Abstract
Background:
Girls’ depressive symptoms typically increase in adolescence, with individual differences in course and severity being key risk factors for impaired emotional functioning in young adulthood. Given the continued brain white matter (WM) maturation that occurs in adolescence, the present study tested whether structural connectivity patterns in late adolescence are associated with variation in the course of depression symptom severity throughout adolescence.
Method:
Participants were girls (N=115) enrolled in a multi-year prospective cohort study of risk for depression. Initial depression severity (intercept) at age 10 and change in severity (linear slope) across ages 10-19 were examined in relation to WM tractography collected at age 19. Network-based statistical analysis was used to identify clusters showing variation in structural connectivity in association with depressive intercept, slope, and their interaction.
Results:
Higher initial depressive severity and steeper positive slope (separately) were associated with greater structural connectivity between temporal, subcortical socio-affective, and occipital regions. Intercept showed more connectivity associations than slope. The interaction effect indicated that higher initial symptom severity and a steeper negative slope (i.e., alleviating symptoms) was related to greater connectivity between cognitive control regions. Moderately severe symptoms that worsened over time were followed by greater connectivity between self-referential and cognitive regions (e.g., posterior cingulate and frontal gyrus).
Conclusions:
Higher depressive severity in early adolescence and increasing symptom severity over time may forecast structural connectivity differences in late adolescence, particularly in pathways involving cognitive and emotion-processing regions. Understanding how clinical course relates to neurobiological correlates may inform new treatment approaches to adolescent depression.
Keywords: Adolescence, Depression, Connectomics, Brain Imaging, Development
Introduction
The onset of depression typically rises in adolescence, especially among girls (Breslau et al., 2017), although decreases in depression symptoms across adolescence have also been observed in all-female cohorts (Keenan, Culbert, Grimm, Hipwell, & Stepp, 2014). Because the temporal course of the disorder shows heterogeneity (e.g., Yaroslavsky, Pettit, Lewinsohn, Seeley, & Roberts, 2013), it is important to characterize variability in both the course and severity of depressive symptoms. Furthermore, adolescents with symptoms that onset early and continue over time face high risk for psychosocial maladjustment in adulthood. For example, adolescents with moderate or high levels of symptom severity that persist across adolescence experience more depressive episodes, higher anxiety, and higher risk for substance use disorders in adulthood (Yaroslavsky et al., 2013).
Adolescence is also a period when white matter (WM) pathways strengthen and stabilize to support coordinated activity among networks of regions involved in higher-level cognitive and emotional functioning (Chen, Liu, Gross, & Beaulieu, 2013). Studies suggest that symptoms emerging earlier in adolescence, during the critical period of WM refinement are associated with atypical neurodevelopment (e.g., volumetric changes in the hippocampus, amygdala, and putamen from early to mid-adolescence) (Whittle et al., 2014) and WM structural connections in late adolescence (Ellis et al., 2017). Thus, when and to what degree adolescents experience depressive symptoms may in part explain heterogeneity in neurobiological correlates and risk for future depression. Determining how variation in symptom course and severity across adolescence relates to WM pathways connecting brain regions (i.e., structural connectivity) in late adolescence, when network properties begin to stabilize (Chen et al., 2013), would advance knowledge about heterogeneous brain-behavior risk profiles that could, in turn, inform person-centered intervention approaches.
Shared across neurodevelopmental models of adolescent depression is the concept of mismatch between the relative development of regions involved in cognitive control and subcortical regions involved in affective processing, such that regulatory systems mature later and render adolescents susceptible to affective dysregulation and psychiatric disorders (see overview in Weir, Zakama, & Rao, 2012). Indeed, lower coupling between emotion-processing and regulatory systems has been found in both adults and adolescents with depression. Relative to healthy controls, adults diagnosed with Major Depressive Disorder (MDD) exhibit lower structural connectivity between cognitive- and motivation-related regions (e.g., frontal cortex, thalamus, and caudate) (Korgaonkar, Fornito, Williams, & Grieve, 2014), as well as between cognitive- and emotion-processing regions (e.g., frontal gyri and hippocampus) (Lu et al., 2017). Additionally, adolescents with MDD show weaker structural connectivity between affective- (e.g., insula, caudate) and cognitive-processing regions (e.g., frontal gyri) compared with typically developing youth (Tymofiyeva et al., 2017). Treatment-naïve young adults with MDD show greater structural connectivity within and between motivation-related (e.g., orbitofrontal cortex [OFC], putamen, caudate) and emotion-processing regions (e.g., hippocampus, fusiform gyrus) (Long et al., 2015). While lower connectivity between cognitive- and emotion-processing regions may reflect ineffective top-down regulation of negative emotions (Fowler, Miernicki, Rudolph, & Telzer, 2017), greater connectivity within affective-processing regions may underlie hyperactivity of affective regions, particularly in response to emotional cues (Connolly et al., 2017; Cullen et al., 2014), as seen in adolescent depression. The few available studies on the relation between structural connectivity and depression in adolescents suggest that brain architectural deviations may contribute to the cognitive, motivational, and affective dysfunction characteristic of the disorder as posited by neurodevelopmental models of depression risk (Foland-Ross & Gotlib, 2012; Weir et al., 2012).
