Abstract
Background
More than 10 years have elapsed since human papillomavirus (HPV) vaccination was implemented. We did a systematic review and meta-analysis of the population-level impact of vaccinating girls and women against human papillomavirus on HPV infections, anogenital wart diagnoses, and cervical intraepithelial neoplasia grade 2+ (CIN2+) to summarise the most recent evidence about the effectiveness of HPV vaccines in real-world settings and to quantify the impact of multiple age-cohort vaccination.
Methods
In this updated systematic review and meta-analysis, we used the same search strategy as in our previous paper. We searched MEDLINE and Embase for studies published between Feb 1, 2014, and Oct 11, 2018. Studies were eligible if they compared the frequency (prevalence or incidence) of at least one HPV-related endpoint (genital HPV infections, anogenital wart diagnoses, or histologically confirmed CIN2+) between pre-vaccination and post-vaccination periods among the general population and if they used the same population sources and recruitment methods before and after vaccination. Our primary assessment was the relative risk (RR) comparing the frequency (prevalence or incidence) of HPV-related endpoints between the pre-vaccination and post-vaccination periods. We stratified all analyses by sex, age, and years since introduction of HPV vaccination. We used random-effects models to estimate pooled relative risks.
Findings
We identified 1702 potentially eligible articles for this systematic review and meta-analysis, and included 65 articles in 14 high-income countries: 23 for HPV infection, 29 for anogenital warts, and 13 for CIN2+. After 5–8 years of vaccination, the prevalence of HPV 16 and 18 decreased significantly by 83% (RR 0·17, 95% CI 0·11–0·25) among girls aged 13–19 years, and decreased significantly by 66% (RR 0·34, 95% CI 0·23–0·49) among women aged 20–24 years. The prevalence of HPV 31, 33, and 45 decreased significantly by 54% (RR 0·46, 95% CI 0·33–0·66) among girls aged 13–19 years. Anogenital wart diagnoses decreased significantly by 67% (RR 0·33, 95% CI 0·24–0·46) among girls aged 15–19 years, decreased significantly by 54% (RR 0·46, 95% CI 0.36–0.60) among women aged 20–24 years, and decreased significantly by 31% (RR 0·69, 95% CI 0·53–0·89) among women aged 25–29 years. Among boys aged 15–19 years anogenital wart diagnoses decreased significantly by 48% (RR 0·52, 95% CI 0·37–0·75) and among men aged 20–24 years they decreased significantly by 32% (RR 0·68, 95% CI 0·47–0·98). After 5–9 years of vaccination, CIN2+ decreased significantly by 51% (RR 0·49, 95% CI 0·42–0·58) among screened girls aged 15–19 years and decreased significantly by 31% (RR 0·69, 95% CI 0·57–0·84) among women aged 20–24 years.
Interpretation
This updated systematic review and meta-analysis includes data from 60 million individuals and up to 8 years of post-vaccination follow-up. Our results show compelling evidence of the substantial impact of HPV vaccination programmes on HPV infections and CIN2+ among girls and women, and on anogenital warts diagnoses among girls, women, boys, and men. Additionally, programmes with multi-cohort vaccination and high vaccination coverage had a greater direct impact and herd effects.
Introduction
More than 10 years after the licensure of the first human papillomavirus (HPV) vaccines, 99 countries and territories have introduced HPV vaccination programmes.1,2 Observational data showing the population-level impact of HPV vaccination from the early adopting countries can be immensely useful for decision makers examining whether to introduce or modify HPV vaccination programmes. This is because such data show the effectiveness of HPV vaccines in real-world settings and can assist in the identification of programme characteristics that lead to the greatest reductions in HPV-related infections and diseases.
In 2015, we did a systematic review and meta-analysis of the population-level impact of HPV vaccination, including data from nine high-income countries up to 4 years after the introduction of HPV vaccination.3 Our meta-analysis showed substantial decreases in HPV 16 and 18 infections and anogenital wart diagnoses among girls and young women targeted for vaccination. Furthermore, in countries with high vaccination coverage (≥ 50%) there was evidence of vaccine cross-protection and herd effects, with significant reductions in HPV 31, 33, and 45 infections among girls targeted for vaccination and anogenital wart diagnoses among unvaccinated boys and older women. However, in our meta-analysis of 2015, the number of years after vaccination was insufficient to examine the impact of HPV vaccination on the occurrence of cervical intraepithelial neoplasia grade 2+ (CIN2+). CIN2+ may take several years to develop, and is the most proximal outcome for cervical cancer.4
We wanted to update our systematic review and meta-analysis for three main reasons. First, the number of countries and studies reporting observational data of the population-level impact of HPV vaccination has increased dramatically since our first review, which will improve both the power and generalisability of the results. Second, the number of years after vaccination has increased, which allows analysis of changes in CIN2+ since the introduction of HPV vaccination. Finally, the WHO Strategic Advisory Group of Experts on Immunization revised its position in 2016 to recommend HPV vaccination of multiple age cohorts of girls (9–14 years old) when the vaccine is introduced in a country, rather than vaccination of a single age cohort.5 Before this recommendation, some high-income countries had implemented vaccination of multiple age cohorts, mainly through catch-up campaigns. A better understanding of the population-level impact of will help inform decisions of policy makers regarding the recent WHO recommendations.
Thus, the aims of this systematic review and meta-analysis are to: (1) update and summarise the most recent evidence about the population-level impact of girls-only HPV vaccination on HPV infections and anogenital wart diagnoses among girls, boys, women, and men; (2) summarise new evidence about the population-level impact of girls-only HPV vaccination on CIN2+ occurrence among screened girls and women; and (3) compare the population-level impact of HPV vaccination on anogenital wart diagnoses and CIN2+ occurrence between countries that have implemented either a single or a multiple age-cohort vaccination strategy.
Methods
Search strategy and selection criteria
In this updated systematic review and meta-analysis, we used the same search strategy as in our previous paper3 and report our methods in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (appendix pp 4–5).6 Studies were eligible if they compared the frequency (prevalence or incidence) of at least one HPV-related endpoint (genital HPV infections, anogenital wart diagnoses, or histologically confirmed CIN2+) between pre-vaccination and post-vaccination periods among the general population and if they used the same population sources and recruitment methods before and after vaccination. For CIN2+, the population was restricted to screened girls and women, to limit the effect of changes in screening recommendations and participation since the introduction of HPV vaccination. Finally, because our aim was to examine the population-level impact of HPV vaccination programmes, we excluded studies if HPV vaccination was administered as part of a randomised trial or if no data were available for the pre-vaccination period.
To update our first systematic review (of studies published between Jan 1, 2007, and Feb 28, 2014), we searched MEDLINE and Embase for studies published between Feb 1, 2014, and Oct 11, 2018, with the same combination of MeSH terms, title, or abstract words: (“papillomavirus vaccine”, “papillomavirus vaccination”, “HPV vaccine”, or “HPV vaccination”) and (“program evaluation”, “population surveillance”, “sentinel surveillance”, “incidence”, or prevalence”), and (“papillomavirus infection”, “condylomata acuminata”, “anogenital warts”, “cervical intraepithelial neoplasia”, “cervical dysplasia”, “uterine cervical neoplasm”, or “HPV related diseases”) (appendix p 6). ÉB or NP and MD independently identified eligible articles on title and abstract first, and then on the full text. Disagreement between reviewers was solved by discussion between those authors. Finally, we searched the reference lists of selected articles.
Data analysis
Our primary assessment was the relative risk (RR) comparing the frequency (prevalence or incidence) of HPV-related endpoints between the pre-vaccination and post-vaccination periods. For HPV infection, we focused on three subgroups of HPV types: (1) HPV 16 and 18, (2) HPV 31, 33, and 45, and (3) all high-risk types except HPV 16 and 18. MD, ÉB, and NP extracted the study characteristics and outcomes using a standardised form. MD, ÉB, NP, and MB assessed the methodological quality of all studies, independently from the authors of the original studies, using the criteria developed for our first systematic review (appendix pp 7–22). Potential bias and risk of confounding were assessed by examining endpoint definitions, algorithms used to identify cases, and procedures used to select or identify participants. We also examined potential confounders specific to each HPV-related endpoint. Then, MD contacted all corresponding authors of eligible studies to request a re-analysis of their data using the same data stratifications (eg, by age group or HPV type) to allow comparison between studies and pooling. All authors were able to provide these data, and in collaboration with authors from the different countries, MD, ÉB, and NP also collected detailed information about the characteristics of HPV vaccination programmes within each country or region (routine programmes and catch-up campaigns), vaccination coverage, and cervical cancer screening recommendations and participation (appendix pp 23–31). Finally, all authors of eligible studies confirmed that the information and data from their study, which we included in our manuscript, were accurate.
