Summary
Neuroimmune interaction is an emerging concept, wherein the nervous system modulates the immune system and vice versa. This concept is gaining attention as a novel therapeutic target in various inflammatory diseases including acute kidney injury (AKI). Vagus nerve stimulation or treatment with pulsed ultrasound activates the cholinergic anti-inflammatory pathway to prevent AKI in mice. The kidneys are innervated by sympathetic efferent and sensory afferent neurons, and these neurons also may play a role in the modulation of inflammation in AKI. In this review, we discuss several neural circuits with respect to the control of renal inflammation and AKI as well as optogenetics as a novel tool for understanding these complex neural circuits.
Keywords: Acute kidney injury, neuroimmune interaction, cholinergic anti-inflammatory pathway, vagus nerve stimulation, optogenetics
Acute kidney injury (AKI) is an important clinical concern because it is highly prevalent and associated with high mortality and morbidity. AKI episodes can lead to chronic kidney disease (CKD) and end-stage renal disease.1–4 Although inflammation by immune cells is undoubtedly a critical step in the pathophysiology of AKI, pharmacologic approaches to decrease inflammation in AKI have been unsuccessful in clinical trials.5,6 The importance of the inter-relationship between the nervous system and the immune system recently was shown, and this neuroimmune interaction is emerging as a therapeutic target for several inflammatory diseases.7,8 In fact, the kidney is densely innervated by the sympathetic efferent nerves that originate from the brain, descend into the spinal cord, and reach the kidney.9 The kidney, the pelvic region in particular, also is innervated by the sensory afferent nerves that transmit various signals from the kidney, mainly via the spinal cord, to the brain.9 In this review, we discuss several neural circuits that are involved in the control of renal inflammation, focusing on AKI, and the therapeutic potential of targeting the neuroimmune interaction in AKI.
NEUROIMMUNE INTERACTION: AN EMERGING MECHANISM TO MODULATE INFLAMMATION
Felten and colleagues showed sympathetic and peptidergic innervation of the primary and secondary lymphoid organs using histologic evaluation with anterograde and retrograde labeling techniques in the 1980s.10–13 These studies clearly showed an anatomic inter-relationship between the nervous system and the immune system. Since then, many studies have been conducted to investigate the interaction between the two seemingly independent systems. It is now well known that the function of afferent (sensory) neurons is modulated by immune cells, while afferent neurons and efferent (motor) autonomic neurons alter the function of the immune cells. These afferent and efferent neurons constitute reflex pathways to regulate immune responses and inflammation.
Somatosensory neurons that innervate the skin, muscles, and joints have cell bodies in the dorsal root ganglia (DRG) that project to the spinal cord. Visceral sensory neurons that innervate all the internal organs, such as the lung, heart, liver, kidney, and gastrointestinal tract, have cell bodies in the DRG or the nodose/jugular ganglia; the latter are called the vagus afferent (sensory) neurons. Most central axons of the vagus afferent neurons project to the nucleus tractus solitarius in the medulla oblongata in the brain.14 In response to infection or tissue injury, peripheral immune cells are activated by pathogen-associated molecular patterns and damage-associated molecular patterns; they then release inflammatory cytokines and chemokines.15,16 In the local sites of infection or tissue injury, sensory neurons that express pattern recognition receptors and cytokine receptors sense inflammation and transmit signals to the central nervous system (CNS).7 Conversely, sensory neurons also can alter the inflammatory state. An activation of sensory neurons causes the release of various neuropeptides, such as calcitonin gene-related peptide (CGRP) and substance P from axon terminals at the site of innervation. These peptides bind to their receptors expressed on immune cells and blood vessels to augment or suppress inflammation.17–19
Efferent (motor) autonomic neurons also alter immune cell function. Cell bodies of the efferent vagus neurons reside in the dorsal motor nucleus of the vagus nerve and nucleus ambiguus in the medulla oblongata.14 The efferent vagus neurons synapse on ganglia located very close to the innervated organs and release acetylcholine. Some postganglionic fibers release acetylcholine that binds to the muscarinic acetylcholine receptors in the thoracic and abdominal organs, such as the heart, liver, and gastrointestinal tract, to modulate physiological functions. However, the anti-inflammatory effects of efferent vagus nerve stimulation require nicotinic acetylcholine receptors, which are stimulated by acetylcholine released by immune cells rather than by vagus nerve terminals, as discussed in the following section.