Connectivity features related to adolescent depression have been found to vary with symptom course heterogeneity. Young adults have been shown to differ in structural connectivity of reward-, affective- and cognitive-processing regions based on whether and when in adolescence they showed elevated depression symptoms (Ellis et al., 2017). Participants with elevated depression symptoms at any point in adolescence exhibited greater connectivity within affective regions than those with consistently low levels of depression. However, those with greater depression symptoms in early adolescence also showed lower connectivity within cognitive-processing regions and between cognitive- and affective-processing regions compared to those with consistently low depression symptoms or those with elevated depression symptoms later in adolescence (Ellis et al., 2017). These findings underscore the need to account for when elevated depressive symptoms emerge and how they change. Knowledge of brain-based differences as they relate to the course of depression not only informs existing theoretical models, but also reveals mechanisms that may perpetuate risk for depression (e.g., kindling effects). Symptoms may be instantiated and/or maintained as the brain’s architecture is still maturing, contributing to lasting individual differences in cognitive, affective, and reward circuitry (Hagan et al., 2015). Indeed, structural networks adapt to environmental demands (Laughlin & Sejnowski, 2003), which is particularly evident in late childhood and early adolescence (Chen et al., 2013). Age of exposure, severity, and course of depressive symptoms, and associated neural activity all likely contribute to WM connections within and between brain regions previously implicated in depression (e.g., cognitive and affective networks) in late adolescence.
Based on existing findings and neurodevelopmental models, the current study investigated whether girls’ courses of depression are associated with subsequent variations in structural connectivity using diffusion-weighted imaging. Longitudinal multilevel modeling was used to test whether severity of symptoms at age 10 (i.e., intercept) and change in severity (i.e., slope from age 10-19) are related to structural connectivity in late adolescence. We hypothesized that higher depression symptom severity at the start of adolescence (higher intercept) would be associated with greater connectivity within affective regions, as well as lower connectivity between cognitive and affective regions, consistent with patterns identified in previous findings and posited by neurodevelopmental models of adolescent depression (Ellis et al., 2017; Tymofiyeva et al., 2017; Weir et al., 2012). Following findings from Ellis et al. (2017), we hypothesized that increasing/worsening severity of symptoms into later ages of adolescence (i.e., steeper positive slope) would be associated with altered connectivity of similar depression-relevant regions, though to a lesser extent, given that whole-brain WM pathways develop profoundly in early adolescence (Chen et al., 2013) and emotional difficulties experienced earlier in adolescence are accompanied by more atypical neurodevelopment (Whittle et al., 2014). As an exploratory aim, we examined the interaction of depressive severity intercept and slope in relation to connectivity patterns. Although no prior research exists upon which to base specific hypotheses related to this interaction, we speculated that higher initial depressive severity that alleviates over time would be associated with more normative connectivity patterns (e.g., greater connectivity between cognitive regions), whereas higher initial severity and non-remitting symptom course would be akin to past findings on adult depression (e.g., lower cognitive connectivity; Long et al., 2015).
Method
Participants
Participants were a subsample of girls (N=115) enrolled in the Pittsburgh Girls Study, a longitudinal community-based study (Keenan et al., 2010). At age 9, girls were enrolled into a sub-study of precursors of depression, where half the participants displayed elevated depressive symptoms and half did not (Appendix S1). Written assent/consent was obtained for all participants and their parents, and monetary compensation was provided. Study procedures were approved by the Human Protections Committee and Institutional Review Board of the study sites.
Measures
Depressive Symptom Severity.
Self-reported depressive symptoms were collected using the Child, Adolescent, and Adult Symptom Inventories- 4th Edition beginning when the girls were age 10 and continuing annually through age 19 (Gadow, 2015). All 9 symptoms of DSM-IV major depressive disorder were assessed with these inventories, which demonstrate high validity, reliability, and clinical utility (Salcedo et al., 2017). Symptom severity, a dimensional measure, provides a significant advantage in identifying youth with mood disorders (Salcedo et al., 2017). Items were rated 0 (never), 1 (sometimes), 2 (often), or 3 (very often) and summed; yes or no items (e.g., change in appetite) were recoded (yes = 2.5, no = 0.5) following the scoring criteria. Reliability at ages 10-19 ranged from α = .72-.86. Research shows that youth self-reported depression, compared to parent-report or multi-informant report, best predicts concurrent depressive episodes as assessed by a clinical diagnostic interview (Cohen, So, Young, Hankin, & Lee, 2019). See Appendix S2 for information about estimated Major Depressive Disorder occurrence.
White Matter Diffusion-Weighted Imaging.
At approximately age 19, girls underwent diffusion-weighted imaging on a 3.0T Siemens Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany). A pulsed-gradient spin-echo sequence was applied in 68 directions, with posterior-to-anterior phase encoding. Scan parameters included repetition time (TR)=8500 ms, echo time (TE)=91 ms, and field of view (FOV)=256 mm2. Sixty-four contiguous slices were acquired with an isotropic voxel size of 2.0 mm3 and a b-value of 1000 s/mm2 (see Appendix S3 for preprocessing steps). A high-resolution T1-weighted anatomical image was acquired with the following parameters: TR=2300 ms; TE=2.98 ms; flip angle=9°; 160 slices; FOV=256 mm; acquisition voxel size=1.0×1.0×1.2 mm.
Tractography and Graph Construction.
Anatomical images were co-registered to diffusion native space and parcellated into 94 contiguous regions of interest based on the Automatic Anatomical Labeling Atlas 2 (AAL2; Rolls, Joliot, & Tzourio-Mazoyer, 2015), currently the most widely used atlas in connectivity studies (Hallquist & Hillary, 2018). Whole-brain probabilistic tractography was run using FSL’s BEDPOSTX and PROBTRACKX (Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007); 5000 streamlines/voxel were sampled for each of the 94 regions. The maximum connective values between pairs of regions were taken to compute per-person connectivity matrices representing the probabilities of connections among regions (i.e., 94×94 undirected and weighted matrices; additional detail in Appendix S3).
