Abstract
Previous reports show that moderate prenatal alcohol exposure (PAE) poses a risk factor for developing neuropathic pain following adult-onset peripheral nerve injury in male rats. Recently, evidence suggests that immune-related mechanisms underlying neuropathic pain in females are different compared to males despite that both sexes develop neuropathy of similar magnitude and duration following chronic constriction injury (CCI) of the sciatic nerve. Data suggest that the actions of peripheral T cells play a greater role in mediating neuropathy in females. The goal of the current study is to identify specificity of immune cell and cytokine changes between PAE and non-PAE neuropathic females by utilizing a well-characterized rodent model of sciatic nerve damage, in an effort to unmask unique signatures of immune-related factors underlying the risk of neuropathy from PAE. Cytokines typically associated with myeloid cell actions such as interleukin (IL)-1β, tumor necrosis factor (TNF), IL-6, IL-4 and IL-10 as well as the neutrophil chemoattractant CXCL1, are examined. In addition, transcription factors and cytokines associated with various differentiated T cell subtypes are examined (anti-inflammatory FOXP3, proinflammatory IL-17A, IL-21, ROR-γt, interferon (IFN)-γ and T-bet). Lymphocyte function associated antigen 1 (LFA-1) is an adhesion molecule expressed on peripheral immune cells including T cells and regulates T cell activation and extravasation into inflamed tissue regions. A potential therapeutic approach was explored with the goal of controlling proinflammatory responses in neuroanatomical regions critical for CCI-induced allodynia by blocking LFA-1 actions using BIRT377. The data show profound development of hindpaw allodynia in adult non-PAE control females following standard CCI, but not following minor CCI, while minor CCI generated allodynia in PAE females. The data also show substantial increases in T cell-associated proinflammatory cytokine mRNA and proteins, along with evidence of augmented myeloid/glial activation (mRNA) and induction of myeloid/glial-related proinflammatory cytokines, CCL2, IL-1β and TNF in discrete regions along the pain pathway (damaged sciatic nerve, dorsal root ganglia; DRG, and spinal cord). Interestingly, the characteristic anti-inflammatory IL-10 protein response to nerve damage is blunted in neuropathic PAE females. Moreover, T cell profiles are predominantly proinflammatory in neuropathic Sac and PAE females, augmented levels of Th17-specific proinflammatory cytokines IL-17A and IL-21, as well as the Th1-specific factor, T-bet, are observed. Similarly, the expression of RORγt, a critical transcription factor for Th17 cells, is detected in the spinal cord of neuropathic females. Blocking peripheral LFA-1 actions with intravenous (i.v.) BIRT377 reverses allodynia in Sac and PAE rats, dampens myeloid (IL-1β, TNF, CXCL1)- and T cell-associated proinflammatory factors (IL-17A and RORγt) and spinal glial activation. Moreover, i.v. BIRT377 treatment reverses the blunted IL-10 response to CCI observed only in neuropathic PAE rats and elevates FOXP3 in pain-reversed Sac rats. Unexpectedly, intrathecal BIRT377 treatment is unable to alter allodynia in either Sac or PAE neuropathic females. Together, these data provide evidence that: 1) fully differentiated proinflammatory Th17 cells recruited at the sciatic nerve, DRGs and lumbar spinal cord may interact with the local environment to shape the immune responses underlying neuropathy in female rats, and, 2) PAE primes peripheral and spinal immune responses in adult females. PAE is a risk factor in females for developing peripheral neuropathy after minor nerve injury.
Keywords: Prenatal alcohol exposure, neuropathic pain, glia, neuroimmune, peripheral immune, T cells, spinal cord, satellite glial cells, Th17, IL-17A
1. Introduction
In utero alcohol exposure can lead to a range of adverse neurobehavioral outcomes known as fetal alcohol spectrum disorder (FASD) [1]. Emerging clinical evidence and findings from animal models now suggest that prenatal alcohol exposure (PAE), even at low to moderate levels, elicits exaggerated adverse neuroimmune responses during early life and throughout adulthood [2–5]. Recent prior reports show that moderate PAE in males induces heightened peripheral myeloid cell responses both in the peripheral and in the central nervous system (PNS and CNS, respectively), and elevates spinal glial activation underlying chronic pathological sensitivity to light touch, referred to as allodynia [6, 7]. These reports to date are striking because chronic allodynia from adult-onset minor injury to the peripheral nerve is observed only in PAE male rats. While sex differences have been observed with respect to proinflammatory cytokine production in the brain and associated adverse neurological outcomes (e.g., cognitive function) following immune challenges (e.g. lipopolysaccharide, LPS) in PAE rats [2, 3], little is understood regarding neuroimmune hypersensitivity in PAE adult female rats.
Animal models used to examine mechanisms underlying chronic neuropathic pain have revealed that heightened excitation of spinal cord pain transmission neurons following peripheral nerve injury involves peripheral immune and spinal glial proinflammatory actions [8, 9]. Specifically, peripheral nerve injury induces heightened glial activation in the spinal cord and satellite glia of the dorsal root ganglia (DRG) that in turn release and further respond to a variety of proinflammatory cytokines; the most widely studied being interleukin (IL)-1β and tumor necrosis factor (TNF). Moreover, chemotactic cytokines such as macrophage chemotactic protein-1 (CCL2) facilitate allodynia while the anti-inflammatory cytokine, IL-10 is well-characterized to suppress allodynia by limiting the actions of IL-1β, TNF and CCL2, as well as a number of other proinflammatory factors that facilitate allodynia [10–14]. Following nerve injury, damaged nociceptive axons increase CCL2 expression in their cell bodies in the DRG and in their nociceptive nerve terminals in the spinal cord [15–17]. CCL2-mediated interactions lead to leukocyte (e.g. macrophages, T cells) accumulation along the pain pathway such as damaged sciatic nerve axons, in the DRGs, and regions corresponding lumbar spinal cord where centrally projecting nociceptors communicate to spinal pain projection neurons [14, 18].
To date, the majority of preclinical pain studies investigating discrete neuroimmune mechanisms have applied male rodent models. Reports from the past five years suggest that neuropathic pain in males is predominantly mediated by spinal microglia, whereas neuropathic pain in females may be mediated by the actions of peripheral T cells [19, 20] that extravasate to the damaged nerve and the lumbar spinal cord via a “leaky” blood-spinal barrier following peripheral nerve injury [21]. While few studies suggest a key role for recruited T cells in the DRGs and the spinal cord in neuropathic females [22–24], the question of whether T cell-mediated responses are proinflammatory (e.g. Th1 or Th17) or anti-inflammatory (T regulatory cells; Treg) have yet to be carefully characterized.
The neuroimmune factors underlying neuropathy between males and females may not involve the same magnitude of cytokine changes or the same immune cell types [6, 7]. Based on the profile of immune and glial cell differences in the spinal cord between females and males that likely underlie chronic allodynia, the neuroimmune priming that occurs as a consequence of PAE may also involve distinctly different neuroimmune factors between PAE females and males [6]. Yet, despite these underlying neuroimmune differences, PAE may remain a risk factor for adult-onset neuropathy in both males and females.
The primary aim of the study is to identify the potential specificity of immune cell and cytokine changes occurring between PAE and non-PAE neuropathic females as consequence of unilateral sciatic nerve injury that may establish unique signatures of immune-related factors underlying the risk of neuropathy from PAE. Specifically, whether PAE increases susceptibility to neuropathy in females occurring in parallel with dysregulated T cell and myeloid cell responses at the damaged sciatic nerve, DRG and spinal cord are examined. Secondary to this aim, a therapeutic approach is taken to explore whether regulating aberrant peripheral and central glial and leukocyte proinflammatory actions could control allodynia in females. To gain such insight, a blood-spinal barrier impermeable small molecule antagonist of Lymphocyte Function-associated Antigen (LFA-1), BIRT377 [25, 26] is used in combination with the minor chronic constriction injury (CCI) model previously utilized with PAE males [6].
LFA-1 is expressed on the surface of a range of peripheral immune cells (including myeloid cells and T cells), and it regulates immune cell migration as well as their function [27–30]. Indeed, blocking LFA-1 actions in vitro with the application of BIRT377 induces a macrophage and T cell bias toward an anti-inflammatory phenotype [26, 27, 31–33]. Effects of systemic BIRT377 treatment on myeloid/glial-derived cytokines, as well as T cell-related factors were examined. This study provides insight into immune mediators present in neuroanatomical regions responsible for the generation and maintenance of neuropathy in females with standard sciatic CCI, and the immune-related factors underlying susceptibility to neuropathy in PAE females. The beneficial effects of BIRT377 as a novel therapeutic to control neuropathy and PAE-related susceptibility to neuropathy provide a new target when considering immune-based intervention strategies to resolve neuroimmune problems in FASD individuals.
2. Materials and methods
2.1. Animals
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of The University of New Mexico Health Sciences Center, and all animal experiments were carried out in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Long-Evans rat breeders purchased from Harlan Industries (Indianapolis, IN) were maintained in a breeding colony on a 12:12-hour reverse light/dark schedule (lights on from 2100 to 0900 hours), and fed Harlan 2920 rodent chow and tap water, available ad libitum.
2.2. Moderate Prenatal Alcohol Exposure Using the Voluntary Drinking Paradigm
Pregnant female rat dams were given either ethanol or saccharin (Sac) throughout pregnancy until birth according to the voluntary drinking paradigm previously described [34, 35]. Briefly, after 1 week of acclimation to the animal facility, all female breeders (3 to 4 month-old, single housed) were allowed to drink tap water containing 0.066% (w/v) saccharin that gradually increased in ethanol content from 0% (v/v) on Days 1-2, to 2.5% (v/v) on Days 3-4, to 5% (v/v) on Day 5 and thereafter for two weeks. Females were allowed to drink four hours each day from 1000 to 1400 hours. It should be noted that regular drinking water is available at all times during housing. Thus, animals could voluntarily choose to drink either Sac-sweetened water containing 5% ethanol or regular water during the four-hour drinking period. Daily four-hour ethanol consumption was monitored for at least 2 weeks and then the mean daily ethanol consumption was determined for each female. Females, whose mean daily ethanol consumption was greater than one standard deviation below the group mean, typically about 10–15% of the entire group, were removed from the study. The remainder of the females were assigned to either a saccharin control or 5% ethanol drinking group and matched such that the mean pre-pregnancy ethanol consumption by each group was similar. Subsequently, the females were placed with proven male rat breeders until pregnant. No alcohol was consumed during the breeding period, which averaged between 1-2 days.
Beginning on gestational day 1, rat dams were given either 0% (Sac) or 5% ethanol (PAE) in Sac water (4 hours/day). Sac control group rats were given a volume of 0% ethanol in Sac water that was matched to the mean volume voluntarily consumed by the PAE group. The amount of ethanol consumed was determined 4 hours after the drinking tubes were introduced into the cages, for each dam through GD21. Ethanol consumption was discontinued at birth. A prior report suggest that, with this paradigm, each dam consumed 1.90 ± 0.06 g ethanol /kg body weight/day throughout gestation which produced a mean peak serum blood alcohol concentration of 60.8 ± 5.8 mg of serum ethanol/dL [35], with no significant differences between prenatal treatment groups in dam weight gain during pregnancy, pup birth weights, or litter size [6, 7, 35, 36]. Offspring were weaned at 24 days of age and female offspring were pair-housed.
After weaning, experimental offspring were transferred to a different room and habituated to a standard light/dark cycle (lights on from 0600 hours to 1800 hours) for at least 28 days and maintained in these conditions for the duration of the study. During early experiments, the effect of different phases of the estrous cycle on hindpaw threshold responses at baseline and after surgical manipulation was determined. Despite female rats entering experiments at different phases of the estrous cycle, hindpaw responses remained stable and predictable, independent of estrus cycle phase. Consequently, the stage of the estrous cycle varied as female rats were entered and examined throughout the chronic neuropathy paradigm. For all experiments, 4-7 month-old female prenatal alcohol or age-matched saccharin exposed (PAE or Sac, respectively) rat offspring derived from 33 litters were used. It should be noted that none of the experimental groups contained more than one subject from a given litter to avoid “litter effects”.
2.3. Behavioral assessment of allodynia
Mechanical allodynia was chosen for investigation because of its daily clinical relevance to chronic pain patients (e.g. light touch from clothes), as nociceptive stimuli are frequently avoided (e.g. nociceptive thermal stimuli). Rats were assessed for allodynia using the von Frey fiber test. After rats were habituated to the testing environment, baseline (BL) responses were assessed as previously described [7, 37]. Briefly, all rats were first habituated to the testing environment by placing rats atop 2-mm thick parallel bars spaced 8-mm apart allowing full access to the plantar surface of hindpaw. Habituation occurred for approximately 45 min/day for 5 sequential days. All behavioral testing was done in a sound-, light-, and temperature-controlled room and was performed within three hours of the light cycle (sleep cycle) to avoid the influence of elevated glucocorticoids under normal circadian rhythms, as this steroid exerts acute anti-inflammatory activity. The von Frey behavioral test utilizes a series of calibrated monofilaments with force indicated in grams, and within parentheses providing the corresponding handle marking which is Log10 of (10 X filament force in mg) as follows: 0.407 g (3.61), 0.692 g (3.84), 1.202 g (4.08), 1.479 g (4.17), 2.041 g (4.31), 3.630 g (4.56), 5.495 g (4.74), 8.511 g (4.93), 11.749 g (5.07), 15.136 g (5.18). These monofilaments were applied systematically to the left and right plantar surface of the hindpaw for a maximum of 8 seconds per application, as indicated by a metronome calibrated to 1 tick per second. The order for testing left and right hindpaws was approached in a random manner. Lifting, licking, or shaking of the paw was considered a response. Baseline (BL) assessment was initiated with the 2.041 g hair presented three times with a minimum interstimulus interval of 30-60 s. In the absence of paw withdrawal responses for 2 or 3 stimulus presentations, the next stronger monofilament, 3.630 g, was presented. The monofilament that elicited a clear response was recorded, and was presented two more times at a minimum of 30–60 s interstimulus intervals. If the animal withdrew its paw on three consecutive trials with the same stiffness value, no further von Frey hairs were tested. In the absence of a response to the monofilament, presentation of monofilaments continued in ascending order until three consecutive responses were elicited from the same monofilament. However, in the event that 2 or 3 paw withdrawals to the 2.041 g hair occurred, the lowest monofilament of the scale was next presented, which was the 0.407 g monofilament. As before, presentation of monofilaments continued in ascending order until three consecutive responses were elicited from the same monofilament. As before, 3 of 3 responses elicited from the same monofilament indicated threshold had been reached and further stimulus presentation was terminated. The typical number of stimulus presentations per timepoint was about 10.