VAGUS NERVE AND THE INFLAMMATORY REFLEX
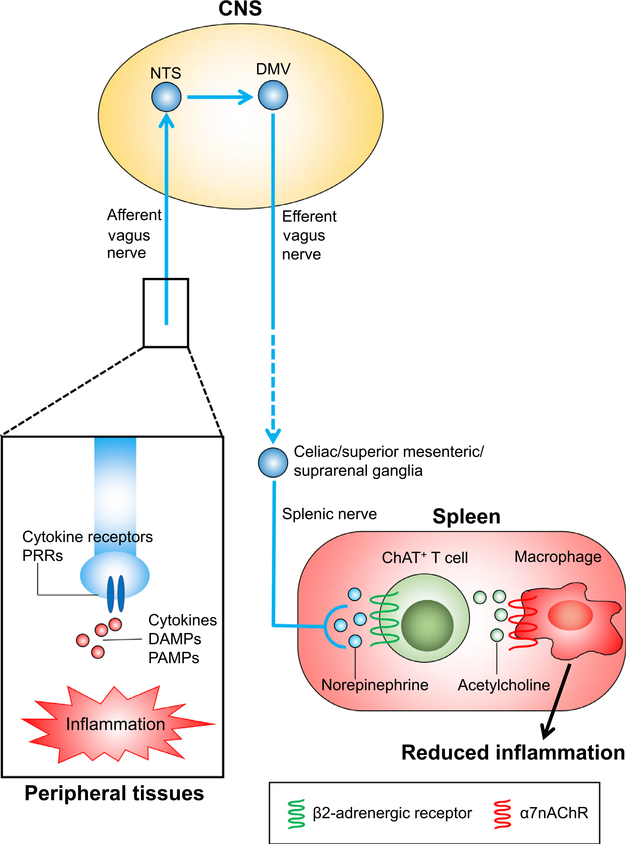
In 1995, it was found that the febrile response elicited by an intraperitoneal injection of interleukin 1β requires the afferent vagus nerve.20 In 2002, Bernik et al21 observed that administering a small amount of a potent anti-inflammatory agent intracerebroventricularly significantly decreased lipopolysaccharide-induced increases in levels of plasma tumor necrosis factor (TNF), which mainly originates from the spleen, despite negligible systemic concentrations of the anti-inflammatory agent. Cutting the vagus nerve nullified the decrease in the plasma TNF level and electrical stimulation of the vagus nerve decreased plasma TNF. These findings indicated the presence of the inflammatory reflex, wherein the afferent vagus nerve senses peripheral inflammation, and the signal is transmitted through the CNS to the efferent vagus nerve and the spleen to alleviate inflammation.22 Thereafter, the mechanism of the inflammatory reflex gained interest and increasingly has been explored (Fig. 1).
Figure 1.
The inflammatory reflex. When inflammation occurs in peripheral tissues, inflammatory cytokines, damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs) bind to cytokine receptors and pattern recognition receptors (PRRs) expressed on the local afferent vagus nerve. The signal is transmitted through the CNS to the efferent vagus nerve and the splenic nerve. Norepinephrine released by splenic nerve terminals binds to β2-adrenergic receptors expressed on choline acetyltransferase (ChAT)-positive T cells, causing acetylcholine release from this specific T cell subpopulation. The released acetylcholine binds to α7nAChRs expressed on macrophages located close to these T cells, resulting in suppressed production of the proinflammatory cytokines by the macrophages and reduced inflammation. Abbreviations: DMV, dorsal motor nucleus of the vagus; NTS, nucleus tractus solitarius.
The inflammatory reflex is initiated through the sensing of local inflammation by the afferent vagus nerve via cytokine receptors and pattern recognition receptors.23 The signal is transmitted through the CNS to the efferent vagus nerve and the splenic nerve.24 The interaction between the efferent vagus nerve and the splenic nerve may occur in the celiac, superior mesenteric, or suprarenal ganglia.10,25–28 Norepinephrine is released by splenic nerve terminals and binds to β2-adrenergic receptors expressed on the choline acetyltransferase—positive T cells in the spleen, resulting in release of acetylcholine from this specific CD4+ CD44high CD62Llow memory T-cell subpopulation.29 Binding of acetylcholine to α7 nicotinic acetylcholine receptors (α7nAChRs) expressed on macrophages that reside close to these T cells leads to suppression of proinflammatory cytokine production (eg, TNFα) by macrophages and reduced inflammation.24,30 The efferent arm of the inflammatory reflex is called the cholinergic anti-inflammatory pathway (CAP).22
INNERVATION OF THE KIDNEY AND INTERACTION BETWEEN THE RENAL SENSORY AFFERENT AND SYMPATHETIC EFFERENT NERVES
The sympathetic innervation reaches all portions of the renal vasculature, with the highest density of innervation in afferent arterioles.31 Tubules also are innervated by sympathetic nerves to a lesser degree. Norepinephrine released from sympathetic terminals is believed to act on the vasculature and tubules. An increase in efferent renal sympathetic nerve activity (ERSNA) increases the renin secretion rate via stimulation of β1-adrenergic receptors on juxtaglomerular granular cells. An increase in ERSNA also decreases renal blood flow via stimulation of α1A-adrenergic receptors on renal arterial vessels as well as sodium excretion in the urine via stimulation of α1B-adrenergic receptors on the tubular epithelial cells. In contrast to the broad innervation by sympathetic nerves to the kidney, the distribution of sensory nerves in the kidney is localized predominantly in the pelvic region.32 The sensory nerves enter the pelvis parallel to the renal artery and ureter, terminating as free nerve endings in the pelvic wall. In contrast, the renal artery and vein are innervated to a lesser degree by the sensory nerves, and there are few sensory fibers in the renal parenchyma. Considerable evidence has shown that the mechanosensitive pelvic nerves are activated by increased pelvic pressure (stretch of the pelvic wall) within the physiological range.33,34 The cell bodies of renal sensory nerves are located mainly in the DRG at the thoracic and lumbar levels,35,36 and neuronal signals are propagated to the spinal cord and then to specific areas of the brain, including the nucleus tractus solitarius and the rostral ventrolateral medulla.37
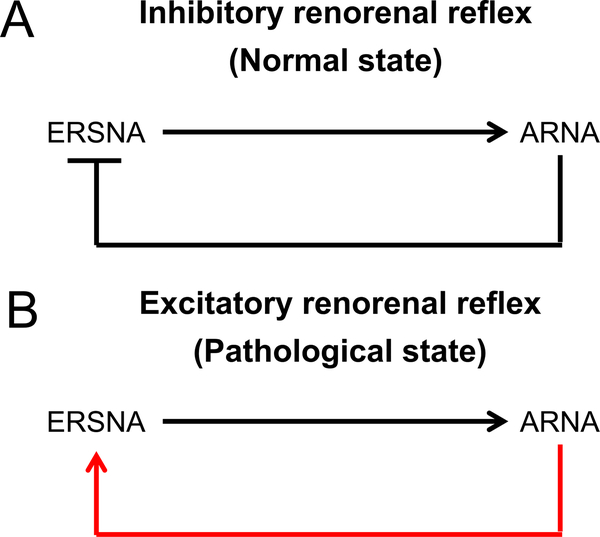
