Abstract
The importance of left ventricular (LV) global longitudinal strain (GLS) is increasingly recognized in multiple clinical scenarios. However, in patients with poor image quality, strain is difficult or impossible to measure without contrast enhancement. The feasibility of contrast-enhanced GLS measurement was recently demonstrated. We sought to determine: (1) whether contrast enhancement improves the accuracy of GLS measurements against cardiac magnetic resonance (CMR) reference, (2) their reproducibility compared to non-enhanced GLS, and (3) the dependence of accuracy and reproducibility on image quality. We prospectively enrolled 25 patients undergoing clinically indicated CMR imaging who subsequently underwent transthoracic echocardiography (TTE) with and without low-dose contrast injection (1–2 mL Optison/3–5 mL saline IV, GE Healthcare). GLS was measured from both non-contrast and contrast-enhanced images using speckle tracking (EchoInsight, Epsilon Imaging). These measurements were compared to each other and to CMR reference values obtained using feature tracking (SuiteHEART, NeoSoft). Inter-technique comparisons included linear regression and Bland–Altman analyses. A random subgroup of 15 patients was used to assess inter- and intra-observer variability using intra-class correlation (ICC). Contrast-enhanced GLS was in close agreement with non-enhanced GLS (r = 0.95; bias: − 0.2 ± 1.5%). Both inter-observer (ICC = 0.88 vs. 0.82) and intra-observer variability (ICC = 0.91 vs. 0.88) were improved by contrast enhancement. The agreement with CMR was better for contrast-enhanced GLS (r = 0.87; bias: 1.1 ± 2.2%) than for non-enhanced GLS (r = 0.80; bias: 1.3 ± 2.7%). In 12/25 patients with suboptimal TTE images that rendered GLS difficult to measure, contrast-enhanced GLS showed better agreement with CMR than non-enhanced GLS (r = 0.88 vs. 0.83) and also improved inter-observer (ICC = 0.83 vs. 0.76) and intra-observer variability (ICC = 0.88 vs. 0.82). In conclusion, contrast enhancement of TTE images improves the accuracy and reproducibility of GLS measurements, resulting in better agreement with CMR, even in patients with suboptimal acoustic windows. This approach may aid in the assessment of LV function in this patient population.
Keywords: Left ventricular function, Myocardial strain, Speckle-tracking echocardiography, Contrast enhancement
Introduction
Global longitudinal strain (GLS) measured using speckle-tracking echocardiography (STE) has been shown to be a strong predictor of morbidity and mortality in patients with heart failure, independent of left ventricular (LV) ejection fraction (EF) [1–7]. It has thus been incorporated into routine clinical practice and has been endorsed by the ASE and EACVI in the recent chamber quantification guidelines [8, 9]. GLS has also been shown to be the optimal parameter of LV deformation for the early detection of subclinical chemotherapy-related LV dysfunction across a wide variety of cancer types [10]. However, in patients with poor image quality, strain is difficult or sometimes even impossible to measure without contrast enhancement. The feasibility of contrast-enhanced GLS measurement was recently demonstrated in patients with a wide range of GLS values, including those with suboptimal image quality [11]. Cardiac magnetic resonance (CMR) imaging is the established reference standard for the quantification of LV size and function. CMR-derived strain measured by feature tracking has been used as a reference technique in several recent studies [12–20]. However, the availability of CMR is limited, and until recently, no commercial STE software has been able to measure strain from contrast-enhanced echocardiographic images.
In this study, we sought to determine whether contrast enhancement would improve the accuracy of GLS measurements compared to non-enhanced GLS measurements, using CMR imaging as a reference. Additionally, we aimed to assess the reproducibility of contrast-enhanced compared to non-enhanced GLS measurements, as well as the dependence of accuracy and reproducibility on image quality.