In a similar manner to BL evaluation, all rats were re-assessed following CCI or sham surgery on Days 3, 10, 15, 18, 23, and 28, prior to receiving intrathecal (i.t.) or intravenous (i.v.) injections. Following BIRT377 or vehicle (Veh) injection, all rats were assessed for allodynia daily for 4 days, at which time, tissue collection occurred. Timepoints for behavioral assessments were chosen to capture potential subtle differences during the development of allodynia and BIRT377-mediated pain reversal while avoiding unnecessary testing potentially altering hindpaw responses. The experimental tester was blind to the treatment groups.
2.4. Chronic constriction injury (CCI)
Three sciatic nerve manipulations were used; sham surgery, single suture CCI (1-suture CCI) referred to as minor CCI and a 4-suture CCI referred to as standard CCI. Female rat offspring from prenatal Sac exposure (control) or PAE conditions were subjected to either sham or minor CCI to determine if female PAE offspring were at greater risk for developing neuropathy following minor injury, compared to Sac exposed rats. In support of this possibility, prior work demonstrates PAE is a risk factor for developing neuropathy from minor injury [6]. Furthermore, given the limited understanding of immune-related factors that underlie neuropathy in female rodent models, T cell-related cytokines and transcription factors were examined in both Sac and PAE female rats. In an attempt to identify specificity of immune and cytokine changes in PAE rats with chronic neuropathic pain, the incorporation of a prenatal alcohol-independent (Sac) chronic neuropathy group was included. This allowed for the potential identification of unique signatures of immune cell type and immune signaling factors in PAE rats with neuropathy. That is, how PAE alters the peripheral and spinal inflammatory milieu with a minor nerve injury (Sac vs PAE rats with minor CCI), and whether PAE-induced allodynia is similar to allodynia from Sac standard injury (PAE rats with minor CCI vs Sac rats with standard CCI) could be addressed.
All surgical procedures were performed under aseptic techniques as previously described [6, 7, 38] with minor modifications [39]. Briefly, under isoflurane anesthesia the sciatic nerve was carefully isolated and ligated with either 4 or 1 segment of 4-0 chromic gut sutures (Ethicon, Somerville, NJ) without pinching into the nerve [6]. Sham surgery involved isolation of the sciatic nerve identical to CCI surgery but without nerve ligation. The overlying muscle was sutured closed with two 3–0 sterile silk sutures (Ethicon, Somerville, NJ). All rats fully recovered from anesthesia within approximately 5 min. Animals were monitored daily after surgery.
2.5. BIRT377, antagonist for LFA-1
LFA-1 is an adhesion molecule expressed on peripheral leukocytes including myeloid and T cells. Upon activation from chemotactic signaling (e.g. CCL2 binding its receptor, CCR2), LFA-1 undergoes a series of conformational changes from a bent inactive position to a straightened active position, thus allowing for a critical binding site of LFA-1 to interact with the surface receptor, intercellular adhesion molecule-1 (ICAM-1) expressed on blood vessel endothelial cells [40]. Upon LFA-1/ICAM-1 interaction, leukocytes are capable of undergoing transendothelial migration and subsequently traffic to regions where damage- or pathogen-associated tissue signals arise (e.g. sciatic nerve, DRG and/or dorsal horn spinal cord). Notably, LFA-1 plays critical roles in immune cell function [26–29, 41, 42]. LFA-1 is a more globally and abundantly expressed integrin of T cells compared to other integrin molecules [43, 44] LFA-1-ICAM1 signaling interacts with TCR (T cell receptor) activation for T cell differentiation and drives T cell pro- or anti-inflammatory function [45, 46] [28, 32]. Moreover, we have previously reported that PAE itself increases surface LFA-1 expression on peripheral leukocytes in the spinal cord, which is further upregulated following neuropathy [7]. Therefore, LFA-1 was chosen to understand the roles of peripheral immune components in PAE-related neuropathic pain susceptibility in females.
The small molecule (R)-5-(4-bromobenzyl)-3-(3,5-dichlorophenyl)-1,5-dimethylimidazolidine-2, 4-dione (BIRT377) is a low molecular weight nonionic compound. BIRT377 was first reported and characterized by Kelly et al. [25]. BIRT377 prevents the formation of the transmembrane β2-integrin adhesion molecule, LFA-1, into its high-affinity conformation. BIRT377 prevents this conformation change through noncovalent binding to CD11a chain, which is a component of LFA-1 [31]. Therefore, upon BIRT377 binding to LFA-1, circulating leukocyte cell adhesion, infiltration and migration to sites of inflammation are prevented [25, 45, 47]. BIRT377 abolishes T cell and antigen presenting cell interactions which is crucial for T cell activation [48]. Moreover, BIRT377 is biocompatible and easier to formulate for oral administration than antibodies against LFA-1 [25, 45]. BIRT377 is highly selective for LFA-1 (not other adhesion molecule such as Mac-1) mediated events [25], and its physical half-life in serum is 10 min in female rabbits with a systemic clearance rate of 130 ml/h/kg [49]. BIRT377 was purchased form Tocris, MN, USA (Cat# 4776) and gifted by CRW. Upon arrival, 10mg BIRT377 was initially reconstituted in 100% ethanol as a stock solution at a concentration of 22.156mg/ml followed by creating 10ul aliquots stored at 4°C. On the day of injection, sterile ddH20 was warmed in a water bath and one aliquot of BIRT377 was added such to reach a concentration of either 2.5 μg/300 μL for i.v injection or 500ng/20 μL for i.t. injection and vortexed for 2 min. Vehicle consisted of sterile ddH2O. Animals were injected within the hour following drug dilution. Our prior reports demonstrate that neuropathic pain (allodynia) is reversed in male rats with either i.t. or i.v. BIRT377 at doses used in the current report [26, 50].
2.6. Intrathecal (i.t.) and intravenous (i.v.) injection
At Day 28 post-surgery, followed by behavior testing rats were given a single i.v. or i.t. injection of BIRT377 or equivolume vehicle (sterile ddH2O). Day 28 was chosen to explore contributions of LFA-1 when cytokine/chemokine signaling and chronic allodynia is established. In addition, a similar timecourse conducted in PAE males could be compared against the current timecourse [7, 50]. Tail vein injections were used for i.v. administration of either BIRT377 or vehicle. Using aseptic procedures, 300 μL of drug or vehicle was collected into individual 1-cc, 27-gauge 5/8 insulin syringes (Becton Dickinson; Cat#: 329412). Under isoflurane anesthesia (3% volume in oxygen), rat weight was recorded followed by tail vein injection. The tail was held firmly in place and the needle was inserted into the lateral tail vein, followed by a small amount of blood efflux into the syringe with a subsequent 10 sec injection. Success of achieving accurate needle placement upon the first attempt was greater than 99%. Following injection, sterile gauze was placed over injection site to stem bleeding and the rat was placed back into its home cage. All rats appeared normal (e.g. moving, grooming, interacting) following injections. The total time required for handling and injection required ~5 minutes.
I.t. injections were performed as described previously [50, 51]. Briefly, under isoflurane anesthesia (3% volume in oxygen), an 18-guage sterile (Bectin Dickson, Franklin Lakes, NJ) needle lacking its plastic hub was inserted between lumbar vertebrae L5-L6. Polyethylene (PE)-10 tubing (Fisher Scientific, Hampton, NH) was fitted with a 30-gauge sterile needle (Bectin Dickson, Franklin Lakes, NJ) attached to a Hamilton syringe. The PE tubing was labeled with a marking at 7.7cm from the opposite open end. 20μl of drug or equivolume vehicle was withdrawn from the open side of the PE-10 tubing. The open end of the tubing was then threaded into the 18-guage needle until the 7.7cm marking was reached. This position corresponds to the lower lumbar L4-L5 region of the spinal cord. Correct subarachnoid placement of the PE-10 was determined by observation of hindleg and/or tail twitching. Occasionally, cerebrospinal fluid efflux was also observed. Injections were administered over a 30 second interval. Following drug or vehicle administration, PE-10 tubing was removed followed by removing the 18-guage needle, and rats were allowed to recover before being placed back in their home cages. Rats fully recovered from anesthesia within 5 min and full motor recovery was observed following i.t. injection.
2.7. Tissue collection for RNA and protein analysis
Specific cytokines for mRNA and their corresponding protein were chosen based on their characterization from prior studies to play critical roles neuropathic pain in the standard CCI model. However, given the sensitivity of mRNA analysis from limited sample amounts (sciatic nerve), a broader panel of mRNA specific to glial and T cell phenotypes was examined without their corresponding protein. In an effort to ensure a balanced protein profile of pro- and anti-inflammatory cytokines where sufficient material remained, protein CXCL1, IL-6 and IL-4 levels were analyzed without their corresponding mRNA, as additional material for mRNA was used for glial and T cell phenotypes, as indicated above. Tissue collection and mRNA and protein analysis was conducted as previously described [7, 52] and modified as described here. Immediately following behavioral analysis on Day 32 after CCI surgery (Day 4 post-injection), rats were deeply anesthetized under isoflurane followed by rapid transcardial perfusion with ice cold 0.1 M phosphate buffered saline (PBS; pH = 7.4; flow rate 10 ml/min). Rats were placed on a frozen gel refrigerant pack (Glacier Ice, Pelton Shepherd Industries) and the lumbar spinal cord (LSC; L3-L6) was dissected with the dorsal spinal cord ipsilateral to the sciatic lesion, and lumbar dorsal spinal cord contralateral to the sciatic lesion stored separately. Additionally, lumbar dorsal root ganglia (DRG; L4-L6) and sciatic nerve (SCN) ipsilateral to the sciatic lesion were dissected. For sciatic nerves a ruler was used to dissect out 1.0 cm nerve tissue, keeping the lesion site approximately in the middle. All tissues were immediately placed in DNase/RNase/Protease-free microtubes (VWR International; Cat#: 47747-358), frozen on dry ice, and stored at −80 °C for future analysis.
2.8. Total RNA isolation
Total RNA was extracted (6-8 samples per session from different experimental conditions), as described previously [52] with minor modifications, as briefly described here. RNA extraction from all tissues was performed using the miRNeasy Micro Kit (Qiagen; Cat#: 217084) per manufacturer’s instructions. Homogenization was performed using a motorized VWR disposable pellet mixer and cordless motor pestle system (VWR; cordless pestle motor: Cat#47747-370; 1.5 ml microtubes: Cat#47747-362; 1.5 ml pestle: Cat#47747-358). DRGs were transferred using Qiazol into microtubes, from pestle-microtube combo (1.5 ml pestle and microtube combo: Cat#47747-366), prior to homogenization. DRG tissues were processed only for total RNA extraction due to a limited amount of material available from the lumbar DRG. SCN and LSC tissues were homogenized in chilled 1x phosphate buffered saline (PBS; Cat# P7059) prior to aliquoting into two microtubes for protein and RNA extraction. For protein analysis, MSD multiplex assay (please see 2.10) was used to allow simultaneous detection of multiple key cytokines from the same tissue lysate sample. The use of total RNA was prioritized to generate a panel inclusive of different T cell-related factors with the remaining total RNA used to verify mRNA levels of key immune factors to corresponding protein changes.
After initial homogenization, 40 μl of the homogenized solution further homogenized with 150 μl of chilled Qiazol. Minor modifications to the mRNA extraction procedure using the miRNeasy kits were incorporated as follows. Samples were vortexed, and incubated at room temperature (RT) for 7 min followed by the addition of 140 μl of chloroform (Sigma-Aldrich; Cat#C2432). Samples were then shaken vigorously for 15 sec, incubated for 4 min at RT and centrifuged in 4 °C at 12,000 g for 15 min. A 300 μl portion of the aqueous layer was placed into a clean RNase/DNase/Protease-free tube and 450 μl of 100% Ethanol (EtOH; Sigma-Aldrich; Cat# E7023) was added to tube, pipetted 4-6x to mix, moved to collection columns, and centrifuged at ~20 °C at 9,000 g for 30 sec, followed by washes with RWT and RPE buffer (provided with Qiagen kit) and 80% EtOH. Collection columns were dried by centrifugation (~20°C at 20,627 g, 12 min) and placed into RNA collection tubes with 14μl sterile water added directly to the column filter, and centrifuged (~20 °C at 20,627 g, 1 min). Concentration and purity of the total RNA was assessed by NanoDrop (Thermofisher Scientific, MA, EISA) immediately after each RNA extraction.
2.9. mRNA Analysis by Quantitative Real-Time PCR
Total RNA samples were diluted to a standardized RNA concentration, 140 ng/ul for sciatic nerve, 70 ng/ul for lumbar dorsal horn, and 200 ng/ul for DRG. Total RNA (0.9-1.2 μg) was used to synthesize cDNA. For reverse transcription (cDNA), Superscript™ IV VILO™ cDNA Synthesis Kit (Invitrogen, catalog #11754050) was used per manufacturer’s instructions. Levels of mRNA transcripts were measured and analyzed, as previously described [52, 53]. The following dilution factors (indicated in parentheses) were applied to cDNA samples for assessment of transcripts of interest in given tissues: ipsilateral and contralateral LSC (1:2.5), ipsilateral SCN (1:2.5) and DRG (1:2). The 1:200 dilutions of cDNA were used for assessment of the normalizer transcripts (18s RNA) for each tissue samples. Levels of mRNAs, as well as 18S rRNA (Rn18s), were assayed in triplicates via quantitative real-time PCR (qRT-PCR) with Taqman Gene Expression Assays (ThermoFisher Scientific, MA, USA). In cases of triplicates with a standard deviation of more than 0.1, the average value of the two closest replicates was used. All selected gene expression assays were identified by the manufacturer to be the “best coverage” assays (unless otherwise noted) and to exclude detection of genomic DNA. mRNA levels were analyzed with the formula C=2^CTNormalizer/2^CTtarget, as previously described [53, 54].