Data Analysis
Depressive Symptom Severity Course.
Unconditional multilevel growth modeling (Hedeker & Gibbons, 2012) in R Statistical Software version 3.4.0 (R Core Team, 2017) was used to measure within- and between-individual change in depressive severity across the ten time points (e.g., annual assessments from ages 10-19). The Level 1 model estimated yearly, within-individual changes in depressive severity during the 10-year period; the Level 2 model estimated the sample’s average growth trajectory. Linear and quadratic models were fit to the data. The intercept of Time was coded as beginning at age 10 and each time-point was coded as integers from 0-9 (age 10-19). The linear and quadratic models’ fit criteria were compared for model selection. Per-person estimates of intercept and slope, derived from multilevel modeling, were extracted for connectivity analyses.
Structural Connectivity Analysis.
Network-Based-Statistic (NBS) analyses (Zalesky, Fornito, & Bullmore, 2010), a robust method to control family-wise error rate during mass univariate testing, were used to test associations between interregional connectivity matrices and depression course variables. NBS analyses identified clusters (i.e., subnetworks) of brain regions where the strength of edges between them were associated with depression course variables. A primary component-forming threshold (p<.001, uncorrected) was applied to form suprathreshold edges of these subnetworks. Size of remaining connected components was computed and its statistical significance evaluated against an empirical null distribution of maximal component size obtained under the null hypothesis of random group membership (1000 permutations). Subnetworks significant at a corrected level of p<.01 were reported (see Appendix S4 for additional details on NBS and Appendix S5 for a list of included brain regions and abbreviations). Resulting p-values were adjusted for multiple comparisons using Bonferroni correction (α=0.05). Depressive severity intercept, linear slope, and their interaction were included predictor variables in one regression model. Given the following variables have been associated with depression and brain connectivity, we included covariates of head motion during the scan (mean frame-wise displacement) (Yendiki, Koldewyn, Kakunoori, Kanwisher, & Fischl, 2014), age 10 verbal IQ (Li et al., 2009), race (Nyquist et al., 2014), and socioeconomic status (SES; number of years receiving public assistance from ages 10-19) (Ursache & Noble, 2016). Given that anxiety may co-occur with depression (e.g., Yaroslavsky et al., 2013), the main effect of anxiety symptoms at age 10 was tested in the presence of depressive symptom intercept; however, given the focus of this study on depression and that different neural systems may be implicated in depression, anxiety and comorbid depression-anxiety, main analyses focused on depressive symptom course.
Results
Table 1 presents means, standard deviations, and correlations among depression measures, race, verbal IQ, head motion, and SES. The distributions of depressive severity scores at all time-points are presented in Figure S1.
Table 1.
Sample means, standard deviations, and correlations.
| Variable | M (SD) | Range | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1. Depressive severity intercept | 7.27 (2.80) | 3.14–17.54 | - | |||||
| 2. Depressive severity linear slope | −0.51 (0.34) | −1.42-.27 | r=−.47* | - | ||||
| 3. Age at scan, years | 19.28 (0.44) | 18.21-20.37 | r=.01 | r=.07 | - | |||
| 4. Race | A/MR (N)=82 |
t=−2.19* A/MR>E |
t=2.60* E>A/MR |
t=2.35* | - | |||
| 5. Verbal IQ | 97.86 (18.08) | 51-141 | −.14 | .43* | r =.15 |
t=6.41* E>A/MR |
- | |
| 6. Head movement (mean volume-to-volume displacement, mm) | 1.09 (0.18) | 0.74-1.67 | −.06 | −.04 | r=−.1 | t=−1.35 | r=0 | - |
| 7. Years of public assistance receipt | 5.17 (4.56) | 0-13 | .19* | −.22* | r=.01 |
t=−6.21* A/MR>E |
r=−.55 | r=.01 |
Note: N=115.
indicates p<.05. M and SD are used to represent mean and standard deviation, respectively. A/MR=African American and Mixed Race; E=European American. T values are indicated for tests of race differences. Pearson correlations among continuous variables are indicated using r.
Depressive Symptom Severity Course
The quadratic model fit the longitudinal data slightly better than the linear model, however there were no differences in model fit between quadratic models with or without freely estimated quadratic terms (Table S1). Based on these results, we selected the quadratic model without the freely estimated quadratic term to parsimoniously conduct structural connectivity analyses. At time 0 (age 10), average depressive severity score was 7.27, t(114)=18.72, p<.0001. With each one year increase in age, participants decreased an average of 0.51 unit in depressive severity score (linear term), t(1012)=3.76, p=.0002, with a 0.05 increase in rate of change of depressive score across time-points (quadratic term), t(1012)=3.39, p=.0007 (Figure 1A, Table S2). A negative correlation was found between depressive severity intercept and linear slope, r=−.47, p<.0001 (Figure 1B). Higher intercept and higher slope were both associated with more MDD diagnoses in adolescence, ps< .05.
Figure 1. Results from multilevel growth modeling of longitudinal change in depressive severity across 10 years of adolescence.
