48A | MQ1–17H12 |
| IL-17** | |||||
| IFNα | B27, 4SB3 | 4S.B3 | B27, MD1 | 45–15 | |
| TNFβ*** | 359–81–11 | ||||
| Chemokines and Proliferation | |||||
| CCR5**** | 3A9 | ||||
| CCR7 | 3D12 | ||||
| ki67 | B56 | ||||
Dako, clone OPD4.
R & D System KIT.
very weak staining.
R & D clone CTC5.
Memory T Cells in Rhesus Macaques
Phenotypic Characterization of T-Cell Memory Subsets
The memory T-cell population in RM has been phenotypically characterized in the blood lymphoid and mucosal tissues of neonate and adult animals.10 Markers that define human T cells subsets have been studied in macaque’s cells using cross-reactive monoclonal antibodies (mAbs) to human lymphocyte surface antigens in flow cytometric analysis (Table 1).1,2,10–12 This method allows simultaneous multi-parametric analysis of the physical and functional characteristics of single cells and both the enumeration and isolation of sorted purified populations.
The optimal separation of memory T cells from the antigen inexperienced naïve T-cell population can be obtained using a pool of mononuclear antibodies that react with different markers simultaneously expressed on the surface of these cells. One study in particular has shown optimal separation of Rhesus macaque CD4 naïve and memory T cells combining markers for the Fas receptor CD95, the costimulator molecule CD28 and β7 integrin. CD8 memory T cells are also characterized using CD28 and CD95 along with the marker for the lymphocyte function-associated antigen 1, or CD11a, instead of β7 integrin.10 Of note, in RM, the transmembrane tyrosine phosphate known as CD45RA, a common marker for human T cells, is highly expressed by both naive CD4+ and CD8+ T cells. While CD95 along with CD45RA efficiently separates naïve T cells from the rest of the lymphocytes, the remaining population shows great phenotypic heterogeneity, suggesting the presence of different subsets within the memory pool.
Central and Effector Memory T Cells
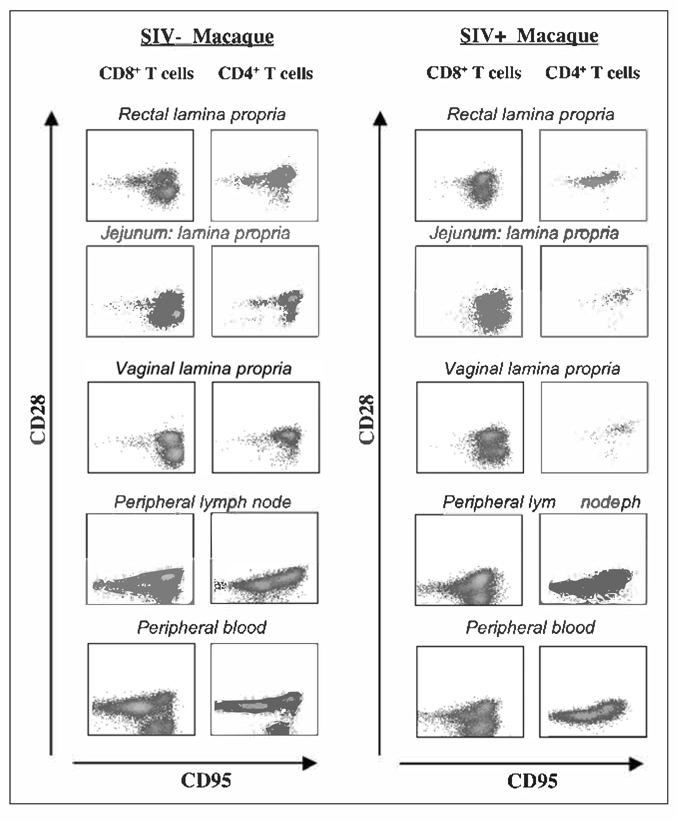
Memory T-lymphocytes contain distinct populations of central memory (TCM) and effector memory (TEM) cells characterized by distinct homing capacity and effector functions.13–15 In humans, TCM express lymph node homing receptors (CD62L and CCR7), whereas TEM are mainly located at the effector sites16 and they express β and β2 integrins, chemokines such as CCR1, CCR3 and CCR5 and homing receptors such as CD103 and CLA.17 In mice and humans, TCM differentiate into effector cells upon secondary stimulation while TEM convert to TCM following antigen clearance.13,18 TCM are the main source of IL-2, a cytokine that induces proliferation of T-lymphocytes, thus displaying greater proliferative potential compared to effector memory T cells. Rhesus macaque’s TCM and TEM have been characterized using mAbs to CD28 costimulatory molecule and CD95 Fas ligand10,19 (Fig. 1). Both the CD4+ and CD8+ TEM lineages express CD95 and low levels of CCR7, a chemokine that controls the migration of memory T cells to the lymph nodes and they also lack CD28 (CD95+/CD28−/CCR7lo). TCM are also CD95+, but they express high levels of both CD28 and CCR7 (CD95+/CD28+/CCR7+). The CD45RA or the CD62L markers, commonly used in humans and in mice to define one or the other subset, have been less extensively used to characterize TCM and TEM in RM. CD45RA marker in combination with CD28 has been used to characterize effector memory (CD28−/CD45RA−) from terminally differentiated effectors (CD28−/CD45RA+) CD8+ T cells, cells that have reached the last stage of their differentiation path, thus lacking proliferative potential and expressing high levels of pro apoptotic markers.20
Figure 1.
Flow cytometric analysis of memory T-cell subsets in healthy and infected macaques. Raw data from one naïve noninfected animal (left panel) and one SIV infected animal (right panel), during the acute phase of infection.
A subset of memory T-lymphocytes that co-expresses CD4 and CD8 has also been identified in RM. This subset expresses high levels of CD4 and low levels of CD8α markers (CD4hi/CD8αlo) and based on analysis of CD28 and CD95 expression, the majority (80%) of CD4hi CD8αlo lymphocytes display an effector memory phenotype (CD28−/CD95+). Only a minor fraction of double positive T cells are central memory. This T-Cell subset is particularly abundant in the intestinal lamina propria where it is capable of producing high levels of cytokines and chemokines and relatively high levels of granzyme B.21,22
T Regulatory Cells and Th17
Heterogeneity is a hallmark of antigen-specific T cells.23–27 Upon antigen stimulation, CD4+ T cells can differentiate into different types of effectors cells: T helper cell 1 and T helper cell 2 (Th1 and Th2) represent well know forms of polarized CD4+ T-cell responses. Th1 produce IL-2, IFN-γ and TNF-α, activating phagocitic cells and CD8+ T cells, thus promoting cell mediated immunity and cytotoxic responses25,26; Th2 cells produce IL-4, IL-5, IL-9 and IL-13, inducing B-cells to produce immunoglobulin IgG1 and IgE. More recently, two different subsets of T cells have been found: under certain stimulatory conditions and depending on the homing tissues, T cells can differentiate in regulatory T cells (Tregs)28–32 or IL-17 producing cells (Th17).