Unilateral renal denervation (both sensory afferents and sympathetic efferents) not only increased ipsilateral urinary sodium excretion, an expected result of the denervation of sympathetic efferents, but also decreased contralateral urinary sodium excretion, accompanied by increased contralateral ERSNA in normal rats.38 Stimulation of renal sensory afferents using various methods (eg, increased renal pelvic pressure and pelvic administration of chemical substances) decreased the contralateral ERSNA and increased the contralateral urinary sodium excretion that was abolished by ipsilateral renal denervation.39–41 These findings suggest that an increase in afferent renal nerve activity (ARNA) suppresses ERSNA, an effect known as the inhibitory renorenal reflex.42 ARNA also modulates the central sympathetic outflow to the periphery. In contrast, an increase in ERSNA increases ARNA, possibly via a synaptic connection between the sympathetic efferent nerves and the sensory afferent nerves in the pelvic wall (binding of norepinephrine released by the sympathetic efferents to α1- and α2-adrenergic receptors expressed on the sensory afferents).43,44 In sum, there is probably a negative feedback system by the sympathetic efferents and sensory afferents in the kidney under normal conditions; an increase in ERSNA increases ARNA, and the increase in ARNA decreases ERSNA through the inhibitory renorenal reflex (Fig. 2A).
Figure 2.
(A) Inhibitory and (B) excitatory renorenal reflexes. An increase in the ERSNA is known to increase the ARNA. (A) In normal kidneys, an increase in ARNA suppresses ERSNA, providing negative feedback to prevent excessive ERSNA (inhibitory renorenal reflex). (B) In contrast, in the kidneys, under pathologic conditions such as hypertension, CKD, and heart failure, the inhibitory renorenal reflex is suppressed and an excitatory reflex prevails. The afferent renal nerves from the damaged kidneys exert an excitatory influence on ERSNA, forming a vicious cycle leading to excessive ERSNA.
In contrast, in pathologic conditions associated with increased sympathetic nervous system activity, such as hypertension, CKD, and heart failure, the inhibitory renorenal reflex is impaired, leading to an excitatory reflex. For example, in two-kidney, one-clip hypertensive rats, denervation of the ipsilateral clipped kidney increased the urinary sodium excretion from the contralateral kidney, accompanied by decreased contralateral ERSNA.45 Removal of the diseased kidneys significantly reduced blood pressure and muscle sympathetic nerve activity in hemodialysis patients and kidney transplant patients,46,47 further supporting the notion that diseased kidneys have an excitatory effect on the sympathetic nervous system (Fig. 2B). Limited information is available about the functional status of renal sensory afferents and the renorenal reflex in AKI, although impaired responsiveness of the renal afferent nerves has been reported in acutely injured kidneys at 24 hours after unilateral ischemia-reperfusion or ureteral obstruction.48,49
RENAL DENERVATION: A POSSIBLE TREATMENT STRATEGY FOR AKI?
Considerable evidence has shown that sympathetic nerve activity is up-regulated in patients with CKD and end-stage renal disease, often accompanied by hypertension.46 Renal denervation in human beings with hypertension has been a popular, albeit controversial, research topic for several years.50–53 Renal denervation was effective in ameliorating kidney inflammation in various animal models, although little information is available regarding its effect in ischemic or septic AKI.54–56 Surgical bilateral renal denervation performed 2 days before inducing glomerulonephritis significantly ameliorated albuminuria, mesangial microaneurysms, and interstitial macrophage infiltration in a rat model of anti—Thy-1.1 nephritis.57 Bilateral renal denervation also was effective in decreasing the urinary albumin and renal cortical expression of monocyte chemotactic protein-1 without affecting the blood pressure in a mouse model of systemic lupus erythematosus.58 The effect of ipsilateral renal denervation with ethanol was investigated in a mouse model of unilateral ureteral obstruction (UUO).59 Renal denervation 2 days before UUO surgery significantly reduced neutrophil and macrophage infiltration and fibrosis in the kidney. Furthermore, continuous infusion of norepinephrine or CGRP, but not neuropeptide Y or substance P, into the cortical region of the denervated kidney via a catheter nullified the protective effect of renal denervation in a dose-dependent manner. Unilateral renal denervation or administration of these neurotransmitters did not alter the systolic blood pressure. In the innervated kidneys, the CGRP level was increased significantly up to 24 hours after UUO. The investigators also showed that UUO-induced kidney inflammation and fibrosis required α2-adrenergic and CGRP receptors and that these receptors are expressed in tubular epithelial cells. Similar findings also were shown in a mouse model of unilateral kidney ischemia-reperfusion injury (IRI).60 In contrast to the protective effect of preventive renal denervation before disease induction, renal denervation at 1 day after UUO and at 3 days after IRI did not ameliorate kidney fibrosis significantly, suggesting that renal nerve activity is important, particularly during the acute phase of injury. These findings suggest that the local actions of both sympathetic efferent and sensory afferent neurons in the kidney play critical roles in kidney inflammation and fibrosis.