For all endpoints we stratified analyses by sex, age, and years since the introduction of HPV vaccination. A priori, we chose to present the RRs stratified into two time categories, to reflect the post-vaccination follow-up period used in our first meta-analysis (1–4 years) and the additional years available for the current update (5–8 years for HPV infections and anogenital warts and 5–9 years for CIN2+). Additionally, we stratified analyses for anogenital warts by the type of vaccine administered, since only the quadrivalent vaccine includes HPV 6 and 11 (associated with 85–95% of anogenital warts).7 We used prevalence or incidence rate ratios as the measure of effect for all HPV-related endpoints (according to the data available from each study). For HPV infections, most studies directly presented crude or adjusted RRs with 95% CIs. We preferably included RRs adjusted for indicators of sexual activity or socioeconomic status in the meta-analysis, but used crude RRs if adjusted estimates were not available. For anogenital warts and CIN2+, studies presented the annual frequency (prevalence or incidence) of the endpoint over time for the pre-vaccination and post-vaccination periods. For these endpoints, we estimated the pre-vaccination frequency by aggregating the data for up to 3 years before vaccination and calculated the crude RR by dividing each post-vaccination year by the pre-vaccination estimate (appendix pp 32–34). We used random-effects models on a log scale in Review Manager (version 5.3.5) to obtain pooled estimates of the effect of HPV vaccination for each HPV-related endpoint.8,9 We used I2 and χ2 statistics to assess heterogeneity across studies, with the p value associated with the χ2 statistic representing the significance of heterogeneity.10
The number of studies available for each HPV-related endpoint was too small for us to do multivariate meta-regression analyses.10 Therefore, we did subgroup analyses to identify the main sources of heterogeneity between studies. First, we examined the effects of vaccination coverage and number of vaccinated cohorts, given that vaccination of single or multiple cohorts is a key policy question. Because HPV endpoints were estimated from different types of studies, the available information about vaccination coverage and number of cohorts vaccinated varied across different types of endpoints. For HPV infections, the vaccination status was directly available for all study participants (except for Dillner and colleagues).11 Hence, we used the age-specific proportion of individuals vaccinated with at least one dose in each study and dichotomised the studies into either high (≥50%) or low (<50%) vaccination coverage. For anogenital warts, most studies were based on population or insurance registries of a country or region. Hence, we used the overall proportion of people vaccinated in the country or region and dichotomised studies into either a medium or high proportion of people vaccinated (if the country or region vaccinated multiple cohorts of girls, with a vaccination coverage ≥50% for at least two doses among the routine cohort) or a low proportion of people vaccinated (if the country or region vaccinated single cohorts of girls or had a coverage for at least two doses <50% among the routine cohort). For CIN2+, studies were based on screened girls and women from screening registries. However, because individual-level data were not available for screened girls and women for all studies, we used the overall country-level or regional-level data and dichotomised studies using the same categories as for anogenital warts (appendix pp 23–28). Second, we examined the effects of data sources (population-based, health provider-based or insurance-based, clinic-based data) and vaccines used (bivalent or quadrivalent) on all endpoints. Finally, we examined relevant endpoint-specific sources of heterogeneity. Studies of HPV infection reported either adjusted or crude RR, so we examined the effect of RR adjustment (yes, no). CIN2+ detection can be influenced by screening recommendations and participation, so we examined the potential effect of a presence or absence of HPV testing during the study period, and the potential effect of an introduction of HPV testing, older age at screening start, and changes in the screening interval during the study period (yes, no).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MB had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
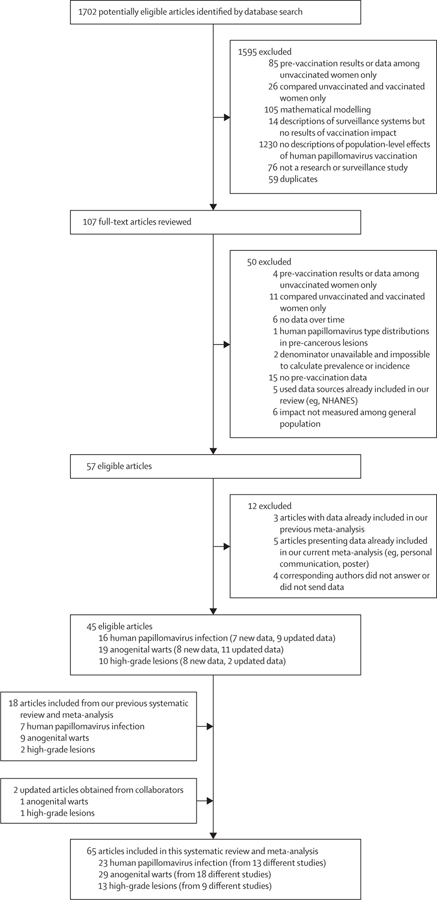
We identified 1702 potentially eligible articles, and included 65 articles (from 40 studies) in this systematic review and meta-analysis: 23 for HPV infection,11–33 29 for anogenital warts,34–62 and 13 for CIN2+ (figure 1).65–77 The studies were done in 14 high-income countries and cumulated data from more than 60 million individuals over 8 years (2007–15; 9 years for CIN2+; appendix pp 35–41). The vaccination programmes, vaccination coverage, and cervical screening recommendations and participation varied substantially between countries (appendix pp 23–31). As of 2015, 12 (86%) of the 14 countries included in this review were vaccinating girls and women only, with three doses of the bivalent or quadrivalent vaccine (appendix pp 23–28). The only exceptions were Australia and the USA; Australia switched to a gender-neutral programme in 2013 (6 years after the implementation of HPV vaccination) and the USA recommended gender-neutral vaccination in 2011 (two-dose vaccination coverage among boys remained below 20% until 2013, 7 years after the implementation of HPV vaccination). The age of girls and women targeted for vaccination also varied between countries (appendix pp 23–28). The age of routine vaccination varied slightly between countries, from 10 to 13 years old. Most countries with multi-cohort vaccination targeted girls up to 18 years of age through routine and catch-up programmes. However, Australia, the USA, and Denmark targeted women up to 26 years of age (with decreasing coverage as age increased). All studies were of sufficiently high methodological quality to be included in the meta-analysis; no studies were found with risk of serious bias (appendix pp 7–22).
Figure 1: Study selection.
Two articles on anogenital warts from our previous systematic review and meta-analysis were not included in this update: Sandø and colleagues63 and Nsouli-Maktabi and colleagues.64 Sandø and colleagues63 was one of two studies we had previously found that analysed the entire Danish population for the same period, but we included the study by Baandrup and colleagues38 in our current analysis because they updated their data and had a longer follow-up. We excluded the study by Nsouli-Maktabi and colleagues64 because it was done among members of the US armed forces and not in the general population. NHANES=National Health and Nutrition Examination Survey.
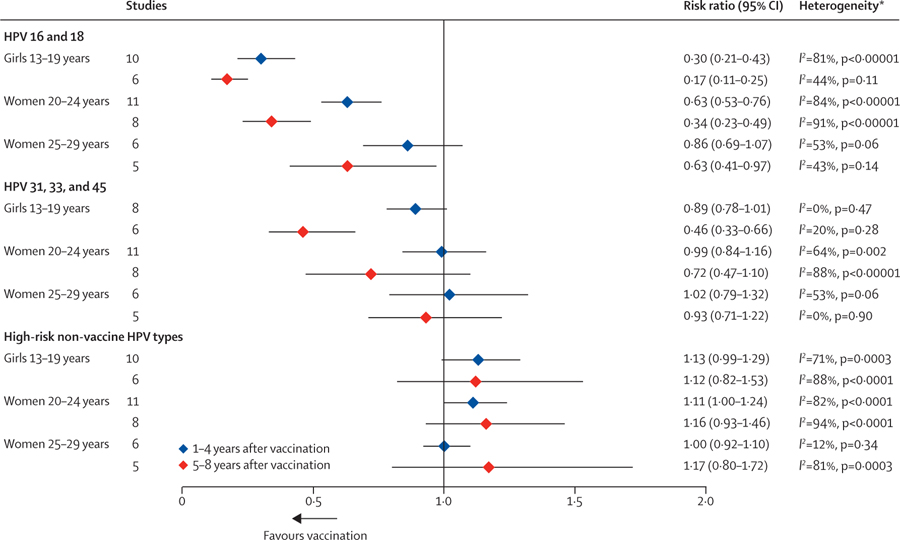
In the first 4 years following the introduction of HPV vaccination, the prevalence of HPV 16 and 18 decreased significantly among girls aged 13–19 years and women aged 20–24 years compared with the pre-vaccination period (figure 2; appendix pp 47–49). After 5–8 years of vaccination, the prevalence of HPV 16 and 18 decreased significantly by 83% (RR 0·17, 95% CI 0·11–0·25) among girls aged 13–19 years, and decreased significantly by 66% (RR 0·34, 95% CI 0·23–0·49) among women aged 20–24 years. Among women aged 25–29 years the prevalence of HPV 16 and 18 did not significantly change in the first 4 years of vaccination, whereas after 5–8 years of vaccination the prevalence decreased significantly by 37% (RR 0·63, 95% CI 0·41–0·97). Most women aged 25–29 years were unvaccinated.