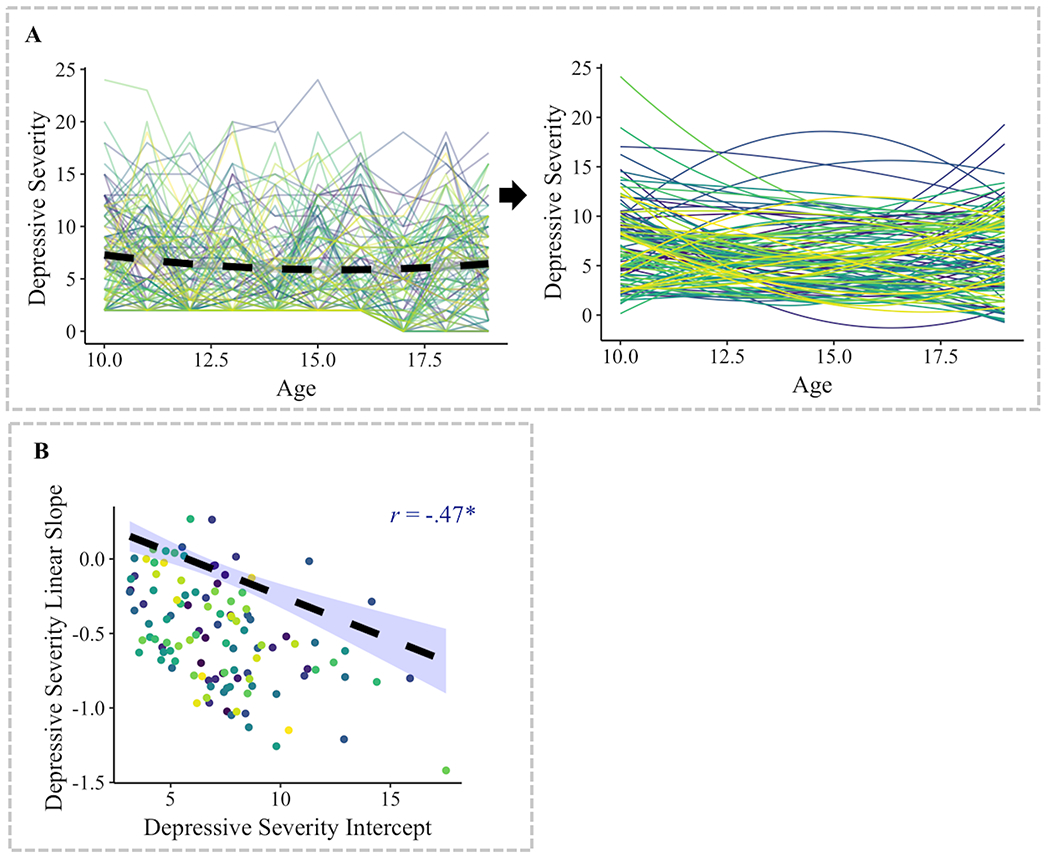
(A) A longitudinal multilevel quadratic model of change (with a fixed effect of the quadratic term) was fit to the data. Intercepts and linear slopes were estimated per individual. Individuals significantly varied in depressive intercept, linear slope, and the association between these variables. (B) There was a significant negative correlation between the initial status (i.e., intercept) and linear slope of depressive severity.
Depressive Severity Course and Structural Connectivity
Higher initial depressive severity was associated with greater structural connectivity of four subnetworks: (A) left amygdala and left rolandic operculum; (B) left inferior temporal gyrus (ITG) and left angular gyrus; (D) right posterior OFC and right supramarginal gyrus; and (E) right fusiform gyrus and right middle occipital gyrus. Conversely, higher initial depressive severity was associated with lower connectivity of one subnetwork (C): the left middle occipital gyrus and left caudate (ps<.001, NBS-corrected; Table 2, Figure 2A). Steeper positive slope of depressive severity was associated with greater connectivity of one subnetwork: (F) left fusiform gyrus and left middle occipital gyrus (Table 2, Figure 2B). Overall, higher initial depressive severity and steeper positive slope of symptom severity across adolescence were separately associated with greater connectivity among temporal, subcortical, and occipital regions.
Table 2.
Results from network-based-statistics of subnetworks associated with intercept and linear slope of depressive severity (derived from quadratic longitudinal model).
| Depressive Severity Effect | Network | Nodes | Link | B | p | Bonferroni p |
|---|---|---|---|---|---|---|
| Intercept | A | 2 | Amygdala (L) – Rolandic Operculum (L) | .40 | <.001 | .002 |
| B | 2 | Inf. Temporal Gyrus (L) – Angular Gyrus (L) | .39 | <.001 | .003 | |
| C | 2 | Caudate (L) – Mid. Occipital Gyrus (L) | −.40 | <.001 | .003 | |
| D | 2 | Fusiform Gyrus (R) – Mid. Occipital Gyrus (R) | .42 | <.001 | .001 | |
| E | 2 | Supramarginal Gyrus (R) – Post. Orbitofrontal Cortex (R) | .37 | <.001 | .008 | |
| Linear Slope | F | 2 | Fusiform Gyrus (L) – Mid. Occipital Gyrus (L) | .35 | <.001 | .007 |
| Intercept X Slope | G | 2 | Sup. Frontal Gyrus (Med. Orbital) (L) – Post. Cingulate Gyrus (R) | −1.02 | <.001 | .007 |
| H | 2 | Med. Orbitofrontal Cortex (R) – Mid. Frontal Gyrus (R) | −1.04 | <.001 | .007 | |
| I | 3 | Inf. Parietal Gyri (R) – Parahippocampal Gyrus (R) | 1.16 | <.001 | .002 | |
| Inf. Parietal Gyri (R) – Lat. Orbitofrontal Cortex (L) | 1.08 | <.001 | .005 |
Note: N=115. Inf. = Inferior; Med. = Medial; Post. = Posterior; Sup. = Superior; Mid. = Middle; Ant. = Anterior. All subnetworks identified with an initial link threshold of p<.001 and a corrected suprathreshold of p<.01. All analyses included race, verbal IQ, socioeconomic status (years of public assistance receipt), and head motion as covariates.
