Abstract
Background:
Tuberculosis preventive therapy (TPT) is recommended for tuberculosis (TB) prevention among people living with HIV (PLHIV) and other high-risk groups. The Nigerian Military HIV Program embarked on TPT-specific ‘direct supportive supervision’ (DSS) in May 2018 to increase TPT initiation and completion rates.
Methods:
Interventional approaches included site visits to conduct root cause analysis, didactic teaching approach on the concepts of quality improvement and mentorship to address barriers. The DSS introduced TPT monitoring tools, sticker reminders on clients’ folders, and bi-weekly data collection and review for decision making.
Results:
TPT initiation increased from a monthly pre-intervention median of 323 clients to monthly medians of 2611 during the ‘surge’ and 1212 clients during the ‘sustained’ phases. Due to an isoniazid stock-out, a ‘dip phase’, with a median of 559 clients was recorded. Overall, 10 463 clients were started on TPT in fiscal year (FY) 2018 and 12 596 in FY2019, with an overall initiation rate of 79%. Completion rates were respectively 73% and 70% for FY2018 and FY2019.
Conclusion:
With the implementation of a tailored DSS, programmatic barriers to TPT were easily identified and quickly addressed to increase initiation and completion rates.
Keywords: tuberculosis, people living with HIV, TPT, isoniazid
Abstract
Contexte :
Le traitement préventif de la tuberculose (TPT) est recommandé pour la prévention de la tuberculose (TB) parmi les personnes vivant avec le VIH (PVVIH) et pour d’autres groupes à risque élevé. Le programme VIH de l’armée au Nigeria a rencontré de nombreux défis dans sa mise en œuvre.
Méthode :
En mai 2018, le programme s’est lancé dans une « supervision directe de soutien » spécifique du TPI (DSS) en vue de son expansion et de la réduction de la transmission de la TB chez les PVVIH. Les approches d’intervention mises en œuvre ont inclus des visites sur le terrain afin d’analyser les causes profondes, une approche didactique d’enseignement relatif aux concepts d’amélioration de la qualité et un tutorat afin de surmonter les obstacles. On a eu recours à l’introduction d’un outil de suivi du TPT, à des rappels sous forme de stickers sur les dossiers des clients et à un recueil de données et à une revue destinée à la prise de décisions bi hebdomadaire.
Résultats :
La mise en route du TPT a augmenté d’une médiane mensuelle avant l’intervention de 323 clients à 2611 et 1212 clients pendant les phases de lancement et de continuation respectivement. En raison d’une rupture de stock d’isoniazide, une phase de chute avec une médiane de 559 clients a été enregistrée. Au total, 10 463 et 12 596 clients ont débuté le TPT en FY2018 et FY2019 respectivement, avec un taux d’ensemble d’initiation de 79%. Les taux d’achèvement ont été de 73% et 70% respectivement pour FY2018 et FY2019.
Conclusion :
Grâce à une stratégie sur mesure de supervision et de soutien et en tenant compte des contextes particuliers du terrain, les obstacles programmatiques à la mise en œuvre du TPI ont été facilement identifiés et rapidement affrontés pour une meilleure prise en charge du patient.
Abstract
Marco de referencia:
El tratamiento preventivo de la tuberculosis (TPT) se recomienda a las personas con infección por el virus de la inmunodeficiencia humana (VIH) y a otros grupos de alto riesgo. El programa contra el VIH de las fuerzas armadas de Nigeria afronta múltiples dificultades en la ejecución del TPT.
Método:
En mayo del 2018, el programa emprendió una iniciativa específica sobre el TPT de «supervisión directa de apoyo», con el fin de ampliar su escala de aplicación y disminuir la transmisión de la TB en las personas infectadas por el VIH. Entre las intervenciones introducidas estaban visitas a los centros para realizar análisis de las causas profundas, enfoques pedagógicos didácticos sobre los conceptos de mejoría de la calidad y orientación para responder a los obstáculos. Se puso en marcha una herramienta de seguimiento del TPT, adhesivos recordatorios en las carpetas de los usuarios y la recogida bimensual de datos con análisis para la toma de decisiones.