Figure 2: Changes in the prevalence of HPV infections between pre-vaccination and post-vaccination periods.
HPV=human papillomavirus. *p values are associated with the χ2 statistic.
For HPV 31, 33, and 45 (cross-protective types), there were substantial but non-significant decreases in prevalence in the first 4 years of vaccination among girls aged 13–19 years. However, after 5–8 years of vaccination, the prevalence of HPV 31, 33, and 45 decreased significantly by 54% (RR 0·46, 95% CI 0·33–0·66) among girls aged 13–19 years, and decreased non-significantly by 28% (RR 0·72, 95% CI 0·47–1·10) among women aged 20–24 years. Among women aged 25–29 years the prevalence of HPV 31, 33, and 45 did not significantly change in the first 4 years of vaccination or after 5–8 years of vaccination. Finally, in all age groups, slight (but non-significant) increases in prevalence of high-risk HPV types not included in the vaccine were observed (figure 2).
In subgroup analyses, studies in which participants had a high vaccination coverage (≥50%) generally had a greater decrease in prevalence of HPV 16 and 18 and HPV 31, 33, and 45 compared with studies in which participants had a low vaccination coverage (<50%). However, the differences in prevalence were not always significant (appendix pp 42–43). Studies that used clinic-based data also showed greater decreases in prevalence of HPV 16 and 18 compared with studies that used population-based data. Among girls aged 13–19 years and during the first 4 years of vaccination, studies that used clinic-based data or had a high vaccination coverage showed greater increases in prevalence of high-risk HPV types other than HPV 16 and 18. However, these changes were not maintained with a longer post-vaccination follow-up period and were not consistent across different age groups.
Only two studies were available for genital HPV infections among boys and men (appendix p 50).13,31 Non-significant decreases in prevalence of HPV 16 and 18 (RR 0·35, 95% CI 0·09–1·40) and HPV 31, 33, and 45 (RR 0·31, 95% CI 0·06–1·58) were observed among boys aged 16–19 years in the first 4 years of girls-only vaccination. The decreases were very similar after 5–8 years of HPV vaccination in the study by Chow and colleagues.13 No significant changes were observed among men aged 20–24 years.
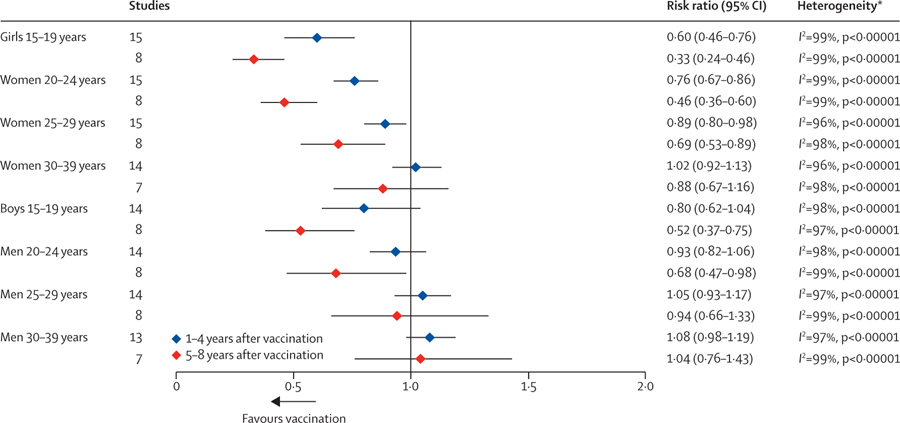
In the first 4 years following the implementation of quadrivalent HPV vaccination, anogenital wart diagnoses decreased significantly among girls and women aged 15–19, 20–24 years, and 25–29 years. Additionally, non-significant but substantial decreases were observed among unvaccinated boys aged 15–19 years (figure 3; appendix pp 51–58). After 5–8 years of HPV vaccination, decreases in anogenital wart diagnoses were significant for girls and women aged 15–29 years and for boys and men aged 15–24 years (figure 3). Anogenital wart diagnoses decreased significantly by 67% (RR 0·33, 95% CI 0·24–0·46) among girls aged 15–19 years, decreased significantly by 54% (RR 0·46, 95% CI 0·36–0·60) among women aged 20–24 years, and decreased significantly by 31% (RR 0·69, 95% CI 0·53–0·89) among women aged 25–29 years. Among boys aged 15–19 years anogenital wart diagnoses decreased significantly by 48% (RR 0·52, 95% CI 0·37–0·75) and among men aged 20–24 years they decreased significantly by 32% (RR 0·68, 95% CI 0·47–0·98). Three studies examined changes in anogenital wart diagnoses following the implementation of bivalent vaccination and results suggest a slight decrease among girls and women aged 15–19 and 20–24 years, and boys aged 15–19 years (appendix pp 51–58).
Figure 3: Changes in anogenital wart diagnoses between pre-vaccination and post-vaccination periods in countries using the quadrivalent vaccine.
*p values are associated with the χ2 statistic.
In subgroup analyses, studies in countries with multi-cohort vaccination and high vaccination coverage showed significantly greater decreases in anogenital wart diagnoses among girls, women, boys, and men aged 14–29 years, compared with studies in countries with cohort vaccination or low vaccination coverage (appendix pp 44–45).
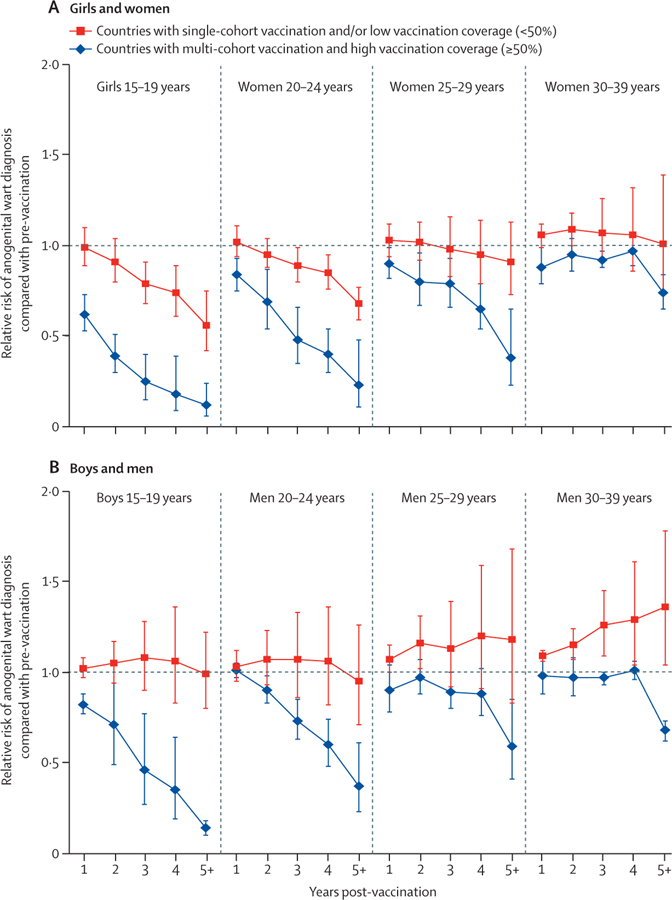
The rapid and significant decrease in anogenital wart diagnoses over time among girls, women, boys, and men aged 15–19, 20–24, and 25–29 years is clearly illustrated in figure 4, in countries vaccinating multiple cohorts of girls and women with high routine vaccination coverage. The decrease was slower in countries vaccinating a single cohort of girls or in countries with low routine vaccination coverage. In these countries, significant decreases in anogenital wart diagnoses among girls and women aged 15–19 and 20–24 years were only observed after 2 years of vaccination. Additionally, increases in anogenital wart diagnoses were observed among the oldest cohorts of men (figure 4). A sensitivity analysis restricted to countries with high vaccination coverage showed that multi-cohort vaccination provided substantial additional reductions in anogenital wart diagnoses compared with single-cohort vaccination (appendix p 61).
