Abstract
Chaperone protein quantity may regulate the balance of proteins involved in invasion and malignancy. BAG3 is a co-chaperone and pro-survival protein that has been implicated in adhesion, migration, and metastasis. We reported that BAG3 overexpression in MDA435 human breast cancer cells results in a significant decrease in migration and adhesion to matrix molecules that is reversed upon deletion of the BAG3 proline-rich domain (dPXXP). We now hypothesize that transcriptional analysis would identify proteins involved in matrix-related processes that are regulated by BAG3 and/or its PXXP domain mutant. Expression array analysis of MDA435 cells overexpressing either wild-type BAG3 (FL) or dPXXP identified CCN1 as a BAG3 target protein. CCN1 is a known AP-1 target. Increased AP-1 transcriptional activity and AP-1 DNA-binding was found in MDA435 dPXXP cells. Consistent with these findings, CCN1 quantity and secretion were increased in dPXXP mutants but suppressed in FL cells; both BAG3 forms resulted in up-regulated CCN1 in HeLa cells. CCN1 silencing in the BAG3 FL overexpressors reduced the already low phospho-integrin β1 in response to attachment on collagen IV. Matrigel invasion of HeLa cells engineered with the BAG3 constructs was enhanced in FL cells and minimal in dPXXP cells. CCN1 silencing blocked a greater percentage of the serum-induced invasion in FL cells than in dPXXP cells. This implies a context-dependent function of BAG3 on CCN1 and thus mesenchymal behaviour. CCN1 may be necessary for adhesion and matrix-related signalling in FL cells, abrogating a negative signal of the PXXP domain when BAG3 is intact. We propose that BAG3 regulates CCN1 expression to regulate tumour cell adhesion and migration.
Keywords: adhesion, BAG3, genomic analysis, CCN1, Cyr61, integrin engagement
Introduction
Tumour cell function is governed by phenotype-disrupting signalling pathways that result in escape from normal physiological balance [1]. Abnormal interactions between the tumour cell and its extracellular environment can enhance a tumour’s aggressiveness [2]. BAG3 is a stress-associated survival co-chaperone protein that is also a marker of aggressive tumour behaviour in pancreatic [3] and thyroid [4] cancer cells. BAG family proteins have been suggested to function primarily via a conserved BAG domain that interacts with heat-shock proteins (HSP70) [5–7]. Paradoxically, we found reduced aggressiveness in BAG3-overexpressing (FL) MDA435 cells but an increase in cells with forced expression of a proline-rich domain deletion mutant (dPXXP). Our results implied that BAG3, through loss of its PXXP domain, augmented cell interaction with the micro-environment through substratum binding and signalling [8]. This suggests a broader and context-dependent role for BAG3.
Cell adhesion to the extracellular matrix and directed migration are in vitro markers of a mesenchymal or invasive phenotype [9]. This behaviour was reduced in FL-BAG3 cells compared with control or dPXXP transfectants [8]. dPXXP cells were more adhesive and had increased stress fibres and spreading. FAK phosphorylation and immunostaining were increased in dPXXP cells and reduced in FL-BAG3 cells; silencing of BAG3 resulted in reactivation of FAK. These data implicated the PXXP domain as a negative regulator of cell adhesion and migration. However, a direct link between BAG3 and specific target proteins in the focal adhesion signalling pathway was not established.
We hypothesized that BAG3 may affect adhesion through (indirect) transcriptional regulation of proteins involved in the focal contact pathway. We queried gene expression of FL, dPXXP, and control cells attached and spread on collagen IV. CCN1 was one of the most up-regulated genes in dPXXP BAG3 cells. CCN1 is a secreted matricellular protein belonging to a set of immediate-early genes induced in response to growth factor signalling [10–12]. It has been implicated in angiogenesis, proliferation, adhesion, and migration, and interacts with integrins to augment their signal activity [13–15]. Here, we provide evidence whereby BAG3 regulates the transcription of CCN1 and elicits an adhesive phenotype.
Materials and methods
Cell culture, plasmids, and adhesion assays
MDA435 human breast cancer and HeLa cells were obtained from ATCC (Manassas, VA, USA) and maintained in DMEM with 10% FCS. FL, BAG domain-deleted (dBAG), and dPXXP MDA435 BAG3 cells have been described previously [6,8,16]; bulk EGFP–BAG fusion protein HeLa clones were generated and validated. Cells were reselected and maintained in 1200 μg/ml G418, except for the passage immediately prior to experiments. Multiple clones of each sub-line showed consistent phenotypes. Protein expression was confirmed regularly by immunoblotting. Cytomatrix extracellular matrix-coated 96-well chambers were purchased from Chemicon (Temecula, CA, USA) and used according to the manufacturer’s instructions [8]. Each was performed at least three times.
cDNA microarray
Total cellular RNA was isolated using ISOGEN (Nippon Gene, Tokyo, Japan), according to the manufacturer’s instructions. RNA was further purified using the RNeasy Mini kit with additional DNase digestion with RNase-free DNase (Qiagen, Valencia, CA, USA). Methods for probe labelling reaction and microarray hybridization have been described previously [17]. Microarrays contained 7680 human cDNA clones and were prepared from the Research Genetics Named Genes set (Huntsville, AL, USA) [17,18]. The Cy5- and Cy3-labelled cDNA hybridized arrays were scanned at 635 and 532 nm. Resulting TIFF images were analysed by GenePix Pro 3.0 software (Axon Instruments, Inc, Foster City, CA, USA). The ratios of sample intensity to reference intensity [green (Cy3)/red (Cy5)] were determined for all targets. The Mann–Whitney test was used to ascertain statistical significance among microarray replicates, since a normal distribution could not be applied to all components of the data set [19]. Well fluorescence was corrected for background fluorescence, and ratios of intensity were established relative to appropriate controls. A 1.5-fold threshold in differences was selected because the multiple repeats in our experimental scheme increase the likelihood of statistical reliability [17,18]. Three additional analytical methods were employed to validate statistical integrity: class comparison; class prediction; and false discovery, at p = 0.05 to validate statistical integrity.