Resultados:
La adopción del TPT aumentó de una mediana mensual de 323 usuarios antes de la intervención, a medianas mensuales de 2611 durante la «fase de aumento rápido» y 1212 durante la «fase sostenida». Debido al desabastecimiento de isoniacida, se produjo una «fase de reducción » con una mediana de 559 usuarios. En general, 10 463 usuarios comenzaron el TPT en el ejercicio económico 2018 y 12 596 en el ejercicio 2019, con una tasa global de inicio de 79%. La tasa de compleción fue 73% en el ejercicio económico 2018 y 70% en el ejercicio 2019.
Conclusión:
Al aplicar una estrategia a la medida de supervisión de apoyo y tener en cuenta el contexto específico del centro, fue sencillo reconocer las barreras programáticas a la ejecución del TPT y se pudieron superar prontamente para prestar una mejor atención de los pacientes.
Globally, tuberculosis (TB) ranks with HIV as one of the most common causes of death by infectious disease.1,2 TB is also the highest cause of morbidity and mortality in people living with HIV (PLHIV), with a global proportion of 40% deaths among PLHIV arising from TB, as evidenced by post-mortem data.3 There are 1.7 million new HIV infections and 1 million AIDS-related deaths annually,4 with 10 million incident cases of TB worldwide, of which 9% were among PLHIV.5 TB and HIV interaction has also been described as a syndemic,6 as individuals infected with HIV are about 20–30 times more likely to develop TB than those without HIV.7
The World Health Organization (WHO) recommends prescribing TB preventive therapy (TPT) medications for PLHIV in high TB burden areas who screen negative for active TB.8 Nigeria has a HIV prevalence rate of 1.4% and an estimated TB burden of 1.9 million individuals nationally.9 Coupled with an incidence rate of 219 TB cases per 100 000 population, this presupposes that the risk posed by TB among PLHIV in Nigeria is substantial.5 The Nigerian National Tuberculosis and Leprosy Control Programme (NTBLCP), through funding from The Global Fund to Fight AIDS, TB, and Malaria (GFATM), and supplementary procurement by US President’s Emergency Plan for AIDS Relief (PEPFAR), provided 6-month TPT in the form of daily 300 mg tablets of isoniazid (INH) as INH preventive therapy (IPT) for adult PLHIV, and 5–10 mg of INH per kg of body weight for HIV-positive children, as well as other children aged <5 years who are contacts of a TB case.10
However, increasing IPT initiation among HIV clients in Nigeria has met with a number of programmatic challenges, including an unstructured IPT distribution and requisition system, lack of supervision by healthcare providers in initiating clients on IPT, myths about IPT, and poor recording and reporting. The Nigerian Military HIV Programme, a partnership program of the Nigerian Ministry of Defence-Health Implementation Programme and the Walter Reed Army Institute of Research (WRAIR), operates within Nigerian military health facilities to provide diagnostic, treatment and care services for HIV, TB and diagnostic services for malaria. In 2015, the Nigerian Military HIV Programme’s IPT initiation rate was only 5% in fiscal year 2016 (FY2016). In 2017, after a review by the PEPFAR in Nigeria, resolved bottlenecks saw IPT initiation improve from 7% in FY2016 to 12% in FY2017 for the overall PEPFAR-Nigeria program.11
Within this period, the IPT coverage of the Nigerian military program also showed some improvement, increasing from 5% to 9% (FY2016–FY2017 annual reports–unpublished). However, as HIV and latent TB increase the lifetime risk of active TB development to 50–70%, against 10% in HIV-negative persons,12 we developed a multi-pronged, provider-focused intervention to increase IPT initiation and completion rates. Here, we describe the IPT scale-up intervention and results of activities implemented at Nigerian military healthcare facilities.
METHODS
Intervention design and population
We implemented an IPT-specific direct supportive supervision (DSS) strategy in May 2018 at 27 Nigerian military health facilities/sites. These sites were chosen purposively depending on the site-specific context and were geographically distributed all over the country. They included a mix of primary healthcare-level clinics and secondary healthcare-level hospitals providing services to servicemen, their dependents and host community members: 28 270 clients received antiretroviral therapy (ART) in FY2017, 30 101 in FY2018, and 31 338 in FY2019.