Methods
Population and study design
We prospectively enrolled 25 patients undergoing clinically indicated CMR imaging. The indications for CMR in our study population were as follows: 14 patients were referred to assess etiology of non-ischemic cardiomyopathy, 6 patients were post-transplant patients referred for surveillance, 3 patients were referred to assess for viability in the setting of ischemic cardiomyopathy, and 2 patients were oncology patients receiving active chemotherapy that were referred to rule out cardiotoxicity. The baseline characteristics of these 25 study patients are listed in Table 1. Each patient subsequently underwent TTE imaging both with and without injection of contrast as soon as possible following the CMR, in the majority of cases (20/25 patients) within 24 h of CMR imaging. GLS was measured from both non-contrast and contrast-enhanced images using echocardiographic speckle tracking technique. These measurements were compared to each other and to reference strain values obtained using CMR feature tracking. Exclusion criteria were: congenital heart disease, arrhythmia during image acquisition, and presence of pacemaker or defibrillator leads. The study was approved by the Institutional Review Board, and informed written consent was obtained from each patient.
Table 1.
Baseline characteristics of the study population (N = 25)
| Age (years) | 47 ± 17 |
| Gender (male %) | 48 |
| BSA (m2) | 2.0 ± 0.3 |
| Coronary artery disease (%) | 24 |
| Hypertension (%) | 76 |
| Diabetes mellitus (%) | 8.0 |
| Dilated cardiomyopathy (%) | 64 |
| Chronic kidney disease (%) | 16 |
| Ejection fraction (%) | 43 ± 17 |
Echocardiographic imaging and strain measurement
Transthoracic echocardiographic imaging was performed in the apical position and 2-, 3-, and 4-chamber LV-focused views were obtained with the patient in the left lateral decubitus position (IE33 or EPIQ systems, Philips Healthcare, Andover, MA) with an X5–1 transducer. Before each acquisition, images were optimized for endocardial visualization by adjusting the gain, compress, and time-gain compensation controls. The same operator acquired images both with and without contrast. Acquisition settings for contrast-enhanced imaging included: (1) approximately half of the manufacturer-recommended dose of a commercial contrast agent (Optison by GE Healthcare Chicago, IL; 1–2 mL diluted in 3–5 mL saline), used to provide partial contrast enhancement with lower bubble density than that typically used for LV opacification, resulting in some degree of visible swirling; (2) higher than usual mechanical indices (0.6–0.7); (3) focus set at the level of the mitral valve annulus to facilitate accurate tracking of the speckles in the far field; and (4) lowest frequency range for maximal penetration.
Images were stored digitally and used for offline analysis. Both contrast-enhanced and non-enhanced echocardiographic images were analyzed using speckle tracking software to measure GLS (EchoInsight, Epsilon Imaging, Ann Arbor, MI). LV boundaries were manually identified at end-diastole and automatically tracked throughout the cardiac cycle by the speckle tracking software. For both contrast-enhanced and non-enhanced images, manual corrections were performed as needed to optimize boundary tracking throughout the cardiac cycle.
CMR imaging and strain measurement
CMR imaging was performed on a 1.5 T scanner (Philips; Best, Netherlands) with a five-channel cardiac coil. A steady-state free-precision (SSFP) pulse sequence was used to obtain cine loops, during approximately 5 s breath holds (repetition time 2.9 ms, echo time 1.5 ms, flip angle 60°, and temporal resolution = 30–40 ms). Images were analyzed using commercial software (SuiteHeart, NeoSoft; Pewaukee, WI) with feature tracking technology to measure GLS from the 2-, 3-, and 4-chamber cine imaging planes. Similar to the echocardiographic analysis, LV boundaries were identified at end-diastole and end-systole and automatically tracked throughout the cardiac cycle. Manual tracings were adjusted as needed to optimize boundary tracking throughout the cardiac cycle.
Statistical analyses
Contrast-enhanced and non-enhanced echocardiographic GLS measurements were compared to one another and to CMR feature tracking-derived GLS. Inter-technique comparisons included linear regression with Pearson correlation coefficients. In addition, Bland–Altman analyses were performed to assess the bias and limits of agreement for each comparison. Continuous variables are reported as means and standard deviations, categorical variables are reported as absolute numbers and percentages.