For gene array listing see http://go.cancer.gov/04162009
Electromobility shift assay (EMSA)
Nuclear extracts were prepared from control, FL, dPXXP, and dBAG [6,8] cells using the previously described method but withholding reducing agents [20]. EMSAs were performed using a 32P-radiolabelled oligonucleotide corresponding to consensus AP-1, NF -κB, or STAT1 DNA-binding sites [21]. Nuclear extracts (10 μg) were incubated with poly dI-dC for 10 min on ice, followed by the addition of radiolabelled oligonucleotide (100000 cpm/probe per reaction) and incubated at 25 °C for 20 min. Samples were electrophoresed on a 6% non-denaturing polyacrylamide gel, dried, exposed to a phosphorimager screen, and analysed using a TYPHOON 860 Phosphorimager (American Biosciences, Piscataway, NJ, USA) with ImageQuant software.
Co-transfections and luciferase assays
FL, dPXXP, dBAG3 or Neo vector control cells were plated at 2 × 106 cells per 100 mm plate and transfected with expression plasmids containing either AP-1-luciferase (p7×-AP-1-tk-LUC) or NFκB-luciferase (p4×-κB-tk-LUC) using FuGene6 (Roche, Germany) [22]. β-Galactosidase expression plasmid (1 μg, pCMV -β-gal) was used as the internal control and carrier DNA (pUC) was added to each sample to maintain a total of 6 mM in each transfection. The total amount of CMV promoter-containing plasmids was adjusted so that the same amount was present in each reaction. Cells were harvested 36 h after transfection and luciferase activity and β-galactosidase activities were determined (Promega, Madison, WI, USA). The relative fold induction of luciferase activity was normalized to β-galactosidase activity. Results are the mean of at least three experiments each done in duplicate (± SEM). The results of individual transfections varied by less than 25%.
Protein isolation and immunoblotting
Cells were plated and grown on collagen IV-coated dishes (25 nM) overnight prior to lysis and immunoblotting [8]. Confluent monolayers of MDA435 or HeLa cells were washed and then incubated in serum-free DMEM (SFM) for 24 h for conditioned medium (CM) experiments. CM was removed, spun free of cells, and stored; cells were harvested independently. Immunoblot antibodies included anti-CCN1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), FAK (Upstate Biotechnology; Charlottesville, VA, USA), phospho-integrin β1 pY783 (Biolegend, San Diego, CA, USA), integrin β1 (BD Transduction Labs, San Jose, CA, USA), phospho-FAK pY861 (Biosource, Camarillo, CA, USA), β-tubulin (Abcam, Cambridge, MA, USA), and polyclonal BAG3 antibodies [16].
Gene silencing siRNA transfection
BAG3, CCN1, and control (non-silencing) siRNAs were purchased from Qiagen, Inc (Valencia, CA, USA). BAG3 (0.2 or 2 μM) or CCN1 (0.4 or 2 μM) or equivalent non-silencing siRNA was suspended in SFM with 8–12 μl/ml siRNA transfection lipid reagent (Bio-Rad, Hercules, CA, USA or Qiagen) for 10–20 min, respectively. The mixture was aliquoted to cells to a final concentration of 20 or 80 nM siRNA per well. Cells were harvested and used at 72 h.
Cell preparation and immunofluorescence
Cells were attached for 3 h to glass coverslips precoated with 25 nM collagen IV and then fixed in 3.7% formaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 1% BSA for 1 h. Coverslips were incubated with anti-CCN1 at 1 : 250 overnight at 4 °C, washed in PBS, and incubated with Alexa-Fluor 488 goat anti-rabbit secondary antibody (Molecular Probes, Eugene, OR, USA) at 1 : 500 for 1 h. Coverslips were washed and mounted using Vectashield with DAPI (Vector Labs, Burlingame, CA, USA).
Matrigel invasion and CCN1 matrix adhesion
The invasion assay used Biocoat Matrigel invasion chambers (Becton Dickinson Biosciences, Bedford, MA, USA) with serum-containing and serum-free medium as the attractant and control, respectively, for 36 h. Methanol-fixed, invaded cells were stained with crystal violet and the number of invaded cells was determined. Results are presented as background-subtracted mean and SEM of four replicate experiments. Plastic plates were coated overnight with recombinant CCN1 (10 μg/ml; Cell Sciences, Can-ton, MA, USA) [23] and/or 0.1% collagen I, and then blocked with 0.1% BSA. Cells were allowed to adhere for 1 h, and were then fixed, stained, and the optical density of the eluted stain was determined.
Statistical analysis
Unless otherwise indicated, Student’s t-test analysis was done (Microsoft ®Excel, Redmond, WA, USA). All reported p values are two-tailed.