Intervention description
The DSS strategy included visits (initial/first visit and then quarterly visits) to the sites (except six sites with ⩽400 ART clients that did not benefit from the initial visit) to conduct root cause analysis of poor IPT implementation, set up a system of collection of bi-weekly IPT initiation data, and provide mentorship and supportive supervision to address barriers to IPT initiation. Technical staff delivered a 1-day didactic teaching syllabus during the initial visits to approximately 6–15 key facility staff to introduce concepts of continuous quality improvement, including, but not limited to performance measurement indicators, model for improvement (Plan, Do, Study and Act cycle), run chart analyses and fish-bone diagrams.13 For sites not already using the IPT monitoring tool and sticker reminders on client folders, we introduced the following process: the IPT monitoring tool was inserted in the clients’ folders and used to document TB screening information, IPT eligibility and drug reactions (if any) prior to and upon IPT completion; sticker reminders were pasted on the front or inside of the folders to give healthcare providers a quick reference of the IPT status of clients at a glance, with information about start and completion dates.
All PLHIV clients seeking care at the intervention sites were routinely screened for TB using the four symptom checklist (cough, fever, night sweats, or weight loss).14 Clients who had any of the symptoms were further evaluated for TB. Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) is the recommended first-line diagnostic tool for TB in PLHIV.10 If Xpert was unavailable, a sputum acid-fast bacilli (AFB) and/or chest X-ray were used. Those with no symptoms were recommended for IPT initiation, barring no other contraindications such as TB treatment, TPT completion within the past 2 years, history of severe drug reactions, including severe cutaneous adverse reaction (SCAR).15 The IPT initiation rate estimate also did not consider clients who developed TB or those who may have died within the study period.
Data collection and analyses
IPT ‘uptake’ indicated three data elements: 1) number of PLHIV expected to complete a course of IPT, ‘Expected to complete’; 2) number of PLHIV who commenced a course of IPT, ‘Commenced’; and 3) number of PLHIV who completed a course of IPT, ‘Completed’. IPT ‘initiation’ indicated a single data element: number of PLHIV who commenced IPT within the reporting period, ‘Commenced’. However, in FY2017 only the ‘Commenced’ data was collected, as the other data elements were yet to be documented and reported at that time.16
Data management and analysis
De-identified, routinely collected program data for evaluation of the intervention were retrieved from the District Health Information System, version 2 (DHIS-2) (https://www.dhis2.org/), which is the repository for the program data. These data were entered at the facility-level into the DHIS-2 system from paper registers every month and data quality assessments were conducted at the sites during the quarterly site visits. We used Excel v2013 (Microsoft; Redmond, VA) to aggregate statistical data and compute percentage, median and interquartile range (IQR). We employed linear regression to develop a trend analysis to compare IPT initiation between the monthly reporting periods.
Ethical considerations
A non-human subjects research determination for this evaluation was obtained from the National Health Research Ethics Committee (NHREC), Abuja, Nigeria, and the Walter Reed Army Institute of Research Institutional Review Board (WRAIR IRB), Silver Spring, MD, United States.
RESULTS
We recorded a monthly median IPT initiation of 326 [IQR 168–423] clients during the pre-intervention period from October 2017 to April 2018. As implementation progressed, the number of clients initiated on IPT increased during the surge phase, reaching a monthly median of 2611 [IQR 1180–2960] from July to November 2018. There was a ‘dip’ in client numbers due to a national-level INH stock-out from December 2018 to April 2019, with a monthly median of 559 [IQR 545–586]. By May 2019, with resumption of INH distribution to sites, there was a resurgence during the sustained phase to a monthly median of 1212 [IQR 1113–1227] between May and September 2019 (Figure 1) .
FIGURE 1.
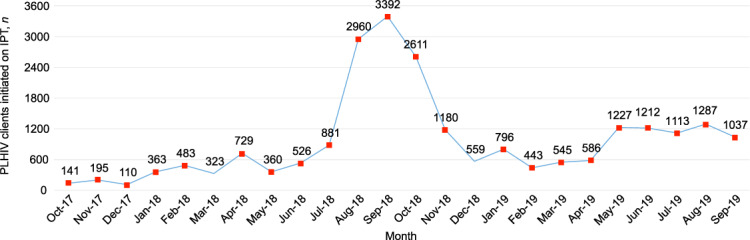
Monthly IPT initiation trends during the pre-intervention, surge, dip and sustained phases, Nigeria, October 2017–September 2019.