Reproducibility assessment
The reproducibility of both contrast-enhanced and non-enhanced echocardiographic GLS measurements was tested using repeated measurements in 15 patients randomly selected from the study group. To determine inter-observer variability, repeated measurements were performed on the same image loops by two independent investigators blinded to all prior measurements, and subsequently compared to one another. To determine intra-observer variability, images were re-analyzed 3 months later by the same investigator, also blinded to all prior measurements. Inter- and intra-observer variability were quantified by calculating intraclass correlation coefficients (ICCs).
Sub-group analysis: suboptimal acoustic windows
All of the aforementioned analyses were repeated in a subset of patients with suboptimal acoustic windows on TTE, defined as poor endocardial border visualization in at least two LV segments (12/25 patients), in order to determine the dependence of accuracy and reproducibility on image quality.
Results
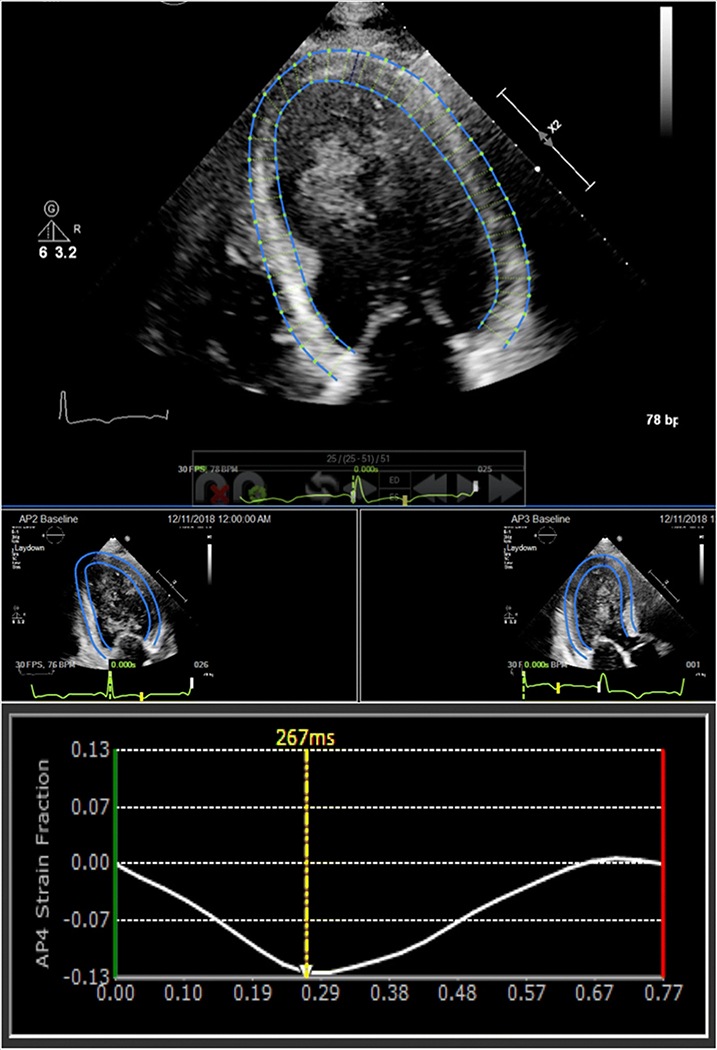
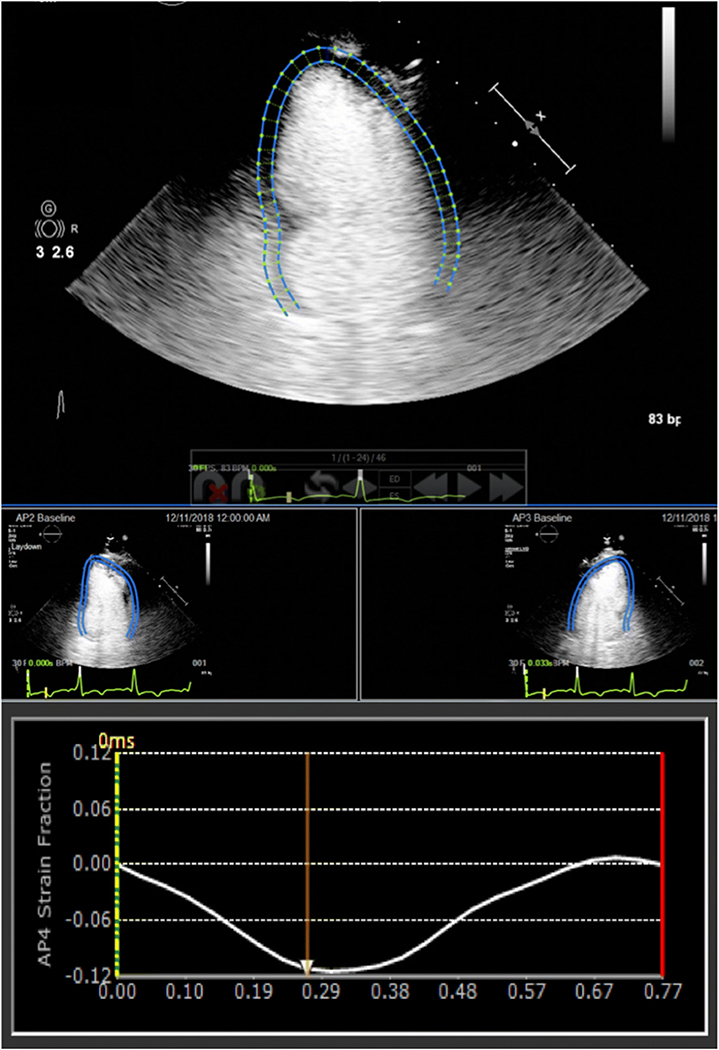
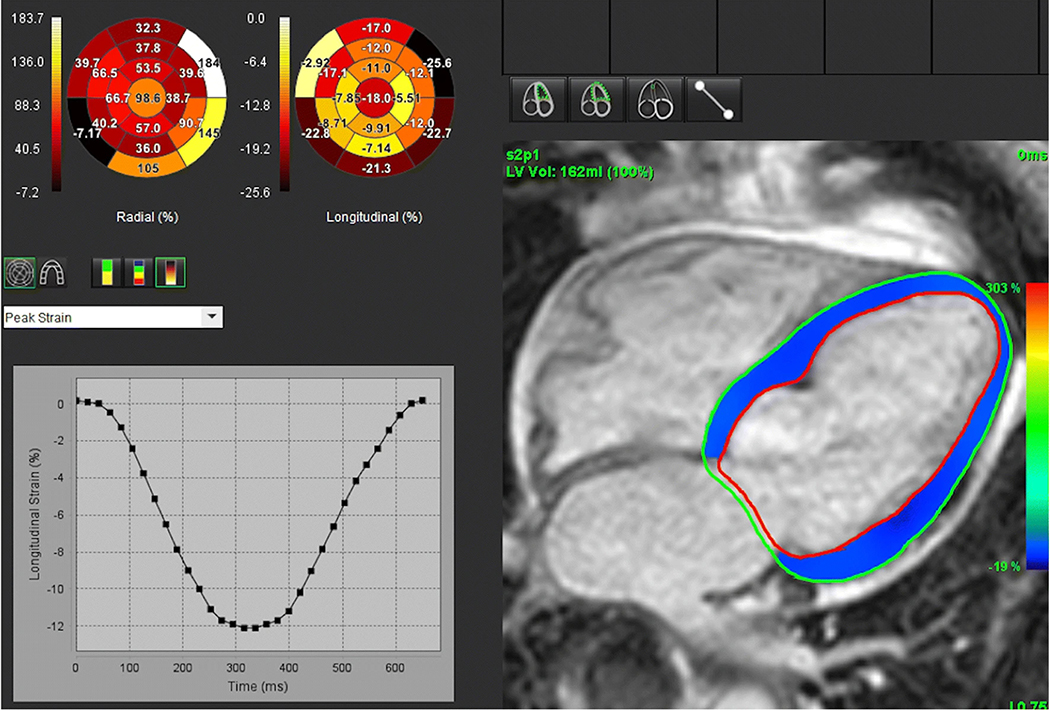
None of the study patients experienced adverse reactions to the ultrasound contrast agent. Examples of GLS measurements obtained from non-enhanced TTE, contrast-enhanced TTE, and CMR in a patient with suboptimal acoustic windows are shown in Figs. 1, 2, 3. The mean GLS values obtained from contrast-enhanced TTE, non-enhanced TTE, and CMR were 13.1 ± 4.7%, 13.2 ± 4.3%, and 12.4 ± 4.5%, respectively.
