Abstract
Reports of neurodegenerative and psychiatric disease in former athletes have increased public concern about the acute and chronic effects of sport-related concussions (SRC). The biological factors underlying individual differences in the psychiatric sequalae of SRC and their role in potential long-term negative outcomes have not been determined. One understudied biological consequence of the known inflammatory response to concussion is the activation of a key immunoregulatory pathway, the kynurenine pathway (KP). Activation of the KP produces several neuroactive metabolites that have been associated with psychiatric and neurodegenerative diseases. We tested the hypothesis that SRC results in an elevation of serum KP metabolites with neurotoxic properties (quinolinic acid [QuinA], 3-hydroxykynurenine [3HK]) together with a reduction in the neuroprotective metabolite kynurenic acid (KynA), and that these metabolites would predict post-concussion psychological symptoms. Additionally, because brain injury is thought to prime the immune system, a secondary goal was to test the hypothesis that athletes with acute SRC and a history of prior SRC would have elevated neurotoxic relative to neuroprotective KP metabolites compared to athletes that were concussed for the first time. High school and collegiate football players (N=1,136) were enrolled at a preseason baseline visit that included clinical testing and blood specimen collection. Athletes that suffered a SRC (N=59) completed follow-up visits within 6-hours (early-acute), at 24–48 hours (late-acute) and at 8, 15, and 45 days post-injury. Uninjured contact sport (CC; N=54) and non-contact sport athletes completed similar visits and served as controls (NCC; N=30). SRC athletes had significantly elevated psychological symptoms, assessed using the Brief Symptom Inventory-18 (BSI), acutely following injury relative to both control groups. There was a group-by-visit interaction on the ratio of KynA to 3HK in serum, a neuroprotective index, with elevated KynA/3HK in athletes with SRC at the early-acute visit relative to later visits. Importantly, athletes with greater elevation in this neuroprotective index at the early-acute visit reported fewer depressive symptoms at the late-acute visit. Finally, SRC athletes with prior concussion had significantly lower serum KynA/QuinA at all visits compared to SRC athletes with no prior concussion, an effect driven by elevated QuinA in SRC athletes with prior concussion. These results suggest that early-acute activation of the KynA branch of the KP may protect against the development of depressive symptoms following concussion. Furthermore, they highlight the potential of serum QuinA as a biomarker for repetitive head injury and provide insight into possible mechanisms linking prior concussion with subsequent injury.
Keywords: concussion, mild traumatic brain injury, kynurenine, mood symptoms
1. Introduction
Millions of sport-related concussions (SRC) are estimated to occur annually in the U.S., with a large percentage of these injuries occurring in high school and collegiate athletes (Langlois et al., 2006). There is increasing public concern about the acute and chronic effects of SRC, largely driven by reports of neurodegenerative and psychiatric disease in former professional athletes (Guskiewicz et al., 2005, 2007; McKee et al., 2013; Montenigro et al., 2017). Despite this concern, the biological factors underlying inter-individual variation in the time course of recovery from SRC and their role in the long-term neurobehavioral and neuroanatomical abnormalities reported in subsets of athletes with multiple concussions has not been determined.
An acute elevation in proinflammatory cytokines and excess glutamate release are known aspects of the neurometabolic cascade of brain injury that has been established in preclinical injury models, patients with moderate to severe traumatic brain injury (TBI), and even recently in patients with more minor injuries including mild TBI (mTBI) and SRC (Faden et al., 1989; Katayama et al., 1990; Kalabalikis et al., 1999; Tasçı et al., 2003; Chiaretti et al., 2005; Giza and Hovda, 2014; Gill et al., 2016, 2018; Jassam et al., 2017; Ritzel et al., 2018; Morganti-Kossmann et al., 2019; Nitta et al., 2019). One under-appreciated potential consequence of the inflammatory response to brain injury is the immune-mediated activation of a key immuno-regulatory network known as the kynurenine (KYN) pathway (Savitz, 2019) (Supplementary Figure 1). Inflammatory cytokines, as well as cortisol, increase the conversion of tryptophan (TRP) to KYN via indoleamine 2,3-dioxygenase (IDO) and tryptophan dioxygenase (TDO), respectively. KYN is subsequently metabolized down two principal branches that yield metabolites with different neuroactive properties. The enzyme kynurenine-3-monooxygenase (KMO) is activated by inflammatory cytokines and increases the metabolism of KYN into 3-hydroxykynurenine (3HK), a potent free radical generator that is eventually converted to quinolinic acid (QuinA), a N-methyl-D-aspartate (NMDA) receptor agonist. QuinA is similarly potent to glutamate at the NMDA receptor, but it remains in the synaptic cleft for a longer period of time due to less-efficient reuptake, and thus its excitotoxic effects are stronger than glutamate once concentrations exceed a threshold (Foster, Miller, Oldendorf, & Schwarcz, 1984). QuinA also exerts neurotoxic effects through multiple additional mechanisms (Guillemin, 2012; Colín-González et al., 2013). Conversely, KYN can be metabolized into kynurenic acid (KynA) by the kynurenine aminotransferase (KAT) enzymes. KynA is often considered to be neuroprotective as it is a competitive antagonist of ionotropic excitatory amino acid receptors (including NMDA) although it has other functional effects being a negative allosteric modulator of the alpha-7-nicotinic receptor, an agonist of an orphan G-protein-coupled receptor, and an agonist of the aryl hydrocarbon receptor (Foster et al., 1984b; Kessler et al., 1989; Carpenedo et al., 2001; Hilmas et al., 2001; Opitz et al., 2011).
Because QuinA is eventually converted into NAD+ and activated immune cells require energy, under inflammatory conditions metabolism down the potentially neurotoxic QuinA pathway is favored (Connor et al., 2008; Zunszain et al., 2012). Importantly, because of their neuroactive properties, KYN pathway (KP) metabolites exert pathological and behavioral effects that extend beyond those of inflammatory cytokines (Savitz, 2019). Preclinical studies have shown that LPS-induced depression can be prevented by genetic deletion or pharmacological inhibition of IDO or KMO, which reduces the production of neurotoxic KP metabolites, but not inflammatory cytokines (O’Connor et al., 2009; Parrott et al., 2016). These data suggest that activation of the Kyn pathway is necessary for the manifestation of depressive-like behavior in rodents even in the presence of high concentrations of inflammatory cytokines.
In humans, an imbalance in KYN pathway (KP) metabolites has been associated with psychiatric disorders, principally mood disorders and schizophrenia (Dantzer et al., 2011; Schwarcz et al., 2012; Savitz, 2019). Specifically, we and others have previously demonstrated that there is a relative decrease in metabolism down the KynA pathway relative to the QuinA pathway in major depressive disorder (MDD) and bipolar disorder (BD), an imbalance associated with anhedonia, sleep disturbance, and brain structure and function (Myint et al., 2007, Savitz et al., 2015a, b, Meier et al., 2016a; Young et al., 2016; Cho et al., 2017; Doolin et al., 2018). This literature is relevant to SRC because multiple prior concussions have been linked with increased risk for MDD (Guskiewicz et al., 2007), and psychiatric symptoms, including depressive symptoms, commonly occur post-concussion (Kontos et al., 2012; Ellis et al., 2015). Similarly, suicide occurs more often in TBI patients relative to the general population (Madsen et al., 2018). Importantly, completed suicide in patients with MDD is associated with increased brain QuinA and decreased KynA postmortem, and QuinA in the cerebrospinal fluid (CSF) is increased up to 18 months following a suicide attempt (Steiner et al., 2011; Bay-Richter et al., 2015).