Figure 4: Changes in anogenital wart diagnoses during the 8 years after the introduction of girls-only human papillomavirus vaccination in countries using the quadrivalent vaccine.
We stratified by number of cohorts vaccinated and routine vaccination coverage. Countries with single-cohort vaccination and/or low coverage (in red) were Canada (Ontario,45 Manitoba49,50) and Italy41 with single-cohort vaccination and high coverage; and Germany,54,55 Belgium,42 Sweden,51,52 and the USA40,43,44 with multi-cohort vaccination and low coverage. Countries with multi-cohort vaccination and high coverage (in blue) were Australia,34,37,46,53,58 Denmark,38,39 New Zealand,56,57 and Canada (Quebec61).
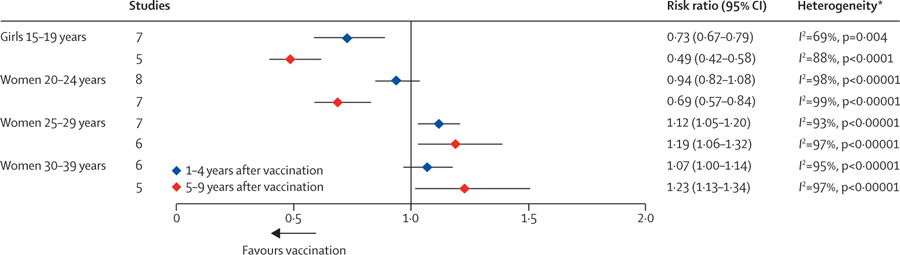
In the first 4 years following the introduction of HPV vaccination, significant decreases in CIN2+ were only observed among screened girls aged 15–19 years (figure 5; appendix pp 59–60). After 5–9 years of HPV vaccination, CIN2+ decreased significantly by 51% (RR 0·49, 95% CI 0·42–0·58) among screened girls aged 15–19 years and decreased significantly by 31% (RR 0·69, 95% CI 0·57–0·84) among women aged 20–24 years. However, in this same period CIN2+ increased significantly by 19% (RR 1·19, 95% CI 1·06–1·32) among screened and mostly unvaccinated women aged 25–29 years and increased significantly by 23% (RR 1·23 [95% CI 1·13–1·34]) among screened and mostly unvaccinated women aged 30–39 years.
Figure 5: Changes in CIN2+ among screened girls and women between the pre-vaccination and post-vaccination periods.
CIN2+=cervical intraepithelial neoplasia grade 2+. *p values are associated with the χ2 statistic.
In subgroup analyses, countries with and high routine vaccination coverage had greater decreases in CIN2+ among girls and women aged 15–24 years than the country with single-cohort vaccination or low routine vaccination coverage (appendix p 46). The study by Pollock and colleagues77 in a country that implemented the bivalent vaccine also showed greater decreases in CIN2+ among women aged 20–24 years, compared with studies in countries that implemented the quadrivalent vaccine; the country using the bivalent vaccine also had very high vaccination coverage. Subgroup analyses also showed that increases in CIN2+ among women aged 25–29 years in the post-vaccination period were significantly greater in the country with single-cohort vaccination or low routine vaccination coverage. None of the variables related to changes in screening recommendations and participation since the introduction of HPV vaccination were clearly associated with changes in CIN2+.
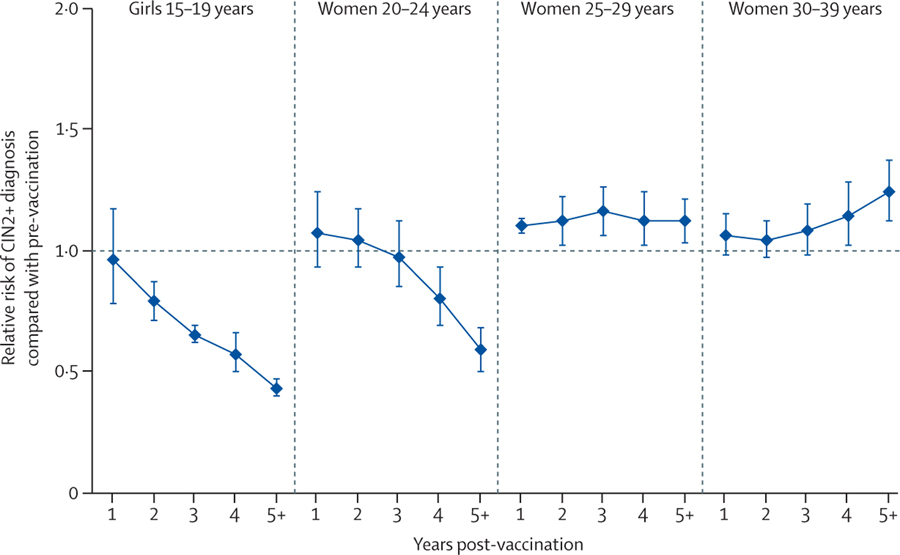
CIN2+ significantly decreased among girls aged 15–19 years after 1 year of vaccination and among women aged 20–24 years after 3 years of vaccination (figure 6). By contrast, CIN2+ significantly increased among mostly unvaccinated women aged 25–29 and 30–39 years.
Figure 6: Changes in CIN2+ among screened girls and women during the first 7 years after the introduction of girls-only human papillomavirus vaccination, in countries with multi-cohort vaccination and high vaccination coverage.
Countries with multi-cohort vaccination and high coverage (≥50%) were Australia,68,70 Canada (British Columbia76), Denmark,65 Scotland,77 and the USA.67,71,72,74 For CIN2+ analysis, the USA was categorised as a country with multi-cohort vaccination and high routine vaccination coverage because several US data indicate an association between screening participation and human papillomavirus vaccination.78,79,80,81 Thus, the vaccination coverage among screened girls and women is likely to be higher than the overall vaccination coverage in the population. We did a sensitivity analysis excluding the USA from countries with multi-cohort and high vaccination coverage and results were unchanged. CIN2+=cervical intraepithelial neoplasia grade 2+.
Discussion
This systematic review and meta-analysis, including data from 14 high-income countries, shows a significant and substantial impact of HPV vaccination on three HPV-related endpoints in the first 9 years after the start of HPV vaccination. Over this period, HPV 16 and 18 infections, anogenital wart diagnoses, and CIN2+ decreased significantly among girls and women. There was also evidence of vaccine cross-protection (HPV 31, 33, and 45 decreased significantly among girls younger than 20 years) and herd effects from girls-only vaccination programmes (anogenital warts diagnoses decreased significantly among boys and men). Finally, our meta-analysis illustrates the greater and faster direct impact and herd effects of HPV vaccination in countries with both multi-cohort vaccination and high routine vaccination coverage, compared with countries with single-cohort vaccination or low routine vaccination coverage. For example, after 5–8 years of HPV vaccination, anogenital wart diagnoses declined by 88% among girls and 86% among boys younger than 20 years in countries with multi-cohort vaccination and high routine vaccination coverage, compared with 44% among girls and 1% among boys in countries with single-cohort vaccination and low routine vaccination coverage.