Fig. 1.
Non-enhanced echocardiographic GLS measured using speckle tracking software from apical four- (top middle), two- (bottom left), and three-chamber windows (bottom right). See text for details
Fig. 2.
Contrast-enhanced echocardiographic GLS measured using speckle tracking software from apical four- (top middle), two- (bottom left), and three-chamber windows (bottom right). See text for details
Fig. 3.
CMR-derived GLS measured using feature tracking software from two-, three-, and four-chamber cine stacks. See text for details
Contrast-enhanced echocardiographic GLS measurements were in close agreement with non-enhanced echocardiographic GLS measurements (r = 0.95; bias: − 0.2 ± 1.5%). Inter- and intra-observer variability of contrast-enhanced GLS was better than for non-enhanced GLS (Table 2).
Table 2.
Inter- and intra-observer variability of echocardiographic measurements of global longitudinal strain with and without contrast enhancement
| Randomly selected subgroup of patients (N = 15) |
Patients with suboptimal acoustic windows (N = 12) |
|||
|---|---|---|---|---|
| Contrast-enhanced GLS |
Non-enhanced GLS |
Contrast-enhanced GLS |
Non-enhanced GLS |
|
| Inter-observer variability (ICC) | 0.88 | 0.82 | 0.83 | 0.76 |
| Intra-observer variability (ICC) | 0.91 | 0.88 | 0.88 | 0.82 |
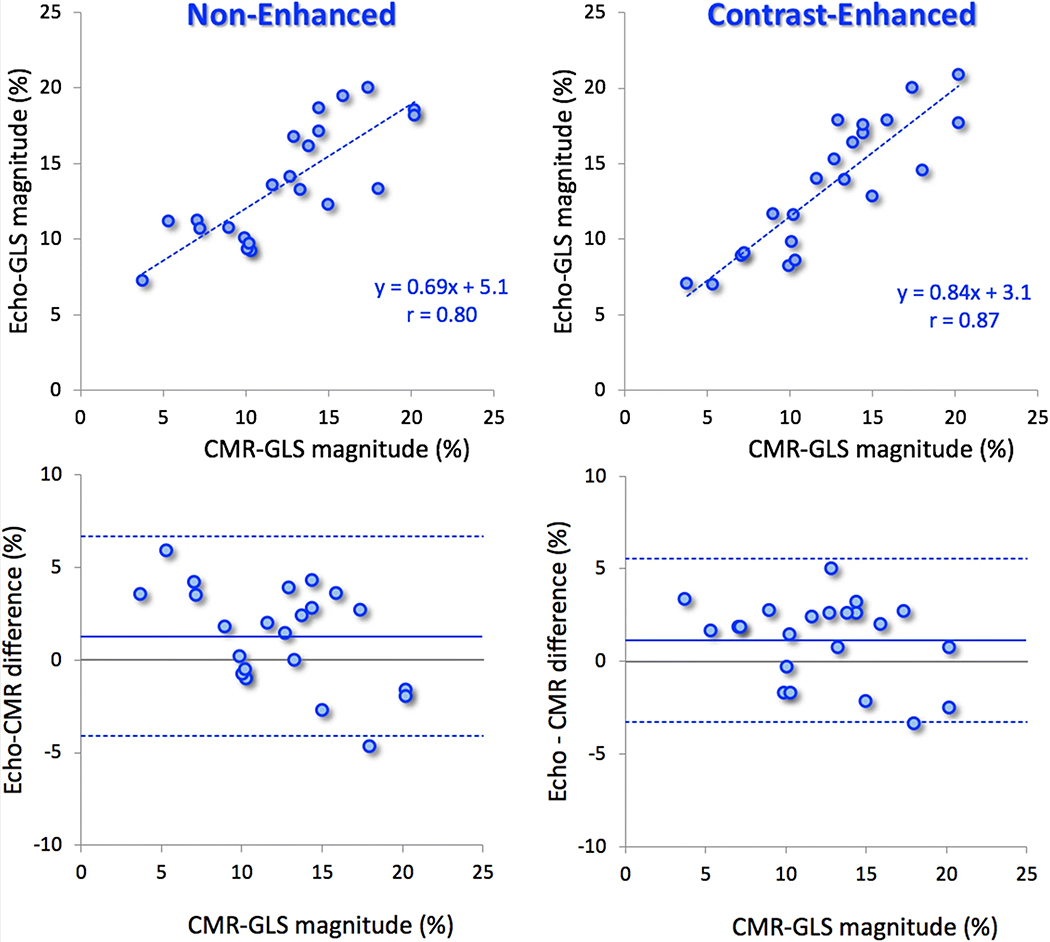
CMR-derived GLS measurement using feature tracking was unsuccessful in 3/25 study patients. In the remaining 22 patients, the agreement between contrast-enhanced GLS and CMR-derived strain (r = 0.87; bias: 1.1 ± 2.2%) was better than for non-enhanced GLS (r = 0.80; bias: 1.3 ± 2.7%) (Fig. 4).
Fig. 4.
Results of linear regression (top) and Bland–Altman (bottom) analyses comparing echocardiographic measurements of global longitudinal strain obtained from non-enhanced (left) and contrast-enhanced (right) images against CMR reference values