Notably, although not extensively studied, abnormalities of the KP have also been documented following brain injury. Compared to controls, severe TBI patients had elevated QuinA in the CSF (Sinz et al., 1998; Bell et al., 1999; Yan et al., 2015), and chronic TBI patients had higher blood levels of KYN relative to tryptophan (KYN/TRP; a marker of KP activity) (Mackay et al., 2006). In addition, we have previously reported elevated plasma concentrations of QuinA, and decreased KynA/QuinA (a neuroprotective index), at approximately one day, one week, and one month following SRC in football players relative to uninjured controls (Singh et al., 2016). Moreover, these alterations were associated with post-injury mood symptoms and outcome (i.e., return-to-play duration). Subsequently, we found elevated QuinA in football players with a history of concussion (on average 10 months post-injury) relative to football players without prior concussion, as well as associations between KP metabolites and hippocampal volume and functional connectivity (Meier et al., 2016b, 2017a).
The current work had two aims. The first aim was to prospectively determine the acute and sub-acute effects of SRC on serum levels of KP metabolites and their association with post-concussion psychological symptoms. Based on our prior work in an independent sample (Singh et al., 2016), we hypothesized that athletes with acute SRC would have elevated neurotoxic metabolites relative to neuroprotective KP metabolites (i.e., lower KynA/3hk and KynA/QuinA) post-injury compared to preinjury and compared to both contact and non-contact control athletes. We also hypothesized that acute alterations in serum neuroactive KP metabolites would predict psychological symptoms following SRC. Because repeated concussions have been associated with a negative clinical outcome (Zemek et al., 2016; Van Pelt et al., 2019) and prior brain injury may prime the immune system (Witcher et al., 2015), the second aim was to differentiate the effects of acute concussion from the cumulative effects of prior concussion on KP metabolites and psychological symptoms in football players. Based on the aforementioned associations between concussion history and KP metabolites in our prior work (Meier et al., 2016b, 2017a), we hypothesized that concussed participants with a history of prior concussion would show decreased KynA/QuinA and elevated QuinA relative to participants who were concussed for the first time.
2. Materials and methods
2.1. Participants
High school and collegiate athlete were enrolled as part of a prospective study of concussion in football players between August 2015 and June 2018. Adult participants and parents of minors provided written informed consent; minors completed written assent. All aspects of this study were approved by the institutional review board at the Medical College of Wisconsin.
A total of 1,136 football players were enrolled and completed a baseline preseason visit consisting of a detailed clinical battery and collection of blood specimens. Football players that sustained a concussion (SRC group) during the study period completed follow-up visits within 6 hours (early-acute) and at 24–48 hours (late-acute), 8 days (8d), 15 days (15d), and 45 days (45d) post-injury with the following exclusion criteria: injury that precluded participation in the study, current psychotic disorder or narcotic use, history or suspicion of conditions known to cause cognitive dysfunction (e.g., epilepsy, moderate-to-severe TBI), and any contraindication to study procedures. Additional exclusion criteria for the current study included a history of or current psychopathology (e.g., mood disorders), migraines or recurrent headaches, attention deficit/hyperactivity disorder or memory difficulties, structural findings on MRI that required clinical follow-up as part of the parent study (Klein et al., 2019), and a history of a potentially confounding illness/disease (e.g., meningitis). Concussions were identified and diagnosed by certified athletic trainers or team physicians at each institution and subsequently screened and triaged by study investigators to confirm they met the study definition and requirements. The study definition of concussion was based on the Centers for Disease Control and Prevention HEADS UP educational initiative, as previously described (Meier et al., 2017b). A total of 59 concussed football players (SRC) met the study criteria and are included in the current study.
Uninjured football players served as contact controls (CC group; n=54) and were selected from enrolled athletes to match injured athletes using the following matching criteria: level of competition (high school or Division III college), institution, team, age, estimated intellectual functioning (word reading performance at baseline), race, handedness, concussion history, and position. In addition, non-contact control athletes (NCC group, n=30) without current or high school football exposure or prior concussion served as additional controls matched on level of competition, age, estimated premorbid intelligence, and race. Exclusion criteria for CC and NCC athletes were identical to SRC athletes with additional criteria: prior concussion in the last 6 months (CC) and prior concussion or football experience in high school or college (NCC). CC and NCC athletes completed the same study protocol at similar follow-up intervals to injured athletes.
2.2. Clinical battery
Health history information, demographic information, and the Wechsler Test of Adult Reading (WTAR; (Wechsler, 2001) as an estimate of intellectual function were collected at baseline. Psychological symptoms were assessed at each visit, except for the early-acute visit, using the Brief Symptom Inventory–18 (BSI-18; (Derogatis, 2000). The BSI-18, a NINDS Common Data Element for SRC, is a validated self-report measure of psychological distress that includes depression, anxiety, and somatization subscales (Derogatis and Fitzpatrick, 2004; Meachen et al., 2008; Broglio et al., 2018). The BSI Global Severity Index (BSI-GSI) comprising all three subscales was used to assess overall psychological symptoms. The clinical battery also included measures of concussion symptom severity (The Sport Concussion Assessment Tool – 3rd Edition symptom; SCAT), balance deficits (Balance Error Scoring System), and neurocognitive performance (Standardized Assessment of Concussion). Detailed concussion and recovery information was collected at follow-up visits.
2.3. Blood biomarker data
Venous blood was collected at every visit using Red Top BD Vacutainer tubes, left to clot at room temperature for 30 minutes, centrifuged at 1,500 RCF for 15 minutes and stored at −80 degrees Celsius. Serum concentrations of tryptophan (TRP), kynurenine (KYN), kynurenic acid (KynA), 3-hydroxykynurenine (3HK), and quinolinic acid (QuinA) were determined blind to diagnosis using high-performance liquid chromatography with tandem mass spectrometry detection by Charles River Laboratories, Inc. according to their standard protocol. Serum concentrations of c-reactive protein (CRP) were determined blind to diagnosis using a Meso Scale Discovery (MSD) QuickPlex SQ 120 instrument and MSD V-PLEX assays following manufacturer’s instructions at the University of Oklahoma Integrative Immunology Center.
2.4. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 24 (Armonk, NY) and visualized using Prism 8.1 (La Jolla, CA). One-way analyses of variance (ANOVA), Kruskal-Wallis tests, chi-square tests, or Fisher’s exact tests were used to compare demographic variables between groups. ANOVA was also used to determine group differences in SCAT, BESS, and SAC scores at the late-acute visit for descriptive purposes. Individual KP metabolites, ratios of KP metabolites, and CRP were natural log-transformed to better approximate a normal distribution. Linear mixed-effects models were used to assess changes in BSI-GSI scores and biomarkers with the fixed factors of group (i.e., SRC, CC, NCC), visit modeled as a repeated factor across participants (i.e., baseline, early-acute, late-acute, 8 day, 15d, and 45d), and the group-by-visit interaction. To differentiate between the potential effects of acute concussion from prior concussion on KP metabolites and psychological symptoms in contact sport athletes, additional linear mixed-effects models were fitted with SRC and CC groups subdivided into groups of athletes with prior concussion (SRC+, CC+) and athletes without at least one prior concussion (SRC−, CC−). As above, models included the effects of visit (i.e., baseline, early-acute, late-acute, 8d, 15d, and 45d), group (i.e., SRC+, SRC−, CC+, CC−), and the visit-by-group interaction. Based on our prior work (see above), a priori group analyses focused on the neuroprotective ratios (i.e., KynA/QuinA and KynA/3HK) and QuinA. Secondary analyses were conducted for KYN, TRP, the KYN/TRP ratio, 3HK, KynA, and CRP with Bonferroni correction for multiple comparisons. Sensitivity analyses were conducted for blood markers with mixed models as above adding demographic variables that differed across groups (i.e., age, years of participation, and body mass index [BMI]; see results) as covariates. For all mixed models, post-hoc testing with sequential Sidak correction was conducted for significant group effects or group-by-visit interactions. Finally, general linear models were fitted to determine if acute concussion-related alterations in KP metabolites (see Results) predicted subsequent psychological symptoms in concussed athletes, covarying the respective baseline KP metabolite and baseline psychological symptoms. An alpha of 0.05 (two-tailed) was used to determine significant effects.