CAP IN AKI
The role of CAP in AKI was explored using vagus nerve stimulation (VNS) in a mouse model of renal IRI.61 Electrical stimulation of the left cervical vagus nerve 24 hours before IRI significantly ameliorated kidney injury, as shown by the decrease in plasma creatinine level and kidney injury molecule-1 expression in the kidney with improved renal histology. As expected, in splenectomized mice or α7nAChR−/− mice, VNS was not protective, indicating that VNS-mediated protection against kidney IRI is caused by CAP activation. It is noteworthy that the stimulation of either the peripheral or central end of the cut vagus nerve also protected the kidneys. Blocking nerve conduction of the right (contralateral) vagus nerve by local anesthesia (bupivacaine) did not inhibit the protective effect of afferent VNS, suggesting the presence of downstream pathway(s) other than the efferent vagus nerve in the protection against AKI. VNS in brain-dead donor rats was effective in attenuating inflammation in the donors, decreasing immune cell infiltration to the tubules and the arteries in the recipients, and improving long-term renal function and survival of the recipients.62,63
Applying pulsed ultrasound to the spleen also appears to exert a protective effect against AKI in a manner similar to that of VNS.64,65 Ultrasound application using a clinical machine 24 hours before IRI attenuated kidney injury in mice. The protective effect by ultrasound was abolished in splenectomized mice, mice with splenic sympathectomy, Rag1−/− mice, α7nAChR−/− mice, and mice treated with an antagonist of α7nAChR. Administration of an α7nAChR agonist mimicked the protective effect of ultrasound. Furthermore, bone marrow chimera experiments showed that α7nAChR expression in bone marrow—derived cells is essential for protection by ultrasound. In sum, these findings suggest that the application of pulsed ultrasound protects the kidneys from IRI by activating the CAP. Pulsed ultrasound also ameliorated septic AKI in a cecal ligation and puncture model. However, direct target(s) of pulsed ultrasound are yet to be determined.
Although all of these studies showed that the spleen plays a critical role in CAP activation to protect the kidneys from AKI, the precise interaction between the spleen and the kidney is unknown. One intriguing finding is that adoptive transfer of splenocytes from ultrasound-treated64 or VNS-treated61 (but not sham) mice to naive mice was sufficient to protect kidneys of recipient mice from IRI. Thus, activation of CAP may have altered the phenotype of splenocytes and conferred protection.
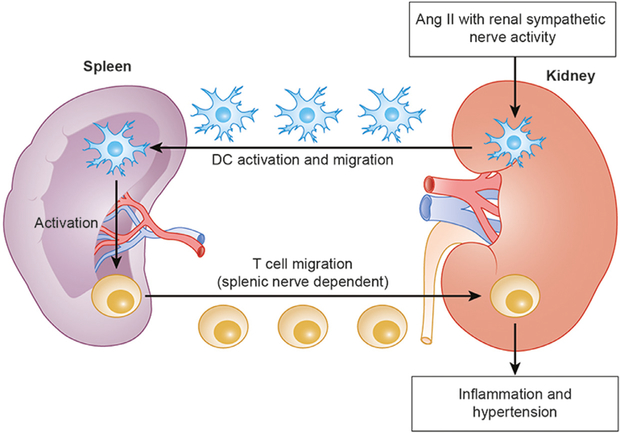
In addition, interaction of spleen and kidneys during protection may include involvement of the renal nerves. Recent findings on the neuroimmune axis in hypertension may provide a clue regarding the missing link between the two organs in the context of CAP activation in AKI. Hypertension is associated with CD4+ and CD8+ T cell infiltration into the kidney.66,67 Xiao et al68 extended their work to investigate the role of renal sympathetic nerves in T cell infiltration into the kidney in angiotensin II—induced hypertension. Bilateral renal denervation with phenol, but not selective renal afferent ablation with capsaicin, ameliorated hypertension and kidney inflammation, as reflected by significant decreases in CD4+ and CD8+ T cell infiltration, fibrosis, and urinary albumin. Moreover, unilateral renal denervation, leading to a partial decrease in blood pressure, ameliorated inflammation only in the denervated kidney, suggesting that suppressed kidney inflammation is not secondary to decreased blood pressure, but directly caused by renal denervation. Moreover, the investigators observed that renal denervation changed the phenotype of dendritic cells in the spleen. Furthermore, mice lacking C—C chemokine receptor type 7, important as a homing signal to secondary lymphoid organs for dendritic cells, showed no CD4+ or CD8+ T cell infiltration into the kidney without activation of dendritic cells in the spleen, while the dendritic cells in the kidney still were activated. These findings, although indirect, support the hypothesis that hypertensive stimuli with renal sympathetic nerve activity activates dendritic cells in the kidney, leading to the migration of these cells to secondary lymphoid organs, such as the spleen, where they, in turn, activate T cells; thereafter, these T cells migrate to the kidney causing inflammation. Another group showed that T cells egress from the spleen and infiltrate the kidney and aorta, eventually causing hypertension in an angiotensin II—infusion model and that the splenic nerve is essential in these steps (Fig. 3).69 The interplay between the spleen and kidney in the context of AKI clearly merits further exploration.