Figure 2. Results from network-based-statistics of connectivity differences associated with depressive severity course across 10 years of adolescence.
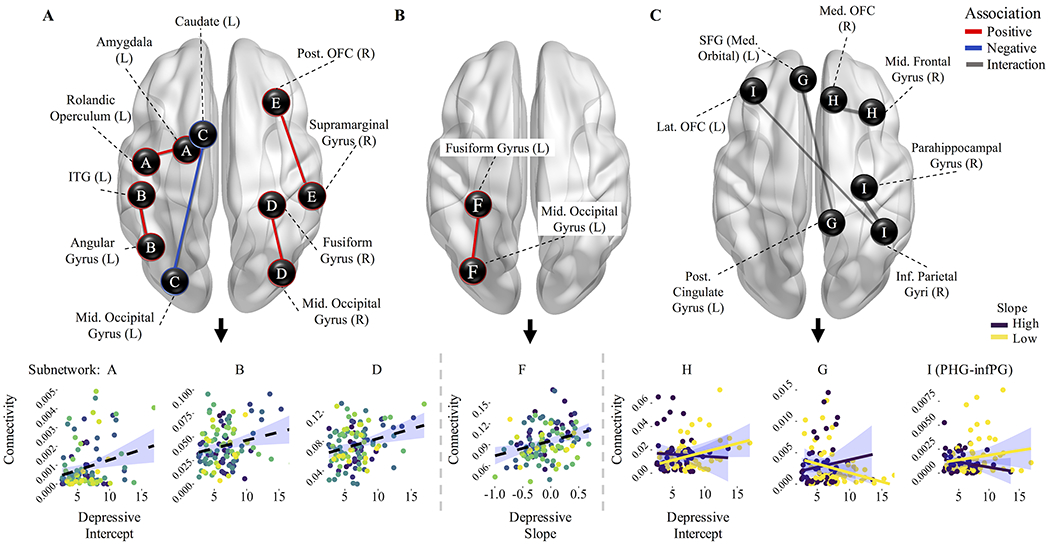
(A) Higher initial depressive severity (i.e., intercept as derived from a quadratic multilevel growth model) was associated with greater structural connectivity of four subnetworks (A, B, D, and E), and lower connectivity of one subnetwork (C). (B) Steeper positive slope of depressive severity (i.e., worsening symptoms) across adolescence (i.e., linear slope derived from a multilevel growth model) was associated with greater structural connectivity of one subnetwork (F). (C) A significant interaction effect of depressive severity intercept and linear slope was found in association with three subnetworks (G-I). Depressive severity slope is grouped (via median split) for the purpose of visualization. Edge-weights of specific connections are depicted in relation to main effects (depressive severity intercept, slope, and interaction).
The interaction of depressive severity intercept and linear slope was observed on connectivity of three subnetworks (G-I). Post-hoc contrasts revealed that higher intercept coupled with steeper negative linear slope was associated with greater connectivity of two subnetworks: (H) right medial OFC and right middle frontal gyrus (MFG); and (I) right inferior parietal gyri (IPG) and right parahippocampal gyrus. A higher intercept with steeper positive slope was associated with greater connectivity between the left superior frontal gyrus (SFG) and left posterior cingulate gyrus (subnetwork G), although higher intercept with steeper negative slope was related to lower connectivity between these regions (ps<.001, NBS- and Bonferroni-corrected) (Table 2, Figure 2C). Overall, initially elevated symptom severity that improved over time was associated with greater connectivity between cognitive control regions, while initially moderate severity that worsened over time was associated with greater connectivity between self-referential processing and cognitive control regions. We additionally tested the association between connectivity and the freely estimated quadratic term from the longitudinal quadratic model. A higher rate of change (i.e., quadratic term) of depressive symptoms was associated with lower connectivity between the right IPG and right posterior OFC (p<.001, NBS-corrected). All results were not contingent upon the inclusion of race, IQ, and/or public assistance variables (Table S3). Including anxiety symptoms in the model also did not affect the results (Table S4).
These results of testing linear model estimates were identical to those found when examining associations between quadratic model estimates and connectivity (Table S5).
Discussion
The current study found that girls’ structural connectivity among regions involved in affective and cognitive processing in late adolescence may partially reflect heterogeneous patterns of the course and degree of emotional functioning, as well as the toll of fluctuating depression, across the decade of adolescence. Our first hypothesis was supported by evidence of elevated depressive severity in early adolescence (i.e., higher intercept) relating to greater structural connectivity of affective-processing regions. Unexpectedly (although consistent with prior research), increasing symptom severity (i.e., steeper positive slope) was also associated with greater connectivity between the fusiform gyrus and middle occipital gyrus. Compared to slope, depressive intercept was associated with connectivity between a greater number of regions, perhaps reflecting more global neural disruptions related to an earlier age of emotional problems. Our exploratory aim revealed the interaction of depressive intercept and slope provided further information about how symptom fluctuations across adolescence forecast later connectivity signatures. Consistent with our speculations, higher initial depressive severity that alleviated over time was associated with greater connectivity among cognitive control regions. The findings advance knowledge of the neurobiological correlates of clinically-relevant patterns of depression progression in an understudied sample of girls who are majority African Americans from predominantly low-income backgrounds.