Our study is the first to show the real-world additional benefit of multi-cohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures. After 5–8 years of girls-only vaccination in countries with multi-cohort vaccination and high routine vaccination coverage, reductions in anogenital wart diagnoses were 44 percentage points greater among girls aged 15–19 years than among girls the same age in countries with single-cohort vaccination or low routine vaccination coverage, and reductions in CIN2+ were more than 100 percentage points greater. Reductions in anogenital wart diagnoses among boys aged 15–19 years were 85 percentage points greater than among boys the same age in countries with single-cohort vaccination or low routine vaccination coverage. The greater impact of multi-cohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage. Our results are also in line with a 2017 mathematical modelling study82 that estimated that 5 years after the introduction of HPV vaccination in Australia, half of the observed declines in anogenital wart diagnoses would be attributable to multi-cohort vaccination (catch-up campaigns for women aged 14–26 years; appendix pp 23–28). In terms of policy implications, our results reinforce WHO’s recently revised position on HPV vaccination. To obtain faster and greater population-level impact, WHO revised its position in 2016 to recommend HPV vaccination of multiple age cohorts of girls (9–14 years old) when the vaccine is introduced in a country, rather than vaccination of a single cohort.5 However, the optimal number of age cohorts to vaccinate remains an open question and might be country-specific. Increasing the number of cohorts will increase the population-level impact, but will have diminishing returns on investment for each additional older cohort included. Number needed to vaccinate (NNV) and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost-effective.82,83 However, vaccine effectiveness per vaccine dose decreases after 18 years as a high proportion of individuals will already have been infected by HPV vaccine types at the time of vaccination, and three doses are recommended in this age group (in contrast with the recent recommendations84,85 of 2 doses for people vaccinated before the age of 15). Hence, decisions and recommendations about the number of age cohorts to be vaccinated is a trade-off between goals of maximising population-level impact (eg, to reach HPV or cervical cancer elimination goals within a specific time frame) and optimising vaccination efficiency and return on investment (eg, NNV and incremental cost-effectiveness ratios). Additionally, several key factors such as competing priorities and vaccine affordability and availability can also affect decisions about multi-cohort vaccination. Finally, our results also have implications for the interpretation of surveillance studies. When comparing HPV vaccination surveillance data between countries, the number of cohorts vaccinated should be considered in addition to vaccination coverage, as the main driver of population-level impact is the overall proportion of the population that is vaccinated.
Importantly, we also present the first pooled estimates of the population-level impact of HPV vaccination on CIN2+, which is the most proximal outcome to cervical cancer and is recognised as a valid proxy for vaccine efficacy against cervical cancer by regulatory agencies worldwide.86–89 The results provide strong evidence of HPV vaccination working to prevent cervical cancer in real-world settings, as both the cause (high-risk HPV infection) and proximal disease endpoint (CIN2+) are significantly declining. The results can also inform potential changes to cervical screening programmes. Substantial declines in high-risk HPV types and CIN2+ might allow for screening to start at an older age and for longer screening intervals. However, when considering any changes in screening recommendations, careful attention will have to be given to unvaccinated cohorts of women. The decreasing HPV prevalence observed in several settings also support a switch from cytology alone to primary HPV testing followed by cytology triage in younger and older cohorts, to benefit from the higher sensitivity of HPV testing to detect pre-cancerous lesions and higher specificity of cytology, without substantially increasing the number of false positive results.90,91 However, CIN2+ surveillance data among screened girls and women should be interpreted with caution. First, the greatest and fastest reductions in CIN2+ are among girls 15–19 years of age—an age group not always recommended for screening. In this age group the proportion of those screened had been declining both before and since the introduction of HPV vaccination efforts, due to improved adherence to guidelines (appendix pp 29–31). Therefore, although we restricted our analysis to screened girls and women, changes towards a lower risk profile among those that are still screened in this age group could partly contribute to decreases in CIN2+. However, to our knowledge there are currently no data supporting changes in risk profiles of screened women in the younger age groups since the introduction of HPV vaccination. Second, several studies have shown that participation in cervical screening and vaccination uptake are associated with the same sociodemographic factors (eg, ethnicity, socioeconomic level, education),78–80,92–94 and therefore vaccination coverage among screened girls and women might be different,81 and potentially higher, than country-level and regional-level vaccination coverage in some settings. Finally, major recent changes in screening recommendations, clinical management recommendations, and participation have been documented in several countries in the years surrounding the introduction of HPV vaccination. For example, the use of HPV testing mainly as triage of low-grade lesions (which has led to increased colposcopy referrals) and longer routine screening intervals have been reported67 in the USA, Denmark, and Norway, and are likely to increase the rate of CIN2+ detection (appendix pp 29–31). As the Scottish study77 of HPV bivalent vaccine uptake reported, future surveillance studies should include, if possible, the vaccination coverage of screened girls and women to more accurately quantify the impact of HPV vaccination on CIN2+.
By examining three main HPV-related endpoints concurrently, we can better understand trends in post-vaccination surveillance data and draw stronger conclusions about the population-level effectiveness and herd effects of HPV vaccination. Of particular interest are the results suggesting increases in HPV-related endpoints among population subgroups not targeted by vaccination: (1) high-risk non-vaccine HPV types; (2) anogenital wart diagnoses among men aged 25–39 years, particularly in countries with single-cohort vaccination or low vaccination coverage of girls; and (3) CIN2+ among screened women aged 25–39 years. Data from several countries suggest that increases in anogenital warts diagnoses34,38,43,47,50,54 and CIN2+65,95 began before the introduction of HPV vaccination. Together, these results suggest that the population-level impact of HPV vaccination could currently be measured within an underlying context of increasing HPV-related endpoints in some countries. Although the reasons for these trends are likely multi-factorial and endpoint-specific, several hypotheses can be made. First, increases in the three HPV-related endpoints could reflect increases in sexual activity. Several data sources indicate that, over the past 10–20 years, the number of sexual partners per individual has increased, and the age at initiation of sexual activity has decreased in several high-income countries.24,96–104 Second, endpoint-specific hypotheses could also explain observed increases. Increases in high-risk non-vaccine HPV types could partly be explained by HPV 16 and 18 unmasking (ie, apparent increased detection of non-vaccine HPV types in a post-vaccination population with fewer HPV 16 and 18 infections, which could have masked detection of other HPV types before vaccination)105 or by type replacement (ie, increased prevalence of non-vaccine HPV types occupying the ecological niche created by prevention of HPV 16 and 18 infections).106 Increases in anogenital wart diagnoses could be partly explained by increased knowledge, awareness, and health-seeking behaviour of the general population about anogenital warts, and by better diagnosis and reporting by health professionals. Finally, as previously discussed, increases in CIN2+ could be attributable to changes in screening recommendations, tests, and participation documented in several countries. More research is needed to better understand the factors affecting the increasing trends in non-HPV vaccine types and HPV-related diseases in older men and women. If they are due to changes in sexual behaviour or increased health-seeking behaviour and diagnoses, the population-level effectiveness of HPV vaccination might be under-estimated when comparing the annual frequency of HPV endpoints in pre-vaccination and post-vaccination periods.
In addition to the epidemiological and public health insights discussed above, our study has important additional strengths. All corresponding authors of the studies we included were contacted in order to have standardised age groups and HPV endpoints, permitting pooling of results. Furthermore, the large pooled sample size of person-time at risk and 8-year follow-up data (9 years for CIN2+) since the introduction of HPV vaccination gave sufficient statistical power to show declines in all three HPV-related endpoints among girls and women targeted for vaccination in both high-coverage and low-coverage settings, and cross-protection and herd effects in countries with high vaccination coverage and multi-cohort vaccination. Our results should however be interpreted considering the following three limitations. The first limitation is that causality between HPV vaccination and the observed changes in HPV-related endpoints cannot be concluded definitively, because this meta-analysis is based on ecological studies. However, the: (1) larger and faster decreases in HPV-related endpoints among cohorts targeted for vaccination and in countries with multi-cohort vaccination and high routine vaccination coverage; (2) larger decreases in HPV-related endpoints with longer follow-up since the introduction of HPV vaccination (as the number of cohorts vaccinated increases); and (3) consistency between the results from the different studies and between the three HPV-related endpoints, strongly suggest that the decreases can be largely attributed to HPV vaccination. The second limitation is that the number of studies available for each HPV-related endpoint was too small for us to do multivariate meta-regression analyses10 in order to simultaneously consider the influence of different programme characteristics or study designs. Additionally, the number of studies within categories is sometimes limited. For example, greater decreases in CIN2+ were observed in the Scottish study77 that used the bivalent vaccine, compared with the studies that used the quadrivalent vaccine. However, it was not possible to measure the effect of the vaccine type, given that Scotland has a very high HPV vaccination coverage, had catch-up vaccination, and had no major change in screening recommendations since the introduction of HPV vaccination. The third limitation is that all studies identified in the systemic review are from high-income countries, so our results should be extrapolated to low-income and middle-income countries with caution. The population-level impact of HPV vaccination, including the impact of multi-cohort vaccination strategies, might be different in countries with substantially different sexual behaviour (eg, age at start of sexual activity, age difference between partners, concurrency in partnerships, percentage of men that are clients of female sex workers) HPV epidemiology, or prevalence of HPV infection and disease cofactors (eg, HIV prevalence).