T regulatory cells are subsets of CD4+ and CD8+ T cells that control immune responses maintaining the balance between immunity and tolerance.28,29 Regulatory T cells expressing CD4 have been most extensively studied in mice and humans and more recently in Rhesus macaques.30,31 Tregs are heterogeneous and can be divided into two subsets, naturally occurring, thymic-derived Tregs, that constitute 5–10% of the total peripheral CD4+ T cells in mice and humans31 and adaptive-Tregs.32
Naturally derived Tregs express the interleukin-2 receptor alpha chain CD2533,34 and they constitutively express several other activation markers, such as the glucocorticoid induced tumor necrosis factor (TNF) receptor-related protein (GITR), OX40 (CD134), L-selectin (CD62L) and the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4 or CD152).35,36 However, none of these markers exclusively identifies Tregs as they can also be expressed on activated T-cell subsets. Forkhead box P3 (FOXP3) is an important transcription factor for the development and functionality of Tregs and it is also a more selective intracellular marker to define this population.37–39 Loss of function mutations in FOXP3, both in mice and men, results in the absence of Tregs, leading to a phenotype with severe autoimmune disorders known as scurfy mice and IPEX (immunedysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) in men.40,41 Also, the Type I glycoprotein CD 127, that is the receptor for IL-7, is down-regulated on Tregs.42 This population originates in the thymus upon strong antigenic stimulation that requires different signals, through the engagement of the T cells receptor (TCR) and co stimulation molecules, such as CD28. Once activated, Tregs inhibit proliferation of T cells primarily through contact dependent mechanisms. However, different studies have shown that this subset can also function in a cytokine dependent way, through the production of IL-10 and transforming growth factor-β(TGF-β).43,44
The origin of the ‘adaptive’ Treg population is less clear: some studies have proposed that this subset is generated in the periphery from the pool of CD4+ CD45RO+ CD25− FOXP3− memory T cells; others suggest that it originates in the thymus and then further differentiates as a consequence of exposure to antigens in distinct immunological contexts.32 Adaptive Tregs express variable levels of CD25, depending on the disease setting or the site of regulatory activity45 and they function in vivo in a cytokine dependent manner.46–48 What remains unclear is the antigen specificity of the adaptive Tregs cells49,50
The suppressive role of Tregs has been studied in the context of many autoimmune diseases as well as cancer51–53 and in HIV.54–58 The hypothesis that Tregs may play a role in HIV infection has been tested in the SIV non-human primate model of AIDS. Tregs can be characterized with the same markers used in humans. Ex vivo studies have shown that depletion of CD25+ cells from human PBMC and monkey’s peripheral lymph nodes leads to a significant enhancement of CD4+ and CD8+ T-cell responses to select pools of HIV and SIV peptides.55,59 These data indicate that CD25+ Tregs exert similar functions in humans and Rhesus macaques and suggest their role in decreasing HIV and SIV specific immune responses. In addition, longitudinal studies on SIV-infected Rhesus macaques have revealed a transient increase in the frequency of Tregs from baseline values following acute infection. Also, during chronic infection T regulatory cells accumulate in tissues of infected macaques, especially in the spleen and in the gut, while the frequency of this population decreases in periphery.56,58 The accumulation of T regulatory cells at these sites has been correlated with disease progression.56,58
More recently, another subset of CD4+ T cells has been identified. These activated CD4+ T cells reside mostly in the gut and at the mucosal sites, where they are able to produce IL-17, a cytokine important in the host defense against extracellular bacteria (Th17).60–66 While the induction and function of CD4 T helper Type 1 and 2 are clear, the full spectrum of function of this subset has not been defined. Together with IL-17, Th17 cells produce IL-22, a cytokine that induces production of antibacterial defensins.63 In Rhesus macaques, this subset is mainly present in the lamina propria of the colon, the jejunum, ileum and the rectum, and less represented in blood, lymph nodes and spleen.64 Th17 can be identified upon in vitro stimulation with CD3 or phorbol myristate acetate (PMA) and ionomicin.
When stimulated in vitro, Th17 cells express CCR5 and CD95 and can be positive or negative for CD27. The frequency of CD4-producing IL-17 significantly declines in the gastrointestinal (GI) tract during the early phase of infection with HIV/SIV. This loss may explain the chronic enteropathy in HIV infection.64–66
Tissue Distribution of T-Cell Memory Subsets in Rhesus Macaques
Many of the cellular and molecular processes involved in forming and maintaining immunological memory are still unknown. Studies in mice have elucidated the distribution of memory T cells in different tissues. In vitro and in vivo imaging in mice have demonstrated that antigen-stimulated memory T cells migrate from the lymphoid tissues to nonlymphoid tissues where they form the first line of defense against re-encountered pathogens.67,68 Memory T cells that remain in the lymphoid tissues constitute a reservoir that can be mobilized again when necessary. The physiological distribution of memory T cells in humans is less known, due to the difficulty of sampling healthy individuals. Therefore, the Rhesus macaque model serves as a valuable tool to study the immunobiology of different lymphoid and nonlymphoid compartments in a model that closely relates to humans.
In macaques, naïve and effector/memory T cells express different trafficking ligands and receptors and consequently have distinct patterns of migration.69,70 Memory T cells are localized in lymphoid tissues and have the ability to traffic to various extra lymphoid tissues of the body, also called effector sites.10 The anatomic location plays an inductive role in the CD8+ T cells memory differentiation program. In fact, it has been shown in mice, that virus-specific intraepithelial lymphocytes in gut resemble neither central nor effector memory CD8+ T cells isolated from spleen or blood, suggesting that memory CD8+ T cells may change phenotype upon changing location.71,72
Peripheral Blood and Lymph Nodes
Naïve and memory T cells coexist in the peripheral blood of Rhesus macaques, as well as in humans. The CD4 memory population in the periphery is mainly CD28 positive (TCM), while both central (CD28+/CD95+) and effector memory (CD28−/CD95+) CD8+ T cells coexist in this compartment (Fig. 1).10
The development of T-cell immune responses starts with delivery of an antigen (Ag) from an exposed tissue site to the draining lymph node. Naive T cells that constantly recirculate from the blood to the lymph nodes are activated and differentiate into effector and memory T cells. Following differentiation T cells express new cell surface molecules that allow them to home to nonlymphoid tissues.73–75 These activated T cells express effector cytokines and are unable to return to the draining lymph nodes or to the pool of circulating lymphocytes.76 Memory CD8+ T cells present in the lymph nodes are important as a first line of defense to pathogenesis as they curb the spread of pathogens from the lymph node to vital organs at very early stages of infection.77 Peripheral lymph nodes of RM contain both CD4+ and CD8+ T cells and the main subsets are naïve and central memory T cells (CD28+/CD95+)(Fig. 1).10
The Gastrointestinal Tract
The gastrointestinal tract is a prominent part of the immune system and is enriched with memory T cells that predominate in the intestinal lamina propria (lamina propria lymphocytes, LPL) and in the epithelium (intraepithelial lymphocytes, IEL).78 The gut associated lymphoid tissues (GALT) of RM has been extensively studied in the context of SIV infection and during disease progression to simian AIDS. In fact, the GALT is the primary site of replication for HIV/SIV. The remarkable similarity in the composition of the GALT between humans and Rhesus macaques has justified the use of these animals as model for human AIDS.79–83
The intra-epithelial lymphocytes (IELs) in the gut of RM are predominantly CD8+ (63–80%) and contain very few CD4+ T cells. CD8+ T cells present a memory phenotype and express the αEβ7, an integrin that mediate T-cell adhesion to epithelial cells through its binding to E-cadherin.79,84,85 Lamina propria lymphocytes (LPLs) are a mixed population of CD4+ and CD8+ T-lymphocytes, with a CD4:CD8 ratio that range from 0.74 to 1.3. Memory phenotypes are present at this site (Fig. 1).79 Both CD8+ and CD4+ T cells express low levels of αEβ7, are mainly positive for the CD95 marker and the CD8 memory population expresses beta7 integrin. The memory pool contains CD4+ and CD8+ TCM and TEM subsets, as indicated by the presence of positive and negative CD28 and CD95 positive T-lymphocytes (Fig. 1).10
In SIV infected macaques, as early as a few weeks from infection with a CCR5 tropic viral strain of SIV, CD4+ T cells in the LP decrease by 50–70% compared to uninfected controls (Fig. 1). The main population that is targeted and killed at this site is activated CD4+ T cells expressing the homing marker CCR5+, which are numerous in the LP but scarce in the periphery.78–82 A near normal level of CD4+ T cells is maintained in lymph nodes and blood.81 The CCR5 coreceptor is selectively expressed at effector site on effector memory T cells that are negative for CCR7.86,87 All CD4+ memory T cells are not equally susceptible to this acute destruction. In SIV infected RMs, the remaining CD4+ T-cell population is predominantly CCR5− TCM cells. This population undergoes a substantial increase in proliferative activity that initially provides sufficient production of TEM cell in the effector sites to maintain clinical immune competence.88 During the chronic phase of infection the frequency of CD4+ TEM cell decreases in the extra-lymphoid immune effector sites of the body, both in humans and RM.89–90
The Lung
Similar to the gut, the RM’s lamina propria of the bronchoalveolar compartment is composed of memory T cells that express CD95, whereas the frequency of T-lymphocytes that express β7 integrin is lower than in the gut. The central memory pool is the main CD4 subset present in the lung, whereas both TCM and TEM CD8 are represented in this compartment.10
The Vaginal Mucosa
The vaginal mucosa of normal juvenile and adult female Rhesus macaques have been examined by flow cytometric analysis and multicolor immunohistochemistry, a process that visualizes antibody-antigen interaction on tissue sections.91–95 The objective of these studies was to characterize the vaginal mucosa as a primary site for HIV transmission and the role of mucosal immune responses in the vagina and cervix in protection from the virus. Lymphocytes of the vaginal mucosa are localized within the epithelial layer and in the lamina propria. Vaginal lamina propria of macaques contains 55 to 65% CD8+ T cells and 28 to 34% CD4+ T cells, while the majority of intra-epithelial cells are CD8+ T cells (75 to 85%).91 54–67% of the CD4+ T cells in the vaginal mucosa express the activation marker CCR5.92 This population resides in the lamina propria, whereas essentially no CD4 or CCR5 expression can be detected within the squamous or keratinized layers of the vaginal epithelium. CCR5 expression is higher in the vaginal lamina propria of mature macaques compared to 1–3-year-old juveniles. The vast majority of CD4+CCR5+ lymphocytes in the vagina also express CD95 and CD28 (CD95+/CD28+) showing a central memory phenotype (Fig. 1). In addition, cytolytic CD8+ T-cell lines derived from the vaginal epithelium are αEβ7 positive and L-selectin negative.93,94 The vaginal lamina propria of SIV infected female macaques is depleted of almost 40–60% of CCR5+CD4+ T cells during the early phase of infection.94,95
In Vivo Manipulation of Memory T Cells in Non-Human Primates
Autologous Transfer
T-lymphocyte migratory circuits in humans remain largely unexplored due to the difficulty of performing cell trafficking in normal volunteers. Recently, NHP have been used as a model to study how T-lymphocytes migrate to different compartments. Experiments of autologous transfer of labeled peripheral blood mononuclear cells (PBMCs) have been performed in RM to study the homing of T-lymphocytes to lymphoid and nonlymphoid compartments. This method uses the carboxyfluorescein diacetate succinimidyl ester (CFSE), a fluorescent cell staining dye that is retained by the cell in the cytoplasm, which does not adversely affect cellular functions and can be detected by flow cytometric analysis.96 Using this technique, Clay and colleagues have shown that, within 48 hours of intravenous transfer of CFSE labeled PBMCs, T-lymphocyte trafficking can be detected to the liver and bone marrow and at a lower level to the thymus and small intestine. The liver contains the highest proportion of stained CD45RA− T-lymphocytes, consistent with homing of activated/memory T-lymphocytes to this nonlymphoid site.97,98
Another recent study established tracking of T cells to various compartments of RM as a preclinical model for the evaluation of T-cell-based immunotherapy.99 In this study, harvested PBMC were either unstimulated or stimulated with antiCD3/antiCD28, then labeled with CFSE and reinjected intravenously into the donor animals. Blood samples, lymph node biopsies and biopsies from duodenum and rectum were collected at various time points and analyzed by flow cytometric analysis for the presence of the reinjected T cells. The authors showed that non-specific in vitro activation changes the in vivo migratory behavior of T cells. In fact, they observed a preferential migration of activated CD8+ T cells to the rectum, while non-specifically activated transferred CD4+ T cells were found in much lower frequencies at this site and also in other compartments.