3. Results
3.1. Demographic comparisons and injury information
The median times for post-injury visits and blood collection were 3 hours [IQR: 2–4], 27.5 hours [20–44.5], 8 days [8–9], 15 days [15–16.5], and 44 days [42–47] post-injury. There were no group differences in race, ethnicity, years of education, premorbid intelligence (WTAR standard scores), or handedness (Table 1). However, NCC were significantly older (ps<0.05), had lower BMI (ps<0.001), had participated longer in their sport (ps<0.001), and by design had significantly fewer prior concussions than SRC or CC athletes (ps<0.005). SRC athletes had more concussion symptoms at the late-acute visit relative to both CC and NCC (ps<0.001) and more balance deficits than CC (p<0.001). NCC had more balance deficits than CC and better performance on the SAC than both SRC and CC (ps<0.05). The median number of days that athletes with concussion reported having symptoms was 7 [IQR: 5–11]. Recovery data for 6 concussed athletes were unavailable as they were lost to follow-up. No concussed athletes reported loss of consciousness, three reported post-traumatic amnesia, and three reported retrograde amnesia in the immediate post-injury period.
Table 1:
Sample Characteristics
| Variable | SRC (N=59) | CC (N=54) | NCC (N=30) | Statistics |
|---|---|---|---|---|
| No. by Visit (BL/EA/LA/8d/15d/45d) | 59/36/56/55/49/44 | 54/54/52/51/49/45 | 30/30/30/27/29/28 | |
| Age | 18.02 (1.58) | 18.30 (1.70) | 19.10(1.75) | F(2, 140)=4.26, p=0.02 |
| Race, No. White/NonWhite* | 42/16 | 41/13 | 25/5 | X2(2)=1.30, p=0.52 |
| Ethnicity, No. Not Hispanic/Hispanic/NR or Unknown | 49/5/5 | 47/3/4 | 26/3/1 | FET, p=0.86 |
| Years of education | 12.97(1.53) | 13.20(1.58) | 13.60(1.48) | F(2,140)=1.69, p=0.19 |
| No. Participants in College | 47 | 42 | 26 | X2(2)=1.00, p=0.60 |
| WTAR Standard Score | 98.52(14.93) | 99.85(12.95) | 104.17(8.74) | F(2,140)=1.88, p=0.16 |
| Household socioeconomic status | 46.03(10.34) | 45.58(11.37) | 50.23(7.48) | F(2,137)=2.23, p=0.11 |
| Body Mass Index | 29.09(5.51) | 28.87 (5.43) | 24.60(2.95) | F(2,140)=8.93, p<0.001 |
| Years of Participation in Sport | 7.66 (3.47) | 8.18 (2.63) | 12.17(4.27) | F(2,139)=19.18, p<0.001 |
| Median No. of Prior Concussions (range) | 1 (0–4) | 0 (0–4) | -- | H(2)= 21.83, p<0.001 |
| Prior Concussion, No. Yes/No | 30/29 | 21/33 | 0/30 | X2(2)=22.80, p<0.001 |
| Position, No. | FET, p=0.28 | |||
| Offensive/Defensive Line | 24 | 20 | ||
| Wide Receiver/Tight End/Quarterback/Running Back | 14 | 18 | ||
| Linebacker/Defensive Back | 21 | 14 | ||
| Kicker | 0 | 2 | ||
| Handedness, No. Right/Left/Ambidextrous | 52/4/3 | 47/5/2 | 23/6/1 | FET, p=0.42 |
| Late-Acute Clinical Measures | ||||
| SCAT Symptom Severity Score | 20.70 (18.01) | 1.50 (2.75) | 1.87(3.04) | F(2, 135)=44.14, p<0.001 |
| BESS Total Score | 12.76 (5.23) | 8.90 (4.06) | 12.17(5.26) | F(2, 132)=9.19, p<0.001 |
| SAC Total Score | 25.91 (2.50) | 26.06 (2.63) | 27.30(1.84) | F(2, 135)=3.52, p=0.03 |
Note: Mean and standard deviation are shown unless otherwise indicated. SRC = sport-related concussion, CC= contact control, NCC= noncontact Control, BL =baseline visit, EA= early-acute visit, LA = late-acute visit, 8d= 8-day visit, 15d= 15-day visit, 45d= 45-day visit, NR = not reported, No. = number, * race not reported for one concussed athlete, FET = Fisher’s Exact Test, SCAT = Sport Concussion Assessment Tool, BESS = Balance Error Scoring System, SAC = Standardized Assessment of Concussion.
3.2. Effects of acute concussion on psychological symptoms
Means, standard deviations, and inferential statistics for the main effects and interactions for BSI scores and biomarker levels are reported in Table 2. The group-by-visit interaction was significant for the overall BSI-GSI score of psychological symptoms (p<0.001; Figure 1). SRC athletes had higher scores at the late-acute visit (i.e., 24–48 hours) relative to all other visits (range of mean difference [MD] (standard error[SE]) =1.93(0.54) to 4.88(0.59); ps<0.005) and relative to both CC and NCC (MD(SE)=4.27(0.73) and 3.40(0.86), respectively; ps<0.001). SRC athletes also had elevated BSI-GSI scores at baseline relative to the 8d, 15d, and 45d visits (range of MD(SE)=1.31(0.54) to 2.96(0.58); ps<0.05) and at the 8d relative to 45d visits (MD(SE)=1.64(0.59); p=0.022). BSI-GSI scores in CC were elevated at baseline relative to the 8d, 15d, and 45d visits (range of MD(SE)=1.66(0.58) to 1.84(0.57); ps<0.05), and also elevated in NCC at baseline relative to 8d (MD(SE)=2.17(0.77); p=0.049).
Table 2.