Previous findings in the larger Pittsburgh Girls Study sample align with the longitudinal changes we present here (Keenan et al., 2014). Specifically, Keenan and colleagues reported that, on average, depression symptoms decreased from ages 10 to 17 in the larger sample of 2,450 girls. Additionally, girls with higher depression symptoms at age 10 showed larger decreases in symptoms across adolescence. The past and current findings of significant interindividual differences in depression course underscore the importance of examining neurobiological correlates of heterogeneous symptom trajectories.
Higher initial depressive severity was associated with higher connectivity of the amygdala and rolandic operculum, connectivity patterns consistent with that of Ellis et al.’s, (2017) report of higher amygdala structural connectivity in individuals with elevated depressive symptoms in early adolescence. The amygdala is involved in emotion and motivation (Janak & Tye, 2015) and the rolandic operculum is involved in self-awareness (Blefari et al., 2017). Other prior work has also shown that earlier onset of depression symptoms is associated with higher functional connectivity of the amygdala with self-referential processing regions (Clark et al., 2018). Of note, the rolandic operculum and adjacent insula are involved in interoceptive awareness and functional connectivity of these regions is implicated in MDD and associated somatic symptoms (Avery et al., 2014).
We also found greater connectivity between the posterior OFC and supramarginal gyrus in association with higher depressive severity intercept. Although the supramarginal gyrus is primarily involved in sensorimotor functions (McDowell, Holmes, Sunderland, & Schürmann, 2018), this region has been implicated in representing others’ emotional states, for example in generating empathic responses (Bernhardt & Singer, 2012) and overcoming egocentric biases in social interactions (Silani, Lamm, Ruff, & Singer, 2013). As the OFC is implicated in subjective emotional and value-based representation of affective stimuli (Rolls, 2019), the finding of greater connectivity between these affect-related regions is consistent with past studies of young adults with depression (Long et al., 2015). Additionally, neurodevelopmental models point to elevated co-activation of affective-processing regions as a risk factor for depression (Weir et al., 2012). The current work adds an individual differences perspective to existing models by showing that structural connectivity between affective and self-referential processing is associated with an adolescent’s course of depression symptoms.
Although the association between higher initial depressive severity and lower caudate-occipital gyrus connectivity was unexpected, lower functional connectivity between these regions has been found previously in adolescents and adult females with depression (Gabbay et al., 2013; Teng et al., 2018). Future work should investigate whether perceptual biases for negative information (Foland-Ross & Gotlib, 2012) are associated with this connectivity signature. Also unexpectedly, we found that higher intercept and steeper positive slope, separately, were related to greater connectivity between the fusiform gyrus and middle occipital gyrus. Activation and connectivity of cortical visual areas is shown to predict treatment response to antidepressants (Furey et al., 2013) and electroconvulsive therapy (Moreno-Ortega et al., 2019), respectively, in patients with depression. The role of visual systems, particularly those implicated in perceptual processing of affective stimuli, in predicting and differentiating depression course should be examined in future work.
Higher initial depressive severity combined with a steeper negative slope (i.e., alleviating symptoms) was associated with greater connectivity between the medial OFC and MFG, regions involved in cognitive and attentional control (Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Cognitive network connectivity is typically lower in adolescents and adults with depression (Korgaonkar et al., 2014; Lu et al., 2017; Tymofiyeva et al., 2017) compared to healthy volunteers. The current findings suggest that a remitting symptom course may re-establish normalcy of connectivity patterns; alternatively, these adolescents may have had greater cognitive connectivity throughout adolescence, a neural signature that may have contributed to, or developed alongside, symptom improvement.
Results also showed that moderately severe symptoms in early adolescence, when accompanied by a steeper positive slope (i.e., worsening throughout adolescence), were associated with greater connectivity between the posterior cingulate gyrus (a default mode region) and SFG (a cognitive control region). Heightened functional connectivity between cognitive and default-mode regions is consistently identified as a marker of depression in adolescents and adults, and implicated in the inability to disengage from internal thought processes in the presence of external demands (e.g., Belleau, Taubitz, & Larson, 2015). Indeed, elevated connectivity of these networks has been found in relation to rumination and severity of depression (e.g., Nejad et al., 2019). Although adolescents with alleviating symptoms may show some connectivity similarities to normative youth, those with moderate symptoms that worsen over time may develop (or already had) connectivity patterns that propagate depression symptoms and maladaptive thought patterns.
Emotional problems emerging earlier in adolescence may be associated with more global brain disruptions that may contribute to the more severe and recurring symptoms seen in earlier, compared to later onset, adolescent depression (Yaroslavsky et al., 2013). A greater number of connectivity perturbations between temporo-parietal and parieto-occipital regions were found in relation to earlier, compared to later, elevated severity in the current and past work (Ellis et al., 2017). As postulated by Ellis and colleagues (2017), the presence of early depressive symptoms may affect development of connections among posterior regions, since WM maturation proceeds in a posterior to anterior direction (Colby, Van Horn, & Sowell, 2011; Ellis et al., 2017). Future work is needed to understand how the locations and coupling of regions implicated in relation to depression symptoms vary with age.