Results
CCN1 mRNA is overexpressed in dPXXP cells
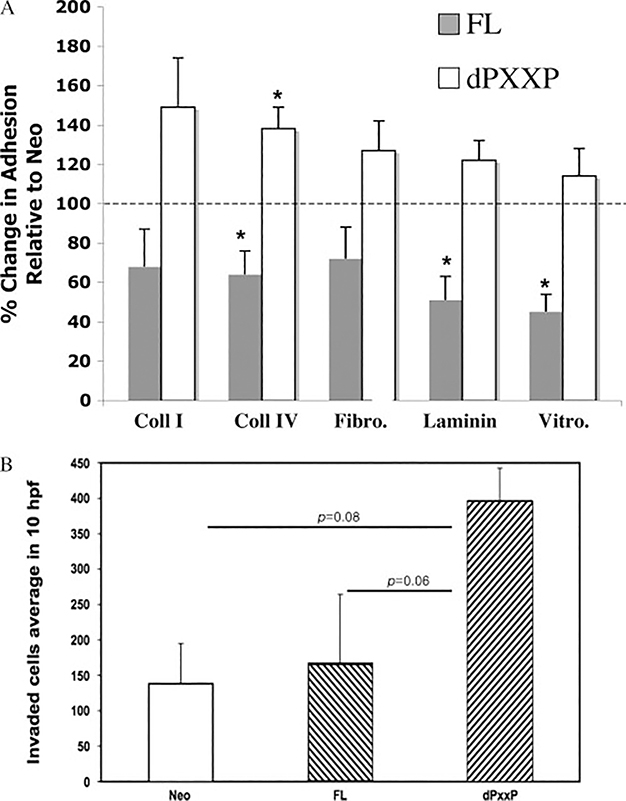
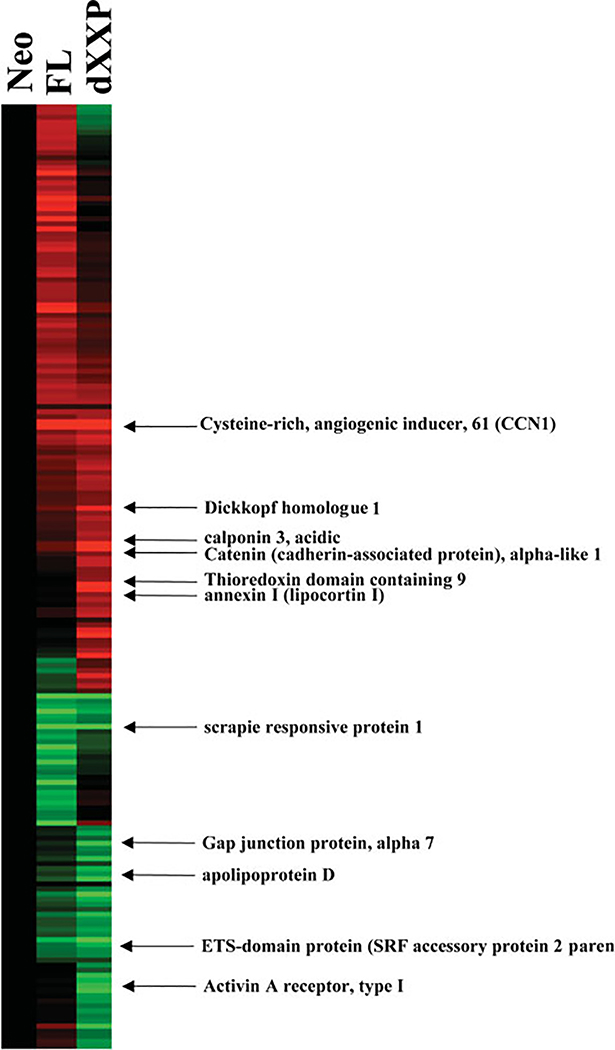
MDA435 FL cells had reduced adhesion to multiple extracellular matrix substrata where dPXXP BAG3 cells displayed increased adhesion; a similar pattern was observed for invasiveness (Figure 1). BAG3 affected adhesion to collagen IV in HeLa cells in a comparable fashion (Supporting information, Supplementary Figure 1). This led to examination of gene expression to identify gene changes accompanying these phenotypic changes. Eleven genes were significantly overexpressed compared with Neo control cells (Figure 2 and Table 1). Expression of CCN1, an angiogenic effector and motogenic inducer, was highest in dPXXP cells. No differences in matrix molecule receptor gene expression were observed. CCN1 has been shown to play a role in the regulation of cellular adhesion [24] and was chosen for validation and mechanistic association to BAG3.
Figure 1.
dPXXP-overexpressing cells are more adhesive and more invasive in MDA435 cells. (A) Vector control, FL, and dPXXP cells were plated onto extracellular matrix-coated plates, allowed to adhere for 3 h, and then stained and assessed. Results represent the % change relative to Neo (100%, represented by a horizontal line) of at least three independent experiments (± SEM). ∗p < 0.05. (B) Net invasion is affected by overexpression of dPXXP. Corrected invasion was highest in dPXXP cells (p= 0.008). A trend towards greater invasion of dPXXP cells over FL is seen (p= 0.06)
Figure 2.
Hierarchical clustering of gene expression. Ratios of gene expression values were generated and clustered hierarchically. The mean gene expression values were determined from experimental replicates, calculated as a ratio in the parental cell lines, and are presented using Cluster® (v. 2.20) and TreeView® (v. 1.60) software [18]. Up- and down-regulated gene expression changes are in red and green, respectively
Table 1.