Means, standard deviations, and associated statistics for the effects of acute concussion on psychological symptoms, kynurenine pathway metabolites, and c-reactive protein
| Measures | BLM (SD) | Early-Acute M (SD) | Late-Acute M (SD) | 8d M (SD) | 15d M (SD) | 45d M (SD) | Group (p-value) | Visit (p-value) | Group by Visit (p-value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| A Priori | ||||||||||
| BSI-GSI (raw score) | SRC | 3.54(6.07) | -- | 5.49(5.86) | 2.27(5.03) | 1.23(3.89) | 0.57(1.99) | 0.03 | <0.001 | <0.001 |
| CC | 2.42 (3.13) | -- | 1.23(2.02) | 0.49 (1.00) | 0.76(1.77) | 0.64(1.35) | ||||
| NCC | 3.60(5.45) | -- | 2.07(3.11) | 1.56(3.00) | 1.52(2.94) | 1.50(3.33) | ||||
| ln KynA/3HK | SRC | 0.50(0.33) | 0.65(0.34) | 0.58(0.33) | 0.50(0.34) | 0.51(0.34) | 0.41(0.26) | 0.95 | 0.003 | 0.01 |
| CC | 0.56(0.28) | 0.51 (0.30) | 0.57(0.35) | 0.54(0.34) | 0.53(0.33) | 0.47(0.25) | ||||
| NCC | 0.59(0.33) | 0.55(0.27) | 0.51(0.27) | 0.56(0.30) | 0.51(0.26) | 0.52(0.29) | ||||
| ln KynA/QuinA | SRC | −1.91(0.40) | −1.89(0.47) | −1.96(0.41) | −1.96(0.42) | −1.94(0.39) | −2.03(0.49) | 0.32 | 0.02 | 0.47 |
| CC | −1.81(0.43) | −1.86(0.37) | −1.91(0.38) | −1.84(0.36) | −1.91(0.37) | −1.85(0.36) | ||||
| NCC | −1.85(0.45) | −1.80(0.39) | −1.92(0.33) | −1.87(0.37) | −1.93(0.40) | −1.84(0.31) | ||||
| ln QuinA (nM) | SRC | 5.96(0.30) | 5.97(0.37) | 5.87(0.32) | 5.94(0.33) | 5.95(0.35) | 5.92(0.40) | 0.42 | 0.01 | 0.35 |
| CC | 5.93(0.31) | 5.86(0.29) | 5.86(0.29) | 5.86(0.27) | 5.89(0.29) | 5.85(0.32) | ||||
| NCC | 5.92(0.44) | 5.91(0.29) | 5.90(0.28) | 5.85(0.26) | 5.97(0.36) | 5.86(0.33) | ||||
| Secondary | ||||||||||
| ln KYN (uM) | SRC | 0.77(0.20) | 0.73(0.25) | 0.74(0.27) | 0.76(0.25) | 0.78(0.31) | 0.72(0.24) | 0.53 | 0.17 | 0.76 |
| CC | 0.81(0.22) | 0.77(0.22) | 0.73(0.24) | 0.78(0.19) | 0.78(0.20) | 0.75(0.21) | ||||
| NCC | 0.79(0.27) | 0.82(0.20) | 0.80(0.24) | 0.81(0.19) | 0.80(0.20) | 0.77(0.23) | ||||
| ln TRP (uM) | SRC | 4.21(0.25) | 4.18(0.23) | 4.17(0.22) | 4.22(0.24) | 4.21(0.24) | 4.20(0.23) | 0.65 | 0.04 | 0.86 |
| CC | 4.20(0.23) | 4.27(0.23) | 4.19(0.21) | 4.24(0.18) | 4.23(0.20) | 4.23(0.20) | ||||
| NCC | 4.22(0.20) | 4.28(0.26) | 4.17(0.24) | 4.22(0.26) | 4.23(0.31) | 4.20(0.30) | ||||
| ln KYN/ TRP | SRC | −3.43(0.23) | −3.44(0.32) | −3.43(0.30) | −3.47(0.32) | −3.43(0.30) | −3.48(0.33) | 0.86 | 0.02 | 0.72 |
| CC | −3.39(0.24) | −3.49(0.23) | −3.46(0.24) | −3.46(0.20) | −3.46(0.22) | −3.48(0.21) | ||||
| NCC | −3.42(0.30) | −3.46(0.28) | −3.37(0.28) | −3.46(0.24) | −3.43(0.32) | −3.44(0.24) | ||||
| ln KynA (nM) | SRC | 4.04(0.41) | 4.07(0.31) | 3.92(0.29) | 3.98(0.31) | 4.01(0.30) | 3.89(0.33) | 0.70 | 0.002* | 0.55 |
| CC | 4.00(0.37) | 4.00(0.38) | 3.94(0.37) | 4.03(0.37) | 3.98(0.37) | 4.00(0.33) | ||||
| NCC | 4.07(0.28) | 4.11(0.38) | |3.98(0.35) | 3.98(0.44) | 4.04(0.40) | 4.01(0.41) | ||||
| ln 3HK (nM) | SRC | 3.55(0.32) | 43.42(0.34) | 3.34(0.31) | 3.48(0.35) | 3.50(0.32) | 3.49 (0.29) | 0.85 | <0.001* | 0.13 |
| CC | 3.56(0.30) | 3.49(0.32) | 3.37(0.33) | 3.49(0.24) | 3.45(0.32) | 3.53(0.26) | ||||
| NCC | 3.48(0.33) | 3.56(0.37) | 3.47(0.35) | 3.42(0.37) | 3.53(0.42) | 3.49(0.37) | ||||
| ln CRP (mg/L) | SRC | −0.60(1.44) | −0.26(1.41) | −0.22(1.38) | 0.71(1.50) | −0.50(1.50) | −0.53(1.50) | 0.006* | 0.26 | 0.67 |
| CC | −0.51(1.20) | −0.37(1.54) | −0.48(1.63) | −0.53(1.42) | −0.41(1.29) | −0.75(1.45) | ||||
| NCC | −1.40(1.36) | −1.24(1.40) | −1.31(1.46) | −1.32(1.23) | −1.17(1.51) | −1.10(1.54) | ||||
Note. ln = natural log, SRC = sport-related concussion, CC= contact control, NCC= noncontact Control, BL= baseline visit, 8d= 8-day visit, 15d= 15-day visit, 45d= 45-day visit, BSI-GSI= Brief Symptom Inventory Global Severity Index, KynA= kynurenic acid, 3HK= 3-hydroxykynurenine, QuinA= quinolinic acid, KYN= Kynurenine, TRP= tryptophan, nM = nanomolar, uM = micromolar, mg/L = milligram per liter.
Indicates significant effects for secondary analyses with Bonferroni correction.
Figure 1: Psychological symptoms and blood biomarkers.
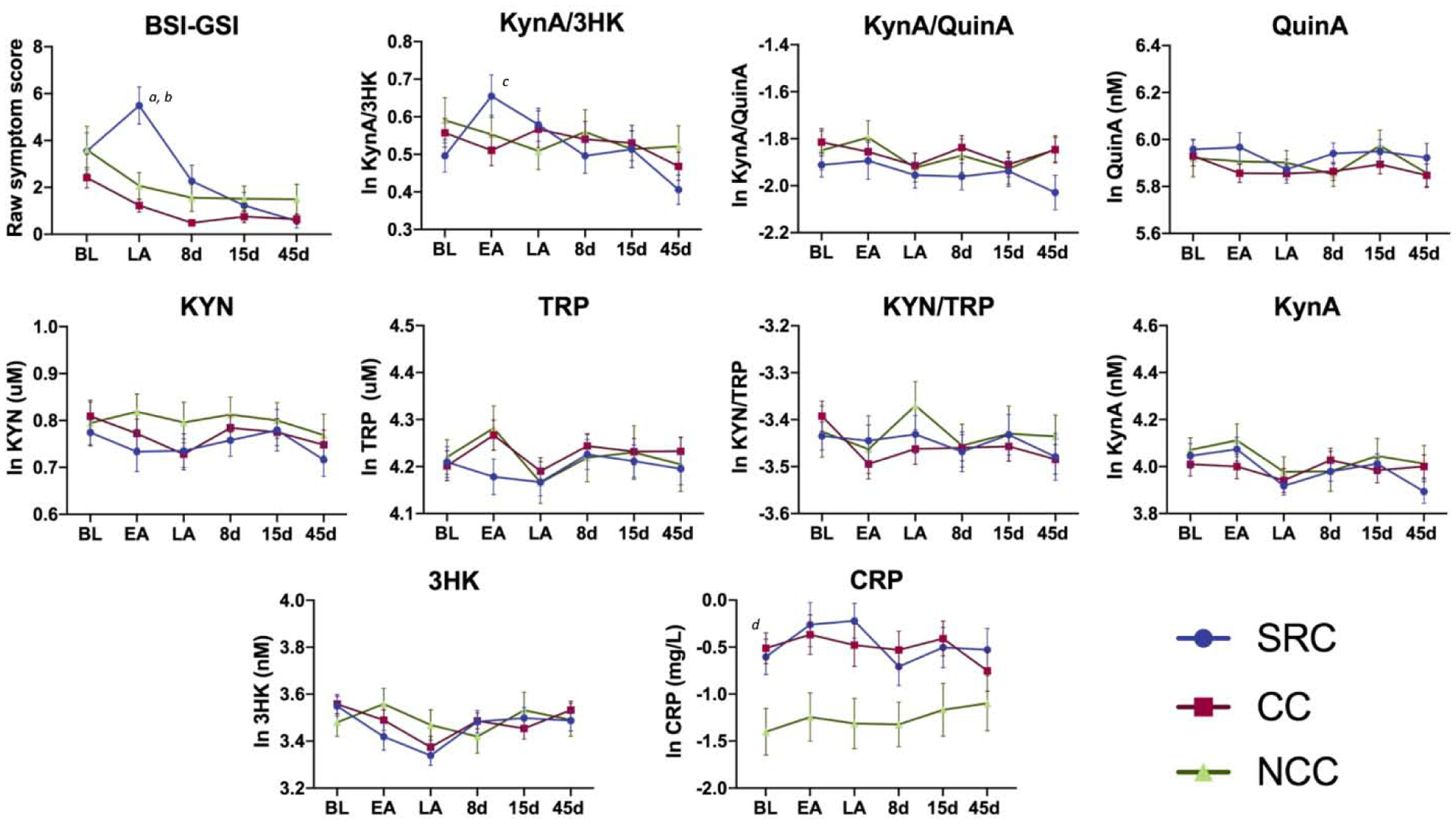
Shown are the mean Brief Symptom Inventory-18 Global Severity Index (BSI-GSI) scores along with a priori (top row) and secondary biomarkers (bottom rows) at the preseason baseline (BL), early-acute (EA), late-acute (LA), 8 day (8d), 15 day (15d), and 45 day (45d) visits in athletes with sport-related concussion (SRC), contact sport controls (CC), and non-contact sport controls (NCC). Error bars are the standard error of the mean. KynA = kynurenic acid, 3HK = 3-hydroxykynurenine, QuinA = quinolinic acid, KYN = kynurenine, TRP = tryptophan, CRP = c-reactive protein, pg/mL = picogram per milliliter, nM = nanomolar, uM = micromolar. Natural log (ln) transformed biomarker values are shown. Letters indicate post-hoc comparisons: ‘a’ = SRC: LA > BL, 8d, 15d, 45d; ‘b’ = LA: SRC > CC, NCC; ‘c’ = SRC: EA > BL, 8d, 45d, ‘d’ = SRC, CC > NCC. The complete list of post-hoc comparisons is provided in the text.