To test whether sciatic nerve damage leads to elevated cytokines and whether BIRT377 treatment influences the inflammatory milieu in collected tissues, mRNA transcripts for the following pain-relevant proinflammatory and anti-inflammatory factors were assessed: C-C motif chemokine ligand 2 (CCL2, Ccl2), interleukin-1β (IL-1β, il1b), tumor necrosis factor (TNF, Tnf,), Interleukin-10 (IL-10). To better understand the contribution of different subsets of T cells and signature cytokines produced by these T cells, the following transcripts were analyzed: Forkhead box P3 (FOXP3, foxp3) for T regulatory (Treg) / anti-inflammatory T cells, T-bet (T-box transcription factor, Tbx21) and IFN-γ expressed by proinflammatory Th1 cells and Interleukin 17A (IL-17A), IL-22 and RAR-related orphan receptor gamma (ROR-γt) expressed by proinflammatory Th17 cells. To assess whether BIRT377 treatment may contribute to pain reversal by modulating glial/immune activation in the lumbar spinal cord, the transcript levels of toll-like receptor 4 (TLR4, tlr4), microglial (and macrophages) activation marker Ionized calcium binding adaptor protein (Iba-1 or Aif-1), integrin alpha M (CD11b, Itgam, common monocyte/macrophage marker) and astrocyte activation marker, GFAP (Gfap, Glial fibrillary acidic protein) were evaluated. All tissue samples and a “no template control” sample for each tissue type were processed for the cDNA preparation or real-time PCRs simultaneously. Assays measuring mRNA transcripts for both ipsilateral and contralateral spinal cord samples were performed in the same 384 well PCR plate (ThermoFisher Scientific, MA, USA).
2.10. Multiplex determination of cytokine and chemokine expression
Frozen sciatic nerves and spinal cord tissues (ipsilateral only) were re-homogenized (initially stored and homogenized as described in section 2.7 – 2.8) in a buffer with protease inhibitors (MSD, MesoScale Discovery) while kept on ice and subsequently sonicated (settings: 5 pulses, at 50%, Fisher Scientific). Tissue samples were then centrifuged at 4,200 x g at 4°C for 10 min to pellet cellular debris. The resultant supernatant from cellular lysates was collected in a new set of tubes and protein concentrations were measured by Quickstart™ Bradford Protein Assay kit (Biorad, CA, USA). Cytokine and chemokine levels were determined using V-Plex™ immunoassays (MesoScale Discovery), as described previously [7, 52]. Briefly, calibrators (provided by the kit) or samples (100 μg protein from each experimental sample per well) were loaded onto a “multi-spot” plate in duplicates. Due to limitations in tissue availability, singlets were also conducted. Samples were read using a Quickplex SQ120 Imager (MesoScale Discovery). Contralateral spinal cord tissues were not examined for protein changes based on the lack of changes observed in the mRNA levels of the cytokines.
2.11. Experimental design and statistical analysis
A total of 64 female offspring (41 Sac and 23 PAE) were used in all experiments. Rats were divided into behavioral experiments, with an n = 4-8 in each experimental condition, as we observed that an n = 4 - 6 per experimental condition was sufficient to yield reliable group differences in similarly conducted studies [6, 19, 52, 55–58]. The first group consisted of 44 (29 Sac and 15 PAE) rats given either i.v. BIRT377 or vehicle treatment. The second group consisted of 20 (12 Sac and 8 PAE) rats given either i.t. BIRT377 or vehicle treatment. To investigate whether young-adult PAE females display increased susceptibility to neuropathic pain, hindpaw sensitivity was measured prior to (at BL) and following surgical manipulations for sham, standard and minor CCI at different time points. Once chronic allodynia was established, BIRT377 was administered i.v. at Day 28 post-surgical manipulations. Upon behavioral verification that i.v. BIRT377 treatment reverses hindpaw sensitivity to levels that were similar to BL levels, on Day 4 post-injection, all the rats were euthanized and tissues were collected for further analysis.
A separate behavioral study was conducted to explore whether spinal blockade of LFA-1 would be sufficient to reverse pain in neuropathic females, as seen in males previously [50]. Therefore, on Day 28 post-surgery, i.t. BIRT377 was administered and hindpaw sensitivity was re-assessed.
All data were graphed in GraphPad Prism version 7.02 (GraphPad Software Inc.; RRID: SCR002798). For all behavioral data presented in Sections 3.1 and 3.9, statistics were conducted using IBM SPSS Statistics version 24 (IBM; RRID: SCR002865). Analysis of Variance (ANOVA) was performed at BL, and on days prior to and after BIRT377 or Veh injection. For post-surgical behavioral timepoints, repeated measures ANOVA were performed to assess group and timecourse differences between drug treatments, as described previously [7]. Intravenous BIRT377 behavioral data was assessed using two-way ANOVA procedures. Rat groups were collapsed based on whether effects of prenatal exposure, surgery or injection were being compared.
Relative mRNA transcript levels and proteins levels were analyzed using One-way ANOVA (unless otherwise mentioned) using GraphPad PRISM version 7.02. The Fisher’s LSD test (reported with adjusted P values) was applied for post hoc examination of possible group differences selected a priori. The threshold for statistical significance for all sets of multiple comparisons was set a priori to α = 0.05. All data are presented as the mean ± Standard Error of the Mean (SEM). For mRNA and protein analyses, within-group outliers were tested for by Grubbs’ Test using the GraphPad QuickCalc Outlier Calculator (https://graphpad.com/quickcalcs/grubbs1/) with α = 0.05.
3. Results
3.1. Voluntary drinking paradigm
Long-Evans rat dams consumed an average of 2.01 ± 0.07 g/kg/day of ethanol throughout gestation (Table 1). This voluntary drinking paradigm did not affect maternal weight gain, litter size and offspring birth weights.
Table 1:
Voluntary maternal drinking paradigm outcomes
| Saccharin Control (Sac) | 5% Ethanol Group (PAE) | |
|---|---|---|
| Daily 4 hour 5% ethanol consumption | NA | 2.01 ± 0.07a (92) |
| Maternal Weight Gain during pregnancy | 100 ± 3b (36) | 108 ± 5 (27) |
| Litter Size | 11.0 ± 0.4c (36) | 11.4 ± 0.5 (27) |
| Pup birth weight | 8.06 ± 0.21d (36) | 7.50 ± 0.19 (27) |
- Mean ± S.E.M. grams ethanol consumed/kg body weight/day
- Mean ± S.E.M. grams increase in body weight from GD 1 through GD 21
- Mean ± S.E.M. number of live births/litter
- Mean ± S.E.M. grams pup birth weight
NA-not applicable, n- Group size
3.2. PAE is a risk factor for developing neuropathy and blocking LFA-1 actions via intravenous (i.v.) administration of BIRT377 reverses neuropathy in PAE rats
Hindpaw sensitivity was assessed to explore whether female PAE rats develop chronic allodynia relative to Sac control rats treated with minor CCI. Additionally we explored whether i.v. BIRT377 was capable of reversing allodynia in Sac and PAE rats with ongoing neuropathy. Hindpaw responses to light mechanical touch was assessed prior to surgery.
Baseline (BL) responses demonstrated no significant differences in all rat groups, with responses occurring at approximately 10 g of touch stimuli (Figure 1). Following sham surgery, Sac and PAE groups maintain normal sensitivity in the ipsilateral and contralateral hindpaws demonstrating that PAE alone, without injury, does not alter hindpaw sensitivity, which remains stable throughout the timecourse despite repeated von Frey testing. Similarly, Sac rats with minor CCI do not develop increases in hindpaw sensitivity. In contrast, Sac rats with standard CCI develop bilateral allodynia through Day 28 post-surgery (Figure 1), with bilateral allodynia observed in this model in numerous prior reports using male rats [6, 7, 51, 59].
Fig 1: Minor CCI results in enduring allodynia in PAE rats and blocking LFA-1 actions reverse allodynia in female PAE rats.

PAE and Sac rats show no significant differences at baseline (BL) (ipsilateral, F1,36 = 0.018, P = 0.895, contralateral, F1,36 = 0.562, P = 0.458). Following surgery, a main effect of exposure in the ipsilateral hindpaw was observed (ipsilateral, F1,36 = 308.383, P < 0.0001, contralateral, F1,36 = 0.153, P = 0.698), an effect of surgery (ipsilateral, F1,36 = 651.198, P < 0.0001, contralateral, F1,36 = 464.695, P < 0.0001), and interaction between exposure and surgery (ipsilateral, F1,36 = 98.005, P < 0.0001, contralateral, F1,36 = 6.345, P = 0.016) is seen. PAE rats with minor CCI develop unilateral allodynia whereas Sac rats with standard CCI develop bilateral allodynia up to day 28 post-surgery (ipsilateral, F1,36 = 103.967, P <0.0001, contralateral, F1,36 = 5.942, P = 0.020). Following BIRT377 injection, allodynic PAE and Sac rats return to normal sensitivity up to day 4 post-injection (ipsilateral, F1,36 = 58.633, P < 0.0001, contralateral, F1,36 = 54.229, P < 0.0001). Number of rats used: Sac/ PAE Sham + Veh (n=6 each), PAE 1 Sut + Veh (n=4), PAE 1 Sut + BIRT377 (n=5), Sac 4 Sut + Veh (n=5), Sac 1 Sut + Veh (n=4), Sac 4 Sut + BIRT377 (n=6) and Sac 1 Sut + BIRT377 (n=8). Minor CCI is a single suture CCI and standard CCI is a 4-suture CCI.
PAE rats with minor CCI develop robust unilateral allodynia similar to our previous observations in male PAE rats with minor CCI [6, 50]. PAE females with minor CCI display allodynia at similar levels to that observed in Sac rats with standard CCI. These data demonstrate that the persistent insidious effects of PAE on immune priming are unmasked following a minor challenge. BIRT377 or vehicle given intravenously at Day 28 post-surgery revealed a robust reversal of allodynia by Day 2 post-injection, with reversal persistent at least through Day 4, which is at a time when the experiment was terminated for tissue collection.
3.3. PAE primes sciatic nerve inflammatory reactions that can be regulated by BIRT377 under neuropathic conditions.
The inflammatory milieu around the injured sciatic nerve (SCN; ipsilateral) was investigated from the same rats used to generate behavioral data presented in Figure 1. Both mRNA and protein levels for the anti-inflammatory cytokine, IL-10 and the proinflammatory cytokines, IL-1β and TNF were examined (Figure 2A–C). Data show IL-10 levels were increased in Sac rats with standard CCI compared to sham manipulations. These data reflect the predicted compensatory endogenous response in an effort to control ongoing inflammation around the injured sciatic nerve [7]. Interestingly, neuropathic PAE females displayed blunted IL-10 responses, as changes in mRNA/protein IL-10 levels were absent (Figure 2A). Therefore, under neuropathic conditions, PAE rats lack IL-10 compensatory responses. However, treatment with BIRT377 increased IL-10 in both pain-reversed Sac and PAE rats.
Fig 2: PAE priming augments sciatic nerve proinflammatory reactions that are regulated by BIRT377.

(A) IL-10 levels were increased neuropathic Sac rats (***P=0.001, mRNA and ***P=0.029, protein). Neuropathic PAE rats displayed lower levels of IL-10 than neuropathic Sac rats (*P=0.007, mRNA and *P=0.04, protein). BIRT377 increased IL-10 levels in pain-reversed Sac (#P=0.006, mRNA, P=0.06, protein) and PAE rats (##P= 0.045, mRNA and ##P=0.014, protein). (B) TNF levels were increased in neuropathic Sac (***P<0.0001) and PAE rats (**P=0.015, mRNA and **P=0.0003, protein). Following minor CCI, increased levels of TNF protein were detected in PAE rats (*P=0.0002). BIRT377 decreased TNF levels in pain-reversed Sac (***P<0.0001) and PAE rats (##P=0.023, mRNA and ##P=0.005, protein). (C) IL-1β levels were increased under neuropathic conditions in Sac (***P<0.0001) and PAE rats (**P=0.002, mRNA and **P<0.0001, protein). Following minor CCI, increased IL-1β was detected in PAE rats (*P=0.006, mRNA and *P=0.01, protein). BIRT377 decreased IL-1β in pain-reversed Sac (#P<0.0001) and PAE rats (##P=0.002, mRNA and ##P=0.004, protein). (D) CXCL1 levels were increased in neuropathic Sac (***P=0.0006) and PAE rats (**P=0.004). Following minor CCI, increased levels of CXCL1 protein (*P=0.01) were detected in PAE rats. BIRT377 decreased CXCL1 levels in pain-reversed Sac (#P=0.004) and PAE rats (##P=0.029). (E) IL-6 protein levels were significantly increased in neuropathic Sac rats (***P=0.003). (F) IL-4 levels did not change during neuropathy. Two-way ANOVA suggests that PAE has a main effect on IL-4 (*P=0.001). Numbers (n) of rat are same as indicated in Figure 1, except, Sac Sham + Veh, n=5 for TNF mRNA; PAE 1 Sut + Veh, n=3 for IL-10 and IL-1β mRNA; PAE 1 Sut + BIRT377, n=4 for TNF mRNA; Sac 4 Sut + Veh, n=4 for IL-1β mRNA; Sac 4 Sut + BIRT377, n=5 for IL-10 mRNA, CXCL1 and Sac 1 Sut + BIRT377, n=7 for IL-1β (mRNA and protein), TNF (protein) and IL-6.
Compared to their corresponding sham-treated controls, IL-1β and TNF mRNA and protein levels were robustly elevated in Sac rats with standard CCI and PAE rat with minor CCI. In contrast to robust proinflammatory responses observed in PAE females, no reliable increases of these cytokines were detected in Sac rats with minor CCI (Figure 2B–C). Other pain-relevant proinflammatory factors such as CXCL1 (chemokine, neutrophil attractant) and IL-6 levels were analyzed at the SCN microenvironment. Interestingly, changes in protein levels of the chemotactic cytokine, CXCL1, displayed a very similar pattern to that observed with IL-1β and TNF (Figure 2B–D), replicating prior reports that this chemokine is upregulated in nervous tissue during chronic neuropathic conditions [60]. BIRT377 reduced IL-1β and TNF (mRNA and protein) and CXCL1 protein levels in all pain-reversed rats.