Total genes increased and decreased in MDA435 breast carcinoma cells
| Gene expression changes dPXXP versus Neo |
|
|---|---|
| Increased/decreased | |
| APOD—apolipoprotein D | 0.397 |
| Homo sapiens cDNA: FLJ22425 fis, clone | 0.425 |
| SCRG1—scrapie responsive protein 1 | 0.460 |
| ACVR1—Ser/Thr protein kinase receptor | 0.465 |
| GJA7—gap junction protein, alpha 7, 4 | 0.473 |
| CTNNAL1—catenin (cadherin-associated) | 2.099 |
| DKK1—dickkopf (Xenopus laevis) homologue | 2.232 |
| CNN3—calponin 3, acidic | 2.287 |
| APACD—ATP binding protein associated | 2.300 |
| ANXA1—annexin 1 (lipocortin 1) | 2.344 |
| CYR61—cysteine-rich, angiogenic inducer | 2.899 |
Total number of genes in which expression increased or decreased in MDA-MB-435 human breast cancer cells that were genetically altered to overexpress either the wild-type BAG-3 gene or a BAG-3 proline-rich domain-deleted (dPXXP) mutant. Numbers indicate genes and the fold increase or decrease.
dPXXP BAG3 cells have increased AP-1 DNA binding
AP-1 transcription factor has been reported to activate the CCN1 promoter [25–27]. Gel-shift assays were conducted to investigate the DNA-binding activity of transcription factors involved in expression of the CCN1 gene. Specific DNA-binding activity to the AP-1 consensus site was increased only in nuclear extracts isolated from the dPXXP cell line (Figure 3A and [dummy] Supporting information, Supplementary Figure 2). In contrast, no discernible difference in DNA-binding activity was demonstrated across sub-lines with NF - κB (Figure 3B) or STAT1 consensus sequences (data not shown). The role of BAG3 in regulating AP-1dependent reporter gene expression was determined with transiently expressed AP-1 or NF -κB reporter plasmids. An increase was seen only in the dPXXP cell line (Figure 3C), consistent with the results for AP-1-dependent DNA-binding activity. No changes were observed in the κB-dependent luciferase reporter cells (Figure 3D). These experiments demonstrate that at least one target of the BAG3 PXXP domain is regulation of AP-1-associated expression, linking BAG3 and CCN1 expression.
Figure 3.
dPXXP cells exhibit increased AP-1 activity. EMSA for AP-1 (A) and NF -κB (B) binding. FL, dPXXP, dBAG, and control (Neo) cells were subjected to subcellular fraction. EMSA proceeded as described in the Materials and methods section with specific 32P-labelled oligonucleotide probes. Luciferase activity from (C) p7×-AP-1-tk-LUC or (D) p4×-κB-tk-LUC was measured in the four cell lines. Results are presented as relative-fold induction over sham-treated control. Luciferase activity was normalized to β-galactosidase activity and results are presented as luciferase/β-galactosidase relative units. All results are the mean of at least three separate experiments done in duplicate (± SEM). ∗pP < 0.05
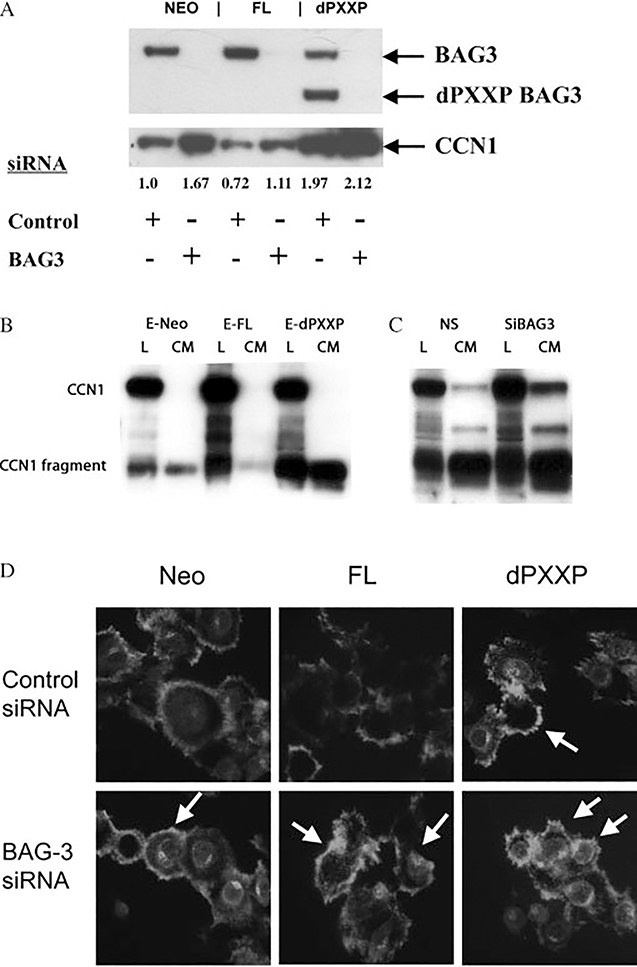
Silencing BAG3 results in increased CCN1 expression
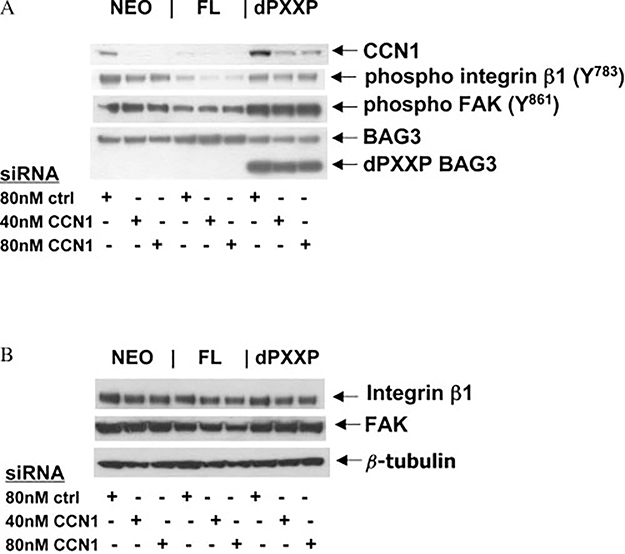