3.3. Effects of acute concussion on kynurenine metabolites
There was a significant group-by-visit interaction for the KynA/3HK ratio (p=0.006; Table 2; Figure 1). Post-hoc testing indicated that this effect was driven by significantly elevated KynA/3HK in SRC at the early-acute visit compared to the BL, 8d, and 45d visits (range of MD(SE)=0.14(0.04) to 0.22(0.04); ps<0.05), and elevated levels at the LA relative to the 45d visit (MD(SE)=0.16(0.04); p=0.001). There were no group or group-by-visit effects for KynA/QuinA, QuinA, or secondary KP markers. Secondary analyses did show group differences in CRP (p=0.006), with elevated CRP in SRC and CC athletes relative to NCC (MD(SE)= 0.80(0.27) and MD(SE)=0.78(0.27); ps<0.01). Sensitivity analyses showed nearly identical results when controlling for BMI, age, and years of participation in sport with the exception that group differences in CRP were not significant when BMI was included in the model (p>0.10; Supplementary Table 1). Similarly, additional sensitivity analyses demonstrated that controlling for the time of blood collection and the number of hours since most recent exertion did not impact results (Supplementary Table 2). The correlation between CRP and KP metabolites are presented in Supplementary Table 3.
3.4. Association between kynurenine metabolites and acute psychological symptoms
Tests for the relationship between psychological symptoms and KP metabolites following concussion were limited to markers showing acute injury effects (i.e., KynA/3HK) and psychological symptoms at the visit with the most elevated symptoms (i.e., late-acute visit). Higher KynA/3HK at the early-acute (i.e., within 6 hours) visit was significantly associated with lower BSI-GSI scores the late-acute (i.e., 24–48 hour) visit when covarying baseline KynA/3HK and psychological symptoms (Wald X2 =5.98, B=−8.67, p=0.014; Figure 2). Exploratory analyses demonstrated that this relationship was driven by the depression subscale of the BSI (Wald X2 =3.93, B=−4.00, p=0.047); the association with the anxiety and somatization subscales was not significant.
Figure 2: Association between KynA/3HK and psychological symptoms.
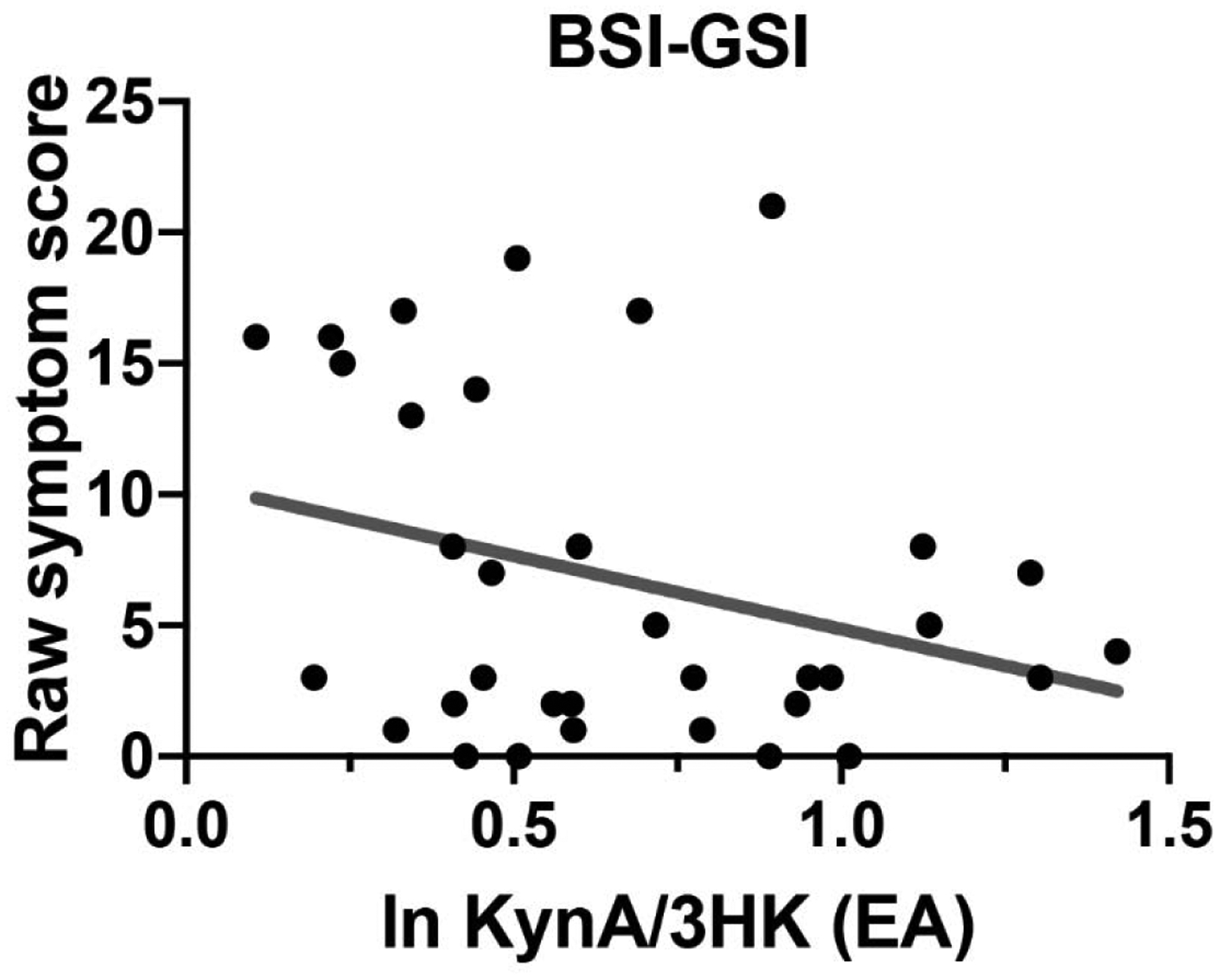
Shown is the association between Brief Symptom Inventory-18 Global Severity Index (BSI-GSI) scores at the late-acute visit and the natural log (ln) transformed kynurenic acid to 3-hydroxykynurenine ratio (KynA/3HK) at the early-acute (EA) visit in athletes with concussion.