Similar to the entire study group, a sub-group analysis of 12/25 patients with suboptimal acoustic windows showed that contrast-enhanced GLS was in better agreement with CMR than non-enhanced GLS (r = 0.88; bias: 1.4 ± 2.4% vs. r = 0.83; bias: 1.6 ± 3.1%), and also more reproducible, as reflected by better inter- and intra-observer variability (Table 2).
Discussion
The results of this study demonstrated for the first time that contrast enhancement improves the accuracy of GLS measurements using echocardiographic speckle tracking when compared to CMR reference, and also improves the reproducibility of these measurements. We also found that these findings hold across the spectrum of image quality and, importantly, apply equally to patients with poor acoustic windows, in whom speckle tracking is difficult to use without contrast enhancement.
In the United States, an estimated 15% of echocardiography studies have poor image quality, and contrast agents are recommended for better visualization of endocardial borders for the purpose of wall motion assessment and to allow the quantification of LV volumes and EF. Although it is possible to measure GLS in a subset of patients with suboptimal image quality [21], in many patients with poor image quality, GLS measurements are not possible, resulting in a diagnostic disadvantage, which may affect clinical management.
This study was designed to determine whether contrast enhancement with low-dose contrast injection improves the accuracy of GLS measurements compared to non-enhanced GLS measurements, using CMR as the gold standard reference. In addition, we sought to assess the inter- and intra-observer reproducibility of contrast-enhanced GLS measurements compared to non-enhanced GLS measurements. We also analyzed a sub-group of patients with suboptimal acoustic windows on echocardiography, to assess the accuracy and reproducibility of contrast-enhanced GLS in this patient population.
