Dear Editor,
The current COVID-19 pandemic presents many unique challenges for occupational health medicine and infection prevention and control (IPC) in hospitals. While many hospital services were postponed during the pandemic's first peak, the resumption of routine services in an environment where patients still present with COVID-19 raises concerns over the potential for significant nosocomial COVID-19 transmission. We read with interest a recent study in this journal that emphasises the importance of identifying COVID-19 cases to prevent onward transmission of SARS-CoV-2.1 While the rapid identification and subsequent testing of symptomatic healthcare workers (HCW) is paramount, we believe there is a role for testing irrespective of symptoms. While HCW can acquire infections and contribute to cross-transmission during hospital outbreaks such as influenza or norovirus, asymptomatic staff are usually not routinely screened as part of the outbreak control measures. The role of COVID-19 surveillance of asymptomatic HCW has recently been highlighted and may become increasingly pertinent with new infection waves.2 , 3 However, the rapid detection of PCR-positive, asymptomatic staff is also crucial in the management of hospital clusters of COVID-19. Here, we discuss the high rate of SARS-CoV-2 PCR positivity among HCW during three inpatient ward outbreaks in Beaumont Hospital, Dublin, Ireland.
In March and April 2020, on three wards with two or more positive COVID-19 patients after three days of admission (designated as potential nosocomial infection), we implemented universal staff SARS-CoV-2 testing on that ward as part of outbreak management. A combined throat/nasopharyngeal swab was taken on HCW who had been working on the designated wards. RT-PCR was performed using Altona Diagnostics RealStar SARS-CoV-2 RT-PCR according to the manufacturer's instructions, on the Roche Light Cycler 480II.4
Demographics including age, sex and job title were collected. All results were disclosed to staff by occupational health and those testing positive were advised to remain off work for minimum 14 days, with appropriate follow up. In addition, details of symptoms, contacts and co-habitation were documented. Asymptomatic, SARS-CoV-2 PCR positive HCW were followed up by telephone for symptom development. Asymptomatic, SARS-CoV-2 PCR negative HCW were advised to self-monitor for symptoms and isolate if they were deemed a close contact of a known positive case.
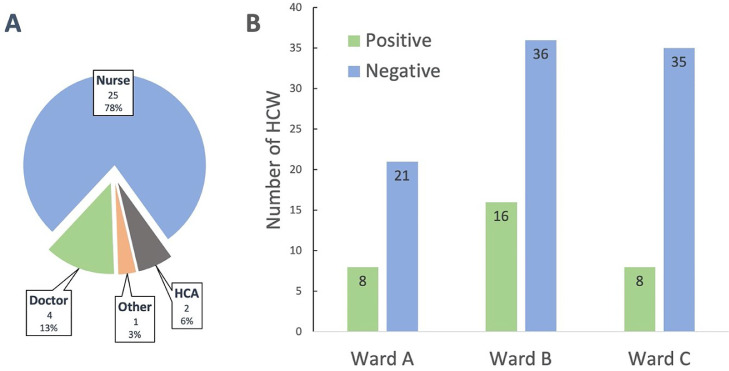
Thirty-two of 124 (26%) staff with a median age of 34 years (range 21 – 65 years) were SARS-CoV-2 PCR positive (see Fig. 1 ). Nine of these (28%) reported cohabitation with another HCW, three of whom worked in another healthcare facility. Symptoms at time of testing were present in 18 (56%), while 14 (44%) were asymptomatic. Symptoms included myalgia (n = 16), cough (n = 13), fever (n = 11), headache (n = 10), fatigue (n = 7), anosmia (n = 2) and diarrhea (n = 1), similar to what has previously been documented.1 Of the asymptomatic PCR-positive HCW, 11 (79%) subsequently developed symptoms, median 36 h later (interquartile range, IQR 24–72 h). Among the 92 PCR-negative HCW, nine (10%) subsequently became symptomatic and tested positive within two weeks, median 11 days later (IQR 7–12 days). Of note, the mean cycle threshold (CT) value of the 32 positive results was 24.7 (95% CI 23.06 – 26.35), although no difference was noted between those who were asymptomatic and those who were symptomatic at time of testing (25.5 in symptomatic PCR-positives compared with 23.8 in asymptomatic PCR-positives, p = 0.24).