In Vivo Studies of T-Cell Turnover
The rates of lymphocyte turnover during health and disease are poorly characterized. This limits the understanding of diseases like HIV infection100,101 that lead to increased rates of cellular turnover and ultimately to deterioration of the immune system. Since the NHP is the primary model for HIV infection of humans, techniques have been developed to study T-cell turnover and loss, in healthy and SIV infected macaques. Proliferative activity of T-lymphocytes can be monitored in ex vivo experiments by flow cytometric analysis through the evaluation of Ki67 antigen, a marker of the cell cycle progression.10 A more advanced technique uses the administration of 5-bromo-2′ -deoxyuridine (BrdU), a thymidine analog measuring the rate at which cells become labeled with this DNA precursor during the S phase of the cell cycle. BrdU can be administered to the animals via drinking water. Cells that contain BrdU are then detected ex vivo using fluorochrome-labeled antiBrdU monoclonal antibody by flow cytometric analysis.102 Various mathematical models have been developed that permit estimates of different parameters from the data generated by this type of experiment.103,104 One of these models has been used to analyze BrdU labeling curves for a number of different lymphocyte populations in healthy and SIV infected Rhesus macaques.104 This method has been used by Luka Čičin-Šain and colleagues to test whether naïve T-cell turnover is increased in aged monkeys. In this study, cohorts of adult and old monkeys were pulsed with BrdU on days O and 3 and assayed for its incorporation/decay kinetic in blood T cells over time. This technique allows the concomitant detection of BrdU positive cells that proliferated at any time during the days of BrdU administration, of actively proliferating cells (Ki67+/BrdU+) and of cells that ceased proliferating by the time of detection (Ki67−/BrdU+).105
Kaur and colleagues have studied the perturbations in lymphocyte dynamics in sooty mangabeys, the natural hosts of nonpathogenic simian immunodeficiency virus (SIV) infection during in vivo administration of BrdU.106 Using the same technique, Picker and colleagues demonstrated that IL-15 dramatically increases in vivo proliferation of RM CD4+ and CD8+ TEM cells, with little effect on the naive or TCM subsets.107
Thymectomy
T-cell maturation partially occurs in the thymus, where lymphocyte precursors from the bone-marrow become thymocytes and mature into T cells. Once mature, T cells emigrate from the thymus and constitute the peripheral T-cell repertoire responsible for directing many facets of the adaptive immune system. Loss of the thymus at an early age through genetic mutation or surgical removal results in severe immunodeficiency and a high susceptibility to infection. To study extra thymic T maturation, a method of thymectomy was developed in macaques by Healy and colleagues.108 The role of the thymus in the pathogenesis of AIDS is a frequently discussed and controversial topic. Therefore, this method has been used to study the role of the thymus in peripheral T-cell homeostasis and to assess the significance of thymic output in SIV infection of RM. By surgical removal of the thymus in juvenile Rhesus macaques, the authors reported that complete abrogation of thymic output in juvenile Rhesus macaques resulted in a faster decay of peripheral CD4+ T cells, but did not cause a substantial shift in CD45RA+ and CD45RA− populations. In conclusion, thymectomy had very little impact on the peripheral T-cell compartment, both in healthy and in SIV-infected macaques.109,110
In Vivo Depletion of T-Cells Subsets
It is difficult to perform studies that assess the role of cell-mediated immune responses during viral infections in humans. Indirect correlations between the frequency of antigen specific CD4+ and CD8+ T cells and virus levels are informative, but they do not directly prove the importance of these responses during the course of infections. Therefore, animal models that permit passive administration of immunoglobulin to naïve hosts have been crucial for demonstrating the contribution of specific components of the immune system in controlling certain infections. Non-human primates provide valuable animal models for human diseases. A Rhesus monkey model of CD8+ cells depletion using a mouse-human chimeric monoclonal antibody has been developed by Schmitz and colleagues111 (Table 2). Administration by the intravenous route of this antibody results in nearly total depletion of CD8+ lymphocytes from the blood and lymph nodes for 2–6 weeks, leaving CD4 cell-mediated immune responses and humoral immune responses intact. In vivo CD8 depletion in RM has been used to study the importance of this population during SIV and other infections. Rhesus monkeys were depleted of CD8+ lymphocytes by monoclonal anti-CD8 antibody infusion and then challenged with wild-type measles virus.112 The CD8+ lymphocyte-depleted animals exhibited a more extensive rash, higher viral loads at the peak of virus replication and a longer duration of viremia than did the control antibody-treated animals, suggesting a central role for CD8+ lymphocytes in the control of measles virus infections. A CD4+ T-cell depleting antibody has first been used in chimpanzee infected with Hepatitis C to demonstrate that memory CD4+ T cells are essential for protection. Indeed, CD4+ T-cell depletion in chimpanzees before re infection impaired the ability of these animals to clear virus despite the presence of functional intra-heapatic CD8+ T cells.113
Table 2.
Antibodies for in vivo depletion assays
| AntiAb | Clone/Commercial Name | Detection mAb | Number of Doses | mg/kg | Route | Ref. |
| aCD8 | cM-T807 | DK25 | 4 doses | 1 × 10, 3 × 5 | s.c./i.v. | 116 |
| DK25/SK1 | 3 doses | 5 | Intravenous | 111/112 | ||
| DK25 | 3 doses | 1 × 10, 2 × 5 | s.c/i.v | 114/117 | ||
| T87PT3F9* | 2/4 doses | 2 | Intravenous | 115 | ||
| aCD4 | OKT4 | L200 | 1 dose | 50 | Intravenous | 114/117 |
| aCD20 | Rituxan** | J4.119 (CD19) | 4 doses/every wk | 20 | Intravenous | 114 |
| aCD16 | 3G8 | DJ130C | 1 dose | 50 | Intravenous | 119, 120 |
|
Antibodies for in vivo blocking assays | ||||||
| AntiAb | Clone/Commercial Name | Detection Ab | Number of Doses | mg/kg | Route | Ref. |
| aCD40L* | 1 dose | 20 | Intravenous | 131 | ||
| CTLA4-Ig* | 1 dose | 20 | Intravenous | 131 | ||
| aCTLA4 | MDX-10** | 2 dose | 10 | Intravenous | 55 | |
| 4 dose | 10 | Intravenous | 133 | |||
s.c. subcutaneus. i.v. intravenous.
Coulter.
Genentech and IDEC Pharmaceuticals.
Baxter Healthcare Corp., Deerfield, Illinois, USA.
Medarex, Inc.