IL-6 has context-specific proinflammatory properties [61]. Interestingly, a strong trend of higher levels of IL-6 proteins was observed in PAE rats than in Sac rats under basal conditions. However, no further increase of IL-6 was observed in PAE females during neuropathy, whereas, IL-6 levels were elevated in the Sac rats with standard CCI compared to sham group. IL-4 is a pleiotropic cytokine, often considered as anti-inflammatory [62]. Recently, IL-10 and IL-4 co-treatment has received significant attention in treating chronic pain [63]. The current data show low levels of IL-4 detected at the SCN, with levels remaining virtually unchanged under neuropathic conditions. Surprisingly, blunted IL-4 levels were observed in PAE rats compared to Sac rats following minor CCI. BIRT377 did not modulate neither IL-4 nor IL-6 protein levels in nerve damaged rats (Figure 2E–F).
3.4. PAE-induced T cell mediated responses during sciatic neuropathy are controlled by BIRT377
Based on emerging evidence that spinal T cells play critical roles during neuropathy in females [6, 19], the contribution of different subsets of activated T cells (proinflammatory Th1 and Th17) and anti-inflammatory T cells (Treg) were examined in anatomical regions of the pain pathway including the SCN. Treg cells produce IL-10, as well as other major sources of IL-10 such as macrophages and monocytes [64, 65]. Therefore, to identify the contribution of Tregs, mRNA for FOXP3 was examined. FOXP3 is T cell-specific and is critical for Treg differentiation [66]. Data suggest that compared to sham controls, both minor and standard CCI leads to increased FOXP3 levels indicating recruitment of Treg cells to the damaged nerve has occurred (Figure 3A). Interestingly, though a strong trend was observed for increased FOXP3 levels in pain-reversed PAE rats, robust increases in FOXP3 mRNA levels only occurred in pain-reversed Sac rats following BIRT377 treatment.
Fig 3: T cell related pro- and anti-inflammatory factors at the level of sciatic nerve are regulated by BIRT377 during neuropathy.
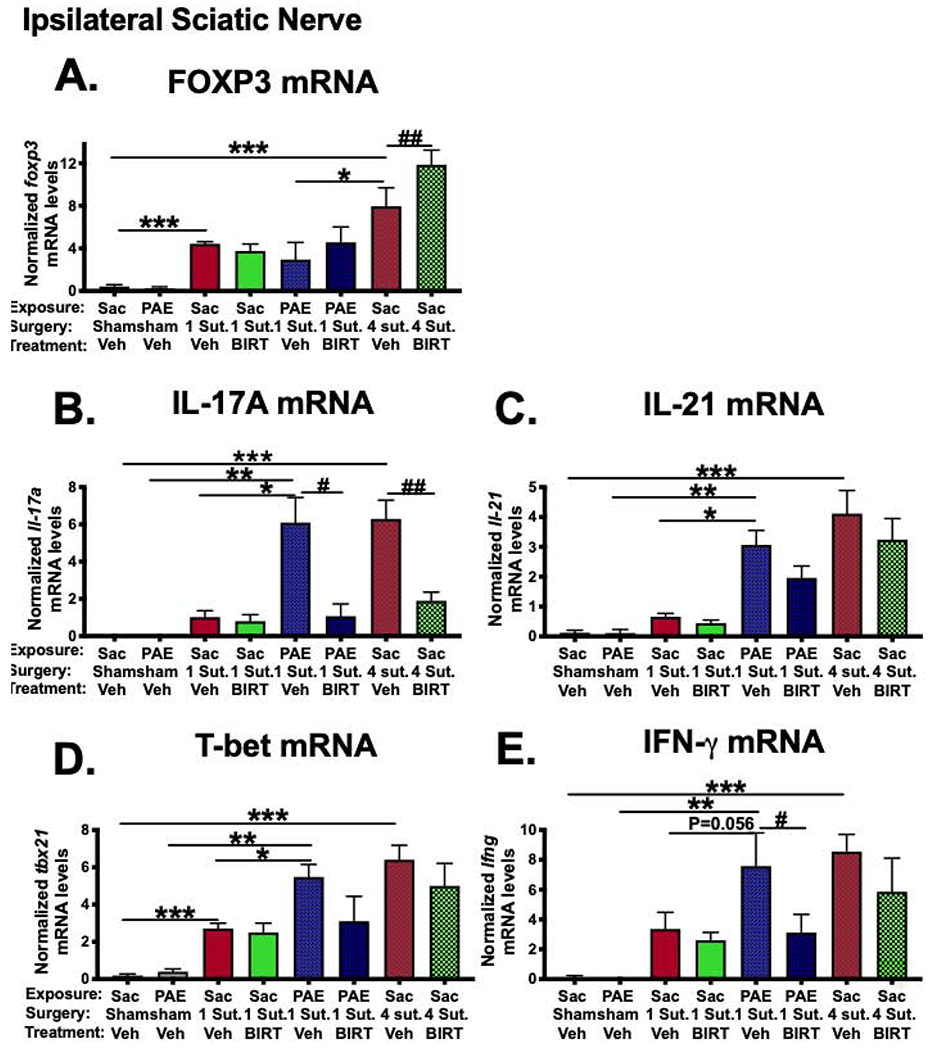
(A) FOXP3 mRNA levels were increased following standard CCI (***P<0.0001) and also, following minor CCI (***P= 0.02) in Sac rats. Under neuropathic conditions, PAE rats displayed significantly lower levels of FOXP3 (*P=0.006). BIRT377 increased FOXP3 levels in pain-reversed Sac rats (##P=0.016). (B) IL-17A mRNA levels were increased in neuropathic Sac (***P<0.0001) and PAE rats (**P<0.0001 and *P<0.0001). IL-17A mRNA levels were reduced in pain-reversed Sac (##P<0.0001) and PAE rats (#P<0.0001). (C) IL-21 levels were increased in neuropathic Sac (***P<0.0001) and PAE rats (**P<0.0001). Following minor CCI, increased levels of IL-21 mRNA were detected in PAE rats (*P<0.0001). (D) T-bet mRNA levels were increased following standard CCI, (***P<0.0001). Minor CCI resulted increased levels of T-bet in Sac (***P=0.04) and PAE (**P=0.0002) rats, greater levels of T-bet mRNA were detected in PAE rats (*P=0.03). (E) IFN-γ levels were significantly increased only under neuropathic conditions in Sac treated rats (***P<0.0001) and PAE rats (**P=0.002). BIRT377 decreased IFN-γ levels (#P=0.034). Numbers of rats are as indicated in Figure 1 except Sac/PAE Sham + Veh, n=5 for T-bet mRNA; Sac 4 Sut + Veh, n=4 for T-bet mRNA, Sac 4 Sut + BIRT377, n=5 for IL-21 mRNA.
T-bet is the master regulator of Th1 differentiation and a major transcription factor for the production of the proinflammatory cytokine, IFN-γ [67]. In a separate T-cell subset, proinflammatory cytokines IL-17A and IL-21 are produced by Th17 cells [68]. Compared to their corresponding sham-treated controls, IL-17A and IL-21 mRNA levels were robustly elevated under neuropathic conditions, both in Sac and PAE rats (Figure 3B–C). In contrast to robust Th17 cell mediated responses observed in neuropathic PAE females (as much as observed in Sac rats with standard CCI), no reliable increases of these cytokines were detected in Sac rats following minor CCI. BIRT377 treatment dramatically decreased IL-17A levels, but not IL-21 levels in pain-reversed rats suggesting that IL-21 may play a less critical role in mediating Th17-driven nociceptive signaling and possibly damage at the sciatic nerve.
Surprisingly, compared to sham conditions, both minor and standard CCI led to increases in T-bet mRNA levels indicating that Th1 type T cells are recruited at the SCN in response to nerve injury (Figure 3D). However, T-bet mRNA levels are much greater in neuropathic Sac and PAE females vs. non-neuropathic Sac rats with minor CCI. In support of these data, significant increases of Th1-specific cytokine, IFN-γ levels were observed in Sac and PAE rats with ongoing neuropathy (Figure 3E). A significant decrease in IFN-γ mRNA was observed in pain-reversed PAE rats with a trend toward this decrease in pain-reversed Sac rats.
3.5. Augmented satellite glial and immune activation and proinflammatory cytokine environment in the DRGs in neuropathic PAE females
Intravenous BIRT377 may exert direct and indirect effects on glial-immune interactions in the DRGs. Changes in mRNA levels of GFAP and CD11b were examined as a marker of satellite glial activation and recruitment of peripheral macrophages to the ipsilateral DRGs following neuropathy [69, 70]. In addition, mRNA levels of CCL2, IL-1β and IL-10 levels were examined to better characterize the cytokine milieu during neuropathy in PAE and Sac control females, and to determine whether BIRT377 treatment influences the inflammatory environment in the DRGs. Data show a significant increase in GFAP and CD11b (Figure 4A–B) mRNA transcriptional activation in all neuropathic groups, indicative of satellite glial activation and peripheral macrophage infiltration (and activation) respectively. Sac rats with minor CCI displayed increased GFAP mRNA levels but not CD11b levels compared to Sac Sham + Veh rats. However, neuropathic PAE rats revealed significantly greater GFAP and CD11b levels than non-neuropathic Sac rats following minor CCI. BIRT377 decreased GFAP and CD11b levels under neuropathic conditions.
Fig 4: BIRT377 reduces PAE-induced augmented satellite glial and myeloid cell activation and alter cytokine profiles in the DRGs in neuropathic PAE females.

Ipsilateral DRGs were examined for glial-immune activation. (A) GFAP mRNA levels were increased following standard CCI in Sac rats, (***P<0.0001) and following minor CCI, ***P=0.029 and **P<0.0001. Greater levels of GFAP mRNA were detected in PAE rats, *P<0.0001). BIRT377 decreased GFAP mRNA levels in Sac (##P=0.001) and PAE rats (#P<0.0001) (B) CD11b mRNA levels were increased with neuropathy in Sac (***P=0.03) and PAE rats (**P=0.013). Minor CCI in PAE rats leads to higher levels of CD11b mRNA than in Sac rats (*P=0.047). CD11b mRNA levels were reduced in pain-reversed Sac rats (##P=0.048). (C) CCL2 mRNA levels were increased in neuropathic Sac (***P=0.004) and PAE (**P=0.001) rats and greater levels of CCL2 were detected in PAE rats than Sac rats following minor CCI (*P=0.039). (D) IL-1β mRNA levels were increased in neuropathic Sac rats (***P<0.0001) and PAE rats (**P=0.0003). Minor CCI in PAE rats leads to higher levels of IL-1β (*P=0.001). IL-1β levels were reduced in pain-reversed Sac (##P=0.0002) and PAE rats (#P<0.0001). (E) IL-10 mRNA levels were increased following standard (***P=0.0006) and minor CCI (***P=0.042) in Sac rats. Greater levels of IL-10 mRNA were detected in neuropathic Sac rats than in PAE rats (*P=0.044). BIRT377 increased IL-10 mRNA levels in pain-reversed PAE rats (#P=0.034). Numbers of rats are as indicated in Figure 1 except Sac Sham + Veh, n=5 for CD11b mRNA.
Supportive of increased macrophages in the DRGs during neuropathy, increased levels of the peripheral macrophage chemoattractant CCL2 were observed only in neuropathic Sac and PAE females but not in Sac rats following minor CCI (Figure 4C). BIRT377 treatment did not alter CCL2 mRNA levels. Similar to the observations made in Figure 2 (sciatic nerve data), augmented levels of IL-1β were observed only in neuropathic PAE and Sac rats, whereas no increases in IL-1β mRNA transcripts were observed in Sac rats with minor CCI. (Figure 4D). Supportive of these data, a significant increase of anti-inflammatory cytokine, IL-10 mRNA was observed following minor CCI in Sac rats (Figure 4E). Though a strong trend of increasing IL-10 mRNA was observed in PAE neuropathic rats (P=0.06, compared to their sham controls), the IL-10 mRNA levels were still significantly less than Sac rats with standard CCI. BIRT377 increased IL-10 levels in pain-reversed PAE rats.
3.6. Augmented T cell mediated proinflammatory cytokine levels in the DRGs of neuropathic PAE females: IL-17 regulation by BIRT377
Data presented in Figure 3 demonstrate the presence of T cell-derived proinflammatory cytokines at the injured sciatic nerve and BIRT377-mediated modulation of T cell responses during neuropathy. However, T cell recruitment and their actions may not be similar in other regions along the CCI pain pathway, such as the DRG. Therefore, mRNA levels of FOXP3, IL-17A, IL-21, and T-bet were examined in the ipsilateral DRG samples.
Compared to their sham controls, FOXP3 mRNA levels were significantly increased in both neuropathic Sac and PAE females, but not in non-neuropathic Sac rats (Figure 5A). In contrast to levels of FOXP3 at the sciatic nerve, similar levels of FOXP3 were present in the DRGs in neuropathic PAE and Sac rats indicating the presence of factors that either facilitate ongoing recruitment of Treg cells or factors that drive further differentiation of T cells to Treg cells once T cells have arrived at the DRG. In contrast to the observations made for the damaged sciatic nerves, BIRT377 given to Sac or PAE rats did not alter FOXP3 levels in the corresponding DRGs.
Fig 5: Characterization of T cell mediated response in the DRGs in PAE neuropathic females and effects of BIRT377.

(A) FOXP3 mRNA levels were increased with neuropathy, ***P=0.021 for Sac and **P= 0.023 for PAE. (B) IL-17A mRNA levels were increased in neuropathic Sac (***P<0.0001) and PAE rats (**P<0.0001). Greater levels of IL-17A were detected in PAE rats than in Sac rats (*P=0.001). IL-17A mRNA levels were reduced in pain-reversed Sac (##P=0.0002) and PAE rats (#P=0.022). (C) IL-21 levels were increased in neuropathic Sac (***P=0.013) and PAE rats (**P=0.037). Following minor CCI, increased levels of IL-21 mRNA were detected in PAE rats (*P=0.039). (D) T-bet mRNA levels were increased in neuropathic Sac (***P=0.046) and PAE rats (**P=0.0008). Minor CCI resulted in greater levels of T-bet in PAE rats (*P=0.004). Numbers of rats are as indicated in Figure 1 except Sac 4 Sut + BIRT377, n=5 for IL-17A mRNA.