We found that contrast-enhanced GLS measurements were in close agreement with non-enhanced GLS measurements, and that both inter- and intra-observer variability were improved by contrast-enhanced GLS compared to non-enhanced GLS. The agreement between contrast-enhanced GLS and CMR was better than for non-enhanced GLS. All of the above results extended to the sub-group with suboptimal acoustic windows on echocardiography.
Previous studies that have used the full manufacturer-recommended dose of contrast for complete LV opacification showed a significant amount of variability in global and regional strain measurements between contrast and non-contrast images, raising questions about the reliability of this methodology for strain analysis [22, 23]. A recent study conducted by our group aimed to identify optimal contrast administration settings for strain analysis, and found that the combination of a higher mechanical index with the partial opacification of the LV cavity resulting in a certain degree of swirling, rather than a homogeneously opacified LV cavity, was found to provide better conditions for contrast-enhanced GLS analysis [11]. We thus used approximately half of the manufacturer-recommended dose of the contrast agent (1–2 mL diluted in 3–5 mL saline) to achieve this swirling effect.
While there is a large body of literature demonstrating the safety of ultrasound enhancing agents, adverse events have been reported and are usually minor and self-limiting, including headache, nausea, altered sense of taste, dry mouth, and back pain. Intolerance to some components of contrast agents can occur and manifest as urticaria, pruritus, or rash. Generalized allergic or anaphylactic reactions are rare (approximately 1 in 10,000) [24]. No adverse events were noted in our study patients.
One advantage of this study compared to previous studies is that the current study used a combination of apical four, three, and two-chamber views to calculate GLS, resulting in a more robust and accurate GLS measurement. Previous feasibility studies calculated GLS using only the apical four-chamber view [11], which may have resulted in less accurate GLS measurements. The methodology used to calculate GLS in our study may also explain the greater degree of reproducibility of GLS compared to previous studies.
Limitations
One limitation of our study is the small sample size. Our study was designed to test whether contrast-enhanced GLS with one commercial contrast agent can be used as an accurate and reproducible measure of LV function. Based on our study’s positive findings, larger studies are needed to test the generalizability of our results using other contrast agents.
In addition, CMR-derived GLS measurement using feature tracking was unsuccessful in 3/25 of our study patients, despite attempted manual corrections to optimize boundary tracking. This was due to the performance of the MRI software used in this study and is unlikely to have had a significant effect on our findings.
Conclusions
This study demonstrated that in a small group of patients with a diverse range of clinical diagnoses, GLS measured from contrast-enhanced TTE was more accurate and reproducible than GLS obtained from non-enhanced images when compared to the CMR reference standard, even in those patients with suboptimal acoustic windows. These results suggest that the routine use of echocardiographic contrast should be considered when measuring GLS in patients with limited echocardiographic windows, who may otherwise be diagnostically disadvantaged.
Acknowledgments
Funding This study was supported by a research Grant from GE Healthcare.