The role of vaccine-induced CD8+, CD4+ T and B cells in protection from monkey pox virus, a virus ortholog of smallpox, has been dissected in Rhesus macaques.114 Neither CD4+ nor CD8+ T-cell depletion in Dryvax vaccinated macaques affected vaccine induced protection from the disease. In contrast, antibody-mediated depletion of B cells during immunization abrogated protection and importantly, passive transfer of immunoglobulines from vaccinated individuals restored protection, indicating that protection from smallpox is antibody-mediated.114
Different groups have shown that in vivo depletion of CD8+ T cells during SIV infection of RMs results in marked increases in plasma viral load, suggestive of a key role for CD8+ T cells in controlling levels of SIV replication.115–117 The main limitation of this model is the fact that the antibody used for depletion is not specifically targeting only adaptive CD8+ T-lymphocytes because a high frequency of natural killers (NK) of RMs also express CD8.118 This leaves an open question about the role of innate responses in the control of SIV replication. Two recent studies have partially ruled out the importance of cytotoxic CD16+ NK cells in controlling AIDS virus replication during primary and chronic infection.119,120 In fact, transient depletion of NK cells from two Rhesus monkeys chronically infected with simian immunodeficiency virus failed to induce changes in virus replication.
A large body of literature in murine models indicates that CD4 help is not required for a generation of specific CD8+ T cells responses, but is essential to maintain a pool of memory CD8 able to expand after a second encounter with an antigen.121–123 In vivo CD4 depletion has also been used in macaques as a mean to investigate the importance of helper T cells on the generation and maintenance of SIV specific CD8+ T cells.117 Depletion of CD4 cells was performed during immunization to decrease the functionality of CD8+ T cells. Treatment with the CD4-depleting antibody resulted in the complete absence of CD4+ T cells from the blood, leaving the frequency of CD8+ T cells and CD20 population intact. The reconstitution of the CD4 population was slow and incomplete, as was previously observed in humans.124 Vaccinated macaques treated with the CD4-depleting antibody developed less functional CD8+ T cells, resulting in lost control of SIV replication earlier than vaccinated macaque controls.117 (see Table 2 for details on treatment with depleting antibodies).
Blocking Antibodies In Vivo
The generation of adaptive immune responses is a highly regulated process that requires the interaction between antigen presenting cells and CD4+ T cells via the major histocompatibility complex (MHC) class 2 and the T-cell receptor (TCR) and also involves numerous costimulatory pathways, aimed to control the balance between immune stimulation and tolerance. One of those pathways involves the binding between the CD40, a protein, expressed by all mature B cells, as well as by dendritic cells, macrophages, fibroblasts, epithelial cells and endothelial cells125,126 and CD40 ligand (CD40L; also known as CD154), expressed by activated T and B cells and activated platelets. The interaction between CD40 and CD40L promotes both humoral and cell-mediated immune responses and is crucial for the induction of effective adaptive immune and inflammatory responses.127 Also, the murine CD40L-CD40 interaction between CD4 T helpers and dendritic cells or CD8+ T cells augments the generation of CD8 memory T cells following viral infections.128
A second pathway involves the binding of the CTLA4 (Cytotoxic T Lymphocyte Antigen 4), a CD28-family receptor constitutively expressed on regulatory CD4+ T cells, to CD80 and CD86 costimulatory molecule expressed on B cells and dendritic cells. CD28 also binds to the CD80 and the CD86, but CTLA4 has higher affinity than the CD28 and in contrast to CD28 which enhances T-cell function, CTLA4 inhibits T-cell activation.129
In mice, the in vivo administration of blocking antibodies to CD40 and to CD28 results in potent and specific immune suppression.130 In Rhesus macaques, administration of CTLA4 immunoglobuline (Ig) and anti CD40 Ligand prevent renal allograft rejection.131
The NHP model has also been used to assess the role of these pathways in the generation of SIV-specific CD4 helper, CD8+ T cells and antibodies.55,132 Garber and colleagues induced the in vivo blockade of CD28 and CD40 T-cell costimulation pathways with the aim to experimentally induce tolerance to SIV antigens in infected Rhesus macaques. Transient administration of CTLA4-Ig and anti-CD40L mAb to SIV-infected macaques resulted in dramatic inhibition of the generation of both SIV-specific cellular and humoral immune responses.132
The impact of immune activation in SIV infection has been addressed directly by inhibiting CTLA4 during the acute and chronic phase of infection.55,133 In vivo CTLA4 blockade significantly increased T-cell activation and viral replication in primary SIV infection, particularly at mucosal sites and increased the expression and activity of the indoleamine 2,3- dioxygenase (IDO), an enzyme that converts tryptophan to N-formyl-kynurenine which suppresses T-cell proliferation.134 Accordingly, protracted anti-CTLA4 treatment of macaques chronically infected with SIV and treated with ART, decreased responsiveness to antiretroviral therapy and abrogated the ability of therapeutic T-cell vaccines to decrease viral replication (see Table 2 for details on treatment with blocking antibodies).
Differentiation of Memory T-Cells Subsets: Lesson from In Vivo Studies in Non-Human Primates
Studies in humans have implicated a family of cytokines, such as IL-2, IL-7 and IL-15 that use the common y chain as part of their receptor, as important regulators of peripheral T-cell homeostasis.135–137 Interleukin-2 (IL-2) exerts a wide spectrum of effects on the immune system and plays crucial roles in regulating both immune activation and homeostasis. IL-2 was identified based on its potent T-cell growth-factor activity and is widely considered to be a key cytokine in T-cell-dependent immune responses both for CD4+ and CD8+ T cells. However, a major nonredundant activity of this cytokine centers on the regulation of T-cell tolerance in the periphery, whereas T-cell immunity to various agents can be readily elicited in the absence of IL-2 in vivo.138,139
IL-2 has been evaluated as a therapeutic in the clinical settings of HIV/SIV infection and cancer.140–143 In vivo administration of IL-2 to Rhesus macaques enhances antigen specific responses. The effects of the administration of IL-2 have been studied as an adjuvant in vaccine strategy for SIV. IL-2 combined with antiretroviral therapy and poxvirus vector based vaccines improves CD4 and CD8 T cells responses and decreases plasma viral load upon ART cessation.141,142 IL-2 administration ameliorates DNA- based vaccines’ efficacy, improving the quantity and the quality of the antigen specific immune responses compared to DNA- based vaccines alone.142 Villinger and colleagues have shown that following primary immunization to tetanus toxoid (TT) or influenza virus, TT specific CD4+ T cells and influenza matrix protein (Flu-MP) specific CD8 effector responses are enhanced by IL-2 administration, but CD8 specific memory responses are not different from cytokine nontreated monkeys.143 In that study, the highest levels of primary effector and memory T cells were observed following alternate administration of both IL-2 and IL-15.
Interleukin-7 (IL-7) is a nonredundant cytokine produced by nonlymphoid cells that is essential for T-cell development in humans and mice and B-cell development in mice144,145 promoting expansion of both thymic and peripheral T-cell populations, the latter including both the CD4+ and CD8+ lineages and both the naive and memory compartments.146,147 IL-7 contributes to the maintenance of the size and subset composition of the peripheral T-cell pool by providing growth and survival signals through the IL-7 receptor.148–150 IL-7 modulates memory CD8+ T cells in response to a virus infection.151
Administration of recombinant human IL-7 (rhIL-7) and IL-15 to NHP has, in part, elucidated the immunologic effects of these cytokines, Following IL-7 therapy, an increase in the absolute number of naive CD4+ and CD8+ T cells has been observed.137,152 Upon treatment peripheral T cells up regulate proliferation markers such as Ki67 and BcL2, a survival marker.153 IL-7 treatment in macaques also induces a transient change in CD 11 a expression on CD8+ cells with the emergence of a dominant population of CD 11amod cells, suggesting a partial conversion of the naive subset to an activated/memory phenotype. Thus, IL-7 treatment alters peripheral T-cell homeostasis and results in a substantial, but reversible, increase of peripheral blood T-cell number due to faster entry of these cells into cell cycle.137 Another study from Moniuszko et al, dissected the effect of IL-7 therapy on different memory T cells subsets of Rhesus macaques.152 IL-7 induced the acquisition of memory cell markers not only in CD8+ T cells but also in CD4+ T-cell subsets that express both CD28 and CD95 markers and are positive for the proliferation marker Ki67, a protein expressed during all phases of the cell cycle (G1, S, G2 and mitosis), but not in resting cells ( GO). Thus, IL-7 increases the frequency of T cells that phenotypically resemble CD4+ TCM The increase of this memory-like population was dose dependent and occurred in blood as well as secondary lymphoid organs. In addiction, IL-7 increased the ability of CD4+ TCM as well as CD4+ TEM to produce tumor necrosis factor alpha (TNF-α) and to a lesser extent, gamma interferon (IFN-γ) following stimulation with cognate antigen.