3.5. Effects of prior concussion on psychological symptoms and kynurenine metabolites
Contact sport athletes were stratified based on whether or not they had a history of at least one prior concussion (i.e., SRC+, SRC−, CC+, CC− to delineate the acute and cumulative effects of concussion. There was a significant group-by-visit interaction effect on BSI-GSI scores (p<0.001; Table 3; Figure 3). Post-hoc tests showed that SRC+ and SRC− groups had higher BSI-GSI scores relative to CC+ and CC− groups at the late-acute visit (range of MD(SE)=3.34(1.09) to 5.28(1.02); ps<0.01). SRC+ athletes had elevated BSI-GSI scores at both baseline and the late-acute visits relative to the 15d and 45d visits (range of MD(SE)=3.26(0.78) to 3.82(0.82); ps<0.001), while BSI-GSI scores in SRC− athletes were elevated at the late-acute visit relative to all other visits, and elevated at baseline relative to the 45d visit (range of MD(SE)=2.25(0.85) to 6.16(0.87); p=0.048)
Table 3.
Means, standard deviations, and associated statistics for the effects of acute concussion in contact sport athletes subdivided based on prior concussion history on psychological symptoms,kynurenine pathway metabolites, and c-reactive protein
| Group by Visit (p-value) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A Priori | ||||||||||
| BSI-GSI (raw score) | SRC− | 2.93(3.87) | -- | 6.28(6.04) | 1.54(2.28) | 0.90(2.30) | 0.38(1.32) | 0.04 | <0.001 | <0.001 |
| SRC+ | 4.13(7.64) | -- | 4.83(5.71) | 2.84(6.39) | 1.48(4.82) | 0.74(2.47) | ||||
| CC− | 2.39(2.97) | -- | 1.06(1.65) | 0.43(0.73) | 0.66(1.47) | 0.68(1.39) | ||||
| CC+ | 2.45(3.47) | -- | 2.45(3.47) | 0.57(1.32) | 0.90(2.17) | 0.59(1.32) | ||||
| ln KynA/3HK | SRC− | 0.57(0.36) | 0.75(0.36) | 0.67(0.34) | 0.53(0.38) | 0.61(0.34) | 0.47(0.27) | 0.25 | <0.001 | 0.14 |
| SRC+ | 0.42(0.29) | 0.55(0.30) | 0.50(0.31) | 0.47(0.32) | 0.44(0.32) | 0.34(0.24) | ||||
| CC− | 0.52(0.29) | 0.47(0.31) | 0.51(0.34) | 0.51(0.36) | 0.54(0.35) | 0.42(0.24) | ||||
| CC+ | 0.61(0.26) | 0.58(0.32) | 0.65(0.37) | 0.58(0.31) | 0.51(0.30) | 0.55(0.26) | ||||
| ln KynA/QuinA | SRC− | −1.82(0.43) | −1.69(0.50) | −1.81(0.44) | −1.86(0.41) | −1.86(0.38) | −1.93(0.53) | 0.02 | 0.06 | 0.23 |
| SRC+ | −2.00(0.34) | −2.13(0.32) | −2.08(0.33) | −2.04(0.42) | −1.99(0.39) | −2.12(0.43) | ||||
| CC− | −1.85(0.46) | −1.92(0.38) | −1.99(0.38) | −1.88(0.35) | −1.93(0.39) | −1.91(0.34) | ||||
| CC+ | −1.76(0.37) | −1.75(0.34) | −1.81(0.36) | −1.78(0.38) | −1.88(0.34) | −1.74(0.36) | ||||
| ln QuinA (nM) | SRC− | 5.91(0.34) | 5.85(0.43) | 5.75(0.35) | 5.82(0.36) | 5.81(0.35) | 5.80(0.47) | 0.04 | 0.02 | 0.40 |
| SRC+ | 6.00(0.25) | 6.09(0.26) | 5.98(0.26) | 6.03(0.28) | 6.05(0.32) | 6.03(0.32) | ||||
| CC− | 5.92(0.34) | 5.84(0.31) | 5.83(0.32) | 5.85(0.29) | 5.89(0.30) | 5.85(0.35) | ||||
| CC+ | 5.93(0.26) | 5.89(0.25) | 5.90(0.26) | 5.89 (0.25) | 5.90(0.27) | 5.85(0.30) | ||||
| Secondary | ||||||||||
| ln KYN (uM) | SRC− | 0.77(0.20) | 0.69(0.31) | 0.68(0.29) | 0.69(0.27) | 0.69(0.29) | 0.66(0.28) | 0.34 | 0.07 | 0.36 |
| SRC+ | 0.78(0.20) | 0.78(0.16) | 0.78(0.24) | 0.81(0.23) | 0.84(0.31) | 0.77(0.18) | ||||
| CC− | 0.82(0.24) | 0.75(0.23 | 0.69(0.23) | 0.77(0.19) | 0.76(0.22) | 0.72(0.22) | ||||
| CC+ | 0.78(0.19) | 0.80(0.22) | 0.78(0.25) | 0.80(0.20) | 0.80(0.18) | 0.79(0.20) | ||||
| ln TRP(uM) | SRC− | 4.21(0.27) | 4.17(0.20) | 4.16(0.24) | 4.20(0.25) | 4.15(0.22) | 4.13(0.27) | 0.65 | 0.27 | 0.39 |
| SRC+ | 4.21(0.24) | 4.19(0.26) | 4.17(0.20) | 4.24(0.23) | 4.26(0.25) | 4.26(0.16) | ||||
| CC− | 4.21(0.25) | 4.25(0.23) | 4.16(0.18) | 4.23(0.18) | 4.24(0.19) | 4.24(0.19) | ||||
| CC+ | 4.19(0.22) | 4.29(0.24) | 4.23(0.24) | 4.26(0.17) | 4.22(0.21) | 4.22(0.23) | ||||
| ln KYN/TRP | SRC− | −3.44(0.26) | −3.48(0.34) | −3.48(0.33) | −3.51(0.34) | −3.46(0.36) | −3.47(0.43) | 0.68 | 0.02 | 0.54 |
| SRC+ | −3.43(0.20) | −3.41(0.30) | −3.39(0.27) | −3.44(0.29) | −3.41(0.26) | −3.49(0.21) | ||||
| CC− | −3.38(0.26) | −3.50(0.24) | −3.48(0.24) | −3.46(0.20) | −3.48(0.25) | −3.52(0.23) | ||||
| CC+ | −3.41(0.20) | −3.48(0.24) | −3.44(0.24) | −3.47(0.21) | −3.42(0.16) | −3.43(0.18) | ||||
| ln KynA (nM) | SRC− | 4.09(0.44) | 4.17(0.32) | 3.94(0.29) | 3.95(0.23) | 3.95(0.28) | 3.89(0.30) | 0.28 | 0.03 | 0.01 |
| SRC+ | 4.00(0.37) | 3.97(0.26) | 3.90(0.30) | 4.00(0.37) | 4.06(0.31) | 3.91(0.36) | ||||
| CC− | 4.04(0.34) | 3.92(0.40) | 3.84(0.37) | 3.97(0.36) | 3.96(0.38) | 3.93(0.32) | ||||
| CC+ | 3.96(0.40) | 4.13(0.32) | 4.09(0.32) | 4.11(0.37) | 4.01(0.35) | 4.11(0.33) | ||||
| ln 3HK (nM) | SRC− | 3.52(0.28) | 3.42(0.37) | 3.28(0.33) | 3.42(0.39) | 3.34(0.31) | 3.40(0.23) | 0.20 | <0.001* | 0.68 |
| SRC+ | 3.58(0.35) | 3.42(0.33) | 3.39(0.29) | 3.53(0.32) | 3.62(0.27) | 3.56(0.32) | ||||
| CC− | 3.55(0.37) | 3.45(0.34) | 3.33(0.30) | 3.45(0.22) | 3.42(0.31) | 3.52(0.27) | ||||
| CC+ | 3.56(0.24) | 3.56(0.29) | 3.44(0.38) | 3.54(0.26) | 3.50(0.32) | 3.56(0.24) | ||||
| ln CRP (mg/L) | SRC− | −0.72(1.46) | −0.53(1.53) | −0.64(1.30) | −1.06(1.43) | −1.08(1.11) | −0.62(1.44) | 0.37 | 0.04 | 0.26 |
| SRC+ | −0.49(1.44) | 0.04(1.22) | 0.13(1.37) | −0.44(1.51) | −0.07(1.61) | −0.44(1.58) | ||||
| CC− | −0.28(1.15) | −0.34(1.38) | −0.46(1.45) | −0.54(1.24) | −0.28(1.25) | −1.11(1.47) | ||||
| CC+ | −0.87(1.23) | −0.41(1.81) | −0.50(1.89) | −0.52(1.69) | −0.59(1.35) | −1.10(1.54) | ||||
Note: ln = natural log, SRC = sport-related concussion, CC= contact control, NCC= noncontact control, + = with prior concussion, − = without prior concussion, BL= baseline visit, 8d= 8-day visit, 15d= 15-day visit, 45d= 45-day visit, BSI-GSI= Brief Symptom Inventory Global Severity Index, KynA= kynurenic acid, 3HK= 3-hydroxykynurenine, QuinA= quinolinic acid, KYN= Kynurenine, TRP= tryptophan, nM = nanomolar, uM = micromolar, mg/L = milligram per liter.