The annual data for FY2017, FY2018, and FY2019 also showed increasing levels of IPT uptake (Figure 2). In FY2018, 78% of the total data accrued from the beginning of the DSS from May to September 2018 ‘commenced’ (initiated). The completion rate for FY2018 and FY2019 was respectively 73% and 70% (Figure 2). Between FY2018 and FY2019, the DSS had reached an overall IPT initiation rate of 79% (23 059/29 303) for all its registered PLHIV clients, with an overall completion rate of 70%. The completion rates for the pre-intervention, surge, dip and sustained phases were respectively 70%, 66%, 66% and 73%.
FIGURE 2.
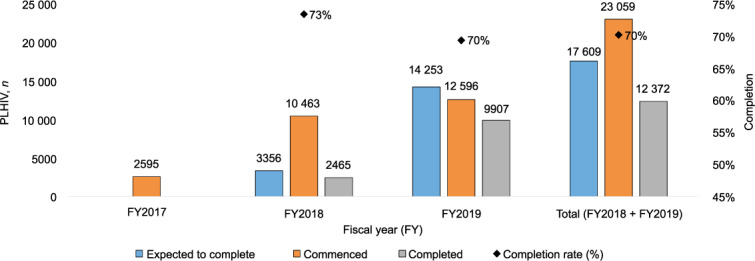
Annual IPT uptake and completion for FY2017, FY2018 and FY2019, Nigeria.
DISCUSSION
The implementation of the DSS strategy and other quality improvement initiatives increased IPT initiation and completion rates. Our data showed programmatic barriers to IPT were identified and addressed, leading to an overall increase of 79% in IPT initiation. The overall completion rate was 70%.
There were four distinct phases identified in our study: pre-intervention, surge, dip, and sustained. The pre-intervention phase was a passive phase, without calculated efforts to ensure increased IPT uptake. As the intervention began, there was a progressive improvement in IPT initiation, reaching a peak in the surge phase, which resulted from a heightened focus at the beginning of the implementation process. This led to utilization of all the stock in most of the sites. As the site-level stock-out was occasioned by a national level stock-out, the program could not resupply the sites. The effect of the stock-out led to initiation levels similar to the pre-intervention level, creating the dip phase in our scale-up process. Following availability of INH again at the national level, supply and distribution resumed at the sites, with a corresponding increase in the number of clients initiated on IPT. Some of the previously registered clients who could not be initiated on IPT because of the stock-out, and some newly registered PLHIV, started receiving their IPT prescriptions. As the monthly initiation levels stabilized, this led to the sustained phase that continued to September 2019. This sustained phase was expected as implementation was nearing saturation, with few previously registered and newly registered PLHIV clients remaining.
Although specific barriers to IPT scale-up, including INH stock-outs, had previously been identified,17 the national-level stock-out encountered during the course of the scale-up efforts was beyond our control. This stock-out incident emphasized the need for a consistent and sustainable system of supply chain for drugs and other commodities for effective program management.18 Poor supply chain systems could hinder disease control and potentially increasing morbidity and mortality, especially in developing countries, due to changes in drug regimens, incomplete course of drugs, or development of resistance.19 To mitigate these issues we ensured that all clients who were initiated on IPT had been allotted a full 6 month’s course, which was then dispensed during monthly refill visits.
The 2014 National TB Treatment Guidelines recommends a repeat course within 2 years of completing a 6-month course of IPT.10 This presupposes that the graphical representation of IPT implementation timeline could see the surge phase repeated because the bulk of these clients would become eligible for a repeat of IPT at the same time. The majority of the client pool that contributed to the surge phase and the sustained phase were previously registered PLHIV clients, as only a monthly average of 170 newly registered PLHIV clients entered the program during these phases. This represented just 6.5% and 14% of the monthly median of clients who started IPT during the surge phase and the sustained phase, respectively, or eight and four times the median number of clients who started on IPT during the pre-intervention phase, respectively. Before beginning scale-up initiatives, adequate preparation should therefore be taken to ensure stock availability to cater for the rapid utilization of INH that may occur in the surge and sustained phases.
Our study had several limitations. Data quality issues could have resulted from unclear documentation, missed documentation or over-reporting; some sites could have erroneously included refill clients as clients newly starting IPT. However, this possibility was minimized with the use of the IPT monitoring tool inserted in the clients’ folders and the IPT registers which document clients’ new drug pick-ups or refill periods, plus the quarterly data quality assessment visits. Also, as our data highlighted some of the dynamics that could exist in the implementation mi-lieu, it is possible that the effects of other quality improvement activities concurrently occurring at some of the sites could have over-estimated our results.