Administration of recombinant IL-15 demonstrated that this cytokine play a role in CD4+ TEM cell development and homeostasis in primates.107,154 IL-15 has proven to be superior to IL-2 in the generation of long-lived antigen specific memory CD4+ and CD8+ T cells in Rhesus macaques.154 Moreover, IL-15 increases the flux of long-lived CD4+ T cells into extra-lymphoid effector sites. The effect of this cytokine on the CD8+ T-cell population is similar to what has been observed for the CD4 T cells: IL-15 potently induces proliferation of CD8+ TEM cells, with little effect on CD8+ TCM cells.107 Thus, IL-15, in contrast to both IL-2 and IL-7, selectively expands the CD4+ and CD8+ TEM cell in the extralymphoid tissues.
Several studies have assessed the impact of IL-7 and IL-15 treatment on viral replication alone,155,156 or in conjunction with vaccines in SIV-infected macaques.157,158 Beq and colleagues investigated the impact of recombinant simian IL-7 on T-cell renewal in Rhesus macaques chronically infected with SIVmac251 and treated with antiretroviral therapy (ART). This treatment resulted in an increase of the number of circulating CD4+ and CD8+ memory T cells expressing activation and proliferation markers and enhanced thymic function, with no effects on the plasma viral load.155 Mueller and colleagues have shown that in vivo treatment of acutely SIV infected Rhesus macaques with IL-15 resulted in an increased number of SIV-specific CD8 T cells and NK cells during the peak of viral load. Interestingly, this increase was not maintained during the set point of viremia; on the contrary, at this time animals that had received IL-15 showed an increased viral set point by 3 logs and accelerated development of simian AIDS.156
Demberg et al have treated Rhesus macaques with SIV plasmid DNA with or without IL-15 DNA, a multigenic replicating Adenovirus based SIV immunization and two boosts with SIV gpl40 and SIV Nef protein.157 Macaques that were treated with the IL-15 DNA showed a higher peak of anti-Nef antibody titer and expanded SIV-specific CD8+ T cells 2 weeks after the challenge with SIVmac251, compared to the DNA-only group. Although, IL-15 treated macaques did not exhibit lower viral replication and better protection from disease. Finally, our group assessed the impact of recombinant IL-7 and IL-15 treatment on viral replication and the immunogenicity of live poxvirus vaccines in SIVmac251 infected macaques.158 Neither cytokine augmented the frequency of vaccine-expanded CD4+ or CD8+ memory T cells, clonal recruitment to the SIV-specific CD8+ T-cell pool, or CD8+ T-cell function. Moreover, while vaccination alone transiently decreased the viral set point following antiretroviral therapy suspension, IL-15 induced massive proliferation of CD4+ effector T cells and abrogated the ability of vaccination to decrease set point viremia. In contrast, IL-7 neither augmented nor decreased the vaccine effect and was associated with a decrease in TGF-β expression.
Aging of T Memory Cells
During aging, the immune system undergoes dramatic changes in both structure and function. Macroscopically, the most evident event is thymic involution, which severly diminishes the production of naïve T cells.159 Consequently, peripheral lymphocyte subset composition is shifted toward the memory phenotype, as an increasing proportion of naïve T cells become exposed to foreign antigens over time. However, the mechanisms linking specific age-related changes in T-cell subset distribution and function to the age-related immunodeficiency are still incompletely understood. Most of the existing data describing the age-related changes in T-cell function come from studies of the rodent’s immune system. 160,161 However, even though this model has been helpful in elucidating some aspect of immune senescence, not all results from rodent models translate to humans, given that these two species diverged approximately 210 million years ago162,163 and they have a different lifespan (10 folds). Moreover, rodents are held in virtually pathogen-free condition during these studies, thus affecting the number of antigens encountered during their existence and therefore affecting the pool of memory T cells generated and accumulated during life. Non-human primates are better suited for immunogerontologic studies, with direct relevance for human T-cell senescence.164,165
With respect to their lifespan, macaques are classified as neonates (first month), juveniles (1–5 years), adult (5–15 years) and old (15–25 years). The memory population in neonates is quite low, but in keeping with the expected result of postnatal Ag exposure, rapidly increases in the first few months of life. Cross-sectional analyses indicate that the average frequency of memory T cells in adult human blood (40–50%) is reached within the first 2–3 years of life in RM.166,167 After this time, the rise in memory frequencies slows and by middle adulthood (10–15 years), memory frequencies average 70% for CD4+ T cells and 80–90% for CD8+ T cells. These results suggest that RM, especially juvenile animals, may be exposed to more diverse pathogens more frequently than humans.10
In vivo and ex vivo studies on cohorts of old RM model have been used as a tool to study the other mechanisms involved in the depletion of naïve T-lymphocytes and in parallel, the accumulation of memory T cells. Through the in vivo administration of BrdU and concomitant analysis of Ki67, a study has shown that in RM, naïve CD8+ and, to a lesser extent, naive CD4+ T cells in old animals exhibit higher proliferation and higher turnover than in young animals.105 Because the relative size of the naïve subset was also decreased, the authors suggest that elevated turnover comes with a naïve T-cell loss that is equal to or surpasses the elevated proliferation. Also, the authors observed a significant increase in CD4+ TCM cells and CD8+ TEM cells in an aged RM cohort with small naive CD8 pools. Cross-age comparisons revealed no age-related differences in proliferation and turnover of the TEM subsets and an age-related decline in proliferation of TCM CD4 and CD8.
Another study has focused on the effector function of RM CD8+ and CD4+ T cells during senescence.168 The authors demonstrated that the percentage of cells capable of immediate TNF-α secretion upon T-cell receptor stimulation increases with age among RMs CD8+ T cells, but not among CD4+ T cells. Also, in this study, age-related loss of CD95− naive cells in RMs did not differ between CD4+ and CD8+ T cells. Therefore, at least among the RMs CD8+ T cells, functional changes within the CD8 memory population appeared to correlate with the aging process better than acquisition of CD95+ CD28− phenotype.
Conclusion
Humans are much indebted to non-human primates. Their use in research has contributed greatly to scientific discoveries that have improved human health worldwide. Studies in NHPs have made possible the development of protective vaccines against important human pathogens, including Poliovirus and Hepatitis and have been essential in developing a plethora of drugs for treatment of human diseases, such as cancer and AIDS. The genetic and immunological similaries between NHP and humans permit the validation of the relevance of some concepts derived from studies in mice to humans. This is particularly important in studies of the immune system. Hopefully, the use of NHP will also facilitate the full understanding of HIV pathogenesis and guide the development of much needed protective vaccines not only for HIV but also for malaria and tuberculosis.
Acknowledgements
The authors thank Gene Shearer and Shari N. Gordon for critical reading and thank Anna Weissman and Teresa Habina for editing.
The authors acknowledge the support of the CCR, National Cancer Institute, NIH.