Similar to sciatic nerve data, mRNA levels of the Th17 cytokines, IL-17A and IL-21 were found elevated during neuropathic conditions but not in Sac rats with minor CCI. Augmented levels of these Th17 cytokines were detected at the DRG in PAE females compared to their Sac-treated counterparts following minor CCI (Figure 5B–C). BIRT377 decreased IL-17A levels but not IL-21 under neuropathic conditions. Interestingly, compared to the sham groups, a significant increase of T-bet mRNA was observed only under neuropathic conditions, with greater levels of T-bet mRNA in PAE females vs. Sac treated females following minor CCI. BIRT377 did not alter T-bet mRNA levels (Figure 5D).
3.7. Proinflammatory lumbar glial activation markers and TLR4 levels following CCI and regulation by BIRT377
The data discussed above demonstrate that BIRT377 corresponds to reduced proinflammatory cytokines while elevating endogenous anti-inflammatory cytokines at the injured sciatic nerve and DRGs. Persistent microglial and astrocyte activation in the spinal cord is often observed following sciatic damage and consequent allodynia. Reducing the proinflammatory cytokine milieu at the peripheral anatomical regions of the pain pathway may likely reduce chronic pain relays to the spinal cord, and in doing so, may reduce spinal glial activation and consequent pathological pain processing. In support of this possibility, the mRNA levels of GFAP, marker of astrocyte activation, and Iba-1, a marker of microglial/peripheral macrophage activation, were examined in the ipsilateral and contralateral spinal cord.
Data revealed that GFAP mRNA levels were significantly increased in the ipsilateral dorsal horn in neuropathic Sac and PAE rats compared to sham rats, consistent with prior reports [71]. Despite a lack of allodynia, a significant increase in GFAP mRNA was observed in Sac rats with minor injury, with Sac rats with standard CCI revealing even greater GFAP increases. However, the greatest increase in GFAP mRNA was observed in neuropathic PAE rats with minor CCI demonstrating that augmented spinal astrocyte activation occurs in PAE rats following minor injury (Figure 6A). In the contralateral dorsal horn, increased GFAP mRNA levels were observed only in neuropathic Sac rats with standard CCI. BIRT377 treatment significantly decreased GFAP mRNA levels in pain-reversed females (Figure 6A).
Fig 6. Lumbar spinal cord glial-immune activation in PAE rats and effects of BIRT377.

(A) In the ipsilateral spinal cord, GFAP mRNA levels were increased following standard CCI (***P<0.0001) and minor CCI (***P=0.015 for Sac rats and **P<0.0001 for PAE rats); greater levels of GFAP were detected in PAE rats compared to Sac rats (*P=0.008). BIRT377 decreased GFAP mRNA levels in pain–reversed Sac (##P=0.012) and in PAE rats (#P=0.0003). In the contralateral side, GFAP mRNA levels were increased only following standard CCI, ***P=0.002 and BIRT377 reduced GFAP mRNA levels (##P=0.016). (B) Interestingly, ipsilateral Iba-1 mRNA levels were higher in PAE than in Sac females under baseline conditions (*P=0.039). Iba-1 mRNA levels were increased in neuropathic Sac rats, ***P=0.001 (ipsilateral) and ***P=0.032 (contralateral). Minor CCI resulted in higher levels of Iba-1 mRNA (ipsilateral only) in Sac rats (***P=0.012). Contralateral Iba-1 mRNA levels were reduced in pain-reversed Sac rats (##P=0.009). (C) Ipsilateral TLR4 mRNA levels were increased following standard CCI in Sac rats, (***P=0.015). Following minor CCI, both Sac and PAE rats displayed increased levels of TLR4, ***P=0.009 for Sac and **P=0.049 for PAE rats. Data are presented on the same scale for Y-axis for ipsilateral and contralateral sides, to indicate the comparative level of these markers in both sides. Numbers of rats are as indicated in Figure 1 except Sac 1 Sut + Veh, n=3 for GFAP mRNA.
Iba-1 mRNA was increased bilaterally in the spinal cord of neuropathic Sac rats compared to Sac sham rats, indicative of increased microglial (and macrophage) activation, as observed in numerous reports and previously in Sac-exposed males [6, 7, 71, 72] (Figure 6B). Interestingly, the Iba-1 increase was also observed in non-neuropathic PAE and Sac rats (PAE Sham + Veh, Sac 1 Sut + Veh). A trend towards increased contralateral Iba-1 mRNA was observed in PAE females with sham or minor CCI. BIRT377 reduced Iba-1 mRNA levels only in pain-reversed Sac rats (Figure 6B).
Pain mediators activate microglia via spinal TLR4 signaling [73]. PAE also causes neuroimmune activation through TLR4-mediated pathways [74–76]. Thus, TLR4 mRNA levels were also assessed as a marker of activated microglial and infiltrating macrophages (Figure 6C). Compared to their corresponding sham controls, a significant increase in TLR4 mRNA was detected in Sac and PAE rats with minor CCI as well as in Sac rats with standard CCI. However, a reduction in TLR4 mRNA levels was not observed in BIRT377-treated rats where allodynia was reversed.
3.8. BIRT377 reduces pain-inducing cytokines and promotes an anti-inflammatory environment in the spinal cord contributing to pain reversal.
The effects of i.v. BIRT377 on modulating spinal astrocyte activation indicate that key inflammatory cytokines released from astrocytes and other cells in the lumbar spinal cord dorsal horn may also be controlled by BIRT377. Levels of multiple cytokines, IL-1β, IL-10, TNF (both protein and mRNA), IL-6, CXCL1, IL-4 and IFN-γ (protein only, ipsilateral spinal cord) were analyzed. These cytokines contribute to pathological pain [77–80]. Bilateral spinal cord mRNA analysis was examined because spinal cord contralateral to the sciatic injury could serve as a within-animal anatomical control region in neuropathic rats with unilateral allodynia observed in PAE rats or further elucidate contributing spinal factors responsible for contralateral allodynia in control rats following standard CCI.
In the ipsilateral dorsal horn, a decrease in IL-10 mRNA and protein was observed in neuropathic Sac rats, with a similar trend observed in the contralateral dorsal horn. These data are consistent with prior observations that IL-10 levels in the spinal cord decrease from basal levels during chronic neuropathy [39, 81]. Similarly, neuropathic PAE females displayed a strong trend in decreased ipsilateral IL-10 mRNA and protein, whereas non-neuropathic Sac-treated rats maintained basal levels of IL-10 following minor CCI (Figure 7A). That is, minor nerve injury in Sac-treated rats revealed significantly higher IL-10 protein levels than neuropathic PAE rats. BIRT377 treatment restored IL-10 protein and mRNA levels in the dorsal horn milieu in pain-reversed rats.
Fig 7. Spinal cord cytokines and the effects of i.v. BIRT377 on these cytokines.
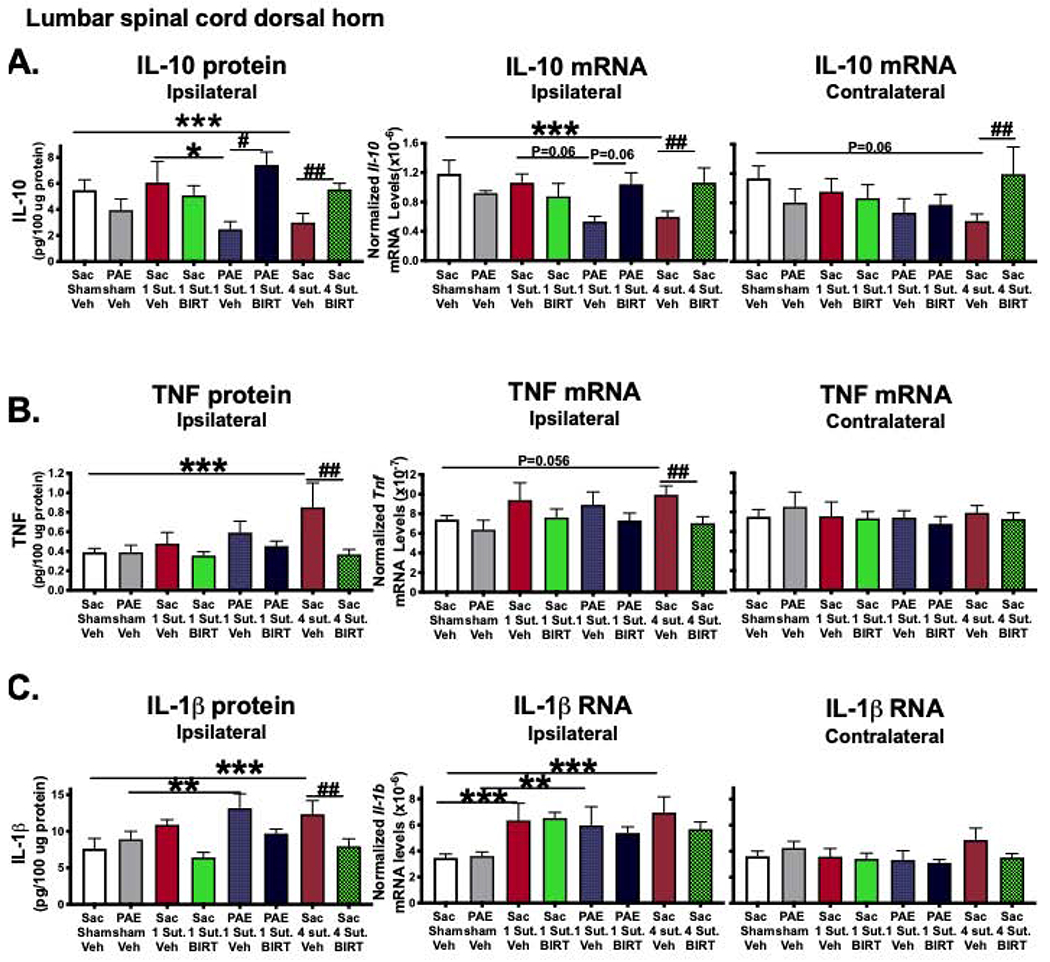
(A) In the ipsilateral spinal cord, IL-10 mRNA and protein levels were decreased under neuropathic conditions in Sac rats (***P=0.013, mRNA and ***P=0.044, protein). Similar trends were observed in neuropathic PAE rats (*P=0.014). BIRT377 increased IL-10 levels in Sac (##P=0.044 for ipsi- and contralateral mRNA and ##P=0.039, protein) and in PAE rats (#P=0.001). (B) In the ipsilateral side, TNF protein levels were increased in neuropathic Sac rats (***P=0.002) and BIRT377 decreased TNF levels (##P=0.029, mRNA and ##P=0.001, protein). (C) Ipsilateral IL-1β levels were increased in neuropathic Sac (***P=0.001, mRNA and ***P=0.008, protein) and PAE rats (**P=0.03, mRNA and **P=0.031, protein). Following CCI, increased levels of IL-1β (mRNA only) were detected in Sac rats (***P=0.013). IL-1β protein levels were decreased in pain-reversed Sac rats (##P=0.014). Numbers of rats are as indicated in Figure 1 except Sac 1 Sut + Veh, n=3 for TNF protein and PAE 1 Sut + Veh, n=3 for TNF mRNA.
As predicted, increases in ipsilateral TNF protein and mRNA were detected following standard CCI in Sac-treated females, (Figure 7B). In contrast to sciatic nerve data (Figure 2B), TNF protein levels in the ipsilateral PAE spinal cord were much lower, lacking significant increases in TNF mRNA or protein following minor CCI in PAE rats. BIRT377 reduced the levels of TNF mRNA and protein in pain-reversed Sac rats. Similar to TNF levels, ipsilateral IL-1β protein and mRNA levels were increased in the dorsal horn under neuropathic conditions in Sac treated rats (Figure 7C). Similar increases of ipsilateral IL-1β protein and mRNA levels were seen in neuropathic PAE females. Interestingly, IL-1β mRNA, but not protein levels, were also significantly greater in Sac rats with minor CCI. Though IL-1β levels appeared lower in most BIRT377-treated groups, a significant decrease in IL-1β protein levels was observed only in pain-reversed Sac rats. In the contralateral dorsal horn, IL-1β mRNA levels were similar across all treatment conditions.
The protein levels of IL-6 were augmented only under neuropathic conditions and greater IL-6 levels were observed in neuropathic PAE rats compared to Sac rats following minor CCI (Figure 8A). BIRT377 reduced IL-6 in pain-reversed Sac rats, along with a similar trend in pain-reversed PAE rats. Similar to IL-6, neuropathic Sac rats displayed higher CXCL1 levels, compared to their corresponding sham controls (Figure 8B). A similar trend was observed in neuropathic PAE rats as well, but absent in Sac rats with minor CCI. BIRT377 reduced CXCL1 in pain-reversed Sac females. Interestingly, while no ipsilateral changes were observed in spinal cord protein levels of the anti-inflammatory cytokine IL-4 during neuropathy, IL-4 protein was significantly increased following BIRT377 treatment (Figure 8C).
Fig 8. Spinal cord protein levels of IL-6, CXCL1 and IL-4 and the effects of i.v. BIRT377.

(A) Ipsilateral IL-6 protein levels were significantly increased in neuropathic Sac (***P=0.02) and PAE rats (**P=0.0003). Greater levels of IL-6 protein were detected in PAE rats (*P=0.033) following minor CCI. IL-6 protein levels were decreased in pain-reversed Sac rats (##P=0.027). (B) BIRT377 decreased CXCL1 levels only in neuropathic Sac rats (##P=0.01). (C) IL-4 protein levels did not change during neuropathy, increases in IL-4 were detected in pain-reversed Sac (##P=0.011) and PAE rats (#P=0.011). Numbers of rats are as indicated in Figure 1 except PAE 1 Sut + Veh, n=3 for IL-4.
3.9. Augmented proinflammatory T cell responses in PAE rats and effects of BIRT377 on T cell related proinflammatory mediators in the spinal cord
Activated T cells, such as Th1 and Th17 cells, migrate from the periphery to lumbar spinal cord, where they can interact with glial cells [67, 82], and likely contribute to allodynia. In this section, the presence of different subsets of differentiated T cells was analyzed to better understand the contribution of infiltrating T cells to the inflammatory environment within the spinal cord, and how BIRT377 indirectly modulates spinal T cell-mediated responses under neuropathic conditions. Proinflammatory cytokines produced by Th1 and Th17 cells can also be produced by glial cells. For example, IL-17A is produced by activated astrocytes [83, 84]. To provide a balanced approach of anti- and proinflammatory-like phenotypes, mRNA changes for the transcription factor for Treg (FOXP3), Th1 (T-bet) and Th17 (RORγt) cells were analyzed.