Figure 3.
The hypothesized interaction between the spleen and the kidney for the activation of dendritic cells (DCs) and T cells in angiotensin II (Ang II)-induced hypertension. Ang II with renal sympathetic nerve activity activates DCs in the kidney, leading to the migration of these cells to the spleen, where they, in turn, activate T cells. Thereafter, these activated T cells egress from the spleen and migrate to the kidney causing inflammation and hypertension. The splenic nerve is essential for T cell migration.
NONCLASSIC CAP AND OTHER NEUROIMMUNE INTERACTIONS TO REGULATE AKI AND INFLAMMATION
Recently, Abe et al70 explored the role of the CNS in the neuroimmune interaction and AKI. C1 neurons that reside in the medulla oblongata innervate the dorsal motor nucleus of the vagus, paraventricular nucleus of the hypothalamus, and sympathetic efferent pathways, and mediate autonomic responses to several stressors, including hypotension and hypoxia.71 The selective stimulation of C1 neurons using the optogenetics technique (described later) protected mice against kidney IRI, which was dependent on the spleen, α7nAChRs, and β2-adrenergic receptors. These results suggest that kidney protection by C1 neuron stimulation involves CAP activation. Interestingly, a short period of physical restraint also protected the kidney from IRI, and selective ablation or inhibition of C1 neurons nullified the protective effect of restraint stress, indicating that renoprotection by restraint stress is mediated by C1 neurons. Furthermore, the investigators attempted to identify the downstream pathway—vagal efferents, hypothalamic-pituitary-adrenal axis, or sympathetic efferents—involved in kidney protection by C1 neuron stimulation. Ganglionic blockade, but not subdiaphragmatic vagotomy or corticosterone-receptor blockade, significantly attenuated the protection, suggesting that a sympathetic, not a vagus route, to the spleen is crucial for kidney protection by C1 neuron stimulation.
Several studies also have shown that AKI can affect CNS function.72 Bilateral kidney IRI increased vascular permeability in the brain and the number of pyknotic neurons; it also activated microglial cells in the hippocampus in mice and was accompanied by reduced locomotor activity.73 Dopamine turnover was decreased in the striatum, mesencephalon, and hypothalamus of rats with bilateral kidney IRI.74 Although circulating factors such as cytokines and damage-associated molecular patterns may be responsible for these brain changes, signal transmission from activated afferent neurons in the injured kidneys to the CNS also may play an important role.
Dopamine released by the adrenal gland also appears to be involved in vagus nerve—mediated anti-inflammation.75 Sciatic nerve activation using electroacupuncture alleviated polymicrobial peritonitis induced by cecal ligation and puncture by increasing dopamine production in the adrenal medulla via vagus nerve activation. Dopamine D1 receptors mediated the suppression in the cytokine production induced by dopamine. It is noteworthy that unlike the classic CAP, this sciatic-to-vagus neural circuit did not need the spleen, α7nAChRs, or β2-adrenergic receptors. Recent studies have suggested that gut macrophages are involved in other types of neuroimmune interactions. Matteoli et al76 showed that efferent vagus nerve interacts with cholinergic myenteric neurons that are in close contact with muscularis macrophages. VNS attenuated surgery-induced intestinal inflammation and improved postoperative intestinal transit. This protective effect of VNS was mediated by α7nAChR on muscularis macrophages and was independent of the spleen and T cells. Gut muscularis macrophages also have been shown to enhance their tissue-protective phenotype in cases of luminal bacterial infection.77 This alteration was attributed to the activation of sympathetic neurons and norepinephrine signaling to β2-adrenergic receptors on these macrophages. Protective neuroimmune interactions other than the classic CAP also may be present in the kidney.
FUTURE CLINICAL APPLICATION OF NEUROIMMUNE INTERACTION IN AKI
Considering the experimental evidence regarding the effectiveness of neuroimmunomodulation in kidney diseases as discussed earlier (Table 1), targeting the neuroimmune interaction appears to be a promising approach for treating human AKI.78 In the 1990s, VNS with a surgically implanted stimulator was approved for treating refractory epilepsy in Europe and the United States. The Food and Drug Administration also approved VNS for treatment-resistant depression in 2005. Recently, the Food and Drug Administration approved a noninvasive vagus nerve stimulator for treating episodic cluster headache pain and migraine pain. In addition to these disorders, many clinical trials of VNS are ongoing for determining the optimal treatment modality for various inflammatory diseases (eg, diabetes and heart failure).79,80 An implanted stimulator (with cuffs placed around the left cervical vagus nerve, a pulse generator implanted on the chest wall, and a subcutaneously tunneled lead connecting the cuffs and pulse generator) was effective in patients with refractory rheumatoid arthritis.81 VNS significantly reduced the disease severity for up to 12 weeks with suppressed TNF production. It also was reported that five of seven patients with active Crohn’s disease achieved significant clinical remission (decreased disease activity index and improved endoscopic findings) by VNS for 6 months.82
Table 1.