Protein expression of CCN1 was determined in the MDA435 BAG3 sub-lines and confirmed the results of the microarray analysis (Figure 4A). CCN1 protein correlated inversely with FL BAG3 and directly with dPXXP expression. Silencing of BAG3 was associated with up-regulation of CCN1 (Figure 4A). CCN1 expression was reduced in FL cells compared with Neo or dPXXP cells (Figure 4A). Secretion of CCN1 was altered by BAG3 expression in both MDA435 and HeLa cells. More CCN1 was secreted by HeLa dPXXP cells, with increased presence of a 28 kD breakdown product (Figure 4B) [28]; similar results were seen with MDA435 cells (data not shown). Silencing of BAG3 in Neo-HeLa cells caused an increase in both total CCN1 and the 28 kD fragment (Figure 4C). Immunofluorescent labelling of CCN1 confirmed its dichotomous intracellular expression and regulation by BAG3 (Figure 4D). An overall reduction in CCN1 was seen in FL cells with an increase in dPXXP cells; silencing of BAG3 increased CCN1 in all MDA435 cells. Localization of CCN1 was not changed with mutants or with BAG3 silencing, suggesting that BAG3 is regulating expression but not function of CCN1.
Figure 4.
Increased CCN1 expression after BAG3 silencing. (A) Differential expression of CCN1 protein in MDA435 cells. Cells were incubated with control siRNA, or 40 nM BAG3 siRNA, followed by incubation on collagen IV. The upper panel is BAG3 expression and the lower panel demonstrates CCN1 expression increases in each setting. (B) Increased secretion and cleavage of CCN1 in dPXXP HeLa cells. CM from vector or bulk FL BAG3-HeLa cells was subjected to immunoblotting for CCN1. (C) Silencing of BAG3 causes an increase in both CCN1 and its 28 kD fragment in EGFP-Neo-HeLa cells. (D) Silencing of BAG3 in MDA435 cells is associated with increased expression of CCN1 and improved attachment and spreading. Cells transfected with either control or BAG3 siRNA were transferred to collagen type IV-coated coverslips and allowed to attach and spread for 3 h. Thereafter, they were fixed, permeabilized, and stained with anti-CCN1 antibody. Arrows indicate cells of interest
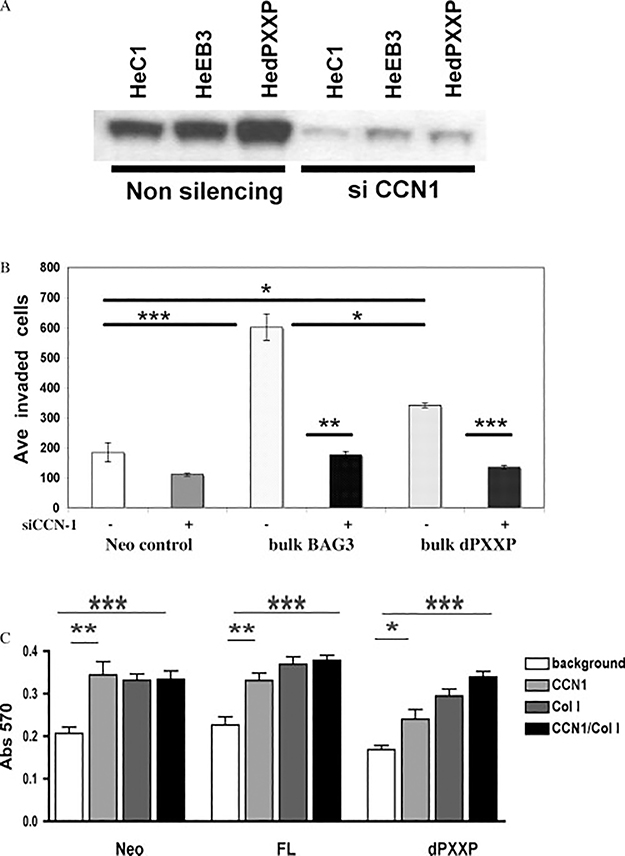
CCN1 on invasion and adhesion
CCN1 has been associated with increased invasion. This was examined using the BAG3 HeLa cells. Increased or silenced CCN1 was confirmed in the passages used for invasion (Figure 5A). FL-HeLa BAG3 cells were two- to three-fold more invasive, with a limited increase in dPXXP cell invasion (Figure 5B). Silencing CCN1 caused a greater loss of invasion in the FL cells compared with dPXXP cells. MDA Neo and FL cells adhered to CCN1 and collagen I similarly. However, dPXXP cells had cooperative adhesion, greatest with both matrices together (Figure 5C). Adhesion of both FL and dPXXP HeLa cells was increased to CCN1 only, with no effect of collagen I (Supporting information, Supplementary Figure 3). The results in both cell lines are suggestive of a regulatory role for the PXXP domain in a CCN1 milieu.