References
- 1.Picker LJ, Siegelman MH. Lymphoid organs and tissues In: Paul WE, ed. Fundamental Immunology, 4th ed. Philadelphia: Lippincott-Raven, 1999:14, 479. [Google Scholar]
- 2.Picker LJ, Terstappen LW, Rott LS et al. Differential expression of homing-associated adhesion molecules by T-cell subsets in man. J Immunol 1990; 145:3247–3255. [PubMed] [Google Scholar]
- 3.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol 2004; 172:2731–2738. [DOI] [PubMed] [Google Scholar]
- 4.ACLAM [American College of Laboratory Animal Medicine]. Public statement: medical records for animals used in research, teaching and testing. ILAR journal 2007; 48(1). Public statement. [DOI] [PubMed] [Google Scholar]
- 5.Conlee KM, Hoffeld EH, Stephens ML. A demographic analysis of primate research in the United States. ATLA (Alternatives to Laboratory Animals) 2004; 32(1):315–322. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol 1990; 8:557–578. [DOI] [PubMed] [Google Scholar]
- 7.Pilcher CD, Wohl DA, Hicks CB. Diagnosing primary HIV infection. Ann Int Med 2002; 136(6):488–489. [DOI] [PubMed] [Google Scholar]
- 8.Pal R, Venzon D, Letvin NL et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*0l) delay simian immunodeficiency virus SIV(mac)-induced immunodeficiency. J Virol 2001; 76(1):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker RA, Regan MM, Reimann KA. Variability of viral load in plasma of Rhesus monkeys inoculated with simian immunodeficiency virus or simian/human immunodeficiency virus: implications for using non-human primate AIDS models to test vaccines and therapeutics. J Virol 2001; 75(22):11234–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitcher CJ, Hagen SI, Walker JM et al. Development and homeostasis of T-cell memory in Rhesus macaque. J Immunol 2002; 168:29–43. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T-cells by phenotype, function and T-cell receptor diversity. Nat Med 2001; 7:245–248. [DOI] [PubMed] [Google Scholar]
- 12.Walker JM, Maecker HT, Maino VC et al. Multicolor flow cytometric analysis in SIV-infected Rhesus macaque. Methods Cell Biol 2004; 75:535–557. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R et al. Two subsets of memory T-lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401(6754):708–712. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T-cell subsets: function, generation and maintenance. Annual Review of Immunology 2004; 22:745–763. [DOI] [PubMed] [Google Scholar]
- 15.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T-cell subsets. Curr Opin Immunol 2005; 17(3):326–332. [DOI] [PubMed] [Google Scholar]
- 16.Masopust D, Vezys V, Marzo AL et al. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001; 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 17.Baron JL, Madri JA, Ruddle NH et al. Surface expression of α4 integrin by CD4 T-cells is required for their entry into brain parenchyma. J Exp Med 1993; 177:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wherry EJ, Teichgräber V, Becker TC et al. Lineage relationship and protective immunity of memory CD8 T-cell subsets. Nat Immunol 2003; 4(3):225–234. [DOI] [PubMed] [Google Scholar]
- 19.Vaccari M, Trindade CJ, Venzon D et al. Vaccine-induced CD8+ central memory T-cells in protection from simian AIDS. J Immunol 2005; 175:3502–3507. [DOI] [PubMed] [Google Scholar]
- 20.Kenneth SC, Kaur A. Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T-lymphocytes in rhesus macaques. J Immunol Methods 2007; 325(1–2):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macchia I, Gauduin MC, Kaur A et al. Expression of CD8 alpha identifies a distinct subset of effector memory CD4+ T-lymphocytes. Immunology 2006; 119(2):232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T-cells are highly activated memory cells with an increased capacity to produce cytokines. Eu J Immunol 2006; 36(3):583–592. [DOI] [PubMed] [Google Scholar]
- 23.Reiner ST, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T-cell fate. Science 2007; 317(5838):622–625. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KM, Reiner SL. The lineage decisions of helper T-cells. Nat Rev Immunol 2002; 2(12):933–44. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Powrie F. Emerging challenges in regulatory T-cell function and biology. Science 2007; 317(5838):627–629. [DOI] [PubMed] [Google Scholar]
- 26.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T-lymphocytes. Nature 1996;383:787–793. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S Lymphokine production by human T-cells in disease states. Annu Rev Immunol 1994; 12:227–257. [DOI] [PubMed] [Google Scholar]
- 28.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T-cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001; 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S Regulatory T-cells: key controllers of immunologic self-tolerance. Cell 2000; 101(5):455–458. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S Naturally arising Foxp3-expressing CD25+CD4+ regulatory T-cells in immunological tolerance to self and non-self. Nat Immunol 2005; 6(4):345–352. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S Naturally arising CD4+ regulatory T-cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22:531–562. [DOI] [PubMed] [Google Scholar]
- 32.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T-cells. Nature Rev Immunol 2003; 3:253–257. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Sakaguchi N, Asano M et al. Immunologic self-tolerance maintained by activated T-cells expressing IL-2 receptor α-chains. J Immunol 1995; 155: 1151–1164. [PubMed] [Google Scholar]
- 34.Malek TR, Yu A, Vincek Vet al. CD4 regulatory T-cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 2002; 17:167–178. [DOI] [PubMed] [Google Scholar]
- 35.Salomon B, Lenschow D, Rhee L et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T-cells that control autoimmune diabetes. Immunity 2000; 12:431–440. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu J, Yamazaki S, Takahashi T et al. Stimulation of CD25+CD4+ regulatory T-cells through GITR breaks immunological self-tolerance. Nature lmmunol 2002; 3:135–142. [DOI] [PubMed] [Google Scholar]
- 37.Hori S, Nomura T, Sakaguchi S. Control of regulatory T-cell development by the transcription factor Foxp3. Science 2003; 299(5609):1057–1061. [DOI] [PubMed] [Google Scholar]
- 38.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T-cells. Nat Immunol 2003; 4:330–336. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto N, Oida T, Hirota K et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T-cells revealed by DNA microarray analysis. Int Immunol 2006; 18(8):1197–1209. [DOI] [PubMed] [Google Scholar]
- 40.Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun 2005; 25: (Suppl)56–62. [DOI] [PubMed] [Google Scholar]
- 41.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 2002; 39(8):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Putnam AL, Xu-Yu Z et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonuleit H, Schmitt E, Stassen M et al. Identification and functional characterization of human CD4(+)CD25(+) T-cells with regulatory properties isolated from peripheral blood. J Exp Med 2001; 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T-cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001; 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez A, Andre-Schmutz I, Carnaud C et al. Damage control, rather than unresponsiveness, effected by protective DX5+ T-cells in autoimmune diabetes. Nature Immunol 2001; 2:1117–1125. [DOI] [PubMed] [Google Scholar]
- 46.Barrat FJ, Cua DJ, Boonstra A et al. In vitro generation of interleukin-10-producing regulatory CD4+ T-cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (TH1)- and TH2-inducing cytokines. J Exp Med 2002; 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self-tolerance in overtly diabetic NOD mice. J Immunol 1997; 158:2947–2954. [PubMed] [Google Scholar]
- 48.Maloy KJ, Powrie F. Regulatory T-cells in the control of immune pathology. Nature Immunol 2001; 2:816–822. [DOI] [PubMed] [Google Scholar]
- 49.Kumanogoh A, Wang X, Lee I et al. Increased T-cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T-cell development. J Immunol 2001; 166:353–360. [DOI] [PubMed] [Google Scholar]
- 50.Pacholczyk R, Kraj P, Ignatowicz L. Peptide specificity of thymic selection of CD4+CD25+ T-cells. J Immunol 2002; 168:613–620. [DOI] [PubMed] [Google Scholar]
- 51.Bohling SD, Allison KH. Immunosuppressive regulatory T-cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol 2008; 21(12):1527–1532. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadzadeh M, Felipe-Silva A, Heemskerk B et al. FOXP3 expression accurately defines the population of intratumoral regulatory T-cells that selectively accumulate in metastatic melanoma lesions. Blood 2008; 112(13):4953–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brivio F, Fumagalli L, Parolini D et al. T-helper/T-regulator lymphocyte ratio as a new immunobiological index to quantify the anticancer immune status in cancer patients. In Vivo 2008; 22(5):647–650. [PubMed] [Google Scholar]
- 54.Estes JD, Li Q, Reynolds MR et al. Premature induction of an immunosuppressive regulatory T-cell response during acute simian immunodeficiency virus infection. J Infect Dis 2006; 193:703–712. [DOI] [PubMed] [Google Scholar]