In conclusion, the results of this meta-analysis show compelling evidence of the substantial impact of three-dose girls-only HPV vaccination programmes with the quadrivalent or bivalent vaccines on infections by HPV 16 and 18 and HPV 31, 33, and 45 as a group, anogenital wart diagnoses, and CIN2+ among women. Furthermore, we found evidence of herd effects among boys and older women. Programmes with multi-cohort vaccination and high vaccination coverage led to a greater and faster direct impact and herd effects. These results should be considered within the rapidly changing landscape of HPV vaccination, with several countries recently switching to two-dose schedules, gender-neutral vaccination, and the nonavalent vaccine, and with research examining one-dose HPV vaccination, two-dose vaccination in older populations, and cervical cancer elimination strategies. Although challenging, it will be crucial to continue monitoring the population-level impact of HPV vaccination to examine the full effect of these changes in vaccination strategies and quantify the effect of vaccination in low-income and middle-income countries.
Supplementary Material
Research in context.
Evidence before this study
Since 2007, 99 countries and territories have introduced human papillomavirus (HPV) vaccination programmes. In 2015, we did a systematic review and meta-analysis to examine the real-world population-level impact of HPV vaccination. The meta-analysis showed substantial decreases in HPV 16 and HPV 18 infections and anogenital wart diagnoses among women targeted for vaccination, and evidence of herd effects among boys and older women, 4 years after the introduction of HPV vaccination. However, at the time of the meta-analysis, the number of years post-vaccination was insufficient to examine the impact of HPV vaccination on cervical intraepithelial neoplasia grade 2+ (CIN2+). Moreover, in 2016, the WHO Strategic Advisory Group of Experts on Immunization revised its position to recommend HPV vaccination of multiple age cohorts of girls, rather than vaccination of a single cohort.
We updated our previous systematic review and meta-analysis to: (1) update and summarise the most recent evidence about the population-level impact of girls-only HPV vaccination on HPV infections and anogenital wart diagnoses among girls, boys, women, and men; (2) summarise new evidence about the population-level impact of girls-only HPV vaccination on CIN2+ occurrence among screened girls and women; and (3) compare the population-level impact of HPV vaccination on anogenital wart diagnoses and CIN2+ occurrence between countries that have implemented either a single or a multiple age-cohort vaccination strategy. We searched MEDLINE and Embase for studies published between Feb 1, 2014, and Oct 11, 2018, with the same combination of MeSH terms, title, or abstract words: (“papillomavirus vaccine”, “papillomavirus vaccination”, “HPV vaccine”, or “HPV vaccination”) and (“program evaluation”, “population surveillance”, “sentinel surveillance”, “incidence”, or “prevalence”), and (“papillomavirus infection”, “condylomata acuminata”, “anogenital warts”, “cervical intraepithelial neoplasia”, “cervical dysplasia”, “uterine cervical neoplasm”, or “HPV related diseases”). We identified 1702 potentially eligible articles for this systematic review and meta-analysis, and included 65 articles in 14 high-income countries: 23 for HPV infection, 29 for anogenital warts, and 13 for CIN2+. We contacted all corresponding authors of eligible studies to request a re-analysis of their data using the same data stratification to allow comparison between studies and pooling.
Added value of this study
The current updated systematic review and meta-analysis, which includes data from 60 million individuals and up to 8 years of post-vaccination follow-up, shows compelling evidence of the substantial impact of HPV vaccination programmes on HPV infections, anogenital wart diagnoses, and CIN2+ among women, and herd effects among boys and older women. Our study also shows greater and faster direct impact and herd effects in countries with multiple age-cohort vaccination and high vaccination coverage, compared with countries with single age-cohort vaccination or low routine vaccination coverage. To our knowledge, our study is the first to present pooled estimates of the population-level impact of HPV vaccination on CIN2+ (the most proximal outcome to cervical cancer), and the first to show the real-world additional benefit of vaccinating multiple age cohorts of girls with high vaccination coverage.
Implications of all the available evidence
Our results provide strong evidence of HPV vaccination working to prevent cervical cancer in real-world settings, as both the cause (high-risk HPV infection) and proximal disease endpoint (CIN2+) are significantly declining. In terms of global policy implications, these results reinforce the recently revised position of WHO recommending HPV vaccination of multiple age cohorts of girls, and provide promising early signs that the WHO call for action on cervical cancer elimination might be possible if sufficient population-level vaccination coverage can be reached.
Acknowledgments
This work was supported by WHO, a Fonds de recherche du Québec – Santé research scholars award to MB, and a foundation scheme grant from the Canadian Institutes of Health Research (FDN-143283). The funding sources of all original studies included in this systematic review are presented in the appendix. The authors would also like to acknowledge Myrto Mondor from the Centre de recherche du CHU de Québec for doing the subgroup analyses and Sara E Oliver from the US Centers for Disease Control and Prevention (CDC) for doing the updated analyses of changes in HPV prevalence from the National Health and Nutrition Examination Survey in the USA. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Funding WHO, Canadian Institutes of Health Research, Fonds de recherche du Québec – Santé.
Footnotes
Declaration of interests
The authors listed in this paper declare no competing interests. Please see the appendix for the declaration of interests of the authors listed as study group members.
Contributor Information
Mélanie Drolet, Centre de recherche du CHU de Québec—Université Laval, Québec, QC, Canada.
Élodie Bénard, Centre de recherche du CHU de Québec—Université Laval, Québec, QC, Canada; Département de médecine sociale et préventive, Faculté de médecine, Université Laval, Québec, QC, Canada.
Norma Pérez, Centre de recherche du CHU de Québec—Université Laval, Québec, QC, Canada.
Marc Brisson, Centre de recherche du CHU de Québec—Université Laval, Québec, QC, Canada; Département de médecine sociale et préventive, Faculté de médecine, Université Laval, Québec, QC, Canada; Department of Infectious Disease Epidemiology, Imperial College, London, UK.
References
- 1.Bruni L, Diaz M, Barrionuevo-Roses L, et al. Worldwide female and male HPV vaccination coverage from 2006 to 2016. International Papillomavirus Conference; Cape Town; Feb 28–March 4, 2017. [Google Scholar]
- 2.Cervical Cancer Action. Global progress in HPV vaccination. http://www.cervicalcanceraction.org/comments/maps.php (accessed May 23, 2018).