Limitations
Because neuroimaging was collected in late adolescence, we cannot determine whether differences in neural indicators were preexisting to, a correlate of, or shaped by internalizing experiences. Future studies incorporating longitudinal neuroimaging and depression assessments would advance understanding of the relation between heterogeneous experiences of depression course and the developing brain. Given the number of within-subject measurements and connectivity method used, we could not fit higher-dimensional models of depression course. Future studies will need to test additional nonlinear models of change. Finally, diffusion imaging and tractography were used to estimate the probability of structural connections between brain regions, however this method is limited in detecting long-range cortical connections (Reveley et al., 2015). Despite being a widely used atlas in connectivity studies, our use of the AAL atlas over others may have precluded detection of some individual differences in structural measures of the brain (Ota, Oishi, Ito, Fukuyama, & SEAD-J Study Group, 2014).
Conclusion
Girls in late adolescence showed variations in structural connectivity in relation to their course of depression throughout adolescence. Symptom severity and changes in severity over a 10-year period across adolescence were related to later differences in WM pathways, particularly those connecting depression-relevant regions (e.g., affective- and cognitive-processing regions). These findings suggest that the underlying neuroanatomical architecture of depression in late adolescence is differentiated based on the developmental course of symptoms.
Supplementary Material
Key Points.
In this study, girls’ brain structural connectivity in late adolescence was examined in relation to the course of depressive symptoms across ten years of adolescence.
Higher initial depressive severity (age 10) was associated with greater connectivity among socio-affective processing regions; increasing depressive severity throughout adolescence (age 10 to 19) was associated with greater connectivity between fusiform and occipital gyri.
High symptom severity that alleviated over time was related to greater connectivity among cognitive control regions, while moderate symptoms that worsened over time were associated with greater connectivity between cognitive and self-referential processing regions.
Depression history-associated structural connectivity patterns may relate to altered affective and cognitive processing in late adolescence.
The present findings contribute to knowledge of the neurobiological correlates of clinically relevant patterns of depression progression.
Acknowledgements
This research was funded by the National Institute of Mental Health grants R01-MH093605 (KK, AEG, EEF), R01-MH066167 (KK), R01-MH056630, and T32 MH100019 (SM), and the National Center for Advancing Translational Sciences grants UL1 TR001860 and linked award TL1 TR001861 (RC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
The authors report no financial relationships with commercial interests.
References
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, & Simmons WK (2014). Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry, 76(3), 258–266. 10.1016/j.biopsych.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, & Woolrich MW (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage, 34(1), 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau EL, Taubitz LE, & Larson CL (2015). Imbalance of default mode and regulatory networks during externally focused processing in depression. Social Cognitive and Affective Neuroscience, 10(5), 744–751. 10.1093/scan/nsu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, & Singer T (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. 10.1146/annurev-neuro-062111-150536 [DOI] [PubMed] [Google Scholar]
- Blefari ML, Martuzzi R, Salomon R, Bello‐Ruiz J, Herbelin B, Serino A, & Blanke O (2017). Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self‐consciousness. European Journal of Neuroscience, 45(10), 1300–1312. 10.1111/ejn.13567 [DOI] [PubMed] [Google Scholar]
- Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, & Miller E (2017). Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Translational Psychiatry, 7(5), e1139 10.1038/tp.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Liu M, Gross DW, & Beaulieu C (2013). Graph theoretical analysis of developmental patterns of the white matter network. Frontiers in Human Neuroscience, 7, 716 10.3389/fnhum.2013.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Konduru N, Kemp A, Bray S, Brown EC, Goodyear B, & Ramasubbu R (2018). The impact of age of onset on amygdala intrinsic connectivity in major depression. Neuropsychiatric Disease and Treatment, 14, 343–352. 10.2147/NDT.S145042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, So FK, Young JF, Hankin BL, & Lee BA (2019). Youth Depression Screening with Parent and Self-Reports: Assessing Current and Prospective Depression Risk. Child Psychiatry and Human Development. 10.1007/s10578-019-00869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JB, Van Horn JD, & Sowell ER (2011). Quantitative in vivo evidence for broad regional gradients in the timing of white matter maturation during adolescence. NeuroImage, 54(1), 25–31. 10.1016/j.neuroimage.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, … Yang TT (2017). Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of Affective Disorders, 207, 86–94. 10.1016/j.jad.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, & Lim KO (2014). Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Seal ML, Adamson C, Beare R, Simmons JG, Whittle S, & Allen NB (2017). Brain connectivity networks and longitudinal trajectories of depression symptoms in adolescence. Psychiatry Research. Neuroimaging, 260, 62–69. 10.1016/j.pscychresns.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, & Gotlib IH (2012). Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Frontiers in Psychology, 3, 489 10.3389/fpsyg.2012.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CH, Miernicki ME, Rudolph KD, & Telzer EH (2017). Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Developmental Cognitive Neuroscience, 27, 99–106. 10.1016/j.dcn.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, & Zarate CA (2013). Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry, 70(3), 280–290. 10.1001/2013.jamapsychiatry.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, … Milham MP (2013). Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 628–641.e13. 10.1016/j.jaac.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD (2015). The Symptom Inventories: An annotated bibliography. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- Hagan CC, Graham JME, Wilkinson PO, Midgley N, Suckling J, Sahakian BJ, & Goodyer IM (2015). Neurodevelopment and ages of onset in depressive disorders. The Lancet. Psychiatry, 2(12), 1112–1116. 10.1016/S2215-0366(15)00362-4 [DOI] [PubMed] [Google Scholar]