- 55.Hryniewicz A, Boasso A, Edghill-Smith Y et al. CTLA-4 blockade decreases TGF-β indoleamine 2.3- dioxygenase and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006; 108:3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boasso A, Vaccari M, Hryniewicz A et al. Regulatory T-cell markers, indoleamine (2.3)-dioxygenase and virus levels in spleen and gut during progressive SIV infection. J Virol 2007; 81:11593–11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinter AL, Hennessey M, Bell A et al. CD25+CD4+ regulatory T-cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T-cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med 2004; 200:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson J, Boasso A, Velilla PA et al. HIV-1 driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 2006; 108:3808–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aandahl EM, Michaelsson J, Moretto WJ et al. Human CD4+ CD25+ regulatory T-cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens J Virol 2004; 78(5):2454–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Acosta-Rodriguez EV, Rivino L, Geqinat J et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat lrnmunol 2007; 8:639–646. [DOI] [PubMed] [Google Scholar]
- 61.Sher A, Coffman RL. Regulation of immunity to parasites by T-cells and T-cell-derived cytokines. Annu Rev lmrnunol 1992; 10:385–409. [DOI] [PubMed] [Google Scholar]
- 62.Ye P, Rodriguez FH, Kanaly S et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment and host defense. J Exp Med 2001; 194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang SC, Tan XY, Luxenberg DP et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cecchinato V, Trindade CJ, Laurence A et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal lmmunol 2008;l(4):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brenchley JM, Paiardini M, Knoxs KS et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008; 112(7):2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raffatellu M, Santos RL, Verhoeven DE et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 2008; 14(4):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reinhardt RL, Khoruts A, Merica R et al. Visualizing the generation of memory CD4 T-cells in the whole body. Nature 2001; 410:101–105. [DOI] [PubMed] [Google Scholar]
- 68.Kodera M, Grailer JJ, Karalewitz AP et al. T Lymphocyte migration to lymph nodes is maintained during homeostatic proliferation Microscopy and Microanalysis. Cambridge University Press; 2008; 14:211–224. [DOI] [PubMed] [Google Scholar]
- 69.Weninger W Crowley MA, Manjunath N et al. Migratory properties of naive, effector and memory CD8+ T-cells. J Exp Med 2001; 194(7):953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends lrnmunol 2006; 27(5):235–243. [DOI] [PubMed] [Google Scholar]
- 71.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996; 272(5258):60–66. [DOI] [PubMed] [Google Scholar]
- 72.Masopust D, Vezys V, Wherry EJ et al. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T-cell population. J lrnmunol 2006; 176:2079–2083. [DOI] [PubMed] [Google Scholar]
- 73.Gowans JL, Knight EJ. The route of recirculation of lymphocytes in the rat. Proc R Soc Lond B 1964; 159:257–282. [DOI] [PubMed] [Google Scholar]
- 74.Picker LJ EC Butcher. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol 1992; 10:561–581. [DOI] [PubMed] [Google Scholar]
- 75.Mackay CR, Marston WL, Dudler L. Naive and memory T-cells show distinct pathways of lymphocyte recirculation. J Exp Med 1990; 171:801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris NL, Watt V, Ronchese F et al. Differential T-cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002; 195(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu RH, Fang M, Klein-Szanto A et al. CD8+ T-cells are gatekeepers of the lymph node draining the site of viral infection. Proc Natl Acad Sci USA 26; 104(26)2007:10992–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veazey RS, Rosenzweig M, Shvetz DE et al. Characterization of gut-associated lymphoid tissue (GALT) of normal Rhesus macaques. Clin Immunol Immunopath 1997; 82(3):230–242. [DOI] [PubMed] [Google Scholar]
- 79.Veazey RS, DeMaria M, Chalifoux LV et al. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 1998; 280:427–431. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Duan L, Estes JD et al. Peak SIV replication in resting memory CD4+ T-cells depletes gut lamina propria CD4+ T-cells. Nature 2005; 434:1148–1152. [DOI] [PubMed] [Google Scholar]
- 81.Mattapallil JJ, Douek DC, Hill B et al. Massive infection and loss of memory CD4+ T-cells in multiple tissues during acute SIV infection. Nature 2005; 434:1093–1097. [DOI] [PubMed] [Google Scholar]
- 82.Mehandru S, Poles MA, Tenner-Racz K et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T-lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004; 200:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Douek DC, Picker LJ, Koup RA. T-cell dynamics in HIV-1 infection. Annu Rev Immunol 2003; 21:265–304. [DOI] [PubMed] [Google Scholar]
- 84.Lundqvist C, Parker CM, Cepek KL et al. Distinct structural and functional epitopes of the αEβ7 integrin. Eur J lrnmunol 1994; 24:2832–2841. [DOI] [PubMed] [Google Scholar]
- 85.Cepek KL, Parker CM, Madara JL et al. Integrin αEβ7 mediates adhesion of T-lymphocytes to epithelial cells. J lmmunol 1993; 150:3459–3470. [PubMed] [Google Scholar]
- 86.Grossman Z, Meier-Schellersheim M, Paul WE et al. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med 2006; 12:289–295. [DOI] [PubMed] [Google Scholar]
- 87.Picker LJ, Hagen SI, Lum R et al. Insufficient production and tissue delivery of CD4+ memory T-cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med 2004; 200:1299–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nishimura YT, Igarashi A, Buckler-White C et al. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in Simian immunodeficiency virus-infected macaques. J Virol 2007; 81:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hellerstein MK, Hoh RA, Hanley MB et al. Subpopulations of long-lived and short-lived T-cells in advanced HIV-1 infection. J Clin Invest 2003; 112:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brenchley JM, Schacker TW. Ruff LE et al. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stevceva L, Kelsall B, Nacsa J et al. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-Lymphocyte responses to Simian Immunodeficiency Virus SIVmac251. J Virol 2002; 76(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T-cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis 2003; 187(5):769–776. [DOI] [PubMed] [Google Scholar]
- 93.Poonia B, Wang X, Veazey RS. Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal Rhesus macaques: implications for virus transmission. J Reprod Immunol 2006; 72(1–2):74–84. [DOI] [PubMed] [Google Scholar]
- 94.Ma Z, Lu FX, Torten M et al. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of Rhesus macaques. Clin Immunol 2001; 100:240–249. [DOI] [PubMed] [Google Scholar]
- 95.McChesney MB, Collins JR, Miller CJ. Mucosal phenotype of antiviral cytotoxic T-lymphocytes in the vaginal mucosa of SIV-infected Rhesus macaques. AIDS Res Hum Retrovir 1998; 14(1):S63–S66. [PubMed] [Google Scholar]
- 96.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods 1990; 133:87–97. [DOI] [PubMed] [Google Scholar]
- 97.Clay CC, Rodrigues DS, Brignolo LL et al. Chemokine networks and in vivo T-lymphocyte trafficking in non-human primates. J Immunol Methods 2004; 293:23–42. [DOI] [PubMed] [Google Scholar]
- 98.Clay CC, Rodrigues DS, Harvey DJ et al. Distinct chemokine triggers and in vivo migratory paths of fluorescein dye-labeled T-lymphocytes in acutely Simian Immunodeficiency Virus SIVmac251-infected and uninfected macaques. J Virol 2005; 79(21):13759–13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allers K, Kunkel D, Moos V et al. Migration patterns of non-specifically activated versus nonactivated non-human primate T-lymphocytes: preferential homing of activated autologous CD8+ T-cells in the rectal mucosa. J Immunother 2008; 31(4):334–344. [DOI] [PubMed] [Google Scholar]
- 100.Ho DD, Neumann AU, Perelson AS et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995; 373(6510):123–126. [DOI] [PubMed] [Google Scholar]
- 101.Mohri H, Bonhoeffer S, Monard S et al. Rapid turnover of T-lymphocytes in SIV-infected Rhesus macaques. Science 1998; 279(5354):1223–1227. [DOI] [PubMed] [Google Scholar]
- 102.Terry NH, White RA. Flow cytometry after bromodeoxyuridine labeling to measure S and G2+M phase durations plus doubling times in vitro and in vivo. Nature Protocols 2006; 1:859–869. [DOI] [PubMed] [Google Scholar]
- 103.Bonhoeffer S, Mohri H, Ho D et al. Qantification of cell turnover kinetics using 5-Bromo-2′-deoxyuridine. J Immunol 2000; 164:5049–5054. [DOI] [PubMed] [Google Scholar]
- 104.De Boer RJ, Hiroshi Mohri H, David D. Ho D et al. Turnover Rates of B-Cells, T-Cells and NK Cells in Simian Immunodeficiency virus-infected and uninfected Rhesus macaques. J Immunol 2003; 170:2479–2487. [DOI] [PubMed] [Google Scholar]
- 105.Cicin-Šain L, Messaoudi I, Park B et al. Dramatic increase in naïve T-cell turnover is linked to loss of naïve T-cells from old primates. Proc Natl Acad Sci USA 2007; 104(50):19960–19965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaur A, Di Mascio M, Barabasz A et al. Dynamics of T- and B-Lymphocyte Turnover in a Natural Host of Simian Immunodeficiency Virus. J Virol 2008; 82:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Picker LJ, Reed-Inderbitzin EF, Hagen SI et al. IL-15 induces CD4+ effector memory T-cell production and tissue emigration in non-human primates. J Clin Invest 2006; 116(6):1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Healy DL, Bacher J, Hodgen GD. A method of thymectomy in macaques. J Med Primatol 1983; 12(2):89–100. [PubMed] [Google Scholar]
- 109.Arron ST, Riberio RM, Gettie A et al. Impact of thymectomy on the peripheral T-cell pool in rhesus macaques before and after infection with simian immunodeficiency virus. Eur J Immunol 2005; 35(1):46–55. [DOI] [PubMed] [Google Scholar]
- 110.Borghans JA, Hazenberg MD, Miedema F. Limited role for the thymus in SIV pathogenesis Eur J Immunol 2005; 35(1):42–45. [DOI] [PubMed] [Google Scholar]