Compared to their corresponding sham-treated controls, FOXP3 mRNA increases were observed in the ipsilateral dorsal horn only following standard CCI in Sac rats. These data indicate that despite ongoing neuropathy, T cells present in spinal cord of PAE neuropathic females do not exhibit a significant increase in Treg cells. Moreover, ipsilateral spinal T-bet and RORγt mRNA levels were greater in neuropathic Sac and PAE females, but not under basal and/or non-neuropathic conditions (Figure 9, A–C). BIRT377 did not alter FOXP3 or T-bet levels. Interestingly, RORγt mRNA levels returned to basal levels in pain-reversed females. Additionally, following minor CCI, augmented IFN-γ protein levels were observed in PAE females compared to Sac rats (Figure 9D). BIRT377 reduced IFN-γ protein levels in neuropathic PAE rats, athough overall spinal IFN-γ protein levels were low and data represent only n=2-5 rats.
Fig 9: BIRT377 reduced T cell-related proinflammatory factors in the spinal cord.

(A) Ipsilateral FOXP3 mRNA levels were significantly increased only in neuropathic Sac rats, (***P=0.022). (B) Ipsilateral RORγt mRNA levels were increased in neuropathic Sac (ipsilateral: ***P=0.011 and contralateral: ***P=0.018), and in PAE rats (**P=0.007). Minor CCI in PAE rats resulted in higher levels of RORγt (*P=0.041). RORγt mRNA levels were reduced in pain-reversed Sac (##P=0.012) and PAE rats (#P=0.033). (C) Ipsilateral T-bet mRNA levels were increased in neuropathic Sac (***P=0.017) and PAE rats (**P<0.0001), with greater levels of T-bet in PAE rats vs. Sac rats (*P=0.005). (D) Ipsilateral IFN-γ protein levels were significantly increased in neuropathic PAE rats (**P=0.01). Following minor CCI, IFN-γ protein levels were greater in neuropathic PAE rats than in Sac rats (*P=0.04). BIRT377 decreased IFN-γ levels in pain-reversed PAE rats (#P=0.003). Numbers (n) of rat in each group are same as indicated in Figure 1 except Sac Sham + Veh, n=5 for RORγt, PAE Sham + Veh, n=5 for contralateral T-bet; Sac 1 Sut + Veh, n=3 for ipsilateral and contralateral T-bet. For IFN-γ data, Sac Sham/1 Sut + Veh (n=3), PAE 1 Sut +Veh / BIRT377 (n=3), Sac 4 Sut + Veh (n=4), Sac 1 Sut + Veh (n=4), Sac 1 Sut + Veh (n=5) and Sac 4 Sut + BIRT377 (n=2); other tissue samples were below the detection limit.
3.10. Female rats (regardless of PAE) displaying allodynia do not return to normal sensitivity following i.t. BIRT377 injection.
Prior reports in male rats have demonstrated that i.t. BIRT377 is sufficient to reverse allodynia to normal sensitivity [50]. Therefore, the effects of i.t. BIRT377 were examined in neuropathic Sac and PAE female rats. Similar to data presented in Figure 1, Sac and PAE rats were assessed for BL hindpaw sensitivity. All groups responded to touch stimuli at ~10g demonstrating normal sensitivity prior to surgery and following sham surgery. As expected, robust allodynia was seen in PAE rats with minor CCI and Sac rats with standard CCI through Day 28 post-surgery (Figure 10). On Day 28 post-surgery, BIRT377 or vehicle was administered i.t. and behavioral responses re-assessed for 4 days after injection. Surprisingly, in contrast to rats given i.v. BIRT377 treatment (Fig. 1), neither neuropathic Sac nor PAE rats responded to i.t. BIRT377, with allodynia remaining consistent over the four days (Figure 10). That is, regardless of PAE, these data indicate that blocking LFA-1 actions at the level of spinal cord was not sufficient to reverse allodynia in females.
Fig 10: Blocking LFA-1 actions at the level of spinal cord via intrathecal (i.t.) administration of BIRT377 does not reverse neuropathic pain in females.

At BL, behavioral responses were similar between Sac and PAE groups. Following standard CCI, Sac rats develop bilateral allodynia, with a main effect of surgery (ipsilateral, F1,6 = 100.131, P < 0.0001, contralateral, F1,6 = 50.070, P < 0.0001). PAE rats with minor CCI develop sensitivity only in the ipsilateral hindpaw, comparable to levels observed in Sac standard CCI rats (ipsilateral, F1,10 = 0.143, P = 0.713, contralateral, F1,9 = 69.905, P < 0.0001). Following i.t. BIRT377 injection, no significant differences observed between vehicle and BIRT377 injected groups (ipsilateral, F1,6 = 5.826, P = 0.052, contralateral, F1,6 = 0.062, P = 0.812). Similarly, Sac rats with standard CCI do not return to normal sensitivity, as no significant differences were observed when compared to PAE rats with BIRT377 in the ipsilateral hindpaw (ipsilateral, F1,6 = 5.124). To minimize unnecessary duplication, PAE Sham + Veh and Sac CCI + Veh groups were not conducted in this study; n=4/group.
4. Discussion
In recent years, neuroimmune dysregulation has received great attention as a possible root cause of neurodevelopmental disorders in individuals with FASD [1–3, 85–87]. Previously, we reported that PAE is a risk factor for aberrant neuroimmune interactions in response to minor challenges such as peripheral nerve injury. While these prior studies were conducted in males, very few studies have examined neuroimmune consequences due to moderate PAE in adult females [2–4]. T cells are considered crucial in terms of driving neuropathy in females [19, 20], yet neuroimmune changes in females have not been investigated in detail. In this study, we examined whether augmented T cell proinflammatory actions enhance susceptibly to neuropathy in adult PAE females and to tease out possible differences in neuroimmune-mediated neuropathy in PAE vs. non-PAE offspring. Attention was focused on the effectors Th1 and Th17, and regulatory T cell involvement. Our findings not only support that augmented myeloid/glial proinflammatory actions occur in PAE females, the data also provide new insights about which cytokines and immune factors are involved during neuropathy in females. We also provide evidence that modulating leukocyte migration and their function via blocking LFA-1 activation is beneficial for controlling neuropathic pain by altering the proinflammatory bias to a more anti-inflammatory bias. To our knowledge, this is the first demonstration that: (1) PAE poses a risk factor for developing adult-onset neuropathy in females (Figure 1), (2) PAE-induced neuropathic susceptibility in females involves augmented proinflammatory cytokines inclusive of T cell actions (Figures 2–3), satellite glial (Figure 4) and spinal astrocytes (Figures 6–8), (3) dysregulated and blunted IL-10 responses may contribute to the proinflammatory bias observed in PAE females following minor injury, (4) Th1-, Th17- and Treg-related transcription factors and cytokines were detected in the sciatic nerve, DRGs and ipsilateral spinal cord in both PAE and control neuropathic females, (5) substantial proinflammatory T cell responses, as evidenced by increased T-bet, IL-17A, ROR-γt without sufficient Treg involvement, likely plays a major role in the development of neuropathy in PAE females following minor injury (Figures 3,5 and 9), (6) blocking activated LFA-1 actions are beneficial in dampening the proinflammatory milieu at the level of sciatic nerve, DRG and spinal cord by decreasing both astrocyte- and myeloid-derived cytokines such as IL-1β, TNF and T cell specific cytokines such as IL-17A, while concurrently increasing anti-inflammatory factors such as IL-10 and FOXP3 (Figures 2–9), (7) independent of PAE, neuropathy in females includes peripheral immune involvement, and blocking LFA-1 actions solely at the spinal level (and possibly at the DRGs) is not sufficient to reverse pain in the female rat model of CCI (Figure 10).
4.1. Minor nerve injury unmasks the PAE induced neuroimnmne effects
While overt PAE-induced abnormal immune function is absent under basal conditions, following pathogenic stimuli or trauma, a significant alteration in the peripheral-immune and CNS-immune interaction occurs [2, 6]. While the mechanisms underlying the PAE-related priming of neuroimmune interactions are currently under investigation, several hypotheses have been proposed (reviewed in [88]), and which have been active areas of research in recent years. Emerging clinical evidence of epigenetic modifications such as long-lasting PAE-induced alterations in miRNA and other non-coding RNA expression provides a possible mechanism for the enduring effects of PAE [89–92]. While evidence of PAE-related epigenetic modification of immune-related gene expression is sparse, PAE-induced hypermethylation of GFAP leads to a reduction in GFAP mRNA stability and expression in fetal brain [93]. Distinct DNA methylation patterns have also been observed in adolescents and children with FASD [94, 95]. In fact, chronic ethanol treatment leads to epigenetic modifications in different brain regions in a TLR4-dependent manner [74]. TLR4 signaling has been associated with heightened peripheral immune and microglial activation and neurodevelopmental alterations in PAE offspring [96]. Independent of peripheral nerve damage, peripheral immune cells, especially myeloid cells, seem to be partially activated in adult PAE males, as indicated by increased MHC class II and LFA-1 expression along with increased proinflammatory cytokine production following ex vivo TLR4 stimulation [7]. To date, a gap in knowledge exists addressing whether PAE alters TLR expression or function on glial or immune cells. While speculative, it is possible that PAE may alter basal levels of these crucial immune modulators rendering glia and peripheral immune cells highly responsive to further immune stimulation with consequent augmentation of proinflammatory responses.
An intriguing aspect of this study was the observation that a standard CCI causes bilateral allodynia, whereas PAE rats with minor CCI leads to unilateral allodynia, replicating prior reports in males [6, 50]. Similarly, immune changes such as glial activation and decreased IL-10 levels and increased Th17 factors were present in the contralateral spinal cord only in Sac control neuropathic rats, supporting the corresponding bilateral allodynia that was observed. Ipsilateral immune-related signaling may drive contralateral spinal cord pain neuron excitability via astrocyte-specific gap junctional communication [97, 98]. Therefore, it is possible that more robust peripheral nerve injuries (e.g. standard CCI) can exceed sufficient thresholds to trigger contralateral astrocyte activation resulting in bilateral allodynia.
4.2. PAE primes peripheral immune cells and satellite glial cells and creates a proinflammatory bias following minor nerve injury
Following nerve injury, peripheral inflammatory reactions are mediated by endothelial cells, Schwann cells and infiltrating leukocytes at the lesion [77]. We find that following minor injury, proinflammatory cytokines and chemokines such as IL-1β, TNF and CXCL1 were increased only in PAE females, with a blunted anti-inflammatory IL-10 response at the injured nerve. Myeloid cells (e.g., macrophages) are major sources of these proinflammatory cytokines [64, 99]. Therefore, these data suggest of augmented peripheral myeloid cell activation toward proinflammatory actions following minor nerve injury, with similar observations made following standard CCI in PAE males [7].
In DRGs, minor nerve injury induces IL-1β and CCL2 expression only in PAE rats, whereas IL-10 mRNA induction is observed only in Sac rats. CCL2 induction co-occurred with myeloid cell recruitment and activation, as evidenced by increases in CD11b mRNA levels in the DRGs of PAE females. Though satellite glial activation was observed in Sac rats, induction of CCL2 and myeloid cell recruitment was not detected. In light of these data from DRG following minor CCI, it is likely that satellite glial cells are major sources of IL-10 detected in Sac rats with minor CCI. Despite myeloid and satellite glial activation in PAE females, IL-10 induction was minimal. These data reflect PAE-related priming of myeloid cells and satellite glia that create a proinflammatory bias following nerve injury.
Robust proinflammatory T cell responses were observed in neuropathic PAE and non-PAE females. Induction of mRNA for the Th17-specific cytokines, IL-17A and IL-21, as well as Th1 specific factor, T-bet was observed in both neuropathic PAE and Sac rats. These observations suggest similar levels of proinflamamtory Th1 and Th17 cells were recruited to the sciatic nerve under neuropathic PAE or Sac conditions, despite the fact that the injury itself was minimal in PAE rats. Supporting these data, induction of IFN-γ, a signature cytokine produced by in Th1 cells, was similar in neuropathic PAE and Sac rats. The emerging hypothesis based on these data is represented in Figure 11.
Figure 11: Proposed schema of naive CD4 T cell differentiation into distinct functional subsets under neuropathic conditions following prenatal alcohol exposure.

In response to peripheral nerve injury, naïve T cells undergo activation and differentiation that can occur at peripheral sites (secondary lymphoid organs, as well as at the injury site). Following antigen presentation by antigen presenting cells (APC), naïve T cells undergo activation (T cell receptor [TCR] engagement and co-stimulation) and rounds of proliferation. Different cytokine cocktails (produced from innate immune cells due to Toll Like Receptor, TLR activation) determine the lineage of the activated T (Th0) cells. For example, the cytokines TGF-β and IL-2 support FOXP3 transcription generating a Treg, which in turn produces TGF-β and IL-10. Transcription factors critical for driving different subtypes of T cell differentiation are indicated in the parenthesis. Notably, some T cell lineages are reciprocally related, with overlapping cytokine profiles. For example, TGF-β drives anti-inflammatory Treg differentiation but TGF-β in combination with proinflammatory IL-6, IL-21 or IL-1 promotes proinflammatory Th17 differentiation. Following differentiation, T cells acquire various homing (chemokine and adhesion molecules) receptors, with cells that then recirculate through inflamed tissue regions and migrate to the spinal cord (responding to the chemokines). In the spinal cord, differentiated Th1 and Th17 cells can further interact with activated astrocytes and pain projection neurons propagating neuroinflammation. Solid arrows indicate cytokines that drive activated T cells into distinct differentiation pathways; broken arrows indicate the signature cytokines produced from various differentiated T cell subsets.
Interestingly, Th1 cells which are identified by T-bet expression, were also observed in Sac rats with minor CCI along with a strong trend toward increased IFN-γ. Elevated IFN-γ may have triggered a counter-adaptive response by the protective Treg generation and recruitment, which was observed in Sac rats following CCI. Importantly, despite greater levels of Th1 and Th17 responses, FOXP3 induction was blunted in PAE rats, indicating dysregulated Treg induction. Therefore, T cell-mediated pathology may contribute to the increased susceptibility for allodynia in PAE females following minor challenges. In fact, myeloid cell-derived cytokines are known to shape adaptive immune responses, with IL-1β and TNF often driving T cell differentiation toward a proinflammatory phenotype [67, 100], which is consistent with our observations in PAE females.