Experimental Evidence for the Effectiveness of Neuroimmunomodulation in AKI and Other Kidney Diseases
| Treatment | Models | Outcomes | References |
|---|---|---|---|
| Renal denervation (both sympathetic and afferent neurons) | Anti–Thy-1.1 nephritis | Decreased urinary albumin, mesangial microaneurysms, and macrophage infiltration | 57 |
| SLE | Decreased urinary albumin and renal cortical expression of MCP-1 | 58 | |
| UUO/unilateral IRI | Decreased neutrophil and macrophage infiltration and fibrosis | 59, 60 | |
| Angiotensin II–induced hypertension | Ameliorated hypertension, renal inflammation (T cell infiltration), renal fibrosis, and albuminuria | 68 | |
| VNS (CAP activation) | Bilateral IRI | Ameliorated AKI | 61 |
| Kidney transplantation | Improved long-term renal function and survival of the recipients with decreased immune cell infiltration | 62, 63 | |
| Pulsed ultrasound (CAP activation) | Bilateral IRI/CLP | Ameliorated AKI | 64, 65 |
| Stimulation of C1 neurons/physical restraint (CAP activation) | Bilateral IRI | Ameliorated AKI | 70 |
| Nicotine/α7nAChR agonists (CAP activation) | Bilateral IRI/LPS/cisplatin | Ameliorated AKI | 65, 83–85 |
Abbreviations: CLP, cecal ligation and puncture; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein-1; SLE, systemic lupus erythematosus.
Pharmacologic activation of CAP or other anti-inflammatory pathways also appears to be a promising treatment strategy for AKI. Clinical trials of GTS-21, an agonist of α7nAChR, currently are ongoing for Alzheimer’s disease and schizophrenia, although the precise mechanisms of its effects still are unclear. In animal studies, administration of nicotine or GTS-21 protects against kidney IRI,83 lipopolysaccharide-induced AKI,84 and cisplatin-induced AKI.85 Torres-Rosas et al75 reported that dopamine D1 receptor agonists rescued mice with adrenal insufficiency from polymicrobial peritonitis by suppressing systemic inflammation.
We believe that the biggest challenge in research regarding AKI treatment that targets neuroimmunomodulation with a nonpharmacologic or pharmacologic approach is the lack of knowledge regarding the precise underlying mechanisms for the amelioration of AKI by neuroimmunomodulation. For example, with respect to VNS, unanswered questions include the downstream effects of afferent VNS and the link between the spleen and the kidney. Understanding the precise mechanisms of the neuroimmune interaction in AKI is critical to ensure its safe and effective clinical application.
OPTOGENETICS: A NOVEL TECHNIQUE TO UNDERSTAND COMPLEX NEURAL CIRCUITS
As discussed previously, a deeper understanding of the complex neural circuits involved in the pathophysiology of AKI is important. However, the lack of methods for selectively stimulating and inhibiting neurons has been a major obstacle. A novel technique called optogenetics helps in selective stimulation and inhibition of target neurons in vivo by light application.
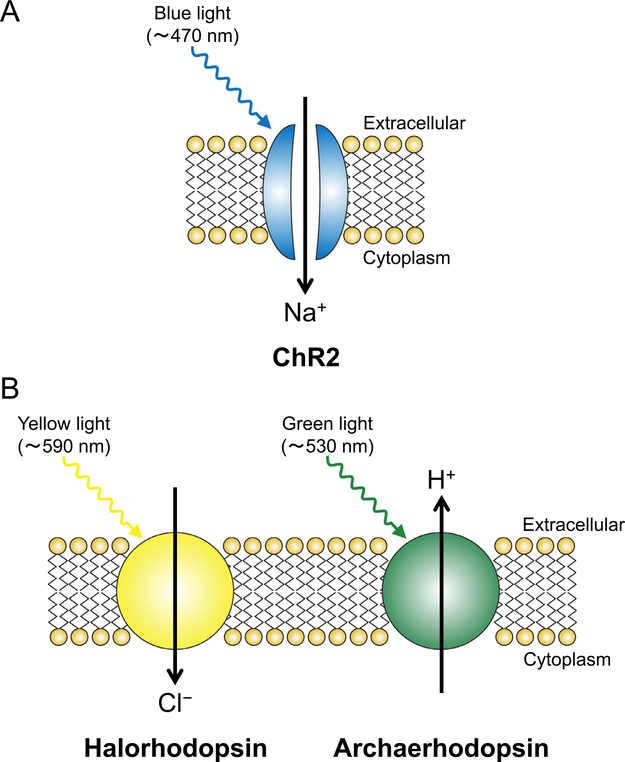
Optogenetics refers to the use of both optics and genetics for controlling cells, typically neurons, which have been genetically manipulated to express light-sensitive opsins. These opsins include excitatory channelrhodopsin-2 (ChR2)86–88 and inhibitory halorhodopsin89 and archaerhodopsin90 (Fig. 4). For example, ChR2, originally discovered from the green alga Chlamydomonas reinhardtii, is a nonselective cation channel and its gate is opened rapidly by the conformational change after blue light application (maximum activation, 470 nm).91 A report on the application of these unique proteins in the field of neuroscience was published in 2005.92 Boyden et al introduced ChR2 to mammalian hippocampal neurons with lentivirus in vitro and successfully evoked action potentials only 1 to 2 ms after blue light application to these cells, and the firing was stopped when the light was switched off. Illuminating ChR2-expressing neurons with blue light opens the ChR2 cation channel, enabling Na+ to enter the cells, which depolarizes the cell membrane and evokes an action potential.