Indicates significant effects for secondary analyses with Bonferroni correction.
Figure 3: Psychological symptoms and blood markers with groups stratified by concussion history.
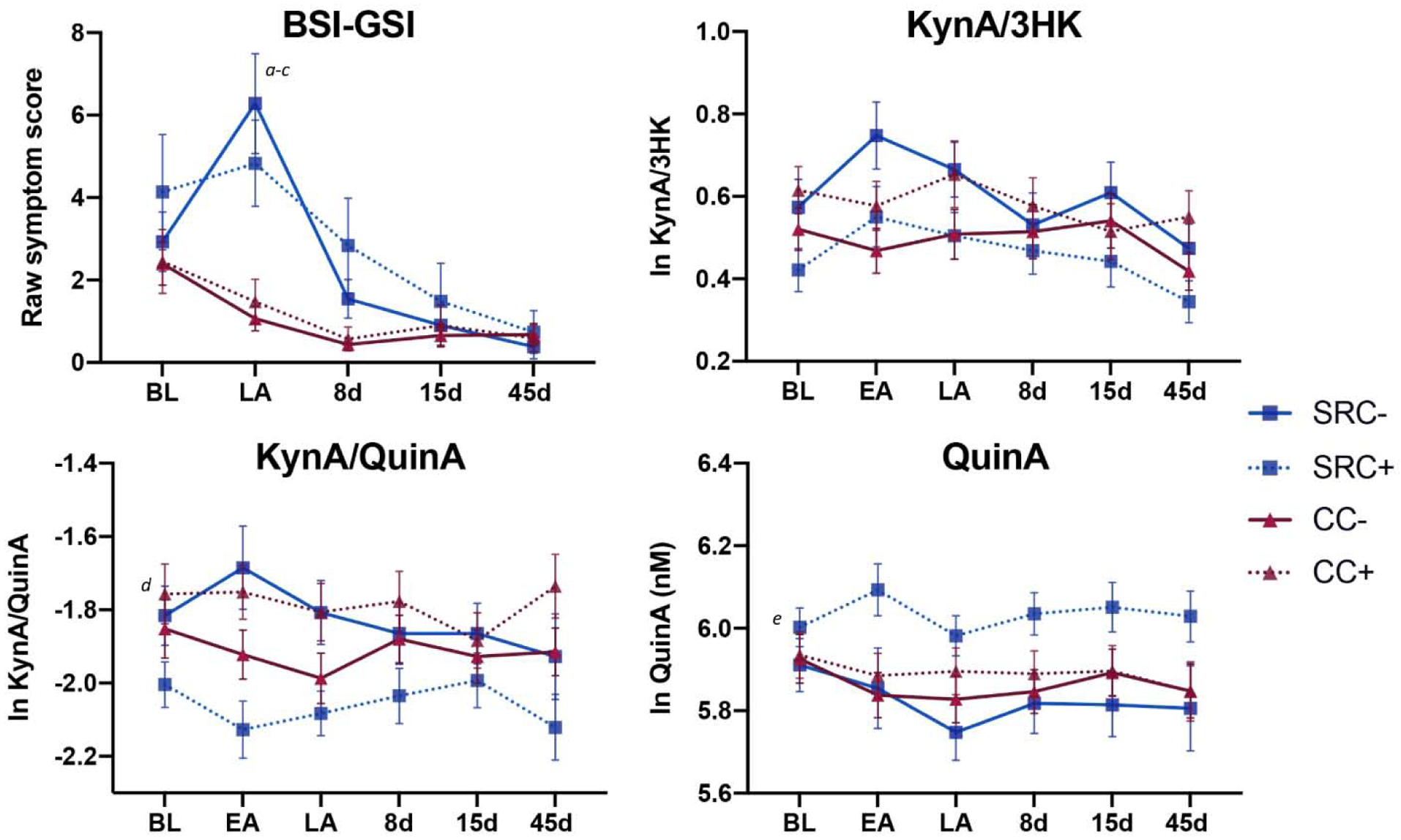
Shown are the mean Brief Symptom Inventory-18 Global Severity Index (BSI-GSI) scores and a priori kynurenine pathway metabolites at the preseason baseline (BL), early-acute (EA), late-acute (LA), 8 day (8d), 15 day (15d), and 45 day (45d) visits in athletes with sport-related concussion and prior concussion (SRC+), athletes with sport-related concussion and no prior concussion (SRC−), contact sport controls with prior concussion (CC+), and contact sport controls with no prior concussion (CC−). Error bars are the standard error of the mean. KynA = kynurenic acid, 3HK = 3-hydroxykynurenine, QuinA = quinolinic acid, nM = nanomolar. Natural log (ln) transformed biomarker values are shown. Letters indicate post-hoc comparisons: ‘a’ = LA: SRC+, SRC− > CC+, CC−; ‘b’ = SRC+: LA > 15d, 45d; ‘c’ = SRC−: LA > BL, 8d, 15d, 45d; ‘d’ = SRC+ < SRC−, CC+; ‘e’ = SRC+ > SRC−, CC− (trend). The complete list of post-hoc comparisons is provided in the text.
For the a priori biomarker outcomes, there was a significant group effect for KynA/QuinA (p=0.015; Table 3; Figure 3), with SRC+ athletes having significantly lower KynA/QuinA across all visits relative to SRC− athletes and CC+ athletes (M(SE)= −0.22(0.08) and M(SE)=−0.28(0.10); ps<0.05). There was also a significant group effect for QuinA (p=0.039) that was driven by non-significant trend for higher QuinA in SRC+ relative to SRC− and CC− (M(SE)=0.18(0.07) and M(SE)= 0.16(0.07); ps<0.10). There were no significant effects for KynA/3HK or for secondary markers including CRP with Bonferroni correction. Sensitivity analyses showed that controlling for BMI, age, and years of participation in sport had little effect on results, though the group effects of KynA/QuinA became non-significant (p=0.06); Supplementary Table 4). Similarly, sensitivity analyses showed that controlling for time of blood collection and number of hours since last physical exertion had limited effect on results, though the group effect on QuinA became a non-significant trend (p=0.06; Supplementary Table 5).