In the DRGs, T cell-related factors (FOXP3, IL-17A, IL-21 and T-bet) were not detected above basal levels in the Sac rats following minor nerve injury. These results, along with a lack of CD11b or IL-1β induction in the DRGs suggest proinflammatory relays from the injured nerve axons to the DRG may not be sufficiently strong to drive myeloid or T cell DRG recruitment at Day 28 post-injury in Sac rats. In contrast, minor injury in PAE rats induced Th1- and Th17-specific cytokines in the DRG at levels comparable to those observed in neuropathic Sac rats with standard CCI. A modest increase in FOXP3 was also detected in neuropathic PAE rats, which may reflect the presence of inflammatory cues facilitating ongoing Treg recruitment to the DRG under neuropathic conditions. However, despite ongoing proinflammatory glial-immune actions, FOXP3 levels in PAE rats were not significantly greater than non-neuropathic Sac rats with minor CCI. Therefore, proinflammatory immune actions with dysregulated anti-inflammatory responses (Treg, IL-10) at the injured axons and DRGs in PAE females are contributing to neuropathic pain susceptibility with a minor nerve injury.
4.3. PAE induces spinal glial proinflammatory actions with peripheral T cell involvement in neuropathic females
Pain studies provide compelling evidence of robust astrocytic activation and T cell involvement with a minimal role for microglial activation during chronic neuropathy in females [19, 71, 101]. In support, following CCI, greater spinal astrocytic activation was observed in PAE compared to Sac rats, while comparable levels of microglial activation with or without sciatic nerve manipulation were observed. Induction of TLR4 and Iba-1 mRNA levels were similar in Sac rats with both minor and standard nerve injury, which suggests that changes in microglial-specific TLR4 mRNA levels in females are minimal and are not a reliable indicator of downstream TLR4-dependent mechanisms underlying neuropathy in females, as reported before [72, 101]. Microglial activation was elevated in PAE rats under non-neuropathic and neuropathic conditions. Thus, microglial activation observed in PAE rats with minor injury does not appear to indicate a compelling microglial role underlying chronic PAE neuropathy. Interestingly, TLR4 upregulation was observed in PAE rat spinal cords concurrent with increased astrocyte activation. Therefore, it is possible that astrocytic TLR4 signaling leads to the induction of proinflammatory cytokines such as IL-1β, IL-6 observed in female PAE rats with minor injury. However, this hypothesis requires further confirmation.
Our data indicate that despite evidence of astrocyte activation in Sac rats, spinal IL-10 production was maintained following minor nerve injury. Sac treated females display similar levels of IL-1β and TNF, as observed in PAE females following minor CCI. However, the presence of these critical proinflammatory cytokines along with augmented production of other proinflammatory cytokines such as IL-6, CXCL1 and IL-17A and importantly, reduced IL-4 (an anti-inflammatory cytokines) and IL-10 levels may create a proinflammatory bias in PAE females following minor nerve injury. Of note, in females whose pain was reversed by BIRT377, alterations in IL-1β levels did not occur in all cases. Similarly, despite the fact that contralateral allodynia is observed in Sac rats with standard CCI, no significant changes in IL-1β and TNF mRNA levels were observed in the contralateral dorsal horn of the spinal cord. These data indicate that diminished IL-10 levels, rather than solely considering increases in IL-1β and TNF, may be critical for eliciting chronic neuropathy in females as well as heightened susceptibility to pain in PAE females.
Results showing increased levels of T-bet, ROR-γt and IFN-γ in the spinal cord supports the involvement of Th1 and Th17 differentiated proinflammatory T cell subpopulations during neuropathy, which were absent in non-neuropathic Sac rats. These data provide evidence that proinflammatory Th17 (IL-17A producing) cells infiltrate the spinal cord and may interact with astrocytes. T cell interaction with astrocytes may induce the production of the feedforward proinflammatory chemokine CCL2 and cytokine such as IL-23 from astrocytes, further propagating the spinal proinflammatory milieu [67, 82] in females. T cells can also directly interact with neurons leading to aberrant neuronal activation [102, 103]. In contrast to our findings in females, peripheral and spinal IL-17A induction was minimal in male mice compared to female mice during neuropathy in the absence of PAE [33]. Interestingly, estrogen has been shown to promote Th17 differentiation [104]. Females are more efficient at initiating adaptive immune responses [105]. Thus, despite astrocytic activation during neuropathy that is common in both sexes, it is possible that microglia in males and infiltrating Th17 cells in females are the predominant cell types responsible for driving chronic excitation of astrocytic-neuronal interactions.
In the contralateral dorsal horn of the spinal cord, higher levels of RORγt (along with a trend towards increased T-bet) were observed only in Sac rats with standard CCI. Spinal cords of BIRT377-treated Sac rats whose allodynia was reversed displayed a trend toward decreasing RORγt levels, while FOXP3 and T-bet mRNA levels remained similar. These data further suggest that infiltrating and activated T cells are not randomly localizing in the spinal cord, but rather, they are responding to inflammatory cues present in the spinal cord, possibly interacting with astrocytes and other immune cells to exert specific cytokine responses, as supported by the data in Figures 7–9. Notably, some of the spinal immune factors presented in Figure 6–9 include small groups of n=3 (indicated in the Figure legends) in Sac or PAE with minor CCI groups due to the presence of outliers, thus data should be interpreted with caution.
4.4. I.v. BIRT377-mediated reversal in neuropathic pain susceptibility in PAE females
LFA-1 is a key integrin molecule involved in leukocyte migration and can control their proinflammatory function during inflammation [26, 27, 31, 106]. LFA-1 interacts with cellular adhesion molecules (e.g. ICAM-1) that regulate T cell extravasation into regions where chemotactic signaling arises, which includes the spinal cord [107]. LFA-1-ICAM1 signaling interacts with TCR (T cell receptor) activation for Th1 cytokine production [45, 46]. Previous studies suggest that blocking LFA-1 action decreases Th17 differentiation and increases FOXP3+ Tregs [28, 32]. LFA-1 signaling on T cells induces TNF and IFN-γ mRNA stabilization [29]. In the current report, we demonstrate that blocking the actions of activated LFA-1 are beneficial for pain reversal in PAE females. Consistent with our prior observations that BIRT377 modulates macrophage activation toward an anti-inflammatory phenotype in vitro [26], reductions in myeloid cell-derived proinflammatory cytokines such as TNF and IL-1β were detected in the sciatic nerves and in the DRGs. BIRT377 reduced the T cell cytokine IL-17A, suggesting that blocking LFA-1 actions reduces Th17 generation [28, 33]. We have observed reduced ROR-γt and IL-17 in the DRGs and spinal cord via peripheral BIRT377 administration. BIRT377 may dampen the ability of Th17 cells to migrate to the spinal cord [26] and also reduce peripheral Th17 generation [33]. It is also possible that BIRT377-mediated reduction of proinflammatory factors including Th17 cells in DRGs and spinal cord is due to the decreased proinflammatory signals relayed from the sciatic nerve that drive excitatory synaptic communication and nearby spinal peri-synaptic glial [108] responses in the dorsal horn of the spinal cord. These data suggests peripheral (i.v.) BIRT377 may modulate: 1) leukocyte extravasation to peripheral sites and may reduce leukocyte transendothelial migration into key spinal regions, 2) macrophage proinflammatory cytokine production and proinflammatory T cell differentiation in the periphery, and consequently 3) neuron-glial and immune-glial communication in the lumbar spinal cord.
The current understanding is that i.t. BIRT377 treatment in males dampens microglial and astrocytic activation via acting on glial LFA-1 without altering immune actions in the periphery [50]. These glial cells are well-characterized contributors of neuropathy in male rodents [71]. However, T cell mediated responses under PAE conditions have not been explored in prior work. The current study suggests that only astrocytic activation, and not microglial activation, is may play a role in the risk for neuropathy following minor injury in PAE rats. Moreover, the presence of proinflammatory Th17 cells is primarily modulated via i.v. BIRT377 treatment in neuropathic females, regardless of prenatal exposure manipulations.
To our surprise, BIRT377 administered intrathecally is not beneficial in reversing neuropathic pain in females. These data further confirm that the spinal cord milieu in females with chronic neuropathy are distinctly different in terms of critical neuroimmune factors mediating neuropathic pain, and a greater reliance on peripheral immune cells may be a distinct process in females than in males [19].
However, the lack of the effects of i.t. BIRT377 to reverse allodynia does not exclude the possibility that fully differentiated spinal T cell subpopulations observed in the current work are critical players underlying neuropathy in females. Fully differentiated spinal T cell subpopulations may reinforce the importance of T cell activation, and their differentiation and migration that occurs at peripheral sites before these cells reach the localized spinal region [67, 109–111]. Thus, while speculative, inhibiting LFA-1 conformational changes on T cells may additionally act to influence ongoing T cell differentiation bias toward anti-inflammatory phenotypes (e.g. less proinflammatory factors and greater anti-inflammatory factors such as IL-10) [32, 33]. Ultimately, these differentiated T cells interacting with spinal astrocytes may influence the spinal cytokine milieu [82]. Blocking LFA-1 actions on fully differentiated T cells (Th1 and Th17) may not allow for subsequent phenotypic changes to occur. That is, it is possible that BIRT377 is unable to further modulate T cell phenotypes that have already fully differentiated and localized to the spinal cord (as discussed above) and to DRGs. In contrast, i.v. BIRT377 exerts its beneficial effects by directly dampening peripheral input through reducing the myeloid and T cell proinflammatory phenotype in the periphery [28, 46], and by indirectly reducing inflammatory cues present in the DRGs that communicate to the spinal cord [67, 100]. In addition, i.v. BIRT377 treatment may reduce the proinflammatory actions of the “activated” trafficking leukocyte pool of macrophages and T cells that respond to the inflammatory cues present in the DRGs and spinal regions following nerve injury.
5. Conclusions
PAE-related priming of immune cells both in the periphery and in the central nervous system have long-term adverse effects involving aberrant innate and adaptive immune components, which may extend beyond chronic pain and involve other neurological disorders [2]. Consistent with prior findings, in utero alcohol exposure, even at moderate levels, is detrimental regardless of sex [50]. We provide compelling evidence that systemic blockade of LFA-1-mediated immune actions has far-reaching therapeutic implications for ameliorating aberrant neuroimmune responses in PAE offspring. However, because of evidence of sex-specific divergent neuroimmune mechanisms in various neurological disorders including PAE, more attention is needed to confirm the beneficial effects and efficient route of administration of these immune modulatory molecules in both sexes.
Highlights:
Prenatal alcohol exposure (PAE) is a risk factor for developing neuropathic pain in female rats.
Augmented T cell proinflammatory actions (IL-17A) along with dysregulated IL-10 responses were evident in female PAE rats following nerve injury.
Augmented peripheral and glial pro-inflammatory responses underlie the susceptibility to develop neuropathic pain following minor nerve injury in PAE rats.
Systemic administration of BIRT377 (LFA-1 antagonist) modulates peripheral immune actions and reverses neuropathic pain susceptibility in female PAE rats.
Spinal blocking of LFA-1 actions is not sufficient to reverse pain in neuropathic female rats; indicative of crucial contribution of peripheral immune interactions underlying neuropathic pain in females.
Acknowledgements:
This study was funded by the National Institutes of Health (R01 AA025967, R21 AA023051, T32-AA014127, P50 AA022534 and R01 AA 019884). The authors would like to sincerely thank Kyle Christensen BS, Morgan Pruitt BS, Andre Moezzi BS, Nicole Graham BS and Kiana Lujan for their animal husbandry work related to the voluntary drinking paradigm. The authors would also like to thank Dr. Karin High, PhD, Department of Anesthesiology and Critical Care, UNM, for providing the laboratory facilities for RNA extraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Competing Interests: The authors declare that they have no competing interest for this study
Availability of data: All data generated and analyzed for the current report is included within the article.