Figure 4.
Schematics of an (A) excitatory light-sensitive opsin, ChR2, and (B) inhibitory light-sensitive opsins, halorhodopsin, and archaerhodopsin. The expression of these opsins does not affect the resting membrane potential because of the lack of ion flux without light application. (A) When ChR2 is illuminated with blue light, the gate of this nonselective cation channel is opened, which allows influx of Na+ and causes depolarization of the ChR2-expressing neurons. If the spike of Na+ entry is large enough for the membrane potential to reach the threshold, an action potential is evoked. (B) When halorhodopsin/archaerhodopsin is illuminated with yellow/green light, it functions as an inward Cl-/outward H+ pump and causes hyperpolarization of the neurons expressing these opsins, thereby exerting an inhibitory effect.
Since a report regarding the control of neurons using optogenetics in 2005, this technique has been broadly used as an intervention tool. Microinjection of viral vectors into the target area and the Cre-LoxP system enable the specific expression of light-sensitive opsins in in vivo studies. This technique has been applied to study subgroups of vagus afferent neurons that express specific markers.93 By applying blue laser to the cervical vagus nerve of transgenic mice, wherein ChR2 specifically was expressed in each subgroup of the vagus afferent neurons, Liberles and colleagues showed that selective stimulation of each subgroup modified the function of various organs (eg, lung, heart, gastrointestinal tract) in a different manner.93,94 For example, the stimulation of P2ry1-positive neurons completely stopped respiration, whereas the stimulation of Npy2r-positive neurons caused rapid/shallow breathing. These findings suggest that subpopulations of vagus neurons may play a critical role in the renoprotective effect of VNS. Thus, optogenetics is a useful tool for studying the roles of complex neural circuits in the neuroimmune interaction in AKI pathophysiology, leading to the clinical application of neuroimmunomodulation to AKI treatment.
CONCLUSIONS
Experimental evidence has suggested that VNS and pulsed ultrasound protect the kidney from AKI by activating CAP, and that renal denervation is effective in ameliorating renal inflammation and possibly AKI. Optogenetics is a useful technique for dissecting complex neural circuits and is applicable in AKI. Further assessment of the neural circuits to control renal inflammation is warranted for future clinical application of neuroimmunomodulation with a nonpharmacologic or pharmacologic approach.
ACKNOWLEDGMENTS
We thank Drs. Diane Rosin, Department of Pharmacology, and Tsuyoshi Inoue, Division of Nephrology/Center for Immunity, Inflammation and Regenerative Medicine, University of Virginia, for very useful discussions and careful reading of the manuscript.
Financial support: Research conducted for this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health awards R01DK085259, R01DK062324, and U18 EB021787 (M.D.O.), and by the Uehara Memorial Foundation Research Fellowship (S.T.).
Footnotes
Conflict of interest statement: none.
REFERENCES
- 1.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. [DOI] [PubMed] [Google Scholar]
- 3.Srisawat N, Hoste EE, Kellum JA. Modern classification of acute kidney injury. Blood Purif. 2010;29:300–7. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol. 2014;307: F1187–95. [DOI] [PubMed] [Google Scholar]
- 5.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmela K, Wramner L, Ekberg H, Hauser I, Bentdal O, Lins LE, et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation. 1999;67:729–36. [DOI] [PubMed] [Google Scholar]
- 7.Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, von Andrian UH. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 2015;36:578–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–66. [DOI] [PubMed] [Google Scholar]
- 9.Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017;13:669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. [DOI] [PubMed] [Google Scholar]
- 11.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. 118–21. [DOI] [PubMed] [Google Scholar]
- 12.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135(Suppl):755s–65s. [PubMed] [Google Scholar]
- 13.Williams JM, Peterson RG, Shea PA, Schmedtje JF, Bauer DC, Felten DL. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull. 1981;6:83–94. [DOI] [PubMed] [Google Scholar]
- 14.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 17.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. [DOI] [PubMed] [Google Scholar]
- 21.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic anti-inflammatory pathway. J Exp Med. 2002;195:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci. 2005;120:104–7. [DOI] [PubMed] [Google Scholar]
- 24.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–69. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–6. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154:66–73. [DOI] [PubMed] [Google Scholar]
- 28.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–90. [DOI] [PubMed] [Google Scholar]
- 29.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 31.Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol. 1992;70:735–49. [DOI] [PubMed] [Google Scholar]
- 32.Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol. 1991;311:389–404. [DOI] [PubMed] [Google Scholar]
- 33.Genovesi S, Pieruzzi F, Wijnmaalen P, Centonza L, Golin R, Zanchetti A, et al. Renal afferents signaling diuretic activity in the cat. Circ Res. 1993;73:906–13. [DOI] [PubMed] [Google Scholar]
- 34.Kopp UC, Smith LA, Pence AL. Na(+)-K(+)-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol. 1994;267:R1109–17. [DOI] [PubMed] [Google Scholar]
- 35.Donovan MK, Wyss JM, Winternitz SR. Localization of renal sensory neurons using the fluorescent dye technique. Brain Res. 1983;259:119–22. [DOI] [PubMed] [Google Scholar]
- 36.Weiss ML, Chowdhury SI. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 1998;812:227–41. [DOI] [PubMed] [Google Scholar]