Figure 5.
CCN1-sensitive invasion and adhesion of BAG3-expressing cells. (A) Expression of CCN1 with and without silencing in HeLa BAG3 cells subjected to invasion. (B) Bulk BAG3 (B3)-HeLa cells had a more robust invasion response to serum, and silencing of CCN1 reduced a greater proportion of B3 cell invasion. Results are mean ± SEM of four replicate experiments. (C) CCN1 matrix augments the adhesive ability of MDA435 dPXXP cells. MDA cells express less CCN1 in their basal or BAG3-transfected state than HeLa. Plates were coated with CCN1 ± collagen I. An additive effect of CCN1 and collagen was seen in dPXXP cells and increased adhesion observed with CCN1 or collagen I in the control and FL cells. (∗p < 0.01; ∗∗p < 0.004; ∗∗∗p= 0.0006)
Integrin β1 phosphorylation is reduced in BAG3-overexpressing cells
CCN1 functions as a cofactor for integrin activation [15,29,30], regulating signalling involved in adhesion and migration [14,31]. Integrins α1β1 and α2β1 are collagen IV receptors and when activated, the β subunits are phosphorylated and signal downstream to FAK either directly or through Src to FAK [32]. We asked whether alteration of CCN1 protein resulted in parallel changes in integrin β1 activity and downstream activation of adhesion signalling in cells grown overnight on collagen IV. Silencing CCN1 in MDA435 FL cells resulted in a 40–60% reduction in p-integrin β1, but only a 20–35% reduction in Neo or dPXXP cells, without a reduction in the already low p-FAK (Figure 6A). Total integrin β1 and FAK protein levels were not altered by CCN1 silencing (Figure 6B) nor was BAG3 lost with CCN1 silencing, indicating that there is no reciprocal regulation of CCN1 on BAG3 (Figure 6A) and confirming that altered CCN1 expression levels are downstream of BAG3. These results connect BAG3 to regulation of adhesion and invasion factors, and strongly suggest that BAG3-mediated reduction of CCN1 is a driver in BAG3-negative regulation of focal adhesion pathways [8].
Figure 6.
BAG3-dependent CCN1 signalling through integrin β1. (A) Total CCN1 and β1 integrin phosphorylation are reduced in FL MDA-435 cells. Neo, FL, and dPXXP MDA435 cells were grown for 72 h in the presence of either non-silencing siRNA (−) or CCN1 siRNA, and transferred to collagen IV-coated plates for adherence, lysis, and immunoblot. Incomplete silencing of CCN1 in dPXXP cells prevented loss of β1 phosphorylation and FAK phosphorylation. (B) Lysates used in A were immunoblotted for total integrin β1, total FAK, and β-tubulin. These results are representative of at least three replicates
Discussion
Our previously reported down-regulation of adhesion and migration in MDA435 cells overexpressing BAG3, and increased activity in dPXXP-BAG3 cells, suggested that deletion of the proline-rich region abrogated signal interdiction, thereby promoting adhesive behaviour [8]. Those results were counterintuitive for a pro-survival and metastasis protein, and differed from results reported for other cell types [33]. We thus hypothesized that expression array analysis would lead to the identification of transcriptional changes that might explain this paradoxical effect in the MDA435 cells and/or identify further components of the interaction of BAG3 and the matricellular micro-environment.
Differential expression of CCN1 was demonstrated. CCN1 expression can be regulated through the AP1 transcription factor [26]. We have shown increased activity of AP-1 in MDA435 dPXXP cells, consistent with the differential expression of CCN1 and supporting a link between BAG3 and CCN1 expression. Subsequent protein validation demonstrated reciprocal expression of CCN1 in MDA435 cells, increased in the more adhesive dPXXP cells and reduced when BAG3 and thus its PXXP domain were over-represented. Experiments in HeLa cells suggest that BAG3 regulation of CCN1 expression is not unique to MDA435 cells, finding up-regulation of CCN1 in both FL and dPXXP cells. The inverse relationship between CCN1 expression in MDA435 FL and dPXXP cells was maintained when BAG3 was silenced, although CCN1 was increased in all cells. Expression differences were not associated with different subcellular localizations, nor was there a reciprocal effect of CCN1 silencing on BAG3 quantity. The expression, production, and secretion of CCN1 in HeLa cells were associated with greater CCN1-dependent invasion in the BAG3 overexpressors. Silencing of CCN1 was associated with reduced phosphorylation of β1 integrin, consistent with the known matricellular signal modulation of CCN1 [31]. These findings show that CCN1 may be affected by BAG3 expression, and may be a mechanism through which the cellular response to matrix stimulation is modified.
We found that CCN1 protein expression patterns in the MDA435 sub-lines correlated closely with our observed adhesion phenotypes [8]. CCN1 is a secreted pro-angiogenic cysteine-rich protein that promotes adhesion through its interaction with integrins and growth factors [24,31]. CCN1 protein was markedly reduced in FL-MDA435 cells, consistent with our previous observations of reduced adhesion, migration, and FAK and paxillin phosphorylation. Conversely, CCN1 was increased in dPXXP cells, again in harmony with our previous findings [8]. The sensitivity of the dPXXP cells with their marked increase in CCN1 in response to BAG3 silencing appears greater than that seen for either Neo or FL cells. The reason for this is unclear. We can postulate that further down-regulation of the dominant negative dPXXP-BAG3 allows for the CCN1 induction in response to loss of the PXXP regulatory domain and then a second induction because of loss of the wild-type BAG3 background, yielding a stronger stimulatory response. The different expression, production, and secretion are consistent with reported roles for CCN1 in modulating matricellular behaviours [10,24].