In conclusion, with the implementation of a tailored DSS, pro-grammatic barriers to IPT were identified and addressed to increase initiation and completion rates. We believe that the improvements seen in this DSS strategy is sustainable and we anticipate that it will translate to better outcomes for the PLHIV clients in the future, although more work still needs to be done to achieve a completion rate of at least 90%.
ACKNOWLEDGEMENTS
This research was supported by the President’s Emergency Plan for AIDS Relief through the US Department of Defense and funded via cooperative agreement (W81XWH-11-2-0174) between the Henry M Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA, and the US Department of Defense (DOD).
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2019. WHO/CDS/TB/2019.15. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 2.MacNeil A, Glaziou P, Sismanidis C, Floyd K, Maloney S. Global Epidemiology of Tuberculosis and Progress Toward Achieving Global Targets–2017. Morb Mortal Wkly Rep. 2019;68:263–266. doi: 10.15585/mmwr.mm6811a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Joint United Nations Program on HIV/AIDS. Fact Sheet World AIDS Day Report. Geneva, Switzerland: UNAIDS; 2019. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf Accessed April 2020. [Google Scholar]
- 5.World Health Organization. Global tuberculosis report, 2018. WHO/CDS/TB/2018.20. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 6.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Recommendation on 36 months isoniazid preventive therapy to adults and adolescents living with HIV in resource-constrained and high TB- and HIV-prevalence settings – 2015 update. Geneva, Switzerland: WHO; 2015. https://www.who.int/tb/publications/2015_ipt_update/en/ Accessed April 2020. [PubMed] [Google Scholar]
- 9.National Agency for the Control of AIDS (NACA). Future directions for the HIV/AIDS response in Nigeria; revised national HIV/AIDS Strategic frame work, 2019–2021. 2019. https://naca.gov.ng/revised-national-hiv-and-aids-strategic-framework-2019-2021/ Accessed May 2019. [Google Scholar]
- 10.National AIDS/STIs Control Programme. Federal Ministry of Health Nigeria. 2014. Integrated national guidelines for HIV prevention, treatment and care. [Google Scholar]
- 11.Odume B, Meribe SC, Odusote T et al. Taking tuberculosis preventive therapy implementation to national scale: the Nigerian PEPFAR Program experience. Public Health Action. 2020;10(1):7–10. doi: 10.5588/pha.19.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation. TB/HIV: a clinical manual for South East Asia. Geneva, Switzerland: WHO; 1997. http://www.who.int/iris/handle/10665/63310 Accessed May 2019. [Google Scholar]
- 13.Ishikawa K. Introduction to quality control. Productivity Press; 1990. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Tuberculosis: TB in specific populations—Children 2014. https://www.cdc.gov/tb/topic/populations/tbinchildren/default.htm Accessed November 2019. [Google Scholar]
- 15.Viswanath BK, Ranka P, Ramanjanayalu M. Severe cutaneous adverse reactions due to isoniazid in a HIV positive patient. Indian J Lepr. 2012;84(3):227–232. [PubMed] [Google Scholar]
- 16.President’s Emergency Plan for AIDS Relief (PEPFAR). MER 2.3 Indicator Reference Guide (FY19) Washington DC, USA: 2018. https://datim.zendesk.com/hc/en-us/articles/360000084446-MER-2-0-Indicator-Reference-Guide Accessed February 2020. [Google Scholar]
- 17.Teklay G, Teklu T, Legesse B, Tedla K, Klinkenberg E. Barriers in the implementation of isoniazid preventive therapy for people living with HIV in Northern Ethiopia: a mixed quantitative and qualitative study. BMC Public Health. 2016;16(1):840. doi: 10.1186/s12889-016-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. HIV/AIDS; Logistics Management and Information System (LMIS) Geneva, Switzerland: WHO; 2007. https://www.who.int/hiv/amds/lmis/en/ Accessed May 2019. [Google Scholar]
- 19.Bam L, McLaren ZM, Coetzee E, von Leipzig KH. Reducing stock-outs of essential tuberculosis medicines: a system dynamic modelling approach to supply chain management. Health Policy Plan. 2017;32(8):1127–1134. doi: 10.1093/heapol/czx057. [DOI] [PubMed] [Google Scholar]