- 111.Schmitz JE, Simon MA, Kuroda MJ et al. A non-human primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Phatol 1999; 154(6):1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Permar SR, Klumpp SA, Mansfield KG et al. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol 2003; 77(7):4396–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grakoui A, Shoukry NH, Woollard DJ et al. HCV Persistence and Immune Evasion in the Absence of Memory T-Cell Help. Science 2003; 302(5645):659–662. [DOI] [PubMed] [Google Scholar]
- 114.Edghill-Smith Y, Golding H, Manischewitz J et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med 2005; 11(7):740–747. [DOI] [PubMed] [Google Scholar]
- 115.Matano T, Shibata R, Siemon C et al. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol 1998; 72(1):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmitz JE, Johnson RP, McClure HM et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated Rhesus macaques. J Virol 2005; 79:8131–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaccari M, Mattapllil J, Song K et al. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T-cells induced by vaccination during CD4+ T-cell deficiency. J Virol 2008; 82(19):9629–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mavilio D, Lombardo G, Kinter A et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA 2005; 102:2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi EI, Wang R, Peterson L et al. Use of an anti-CD16 antibody for in vivo depletion of natural killer cells in rhesus macaques. Immunology; 2008; 124(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi EI, Reimann KA, Letvin NL. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol 2008; 82(13):6758–6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shedlock DJ, Shen H. Requirement for CD4 T-cell help in generating functional CD8 T-cell memory. Science 2003; 300:337–339. [DOI] [PubMed] [Google Scholar]
- 122.Sun JC, Williams MA, Bevan MJ. CD4+ T-cells are required for the maintenance, not programming, of memory CD8+ T-cells after acute infection. Nat Immunol 2004; 5:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Janessen EM, Lemmens EE, Wolfe T et al. CD4+ T-cells are required for secondary expansion and memory in CD8+ T-lymphocytes. Nature 2003; 178:3492–3504. [DOI] [PubMed] [Google Scholar]
- 124.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 2001; 98:3192–3204. [DOI] [PubMed] [Google Scholar]
- 125.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol 2000; 67(1):2–17. [DOI] [PubMed] [Google Scholar]
- 126.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998; 16:111–135. [DOI] [PubMed] [Google Scholar]
- 127.Melter M, Reinders ME, Sho M et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood 2000; 96:3801–3808. [PubMed] [Google Scholar]
- 128.Reinders ME, Sho M, Robertson SW et al. Proangiogenic function of CD40 ligand-CD40 interactions. J Immunol 2003; 171:1534–1541. [DOI] [PubMed] [Google Scholar]
- 129.Waterhouse P, Penninger JM, Timms E et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 1995; 270(5238):985–988. [DOI] [PubMed] [Google Scholar]
- 130.Larsen CP, Elwood ET, Alexander DZ et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381:434–438. [DOI] [PubMed] [Google Scholar]
- 131.Kirk AD, Harlan DM, Armstrong NN et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA 1997; 94:8789–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garber DA, Guido Silvestri G, Barry AP et al. Blockade of T-cell costimulation reveals interrelated actions of CD4+ and CD8+ T-cells in control of SIV replication. J Clin Invest 2004; 113(6):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cecchinato V, Tryniszewska E, Ma ZM et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol 2008; 180:5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4:762–774. [DOI] [PubMed] [Google Scholar]
- 135.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma (c)-dependent cytokines interleukins 2, 4, 7, 9, 15 and 21 and their signaling pathways. Immunol Rev 2004; 202:67–83. [DOI] [PubMed] [Google Scholar]
- 136.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol 2005; 26:56–64. [DOI] [PubMed] [Google Scholar]
- 137.Fry TJ, Moniuszko M, Creekmore S et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected non-human primates. Blood 2003; 101:2294–2299. [DOI] [PubMed] [Google Scholar]
- 138.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 2004; 4:665–674. [DOI] [PubMed] [Google Scholar]
- 139.Khaled AR, Durum SK. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat Rev Immunol 2002; 2:817–830. [DOI] [PubMed] [Google Scholar]
- 140.Tryniszewska E, Nacsa J, Lewis MG et al. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J Immunol 2002; 169(9):5347–5357. [DOI] [PubMed] [Google Scholar]
- 141.Nacsa J, Edghill-Smith Y, Tsai WP et al. Contrasting effects of low-dose IL-2 on vaccine-boosted simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T-cells in macaques chronically infected with SIVmac251. J Immunol 2005; 174(4):1913–1921. [DOI] [PubMed] [Google Scholar]
- 142.Barouch DH, Letvin NL, Seder RA. Expression kinetics of the interleukin-2/immunoglobuIin (IL-2/Ig) plasmid cytokine adjuvant. Vaccine 2004; 22(23–24):3092–3097. [DOI] [PubMed] [Google Scholar]
- 143.Villinger F, Miller R, Mori K et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T-cells in rhesus macaques. Vaccine 2004; 22(25–26):3510–3521. [DOI] [PubMed] [Google Scholar]
- 144.von Freeden-Jeffry U, Vieira P, Lucian LA et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 1995; 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peschon JJ, Morrissey PJ, Grabstein KH et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 1994; 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kieper WC, Tan JT, B. Bondi-Boyd B et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T-cells. J Exp Med 2002; 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schluns KS, Kieper WC, Jameson SC et al. Interleukin-7 mediates the homeostasis of naive and memory CD8 T-cells in vivo. Nat Immunol 2000; 1:426–432. [DOI] [PubMed] [Google Scholar]
- 148.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol 2003; 3:269–279. [DOI] [PubMed] [Google Scholar]
- 149.Lantz O, Grandjean I, Matzinger P et al. Gamma chain required for naive CD4+ T-cell survival but not for antigen proliferation. Nat Immunol 2000; 1:54–58. [DOI] [PubMed] [Google Scholar]
- 150.Tan JT, Dudl E, LeRoy E et al. IL-7 is critical for homeostatic proliferation and survival of naive T-cells. Proc Natl Acad Sci USA 2001; 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wiryana P, Bui T, Faltcynek CR et al. Augmentation of cell-mediated immunotherapy against herpes simplex virus by interleukins: comparison of in vivo effects of IL-2 and IL-7 on adoptively transferred T-cells. Vaccine 1997; 15:561–563. [DOI] [PubMed] [Google Scholar]
- 152.Moniuszko M, Fry T, Tsai WP et al. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T-Cells in vivo. J Virol 2004; 78(18):9740–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dereuddre-Bosquet N, Vaslin B, Delache B et al. Rapid modifications of peripheral T-cell subsets that express CD127 in macaques treated with recombinant IL-7. J Med Primatol 2007; 36(4–5):228–237. [DOI] [PubMed] [Google Scholar]
- 154.Villinger F, Miller R, Mori K et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T-cells in Rhesus macaques. Vaccine 2004; 22:3510–3521. [DOI] [PubMed] [Google Scholar]
- 155.Beq S, Nugeyre MT, Fang RHT et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol 2006; 176:914–922. [DOI] [PubMed] [Google Scholar]
- 156.Mueller YM, Do DH, Altork SR et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T-cell responses. J Immunol 2008; 180(1):350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Demberg T, Boyer JD, Malkevich N et al. Sequential priming with simian immunodeficiency virus (SIV) DNA vaccines, with or without encoded cytokines and a replicating adenovirus-SIV recombinant followed by protein boosting does not control a pathogenic SIVmac251 mucosal challenge. J Virol 2008; 82(21):10911–10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hryniewicz A, Price DA, Moniuszko M et al. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virusmac 251-infected macaques. J Immunol 2007; 178:3492–3504. [DOI] [PubMed] [Google Scholar]
- 159.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004; 5:133–139. [DOI] [PubMed] [Google Scholar]
- 160.Miller RA, Garcia G, Kirk CJ et al. Early activation defects in T-lymphocytes from aged mice. Immunol Rev 1997; 160:79–90. [DOI] [PubMed] [Google Scholar]
- 161.Eisenbraun M, Tamir A, Miller RA. Altered composition of the immunological synapse in an anergic, age-dependent memory T-cell subset. J Immunol 2000; 164:6105–6112. [DOI] [PubMed] [Google Scholar]
- 162.Flajnik MF, Kasai M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity 2001; 15:351–362. [DOI] [PubMed] [Google Scholar]
- 163.Murphy WJ, Stanyon R, O’Brien SJ. Evolution of mammalian genome organization inferred from comparative gene mapping. Genome Biol 2001; 2:0005.1–0005.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roth GS, Mattison JA, Ottinger MA et al. Aging in rhesus monkeys: relevance to human health Interventions. Science 2004; 305(5689); 1423–1426. [DOI] [PubMed] [Google Scholar]
- 165.Messaoudi I, Warner J, Fischer M et al. Delay of T-cell senescence by caloric restriction in aged long-lived non-human primates. Proc Natl Acad Sci USA 2006; 103:19448–19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Douek DC, McFarland RD, Keiser PH et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396:690–695. [DOI] [PubMed] [Google Scholar]
- 167.McFarland RD, Douek DC, Koup RA et al. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci USA 2000; 97:4215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jankovié V Messaoudi I, Nikolich-Žugich J. Phenotypic and functional T-cell aging in Rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood 2003; 102(9):3244–3251. [DOI] [PubMed] [Google Scholar]