Cell adhesion is an integral component of tumour progression and invasion [2]. Adhesion and the signalling pathways that govern it are critical in at least two phases of invasion: attachment to the extracellular matrix and cell translocation. CCN1 expression, production, and secretion follow the adhesion and focal contact signalling phenotypic dichotomy between MDA435 FL and dPXXP cells [8]. Paradoxically, we also found increased invasion (Figure 5) in engineered HeLa cells, albeit with little change in matrix adhesion as a function of BAG construct expression (data not shown). This suggests that BAG3’s effects on adhesion, migration, and invasion may be related to cellular or matrix context. Consistent with our findings with the HeLa cells, Iwasaki reported that BAG3 overexpression induced the motility of MCF7, DU145, and ALVA31 cells that was reduced after BAG3 silencing [33]. Rac1 activity was also reduced in this setting; its downstream role in integrin and FAK signalling links those findings with ours [31,34].
A direct association between integrins and CCN1 has been described [24,27,35,36]. Our prior results coupled with the present identification of concomitant regulation and activity of CCN1 link integrin and matrix behaviour with BAG3-related behaviour. The change in integrin β1 phosphorylation correlated with both CCN1 expression and FAK phosphorylation patterns [8], reduced in FL- but not dPXXP-overexpressing cells. These results are also consistent with our previous studies showing that BAG3 silencing was accompanied by a significant rise in the number of focal adhesions in FL cells. CCN1 thus appears to be a factor linking the differential adhesion, migration, and invasion phenotypes of the BAG3 mutants [23].
AP-1 has been shown to increase CCN1 promoter function [26,27]. Stimulation of AP-1 with subsequent induction of CCN1 may be a mechanism through which absence of the BAG3 proline-rich domain alters the adhesive cellular phenotype. Our data infer that the BAG3 PXXP domain negatively regulates the AP-1 complex transcription factor that is responsible for induction of CCN1. This is consistent with our results showing that cell lines expressing the dPXXP BAG3 mutant both overexpress CCN1 mRNA and protein, and contain increased AP-1 DNA-binding and transcriptional activity. Transcriptional trans-regulation of CCN1 by domains of BAG3 may be a mechanism for BAG3 direction of a mesenchymal transition [9,37].
We postulate that AP-1 regulation by BAG3 occurs in a stepwise fashion through a heat-shock response mechanism. We have demonstrated that BAG3 is a co-chaperone for and binds to inducible and constitutive heat-shock proteins [6,16]. HSP70 has been shown to suppress AP-1 activation [38], and cellular inflammatory injury results in heat-shock protein up-regulation that in turn inhibits inflammatory mediators including NF -κB and AP-1 [39]. Closing this loop is recent work by Turco and co-workers showing that heat-shock factor-1 transcription factor (HSF-1) directly binds to heat-shock-responsive elements in the BAG3 promoter, activating BAG3 transcription [40]. Together, this implicates BAG3 in heat-shock protein regulation of AP-1, suggesting that full-length BAG3 may function as a co-repressor, with heat-shock proteins, of AP-1/NF -κB activity in stressed cells.
Our results confirm and expand on previously reported findings leading to a model whereby BAG3 influences the transcription regulation of CCN1, a pro-adhesion signalling protein that is active both cytoplasmically and extracellularly. CCN1 levels may regulate the degree of activity of adhesion signalling pathways as determined by the integrin β1/FAK/paxillin signalling cascades and their downstream effector molecules [8,27,31]. This study provides insight into the regulatory mechanism of co-chaperones such as BAG3.
Supplementary Material
Acknowledgements
EAG was a Howard Hughes Medical Institute/National Institutes of Health Research Scholar. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors have no conflicts of interest to declare.