- 3.Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15: 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco E, Schiffman M, Wacholder S, Stanley M. Methodological issues for trials of vaccine efficacy against HPV types 16 and 18 In: ARC HPV Working Group. Primary end-points for prophylactic vaccine trials. Lyon: International Agency for Research on Cancer, 2014: 39–45. [Google Scholar]
- 5.WHO. Human papillomavirus vaccines: WHO position paper, May 2017–recommendations. Vaccine 2017; 35: 5753–55. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199: 805–14. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 9.Deeks JJ, Higgins JPT, on behalf of the Statistical Methods Group of the Cochrane Collaboration. Statistical algorithms in Review Manager 5 https://training.cochrane.org/handbook/statistical-methods-revman5 (accessed May 23, 2014). [Google Scholar]
- 10.Deeks JJ, Higgins PT, Altman DG. Analysing data and undertaking meta-analyses In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
- 11.Dillner J, Nygård M, Munk C, et al. Decline of HPV infections in Scandinavian cervical screening populations after introduction of HPV vaccination programs. Vaccine 2018; 36: 3820–29. [DOI] [PubMed] [Google Scholar]
- 12.Chow EP, Danielewski JA, Fehler G, et al. Human papillomavirus in young women with Chlamydia trachomatis infection 7 years after the Australian human papillomavirus vaccination programme: a cross-sectional study. Lancet Infect Dis 2015; 15: 1314–23. [DOI] [PubMed] [Google Scholar]
- 13.Chow EPF, Machalek DA, Tabrizi SN, et al. Quadrivalent vaccine-targeted human papillomavirus genotypes in heterosexual men after the Australian female human papillomavirus vaccination programme: a retrospective observational study. Lancet Infect Dis 2017; 17: 68–77. [DOI] [PubMed] [Google Scholar]
- 14.Cummings T, Zimet GD, Brown D, et al. Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine 2012; 30: 5496–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunne EF, Naleway A, Smith N, et al. Reduction in human papillomavirus vaccine type prevalence among young women screened for cervical cancer in an integrated US healthcare delivery system in 2007 and 2012–2013. J Infect Dis 2015; 212: 1970–75. [DOI] [PubMed] [Google Scholar]
- 16.Grün N, Ährlund-Richter A, Franzén J, et al. Follow-up on oral and cervical human papillomavirus prevalence 2013–2015 in youth at a youth clinic in Stockholm, Sweden. Infect Dis (Lond) 2016; 48: 169–70. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh K, Pollock KG, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 2014; 110: 2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron RL, Kavanagh K, Pan J, et al. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 2016; 22: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17: 1293–302. [DOI] [PubMed] [Google Scholar]
- 20.Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics 2012; 130: e249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn JA, Widdice LE, Ding L, et al. Substantial decline in vaccine-type human papillomavirus (HPV) among vaccinated young women during the first 8 years after HPV vaccine introduction in a community. Clin Infect Dis 2016; 63: 1281–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis 2018; 217: 1590–600. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013; 208: 385–93. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137: e20151968. [DOI] [PubMed] [Google Scholar]
- 25.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open 2016; 6: e009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine 2013; 32: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesher D, Panwar K, Thomas SL, et al. The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type-specific HPV in young females, 2010–2016. J Infect Dis 2018; 218: 911–21. [DOI] [PubMed] [Google Scholar]
- 29.Purriños-Hermida MJ, Santiago-Pérez MI, Treviño M, et al. Direct, indirect and total effectiveness of bivalent HPV vaccine in women in Galicia, Spain. PLoS One 2018; 13: e0201653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Söderlund-Strand A, Uhnoo I, Dillner J. Change in population prevalences of human papillomavirus after initiation of vaccination: the high-throughput HPV monitoring study. Cancer Epidemiol Biomarkers Prev 2014; 23: 2757–64. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenberg P, Clifton S, Beddows S, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013; 382: 1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabrizi SN, Brotherton JML, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 2012; 206: 1645–51. [DOI] [PubMed] [Google Scholar]
- 33.Tabrizi SN, Brotherton JML, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014; 14: 958–66. [DOI] [PubMed] [Google Scholar]
- 34.Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013; 346: f2032. [DOI] [PubMed] [Google Scholar]
- 35.Ali H, McManus H, O’Connor CC, et al. Human papillomavirus vaccination and genital warts in young Indigenous Australians: national sentinel surveillance data. Med J Aust 2017; 206: 204–09. [DOI] [PubMed] [Google Scholar]
- 36.Chow EP, Read TR, Wigan R, et al. Ongoing decline in genital warts among young heterosexuals 7 years after the Australian human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2015; 91: 214–19. [DOI] [PubMed] [Google Scholar]
- 37.Callander D, Ali H, Donovan B. Genital Warts Surveillance Network report 2004–2015. Sydney, NSW: The Kirby Institute, 2016. [Google Scholar]
- 38.Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 2013; 40: 130–35. [DOI] [PubMed] [Google Scholar]
- 39.Bollerup S, Baldur-Felskov B, Blomberg M, Baandrup L, Dehlendorff C, Kjaer SK. Significant reduction in the incidence of genital warts in young men 5 years into the Danish human papillomavirus vaccination program for girls and women. Sex Transm Dis 2016; 43: 238–42. [DOI] [PubMed] [Google Scholar]
- 40.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. Am J Public Health 2012; 102: 833–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cocchio S, Baldovin T, Bertoncello C, et al. Decline in hospitalization for genital warts in the Veneto region after an HPV vaccination program: an observational study. BMC Infect Dis 2017; 17: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominiak-Felden G, Gobbo C, Simondon F. Evaluating the early benefit of quadrivalent HPV vaccine on genital warts in Belgium: a cohort study. PLoS One 2015; 10: e0132404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103: 1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health 2018; 108: 112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerra FM, Rosella LC, Dunn S, Wilson SE, Chen C, Deeks SL. Early impact of Ontario’s human papillomavirus (HPV) vaccination program on anogenital warts (AGWs): a population-based assessment. Vaccine 2016; 34: 4678–83. [DOI] [PubMed] [Google Scholar]
- 46.Harrison C, Britt H, Garland S, et al. Decreased management of genital warts in young women in Australian general practice post introduction of national HPV vaccination program: results from a nationally representative cross-sectional general practice study. PLoS One 2014; 9: e105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell-Jones R, Soldan K, Wetten S, et al. Declining genital warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis 2013; 208: 1397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canvin M, Sinka K, Hughes G, Mesher D. Decline in genital warts diagnoses among young women and young men since the introduction of the bivalent HPV (16/18) vaccination programme in England: an ecological analysis. Sex Transm Infect 2017; 93: 125–28. [DOI] [PubMed] [Google Scholar]
- 49.Kliewer EV, Mahmud SM, Demers AA, Lambert P, Musto G. Quadrivalent HPV vaccination and the incidence of anogenital warts in Manitoba, Canada. 28th International Papillomavirus Conference; San Juan, Puerto Rico; Nov 30–Dec 6, 2012 Abstract E07–663. [Google Scholar]
- 50.Thompson LH, Nugent Z, Blanchard JF, Ens C, Yu BN. Increasing incidence of anogenital warts with an urban-rural divide among males in Manitoba, Canada, 1990–2011. BMC Public Health 2016; 16: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leval A, Herweijer E, Arnheim-Dahlström L, et al. Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis 2012; 206: 860–66. [DOI] [PubMed] [Google Scholar]
- 52.Herweijer E, Ploner A, Sparén P. Substantially reduced incidence of genital warts in women and men six years after HPV vaccine availability in Sweden. Vaccine 2018; 36: 1917–20. [DOI] [PubMed] [Google Scholar]
- 53.Liu B, Donovan B, Brotherton JM, Saville M, Kaldor JM. Genital warts and chlamydia in Australian women: comparison of national population-based surveys in 2001 and 2011. Sex Transm Infect 2014; 90: 532–37. [DOI] [PubMed] [Google Scholar]
- 54.Mikolajczyk RT, Kraut AA, Horn J, Schulze-Rath R, Garbe E. Changes in incidence of anogenital warts diagnoses after the introduction of human papillomavirus vaccination in Germany—an ecologic study. Sex Transm Dis 2013; 40: 28–31. [DOI] [PubMed] [Google Scholar]
- 55.Thöne K, Horn J, Mikolajczyk R. Evaluation of vaccination herd immunity effects for anogenital warts in a low coverage setting with human papillomavirus vaccine-an interrupted time series analysis from 2005 to 2010 using health insurance data. BMC Infect Dis 2017; 17: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliphant J, Perkins N. Impact of the human papillomavirus (HPV) vaccine on genital wart diagnoses at Auckland Sexual Health Services. N Z Med J 2011; 124: 51–58. [PubMed] [Google Scholar]
- 57.Oliphant J, Stewart J, Saxton P, Lo M, Perkins N, Ward D. Trends in genital warts diagnoses in New Zealand five years following the quadrivalent human papillomavirus vaccine introduction. N Z Med J 2017; 130: 9–16. [PubMed] [Google Scholar]
- 58.Smith MA, Liu B, McIntyre P, Menzies R, Dey A, Canfell K. Fall in genital warts diagnoses in the general and indigenous Australian population following implementation of a national human papillomavirus vaccination program: analysis of routinely collected national hospital data. J Infect Dis 2015; 211: 91–99. [DOI] [PubMed] [Google Scholar]
- 59.Smith MA, Liu B, McIntyre P, Menzies R, Dey A, Canfell K. Trends in genital warts by socioeconomic status after the introduction of the national HPV vaccination program in Australia: analysis of national hospital data. BMC Infect Dis 2016; 16: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonnenberg P, Tanton C, King E, et al. Genital warts & HPV 6/11 in the British population: data from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). 31st International Papillomavirus Conference; Cape Town; Feb 28–March 4, 2017 Abstract 454. [Google Scholar]
- 61.Steben M, Ouhoummane N, Rodier C, Sinyavskaya L, Brassard P. The early impact of human papillomavirus vaccination on anogenital warts in Québec, Canada. J Med Virol 2018; 90: 592–98. [DOI] [PubMed] [Google Scholar]
- 62.Woestenberg PJ, King AJ, van der Sande MA, et al. No evidence for cross-protection of the HPV-16/18 vaccine against HPV-6/11 positivity in female STI clinic visitors. J Infect 2017; 74: 393–400. [DOI] [PubMed] [Google Scholar]
- 63.Sandø N, Kofoed K, Zachariae C, Fouchard J. A reduced national incidence of anogenital warts in young Danish men and women after introduction of a national quadrivalent human papillomavirus vaccination programme for young women – an ecological study. Acta Derm Venereol 2014; 94: 288–92. [DOI] [PubMed] [Google Scholar]
- 64.Nsouli-Maktabi H, Ludwig SL, Yerubandi UD, Gaydos JC. Incidence of genital warts among U.S. service members before and after the introduction of the quadrivalent human papillomavirus vaccine. MSMR 2013; 20: 17–20. [PubMed] [Google Scholar]
- 65.Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control 2014; 25: 915–22. [DOI] [PubMed] [Google Scholar]
- 66.Baldur-Felskov B, Munk C, Nielsen TSS, et al. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997–2012. Cancer Causes Control 2015; 26: 1105–16. [DOI] [PubMed] [Google Scholar]
- 67.Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3: 833–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377: 2085–92. [DOI] [PubMed] [Google Scholar]
- 69.Australian Institute of Health and Welfare. Cervical screening in Australia 2013–2014. National Cervical Screening Program. https://www.aihw.gov.au/getmedia/c5e7776b-907e-4467-b435-8d6863ef33e7/19750.pdf.aspx?inline=true (accessed Nov 2, 2018).