Abbreviations
- CMR
Cardiac magnetic resonance
- EF
Ejection fraction
- GLS
Global longitudinal strain
- ICC
Intraclass correlation
- LV
Left ventricular
Footnotes
Compliance with ethical standards
Conflict of interest All authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ (2009) Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 54:618–624 [DOI] [PubMed] [Google Scholar]
- 2.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M et al. (2004) Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17:1021–1029 [DOI] [PubMed] [Google Scholar]
- 3.Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C et al. (2010) Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 23:1019–1024 [DOI] [PubMed] [Google Scholar]
- 4.Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R et al. (2006) Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol 47:1175–1181 [DOI] [PubMed] [Google Scholar]
- 5.Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2:356–364 [DOI] [PubMed] [Google Scholar]
- 6.Syeda B, Hofer P, Pichler P, Vertesich M, Bergler-Klein J, Roedler S et al. (2011) Two-dimensional speckle-tracking strain echocardiography in long-term heart transplant patients: a study comparing deformation parameters and ejection fraction derived from echocardiography and multislice computed tomography. Eur J Echocardiogr 12:490–496 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Harada N, Isozaki Y, Lee K, Yajima R, Kataoka A et al. (2013) Efficiency of quantitative longitudinal peak systolic strain values using automated function imaging on transthoracic echocardiogram for evaluating left ventricular wall motion: new diagnostic criteria and agreement with naked eye evaluation by experienced cardiologist. Int J Cardiol 167:1625–1631 [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1–39):e14. [DOI] [PubMed] [Google Scholar]
- 9.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G et al. (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 24:277–313 [DOI] [PubMed] [Google Scholar]
- 10.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al. (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 27:911–939 [DOI] [PubMed] [Google Scholar]
- 11.Medvedofsky D, Lang RM, Kruse E, Guile B, Weinert L, Ciszek B et al. (2018) Feasibility of left ventricular global longitudinal strain measurements from contrast-enhanced echocardiographic images. J Am Soc Echocardiogr 31:297–303 [DOI] [PubMed] [Google Scholar]
- 12.Andre F, Robbers-Visser D, Helling-Bakki A, Foll A, Voss A, Katus HA et al. (2016) Quantification of myocardial deformation in children by cardiovascular magnetic resonance feature tracking: determination of reference values for left ventricular strain and strain rate. J Cardiovasc Magn Reson 19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y et al. (2015) Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurich M, Keller M, Greiner S, Steen H, Aus dem Siepen F, Riffel J et al. (2016) Left ventricular mechanics assessed by two-dimensional echocardiography and cardiac magnetic resonance imaging: comparison of high-resolution speckle tracking and feature tracking. Eur Heart J Cardiovasc Imaging 17:1370–1378 [DOI] [PubMed] [Google Scholar]
- 15.de Siqueira ME, Pozo E, Fernandes VR, Sengupta PP, Modesto K, Gupta SS et al. (2016) Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick A, Schmidt B, Michels G, Bunck AC, Maintz D, Baessler B (2017) Left and right atrial feature tracking in acute myocarditis: a feasibility study. Eur J Radiol 89:72–80 [DOI] [PubMed] [Google Scholar]
- 17.Obokata M, Nagata Y, Wu VC, Kado Y, Kurabayashi M, Otsuji Y et al. (2016) Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging 17:525–532 [DOI] [PubMed] [Google Scholar]
- 18.Pandey T, Alapati S, Wadhwa V, Edupuganti MM, Gurram P, Lensing S et al. (2017) Evaluation of myocardial strain in patients with amyloidosis using cardiac magnetic resonance feature tracking. Curr Probl Diagn Radiol 46:288–294 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt B, Dick A, Treutlein M, Schiller P, Bunck AC, Maintz D et al. (2017) Intra- and inter-observer reproducibility of global and regional magnetic resonance feature tracking derived strain parameters of the left and right ventricle. Eur J Radiol 89:97–105 [DOI] [PubMed] [Google Scholar]
- 20.Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B et al. (2015) Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 16:871–881 [DOI] [PubMed] [Google Scholar]
- 21.Macron L, Lairez O, Nahum J, Berry M, Deal L, Deux JF et al. (2011) Impact of acoustic window on accuracy of longitudinal global strain: a comparison study to cardiac magnetic resonance. Eur J Echocardiogr 12:394–399 [DOI] [PubMed] [Google Scholar]
- 22.Lee KS, Honda T, Reuss CS, Zhou Y, Khandheria BK, Lester SJ (2008) Effect of echocardiographic contrast on velocity vector imaging myocardial tracking. J Am Soc Echocardiogr 21:818–823 [DOI] [PubMed] [Google Scholar]
- 23.Malm S, Frigstad S, Stoylen A, Torp H, Sagberg E, Skjarpe T (2006) Effects of ultrasound contrast during tissue velocity imaging on regional left ventricular velocity, strain, and strain rate measurements. J Am Soc Echocardiogr 19:40–47 [DOI] [PubMed] [Google Scholar]
- 24.Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM et al. (2008) American society of echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr 21:1179–1201 [DOI] [PubMed] [Google Scholar]