Supporting information
Supporting information may be found in the online version of this article.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Kohn EC. The microenvironment of the tumour–hostinterface. Nature 2001;411:375–379. [DOI] [PubMed] [Google Scholar]
- 3.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, et al. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett 2001;503:151–157. [DOI] [PubMed] [Google Scholar]
- 4.Chiappetta G, Ammirante M, Basile A, Rosati A, Festa M, Monaco M, et al. The anti-apoptotic protein BAG3 is expressed in thyroid carcinomas and modulates apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Clin Endocrinol Metab 2007;92:1159–1163. [DOI] [PubMed] [Google Scholar]
- 5.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’? — Afunctional domain analysis of the BAG-family proteins. Cancer Lett 2002;188:25–32. [DOI] [PubMed] [Google Scholar]
- 6.Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem 2003;278:28490–28500. [DOI] [PubMed] [Google Scholar]
- 7.Pagliuca MG, Lerose R, Cigliano S, Leone A. Regulation byheavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett 2003;541:11–15. [DOI] [PubMed] [Google Scholar]
- 8.Kassis JN, Guancial EA, Doong H, Virador V, Kohn EC. CAIR-1/BAG-3 modulates cell adhesion and migration by down-regulating activity of focal adhesion proteins. Exp Cell Res 2006;312:2962–2971. [DOI] [PubMed] [Google Scholar]
- 9.Guarino M, Rubino B, Ballabio G. The role of epithelial–mesenchymal transition in cancer pathology. Pathology 2007;39:305–318. [DOI] [PubMed] [Google Scholar]
- 10.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol 2003;178:169–175. [DOI] [PubMed] [Google Scholar]
- 11.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, aproduct of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A 1998;95:6355–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61,a growth factor-inducible immediate-early gene. Mol Cell Biol 1990;10:3569–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Leu SJ, Todorovic V, Lam SC, Lau LF. Identificationof a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem 2004;279:44166–44176. [DOI] [PubMed] [Google Scholar]
- 14.Lau LF, Lam SC. The CCN family of angiogenic regulators: theintegrin connection. Exp Cell Res 1999;248:44–57. [DOI] [PubMed] [Google Scholar]
- 15.Leu SJ, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identificationof a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61). J Biol Chem 2003;278:33801–33808. [DOI] [PubMed] [Google Scholar]
- 16.Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA,et al. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene 2000;19:4385–4395. [DOI] [PubMed] [Google Scholar]
- 17.Gius D, Cui H, Bradbury CM, Cook J, Smart DK, Zhao S, et al. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell 2004;6:361–371. [DOI] [PubMed] [Google Scholar]
- 18.Eisen MB, Brown PO. DNA arrays for analysis of gene expression. Methods Enzymol 1999;303:179–205. [DOI] [PubMed] [Google Scholar]
- 19.Troyanskaya OG, Garber ME, Brown PO, Botstein D, Altman RB. Nonparametric methods for identifying differentially expressed genes in microarray data. Bioinformatics 2002;18:1454–1461. [DOI] [PubMed] [Google Scholar]
- 20.Dignam JD. Preparation of extracts from higher eukaryotes.Methods Enzymol 1990;182:194–203. [DOI] [PubMed] [Google Scholar]
- 21.Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, et al. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res 2000;60:6688–6695. [PubMed] [Google Scholar]
- 22.Smart DK, Ortiz KL, Mattson D, Bradbury CM, Bisht KS, Sieck LK, et al. Thioredoxin reductase as a potential molecular target for anticancer agents that induce oxidative stress. Cancer Res 2004;64:6716–6724. [DOI] [PubMed] [Google Scholar]
- 23.Walsh CT, Radeff-Huang J, Matteo R, Hsiao A, Subramaniam S,Stupack S, et al. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J 2008;22:4011–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Du XY. Functional properties and intracellular signalingof CCN1/Cyr61. J Cell Biochem 2007;100:1337–1345. [DOI] [PubMed] [Google Scholar]
- 25.Grote K, Bavendiek U, Grothusen C, Flach I, Hilfiker-Kleiner D,Drexler H, et al. Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem 2004;279:55675–55681. [DOI] [PubMed] [Google Scholar]
- 26.Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Eur J Biochem 2003;270:3408–3421. [DOI] [PubMed] [Google Scholar]
- 27.Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, et al. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem 2007;282:34594–34604. [DOI] [PubMed] [Google Scholar]
- 28.Pendurthi UR, Tran TT, Post M, Rao LV. Proteolysis of CCN1 byplasmin: functional implications. Cancer Res 2005;65:9705–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimura T, Esteban R. The bipartite 3-cis-acting signal for replication is required for formation of a ribonucleoprotein complex in vivo between the viral genome and its RNA polymerase in yeast 23 S RNA virus. J Biol Chem 2004;279:44219–44228. [DOI] [PubMed] [Google Scholar]
- 30.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 2002;277:46248–46255. [DOI] [PubMed] [Google Scholar]
- 31.Yeger H, Perbal B. The CCN family of genes: a perspective onCCN biology and therapeutic potential. J Cell Commun Signal 2007;1:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eble JA, Golbik R, Mann K, Kuhn K. The alpha 1 beta 1 integrinrecognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV). EMBO J 1993;12:4795–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki M, Homma S, Hishiya A, Dolezal SJ, Reed JC, TakayamaS. BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res 2007;67:10252–10259. [DOI] [PubMed] [Google Scholar]
- 34.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol 2007;19:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leu SJ, Chen N, Chen CC, Todorovic V, Bai T, Juric V, et al. Targeted mutagenesis of the angiogenic protein CCN1 (CYR61). Selective inactivation of integrin alpha6beta1-heparan sulfate proteoglycan coreceptor-mediated cellular functions. J Biol Chem 2004;279:44177–44187. [DOI] [PubMed] [Google Scholar]
- 36.Brakebusch C, Fassler R. Beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev 2005;24:403–411. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Weinberg RA. Epithelial–mesenchymal transition: Atthe crossroads of development and tumor metastasis. Dev Cell 2008;14:818–829. [DOI] [PubMed] [Google Scholar]
- 38.He H, Chen C, Xie Y, Asea A, Calderwood SK. HSP70 andheat shock factor 1 cooperate to repress Ras-induced transcriptional activation of the c-fos gene. Cell Stress Chaperones 2000;5:406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Currie RW. Small interfering RNA knocks down heat shock factor-1 (HSF-1) and exacerbates pro-inflammatory activation of NF-kappaB and AP-1 in vascular smooth muscle cells. Cardiovasc Res 2006;69:66–75. [DOI] [PubMed] [Google Scholar]
- 40.Franceschelli S, Rosati A, Lerose R, De Nicola S, Turco MC,Pascale M. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol 215:575–577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.