- Hallquist MN, & Hillary FG (2018). Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small-world. 0(0), 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, & Gibbons RD (2012). Longitudinal Data Analysis. New York: Wiley. [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Culbert K, Grimm KJ, Hipwell A, & Stepp S (2014). Timing and tempo: Exploring the complex association between pubertal development and depression in African American and European American girls. Journal of Abnormal Psychology, 123(4), 725–736. 10.1037/a0038003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, & McTigue K (2010). The Pittsburgh Girls Study: Overview and Initial Findings. Journal of Clinical Child & Adolescent Psychology, 39(4), 506–521. 10.1080/15374416.2010.486320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Fornito A, Williams LM, & Grieve SM (2014). Abnormal structural networks characterize major depressive disorder: A connectome analysis. Biological Psychiatry, 76(7), 567–574. 10.1016/j.biopsych.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Laughlin S, & Sejnowski T (2003). Communication in Neuronal Networks | Science. Science, 301(5641), 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, & Jiang T (2009). Brain anatomical network and intelligence. PLoS Computational Biology, 5(5), e1000395 10.1371/journal.pcbi.1000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Duan X, Wang Y, Liu F, Zeng L, Zhao J-P, & Chen H (2015). Disrupted structural connectivity network in treatment-naive depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 56, 18–26. 10.1016/j.pnpbp.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Lu Y, Shen Z, Cheng Y, Yang H, He B, Xie Y, … Han D (2017). Alternations of White Matter Structural Networks in First Episode Untreated Major Depressive Disorder with Short Duration. Frontiers in Psychiatry, 8 10.3389/fpsyt.2017.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, & Chahal R (2015). An integrative model of the maturation of cognitive control. Annual Review of Neuroscience, 38, 151–170. 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell T, Holmes NP, Sunderland A, & Schürmann M (2018). TMS over the supramarginal gyrus delays selection of appropriate grasp orientation during reaching and grasping tools for use. Cortex, 103, 117–129. 10.1016/j.cortex.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Moreno-Ortega M, Prudic J, Rowny S, Patel GH, Kangarlu A, Lee S, … Javitt DC (2019). Resting state functional connectivity predictors of treatment response to electroconvulsive therapy in depression. Scientific Reports, 9(1), 5071 10.1038/s41598-019-41175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Rotgé J-Y, Valabregue R, Guérin-Langlois C, Hoertel N, Gorwood P, … Lemogne C (2019). Medial prefrontal disengagement during self-focus in formerly depressed patients prone to rumination. Journal of Affective Disorders, 247, 36–44. 10.1016/j.jad.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Nyquist P, Bilgel M, Gottesman R, Yanek L, F Moy T, C Becker L, … Vaidya D (2014). Extreme Deep White Matter Hyperintensity Volumes Are Associated with African American Race. Cerebrovascular Diseases (Basel, Switzerland), 37, 244–250. 10.1159/000358117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K, Oishi N, Ito K, Fukuyama H, & SEAD-J Study Group. (2014). A comparison of three brain atlases for MCI prediction. Journal of Neuroscience Methods, 221, 139–150. 10.1016/j.jneumeth.2013.10.003 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/.
- Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, … Ye FQ (2015). Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proceedings of the National Academy of Sciences of the United States of America, 112(21), E2820–2828. 10.1073/pnas.1418198112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2019). The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia, 128, 14–43. 10.1016/j.neuropsychologia.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Joliot M, & Tzourio-Mazoyer N (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage, 122, 1–5. 10.1016/j.neuroimage.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Salcedo S, Chen Y-L, Youngstrom EA, Fristad MA, Gadow KD, Horwitz SM, … Findling RL (2017). Diagnostic Efficiency of the Child and Adolescent Symptom Inventory (CASI-4R) Depression Subscale for Identifying Youth Mood Disorders. Journal of Clinical Child & Adolescent Psychology, 0(0), 1–15. 10.1080/15374416.2017.1280807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff CC, & Singer T (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(39), 15466–15476. 10.1523/JNEUROSCI.1488-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C, Zhou J, Ma H, Tan Y, Wu X, Guan C, … Zhang N (2018). Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry, 18(1), 370 10.1186/s12888-018-1955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymofiyeva O, Connolly CG, Ho TC, Sacchet MD, Henje Blom E, LeWinn KZ, … Yang TT (2017). DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. Journal of Affective Disorders, 207, 18–25. 10.1016/j.jad.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, & Noble KG (2016). Socioeconomic status, white matter, and executive function in children. Brain and Behavior, 6(10). 10.1002/brb3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir JM, Zakama A, & Rao U (2012). Developmental Risk I: Depression and the Developing Brain Child and Adolescent Psychiatric Clinics of North America, 21(2), 237–259. 10.1016/j.chc.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, … Allen NB (2014). Structural Brain Development and Depression Onset During Adolescence: A Prospective Longitudinal Study. American Journal of Psychiatry, 171(5), 564–571. 10.1176/appi.ajp.2013.13070920 [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Pettit JW, Lewinsohn PM, Seeley JR, & Roberts RE (2013). Heterogeneous trajectories of depressive symptoms: Adolescent predictors and adult outcomes. Journal of Affective Disorders, 148(0), 391–399. 10.1016/j.jad.2012.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, & Fischl B (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. 10.1016/j.neuroimage.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, & Bullmore ET (2010). Network-based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