References
- 1.Drew PD and Kane CJ, Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol, 2014. 118: p. 41–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terasaki LS and Schwarz JM, Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J Neuroimmune Pharmacol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terasaki LS and Schwarz JM, Impact of Prenatal and Subsequent Adult Alcohol Exposure on Pro-Inflammatory Cytokine Expression in Brain Regions Necessary for Simple Recognition Memory. Brain Sci, 2017. 7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar TS, Hill LA, and Weinberg J, Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain Behav Immun, 2016. 58: p. 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenzuela CF, et al. , Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci, 2012. 35(5): p. 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez JJ, et al. , Prenatal alcohol exposure is a risk factor for adult neuropathic pain via aberrant neuroimmune function. J Neuroinflammation, 2017. 14(1): p. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noor S, et al. , Prenatal alcohol exposure potentiates chronic neuropathic pain, spinal glial and immune cell activation and alters sciatic nerve and DRG cytokine levels. Brain Behav Immun, 2017. 61: p. 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan ED and Watkins LR, Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci, 2009. 10(1): p. 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grace PM, Rolan PE, and Hutchinson MR, Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun, 2011. 25(7): p. 1322–32. [DOI] [PubMed] [Google Scholar]
- 10.Khan J, et al. , Interleukin-10 levels in rat models of nerve damage and neuropathic pain. Neurosci Lett, 2015. 592: p. 99–106. [DOI] [PubMed] [Google Scholar]
- 11.DeLeo JA, Colburn RW, and Rickman AJ, Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res, 1997. 759(1): p. 50–7. [DOI] [PubMed] [Google Scholar]
- 12.Lee HL, et al. , Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport, 2004. 15(18): p. 2807–11. [PubMed] [Google Scholar]
- 13.Zhang J, et al. , Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci, 2007. 27(45): p. 12396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki Y, et al. , Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci, 2008. 28(20): p. 5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biber K and Boddeke E, Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front Cell Neurosci, 2014. 8: p. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J and De Koninck Y, Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem, 2006. 97(3): p. 772–83. [DOI] [PubMed] [Google Scholar]
- 17.White FA, Bhangoo SK, and Miller RJ, Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov, 2005. 4(10): p. 834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbadie C, et al. , Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A, 2003. 100(13): p. 7947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorge RE, et al. , Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci, 2015. 18(8): p. 1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen S, Ham B, and Mogil JS, Sex differences in neuroimmunity and pain. J Neurosci Res, 2017. 95(1-2): p. 500–508. [DOI] [PubMed] [Google Scholar]
- 21.Echeverry S, et al. , Peripheral nerve injury alters blood-spinal cord barrier Junctional and molecular integrity through a selective inflammatory pathway. J Neurosci, 2011. 31(30): p. 10819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draleau K, et al. , Phenotypic Identification of Spinal Cord-Infiltrating CD4(+) T Lymphocytes in a Murine Model of Neuropathic Pain. J Pain Relief, 2014. Suppl 3: p. 003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu P and McLachlan EM, Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience, 2002. 112(1): p. 23–38. [DOI] [PubMed] [Google Scholar]
- 24.Lopes DM, et al. , Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep, 2017. 7(1): p. 16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly TA, et al. , Cutting edge: a small molecule antagonist of LFA-1-mediated cell adhesion. J Immunol, 1999. 163(10): p. 5173–7. [PubMed] [Google Scholar]
- 26.Lam NCK, et al. , The role of leukocyte accumulation and diminshed spinal IL-10 expression in chronic neuropathy. Poster session presented at : Society for Neuroscience meeting; Washington DC, 2014(November 15-19). [Google Scholar]
- 27.Emoto M, et al. , Increased resistance of LFA-1-deficient mice to lipopolysaccharide-induced shock/liver injury in the presence of TNF-alpha and IL-12 is mediated by IL-10: a novel role for LFA-1 in the regulation of the proinflammatory and anti-inflammatory cytokine balance. J Immunol, 2003. 171(2): p. 584–93. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, et al. , A critical role of LFA-1 in the development of Th17 cells and induction of experimental autoimmune encephalomyelytis. Biochem Biophys Res Commun, 2007. 353(4): p. 857–62. [DOI] [PubMed] [Google Scholar]
- 29.Wang JG, et al. , LFA-1-dependent HuR nuclear export and cytokine mRNA stabilization in T cell activation. J Immunol, 2006. 176(4): p. 2105–13. [DOI] [PubMed] [Google Scholar]
- 30.Fantini MC, et al. , In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc, 2007. 2(7): p. 1789–94. [DOI] [PubMed] [Google Scholar]
- 31.Evans R, et al. , Integrins in immunity. J Cell Sci, 2009. 122(Pt 2): p. 215–25. [DOI] [PubMed] [Google Scholar]
- 32.Verhagen J and Wraith DC, Blockade of LFA-1 augments in vitro differentiation of antigen-induced Foxp3(+) Treg cells. J Immunol Methods, 2014. 414: p. 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noor S, S. M, Vanderwall AG, Havard MA, Sanchez JE, Harris NW, Nysus MV, Norenberg JP, West HT, Wagner CR, Jantzie LL, Mellios N, Milligan ED, LFA-1 antagonist (BIRT377) similarly reverses peripheral neuropathic pain in male and female mice with underlying sex divergent peripheral immune proinflammatory phenotypes. Neuroimmunol Neuroinflammation, 2019. 6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton DA, et al. , Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res, 2010. 207(2): p. 290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies S, et al. , Impact of moderate prenatal alcohol exposure on histaminergic neurons, histidine decarboxylase levels and histamine H2 receptors in adult rat offspring. Alcohol, 2019. 76: p. 47–57. [DOI] [PubMed] [Google Scholar]
- 36.Savage DD, et al. , Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res, 2010. 34(10): p. 1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milligan ED, et al. , Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res, 2000. 861(1): p. 105–16. [DOI] [PubMed] [Google Scholar]
- 38.Bennett GJ and Xie YK, A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 1988. 33(1): p. 87–107. [DOI] [PubMed] [Google Scholar]
- 39.Wilkerson JL, et al. , Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia. Brain Behav, 2012. 2(2): p. 155–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giblin PA and Lemieux RM, LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des, 2006. 12(22): p. 2771–95. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y and Wang H, Integrin signalling and function in immune cells. Immunology, 2012. 135(4): p. 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki J, et al. , The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood, 2007. 109(1): p. 168–75. [DOI] [PubMed] [Google Scholar]
- 43.Crucian B, Nelman-Gonzalez M, and Sams C, Rapid flow cytometry method for quantitation of LFA-1-adhesive T cells. Clin Vaccine Immunol, 2006. 13(3): p. 403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen JE, et al. , CD11b expression as a marker to distinguish between recently activated effector CD8(+) T cells and memory cells. Int Immunol, 2001. 13(4): p. 593–600. [DOI] [PubMed] [Google Scholar]
- 45.Woska JR Jr., et al. , A small-molecule antagonist of LFA-1 blocks a conformational change important for LFA-1 function. J Leukoc Biol, 2001. 70(2): p. 329–34. [PubMed] [Google Scholar]
- 46.Varga G, et al. , LFA-1 contributes to signal I of T-cell activation and to the production of T(h)1 cytokines. J Invest Dermatol, 2010. 130(4): p. 1005–12. [DOI] [PubMed] [Google Scholar]
- 47.Woska JR Jr., et al. , Small molecule LFA-1 antagonists compete with an anti-LFA-1 monoclonal antibody for binding to the CD11a I domain: development of a flow-cytometry-based receptor occupancy assay. J Immunol Methods, 2003. 277(1-2): p. 101–15. [DOI] [PubMed] [Google Scholar]
- 48.Hosseini BH, et al. , Immune synapse formation determines interaction forces between T cells and antigen-presenting cells measured by atomic force microscopy. Proc Natl Acad Sci U S A, 2009. 106(42): p. 17852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wasan KM, et al. , Rat and rabbit plasma distribution of free and chylomicron-associated BIRT 377, a novel small molecule antagonist of LFA-1-mediated ceii adhesion. Pharm Res, 2001. 18(4): p. 510–9. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez JJ, et al. , Targeting the beta2-integrin LFA-1, reduces adverse neuroimmune actions in neuropathic susceptibility caused by prenatal alcohol exposure. Acta Neuropathol Commun, 2019. 7(1): p. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milligan ED, et al. , Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci, 2005. 21(8): p. 2136–48. [DOI] [PubMed] [Google Scholar]
- 52.Vanderwall AG, et al. , Effects of spinal non-viral interleukin-10 gene therapy formulated with d-mannose in neuropathic interleukin-lO deficient mice: Behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain Behav Immun, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellios N, et al. , beta2-Adrenergic receptor agonist ameliorates phenotypes and corrects microRNA-mediated IGF1 deficits in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A, 2014. 111(27): p. 9947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
- 55.Taves S, et al. , Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun, 2016. 55: p. 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siqueira Mietto B, et al. , Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J Neurosci, 2015. 35(50): p. 16431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleinschnitz C, et al. , T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol, 2006. 200(2): p. 480–5. [DOI] [PubMed] [Google Scholar]
- 58.Kim CF and Moalem-Taylor G, Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain, 2011. 12(3): p. 370–83. [DOI] [PubMed] [Google Scholar]
- 59.Milligan ED, et al. , Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain, 2005. 1: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva RL, et al. , CXCL1/CXCR2 signaling in pathological pain: Role in peripheral and central sensitization. Neurobiol Dis, 2017. 105: p. 109–116. [DOI] [PubMed] [Google Scholar]
- 61.Hunter CA and Jones SA, IL-6 as a keystone cytokine in health and disease. Nat Immunol, 2015. 16(5): p. 448–57. [DOI] [PubMed] [Google Scholar]
- 62.Hart PH, et al. , Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A, 1989. 86(10): p. 3803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eijkelkamp N, et al. , IL4-10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J Neurosci, 2016. 36(28): p. 7353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arango Duque G and Descoteaux A, Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol, 2014. 5: p. 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nalbant A and Saygili T, IL12, IL10, IFNgamma and TNFalpha Expression in Human Primary Monocytes Stimulated with Bacterial Heat Shock GroEL (Hsp64) Protein. PLoS One, 2016. 11(4): p. e0154085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teh PP, Vasanthakumar A, and Kallies A, Development and Function of Effector Regulatory T Cells. Prog Mol Biol Transl Sci, 2015. 136: p. 155–74. [DOI] [PubMed] [Google Scholar]
- 67.Sonar SA and Lal G, Differentiation and Transmigration of CD4 T Cells in Neuroinflammation and Autoimmunity. Front Immunol, 2017. 8: p. 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez GJ, et al. , Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci, 2008. 1143: p. 188–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu FY, et al. , Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res, 2012. 1427: p. 65–77. [DOI] [PubMed] [Google Scholar]
- 70.Kim D, et al. , Toll-like receptor 2 contributes to chemokinegene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol Pain, 2011. 7: p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen G, et al. , Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci Bull, 2018. 34(1): p. 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorge RE, et al. , Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci, 2011. 31(43): p. 15450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, et al. , Lentiviralmediated inducible silencing of TLR4 attenuates neuropathic pain in a rat model of chronic constriction injury. Mol Med Rep, 2018. 18(6): p. 5545–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pascual M, et al. , Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun, 2011. 25 Suppl 1: p. S80–91. [DOI] [PubMed] [Google Scholar]
- 75.Crews FT, et al. , Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res, 2015. 37(2): p. 331–41, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alfonso-Loeches S, et al. , Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci, 2010. 30(24): p. 8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scholz J and Woolf CJ, The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci, 2007. 10(11): p. 1361–8. [DOI] [PubMed] [Google Scholar]
- 78.Kim CF and Moalem-Taylor G, Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res, 2011. 1405: p. 95–108. [DOI] [PubMed] [Google Scholar]
- 79.Zhang ZJ, et al. , Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain, 2013. 154(10): p. 2185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao DL, et al. , Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol, 2014. 261: p. 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilkerson JL and Milligan ED, The Central Role of Glia in Pathological Pain and the Potential of Targeting the Cannabinoid 2 Receptor for Pain Relief. ISRN Anesthesiol, 2011. 2011(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prajeeth CK, et al. , Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J Neuroinflammation, 2017. 14(1): p. 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tzartos JS, et al. , Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol, 2008. 172(1): p. 146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li GZ, et al. , Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol, 2005. 62(5): p. 481–6. [DOI] [PubMed] [Google Scholar]
- 85.Kane CJ, et al. , Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res, 2014. 38(2): p. 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kane CJ, Phelan KD, and Drew PD, Neuroimmune mechanisms in fetal alcohol spectrum disorder. Dev Neurobiol, 2012. 72(10): p. 1302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drew PD, et al. , Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res, 2015. 39(3): p. 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noor S and Milligan ED, Lifelong Impacts of Moderate Prenatal Alcohol Exposure on Neuroimmune Function. Front Immunol, 2018. 9: p. 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lussier AA, Weinberg J, and Kobor MS, Epigenetics studies of fetal alcohol spectrum disorder: where are we now? Epigenomics, 2017. 9(3): p. 291–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balaraman S, et al. , Maternal and neonatal plasma microRNA biomarkers for fetal alcohol exposure in an ovine model. Alcohol Clin Exp Res, 2014. 38(5): p. 1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laufer BI, et al. , Long-lasting alterations to DNA methyiation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Dis Model Mech, 2013. 6(4): p. 977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang CR, et al. , Prenatal ethanol exposure alters adult hippocampal VGLUT2 expression with concomitant changes in promoter DNA methylation, H3K4 trimethylation and miR-467b-5p levels. Epigenetics Chromatin, 2015. 8: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valles S, et al. , Ethanol exposure affects glial fibrillary acidic protein gene expression and transcription during rat brain development. J Neurochem, 1997. 69(6): p. 2484–93. [DOI] [PubMed] [Google Scholar]
- 94.Portales-Casamar E, et al. , DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin, 2016. 9: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laufer BI, et al. , Associative DNA methylation changes in children with prenatal alcohol exposure. Epigenomics, 2015. 7(8): p. 1259–74. [DOI] [PubMed] [Google Scholar]
- 96.Pascual M, et al. , TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J Neuroinflammation, 2017. 14(1): p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koltzenburg M, Wall PD, and McMahon SB, Does the right side know what the left is doing? Trends Neurosci, 1999. 22(3): p. 122–7. [DOI] [PubMed] [Google Scholar]
- 98.Spataro LE, et al. , Spinal gap junctions: potential involvement in pain facilitation. J Pain, 2004. 5(7): p. 392–405. [DOI] [PubMed] [Google Scholar]
- 99.Mosser DM and Zhang X, Activation of murine macrophages. Curr Protoc Immunol, 2008. Chapter 14: p. Unit 14 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng Y, et al. , TNFalpha promotes Th17 cell differentiation through IL-6 and IL-1beta produced by monocytes in rheumatoid arthritis. J Immunol Res, 2014. 2014: p. 385352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mapplebeck JCS, et al. , Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain, 2018. 159(9): p. 1752–1763. [DOI] [PubMed] [Google Scholar]
- 102.Nitsch R, et al. , Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J Neurosci, 2004. 24(10): p. 2458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yshii L, et al. , Neurons and T cells: Understanding this interaction for inflammatory neurological diseases. Eur J Immunol, 2015. 45(10): p. 2712–20. [DOI] [PubMed] [Google Scholar]
- 104.Khan D, et al. , Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur J Immunol, 2010. 40(9): p. 2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weinstein Y, Ran S, and Segal S, Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol, 1984. 132(2): p. 656–61. [PubMed] [Google Scholar]
- 106.Smith A, et al. , The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev, 2007. 218: p. 135–46. [DOI] [PubMed] [Google Scholar]
- 107.Engelhardt B, Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm (Vienna), 2006. 113(4): p. 477–85. [DOI] [PubMed] [Google Scholar]
- 108.Glatigny S, et al. , Integrin alpha L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin alpha 4. Sci Rep, 2015. 5: p. 7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou L, Chong MM, and Littman DR, Plasticity of CD4+ T cell lineage differentiation. Immunity, 2009. 30(5): p. 646–55. [DOI] [PubMed] [Google Scholar]
- 110.Zhu J, Yamane H, and Paul WE, Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol, 2010. 28: p. 445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reiner SL, Decision making during the conception and career of CD4+ T cells. Nat Rev Immunol, 2009. 9(2): p. 81–2. [DOI] [PubMed] [Google Scholar]