- 70.Australian Institute of Health and Welfare. Cervical screening in Australia: 2018. National Cervical Screening Program. https://www.aihw.gov.au/getmedia/8a26b34d-a912-4f01-b646-dc5d0ca54f03/aihw-can-111.pdf.aspx?inline=true (accessed Jan 25, 2019).
- 71.Flagg EW, Torrone EA, Weinstock H. Ecological Association of Human Papillomavirus Vaccination with Cervical Dysplasia Prevalence in the United States, 2007–2014. Am J Public Health 2016; 106: 2211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gargano JW, Park IU, Griffin MR, et al. Trends in high-grade cervical lesions and cervical cancer screening in five states, 2008–2015. Clin Infect Dis 2018; 10.1093/cid/ciy707. [DOI] [PMC free article] [PubMed]
- 73.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiol Biomarkers Prev 2013; 22: 1446–50. [DOI] [PubMed] [Google Scholar]
- 74.Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in human papillomavirus (HPV)–associated high-grade cervical lesions after introduction of HPV vaccines in Connecticut, United States, 2008–2015. Clin Infect Dis 2017; 65: 884–89. [DOI] [PubMed] [Google Scholar]
- 75.Liaw KL, Kjaer S, Nygard M, Dillner J. Utilization of nordic countries national registries to monitor the impact of HPV vaccination. Pharmacoepidemiol Drug Saf 2014; 23: 356. [Google Scholar]
- 76.Ogilvie GS, Naus M, Money DM, et al. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: an ecological analysis. Int J Cancer 2015; 137: 1931–37. [DOI] [PubMed] [Google Scholar]
- 77.Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111: 1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen HY, Kessler CL, Mori N, Chauhan SP. Cervical cancer screening in the United States, 1993–2010: characteristics of women who are never screened. J Womens Health (Larchmt) 2012; 21: 1132–38. [DOI] [PubMed] [Google Scholar]
- 79.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105: 175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiro JA, Tsui J, Bauer HM, Yamada E, Kobrin S, Breen N. Human papillomavirus vaccine use among adolescent girls and young adult women: an analysis of the 2007 California Health Interview Survey. J Womens Health (Larchmt) 2012; 21: 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chao C, Silverberg MJ, Becerra TA, et al. Human papillomavirus vaccination and subsequent cervical cancer screening in a large integrated healthcare system. Am J Obstet Gynecol 2017; 216: 151.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drolet M, Laprise JF, Brotherton JML, et al. The impact of human papillomavirus catch-up vaccination in Australia: implications for introduction of multiple age cohort vaccination and postvaccination data interpretation. J Infect Dis 2017; 216: 1205–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaiyakunapruk N, Ng SS. Summary of evidence. Human papillomavirus (HPV) vaccination: an updated systematic review of cost-effectiveness analyses. Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE); Geneva; October, 2016. [Google Scholar]
- 84.WHO. Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec 2014; 89: 465–91. [PubMed] [Google Scholar]
- 85.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination —updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65: 1405–08. [DOI] [PubMed] [Google Scholar]
- 86.Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004; 23: 569–78. [DOI] [PubMed] [Google Scholar]
- 87.National Advisory Committee on Immunization (NACI). Statement on human papillomavirus vaccine. An advisory committee statement (ACS). Can Commun Dis Rep 2007; 33 (ACS-2): 1–31. [PubMed] [Google Scholar]
- 88.US Food and Drug Administration. Vaccines, blood & biologics: Gardasil. https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094042.htm (accessed May 2, 2019).
- 89.European Centre for Disease Prevention and Control. Guidance for the introduction of HPV vaccines in EU countries. Stockholm: January, 2008. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0801_GUI_Introduction_of_HPV_Vaccines_in_EU.pdf (accessed May 2, 2019). [Google Scholar]
- 90.Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol 2011; 6: 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the compass pilot randomised trial. PLoS Med 2017; 14: e1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drolet M, Boily MC, Greenaway C, et al. Sociodemographic inequalities in sexual activity and cervical cancer screening: implications for the success of human papillomavirus vaccination. Cancer Epidemiol Biomarkers Prev 2013; 22: 641–52. [DOI] [PubMed] [Google Scholar]
- 93.Herweijer E, Feldman AL, Ploner A, et al. The participation of HPV-vaccinated women in a national cervical screening program: population-based cohort study. PLoS One 2015; 10: e0134185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanton C, Soldan K, Beddows S, et al. High-risk human papillomavirus (HPV) infection and cervical cancer prevention in Britain: Evidence of differential uptake of interventions from a probability survey. Cancer Epidemiol Biomarkers Prev 2015; 24: 842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brotherton JML, Gertig DM, May C, Chappell G, Saville M. HPV vaccine impact in Australian women: ready for an HPV-based screening program. Med J Aust 2016; 204: 184. [DOI] [PubMed] [Google Scholar]
- 96.Liu G, Hariri S, Bradley H, Gottlieb SL, Leichliter JS, Markowitz LE. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 years, United States. Sex Transm Dis 2015; 42: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis R, Tanton C, Mercer CH, et al. Heterosexual practices among young people in Britain: evidence from three national surveys of sexual attitudes and lifestyles. J Adolesc Health 2017; 61: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013; 382: 1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rissel C, Badcock PB, Smith AM, et al. Heterosexual experience and recent heterosexual encounters among Australian adults: the Second Australian Study of Health and Relationships. Sex Health 2014; 11: 416–26. [DOI] [PubMed] [Google Scholar]
- 100.Jensen KE, Munk C, Sparen P, et al. Women’s sexual behavior. Population-based study among 65,000 women from four Nordic countries before introduction of human papillomavirus vaccination. Acta Obstet Gynecol Scand 2011; 90: 459–67. [DOI] [PubMed] [Google Scholar]
- 101.Stenhammar C, Ehrsson YT, Åkerud H, Larsson M, Tydén T. Sexual and contraceptive behavior among female university students in Sweden—repeated surveys over a 25-year period. Acta Obstet Gynecol Scand 2015; 94: 253–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herlitz CA, Forsberg M. Sexual behaviour and risk assessment in different age cohorts in the general population of Sweden (1989–2007). Scand J Public Health 2010; 38: 32–39. [DOI] [PubMed] [Google Scholar]
- 103.Cocchio S, Bertoncello C, Baldovin T, Buja A, Majori S, Baldo V. Self-reported genital warts among sexually-active university students: a cross-sectional study. BMC Infect Dis 2018; 18: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marino C, Vieno A, Lenzi M, Santinello M. Time trends in adolescent sexual behaviour in Italy. Sex Health 2014; 11: 379–80. [DOI] [PubMed] [Google Scholar]
- 105.Mori S, Nakao S, Kukimoto I, Kusumoto-Matsuo R, Kondo K, Kanda T. Biased amplification of human papillomavirus DNA in specimens containing multiple human papillomavirus types by PCR with consensus primers. Cancer Sci 2011; 102: 1223–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol 2013; 178: 625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.