Fig. 1.
SARS-CoV-2 PCR results from 124 staff members during an outbreak investigation. HCW positive by profession (A) and ward breakdown of PCR results (B). HCW; healthcare worker, HCA; healthcare assistant.
Staff screening is now part of our hospital's COVID-19 response on wards with a high burden of infection. While the burden of COVID-19 on the three surveyed wards may have influenced the high prevalence of HCW infection, community and household acquisition cannot be excluded. Although 44% of those who tested positive were asymptomatic at the time of testing, the majority developed symptoms within three days, suggesting that the test was carried out during an incubation period. This nonetheless makes IPC problematic, especially in clinical areas where physical distancing is often difficult to achieve when providing routine clinical care. The issue of universal mask use in clinical areas has been discussed as a measure to prevent transmission from asymptomatic and minimally symptomatic HCW5. In this review, the level of asymptomatic staff carriage and the smaller number of staff in the pre-symptomatic stage, where viral shedding may commence three days before symptoms,6 supports the use of masks, in addition to hand hygiene and other routine IPC measures. On March 31st the hospital introduced a policy that in all clinical areas, if physical distancing could not be achieved, surgical masks should be worn by HCW.
Interestingly, 11 (61%) of the symptomatic, PCR-positive HCW worked while symptomatic, similar to other reports.7 This may reflect HCW presenteeism which has been reported previously during influenza seasons.8 As asymptomatic (or indeed, pre-symptomatic) HCW may have similar viral loads and may be capable of transmission as much as symptomatic individuals,9 their detection and subsequent exclusion from work is an important aspect of a hospital's COVID-19 strategy.
In conclusion, as hospitals begin to reopen to routine non-COVID-19 services, HCW SARS-CoV-2 testing irrespective of symptoms should be considered, particularly as part of outbreak management to rapidly prevent onward transmission to patients and other staff. Detecting SARS-CoV-2 positive staff will benefit not only public health measures, but in the case of staff cohabiting with other HCW, will also benefit other healthcare institutions.
References
- 1.Jary A., Flandre P., Chabouis A., et al. Clinical presentation of Covid-19 in health care workers from a French University Hospital. J Infect;0. DOI:10.1016/J.JINF.2020.06.048. [DOI] [PMC free article] [PubMed]
- 2.TA T., C M., M B. COVID-19: PCR Screening of Asymptomatic Health-Care Workers at London Hospital. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil A., Hill R., Ladhani S., Pattisson K., O'Brien P.COVID-19 screening of health-care workers in a London maternity hospital. Lancet Infect Dis DOI:10.1016/S1473-3099(20)30403-5. [DOI] [PMC free article] [PubMed]
- 4.Altona Diagnostics; RealStar ® SARS-CoV-2 RT-PCR Kit 1.0. https://altona-diagnostics.com/files/public/ContentHomepage/- 02 RealStar/INS - RUO - EN/RealStar SARS-CoV-2 RT-PCR Kit 1.0_WEB_RUO_EN-S02.pdf (accessed June 21, 2020).
- 5.Klompas M., Morris C.A., Sinclair J., Pearson M., Shenoy E.S. Universal Masking in Hospitals in the Covid-19 Era. N Engl J Med. 2020 doi: 10.1056/NEJMp2006372. NEJMp2006372. [DOI] [PubMed] [Google Scholar]
- 6.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020:1–4. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 7.Kluytmans M., Buiting A., Pas S. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv. 2020 2020.03.23.20041913. [Google Scholar]
- 8.S C., CL B., X Y. Working With Influenza-Like Illness: presenteeism Among US Health Care Personnel During the 2014-2015 Influenza Season. Am J Infect Control. 2017;45 doi: 10.1016/J.AJIC.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou L., Ruan F., Huang M. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]