- 37.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–19. [DOI] [PubMed] [Google Scholar]
- 38.Colindres RE, Spielman WS, Moss NG, Harrington WW, Gottschalk CW. Functional evidence for renorenal reflexes in the rat. Am J Physiol. 1980;239:F265–70. [DOI] [PubMed] [Google Scholar]
- 39.Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol. 1984;246:F67–77. [DOI] [PubMed] [Google Scholar]
- 40.Kopp UC, Smith LA. Inhibitory renorenal reflexes: a role for substance P or other capsaicin-sensitive neurons. Am J Physiol. 1991;260:R232–9. [DOI] [PubMed] [Google Scholar]
- 41.Kopp UC, Smith LA. Role of prostaglandins in renal sensory receptor activation by substance P and bradykinin. Am J Physiol. 1993;265:R544–51. [DOI] [PubMed] [Google Scholar]
- 42.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopp UC, Cicha MZ, Smith LA, Mulder J, Hokfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2-adrenoceptors on renal sensory nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1561–72. [DOI] [PubMed] [Google Scholar]
- 44.Kopp UC, Cicha MZ, Smith LA, Ruohonen S, Scheinin M, Fritz N, et al. Dietary sodium modulates the interaction between efferent and afferent renal nerve activity by altering activation of alpha2-adrenoceptors on renal sensory nerves. Am J Physiol Regul Integr Comp Physiol. 2011;300:R298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension. 1989;14:445–52. [DOI] [PubMed] [Google Scholar]
- 46.Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8. [DOI] [PubMed] [Google Scholar]
- 47.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–9. [DOI] [PubMed] [Google Scholar]
- 48.Ma MC, Huang HS, Chen CF. Impaired renal sensory responses after unilateral ureteral obstruction in the rat. J Am Soc Nephrol. 2002;13:1008–16. [DOI] [PubMed] [Google Scholar]
- 49.Ma MC, Huang HS, Wu MS, Chien CT, Chen CF. Impaired renal sensory responses after renal ischemia in the rat. J Am Soc Nephrol. 2002;13:1872–83. [DOI] [PubMed] [Google Scholar]
- 50.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. [DOI] [PubMed] [Google Scholar]
- 51.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–82. [DOI] [PubMed] [Google Scholar]
- 52.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–7. [DOI] [PubMed] [Google Scholar]
- 54.Calzavacca P, May CN, Bellomo R. Glomerular haemodynamics, the renal sympathetic nervous system and sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2014;29:2178–84. [DOI] [PubMed] [Google Scholar]
- 55.Lambert E, Schlaich M. The role of renal sympathetic nerves in ischemia reperfusion injury. Auton Neurosci. 2017;204:105–11. [DOI] [PubMed] [Google Scholar]
- 56.May CN, Calzavacca P, Ishikawa K, Langenberg C, Wan L, Ramchandra R, et al. Novel targets for sepsis-induced kidney injury: the glomerular arterioles and the sympathetic nervous system. Exp Physiol. 2012;97:1168–77. [DOI] [PubMed] [Google Scholar]
- 57.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, et al. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol. 2008;19:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathis KW, Venegas-Pont M, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, et al. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol. 2013;305:R711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015;87:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126:1939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoeger S, Bergstraesser C, Selhorst J, Fontana J, Birck R, Waldherr R, et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am J Transplant. 2010;10:477–89. [DOI] [PubMed] [Google Scholar]
- 63.Hoeger S, Fontana J, Jarczyk J, Selhorst J, Waldherr R, Kramer BK, et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol Dial Transplant. 2014;29:544–9. [DOI] [PubMed] [Google Scholar]
- 64.Gigliotti JC, Huang L, Bajwa A, Ye H, Mace EH, Hossack JA, et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J Am Soc Nephrol. 2015;26:2470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24:1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res. 2015;117:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41:737–52. [DOI] [PubMed] [Google Scholar]
- 70.Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidneybrain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–19. [DOI] [PubMed] [Google Scholar]
- 73.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adachi N, Lei B, Deshpande G, Seyfried FJ, Shimizu I, Nagaro T, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27:1655–60. [DOI] [PubMed] [Google Scholar]
- 75.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–48. [DOI] [PubMed] [Google Scholar]
- 77.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka S, Inoue T, Hossack JA, Okusa MD. Nonpharmacological, biomechanical approaches to control inflammation in acute kidney injury. Nephron. 2017;137:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22:1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014;1:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113:8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28:948–53. [DOI] [PubMed] [Google Scholar]
- 83.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7:e35361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chatterjee PK, Yeboah MM, Solanki MH, Kumar G, Xue X, Pavlov VA, et al. Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS One. 2017;12:e0188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–8. [DOI] [PubMed] [Google Scholar]
- 87.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2002;99:8689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki T, Yamasaki K, Fujita S, Oda K, Iseki M, Yoshida K, et al. Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem Biophys Res Commun. 2003;301:711–7. [DOI] [PubMed] [Google Scholar]
- 89.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. [DOI] [PubMed] [Google Scholar]
- 90.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. [DOI] [PubMed] [Google Scholar]
- 93.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]