4. Discussion
This prospective study assessed the effects of SRC on neuroactive KYN pathway metabolites in serum in high school and collegiate football players and their association with post-injury psychological symptoms and prior concussion history. There were three main findings. First, concussed athletes reported elevated psychological symptoms acutely following injury relative to contact and non-contact sport controls. Second, surprisingly, concussed athletes had higher levels of KynA/3HK in serum, a neuroprotective index, at the early-acute window (i.e., within 6 hours post-injury) relative to baseline and sub-acute periods. However, consistent with the hypothesized neuroprotective effects of KynA, in athletes with SRC, elevated KynA/3HK levels at the early-acute visit (i.e., within 6 hours of injury) were associated with fewer psychological symptoms, specifically depression symptoms, at the late-acute period (i.e., 24–48 hours post-injury). Third, stratifying football players based on prior concussion history showed an interactive effect of prior and acute concussion, i.e. concussed athletes who also had a history of a prior concussion had lower serum KynA/QuinA and elevated concentrations of the potential neurotoxin, QuinA, at all timepoints. We discuss each of these three findings in turn, below.
Regarding the psychiatric sequalae of concussion – psychological symptoms (e.g., depression, anxiety) are common following brain injury of all severity (Bombardier et al., 2010; Kontos et al., 2012). Our group and other researchers have previously shown that psychological symptoms emerge acutely following injury and can persist for weeks, often lasting longer than self-reported concussion symptoms or neurocognitive deficits (Kontos et al., 2012, 2016; Ellis et al., 2015; Singh et al., 2016; McCuddy et al., 2018; Meier et al., 2019). Previous work has also documented subtle differences in baseline psychological symptoms based on concussion history (Brett et al., 2019) although in the current study, prior concussion did not significantly affect the severity of acute psychological symptoms following concussion.
The early-acute (within 6 hours of injury) increase in serum KynA/3HK observed in athletes with SRC was not hypothesized. The earliest post-injury time point in our previous pilot study was approximately one-day post-injury (Singh et al., 2016); thus, this is the first study in which the early-acute effects of injury on these markers have been examined. The fact that acute serum levels of KynA/3HK were inversely related to psychological symptoms at the late-acute visit suggests that acute increases in KynA/3HK may be neuroprotective against post-injury depression symptoms. Extensive work has been done to identify markers of adverse responses to concussion (e.g., glial fibrillary acidic protein levels) (Zetterberg et al., 2016). However, to our knowledge this is the first study to identify a potential protective biomarker. This is important because the KP is a target for pharmacologic intervention. Inhibitors of the enzyme (KMO) that drives the metabolism of KYN down the QuinA pathway have shown therapeutic potential in preclinical models of neurodegenerative disorders. Further, clinical trials for MDD are currently underway with the KynA analogue, 4-chlorokynurenine or AV-101 (NCT02484456 and NCT03078322). Thus, our results raise the possibility that the psychiatric sequalae of SRC can be treated with medications that modulate KP activity.
A history of prior concussion has been associated with an increased risk for a subsequent concussion and also a worse clinical outcome (e.g., persistent symptoms) following a subsequent concussion (Zemek et al., 2016; Van Pelt et al., 2019). However, our understanding of the biological mechanisms underlying these effects is limited. One potential factor is immune system priming - changes in the reactive state of immune cells that prime the cells to respond more aggressively to subsequent stimuli. It has been hypothesized that chronic inflammation may sensitize the immune system to secondary inflammatory triggers, resulting not only in an overall peripheral immune response that is greater in magnitude than the sum of the responses to the individual stimuli alone, but at least in animal models, elicits a discordant central immune response as a result of microglial priming (Dilger and Johnson, 2008; Perry and Holmes, 2014; Witcher et al., 2015). QuinA is thought to be made by microglia in the brain and macrophages in the periphery (Espey et al., 1997; Guillemin et al., 2005), and thus the elevation in circulating concentrations of QuinA in the SRC group with a concussion history is consistent with the possibility that prior concussions result in the long-term priming of monocyte-lineage cells. Additional research is needed to test this hypothesis by directly quantifying immune cell priming in vitro.
The elevation in serum QuinA (and the reduction in the KynA/QuinA ratio) at multiple visits in the SRC+ group is also consistent with prior work in a variety of psychiatric disorders as well as neurodegenerative diseases for which brain injury has been posited as a potential risk factor (Guskiewicz et al., 2007; Amaral et al., 2013, Savitz et al., 2015a, b, Meier et al., 2016a; Perry et al., 2016; Young et al., 2016; Cho et al., 2017; Savitz, 2019). QuinA is thought to exert neurotoxic effects through a variety of mechanisms, including excitotoxicity, the generation of reactive oxygen species, blood brain barrier disruption, and the destabilization of cellular cytoskeleton (Guillemin, 2012). Moreover, QuinA co-localized with hyperphosphorylated tau in cortical neurons of Alzheimer’s Disease patients, post-mortem, and was shown to promote the phosphorylation of tau in neuronal cultures (Rahman et al., 2009). Hyperphosphorylated tau is a hallmark of chronic traumatic encephalopathy, a neurodegenerative disease that has been linked to repeated head injury (McKee et al., 2016), though the link between hyperphosphorylated tau and QuinA in the context of brain injury has not yet been established. Nevertheless, our current and prior results suggest that QuinA might be a biomarker for repeat concussion and also highlight the need for additional research to determine if circulating concentrations of QuinA are markers of risk for negative outcomes following repetitive concussion (Meier et al., 2016b, 2017a; Singh et al., 2016).
Finally, the aforementioned differences in KP metabolites were observed without similar differences in CRP. Although group differences in CRP were present in the primary analyses (i.e., football players > non-contact athletes), supplementary analyses demonstrated that these were driven by group differences in BMI. An acute inflammatory response is a well-established consequence of brain injury, including SRC (Faden et al., 1989; Katayama et al., 1990; Kalabalikis et al., 1999; Tasçı et al., 2003; Chiaretti et al., 2005; Giza and Hovda, 2014; Gill et al., 2016, 2018; Jassam et al., 2017; Ritzel et al., 2018; Morganti-Kossmann et al., 2019; Nitta et al., 2019). However, endocrine dysfunction can also occur following brain injury (Schneider et al., 2007; Yang et al., 2016; Ritchie et al., 2018), and corticosteroids can mediate KP activity via TDO. Future studies are needed to determine the independent effects of endocrine dysfunction and inflammation following brain injury on KP metabolites.
4.1. Limitations
The current study significantly expands upon our prior work investigating the association between concussion and KP metabolites by the use of a prospective design with pre-injury blood collection, the inclusion of an early-acute blood sample, a more richly characterized sample with detailed concussion history, and the inclusion of non-contact athletes as an additional control group (Meier et al., 2016b, 2017a; Singh et al., 2016). The study does, however, have limitations that should be considered. First, concussed athletes in this study were limited to high school and collegiate football players; it is unclear if these results generalize to female athletes, athletes of different ages, or athletes from other sports. In addition, the number of prior concussions was based on self-report, which could be biased. Other factors could affect serum levels of KP metabolites including the timing of blood draws and time since most recent exercise (Agudelo et al., 2014). These factors were not a priori matched between injured and non-injured athletes; however, results from sensitivity analyses controlling for these factors were consistent with those from the primary analyses. Finally, KP metabolites were measured in serum and it is unclear if they are reflective of central KP levels. Nevertheless, KYN and 3HK can cross the blood brain barrier and be metabolized into QuinA in the brain, perhaps explaining why a number of studies have reported significant correlations between QuinA concentrations in the blood and brain or CSF (Heyes et al., 1992; Heyes and Morrison, 1997; Raison et al., 2010).
4.2. Conclusion
Evidence from this prospective study of the association between SRC and neuroactive KP metabolites reinforces the link between SRC and psychological symptoms. The results suggest that acutely elevated serum KynA/3HK may protect against the development of depressive symptoms following concussion. Additionally, higher QuinA and lower KynA/QuinA in serum were evident at all visits in athletes with both acute SRC and a history of prior concussion, highlighting their potential as biomarkers for repetitive head injury and providing insight into possible mechanisms linking prior concussion with subsequent repeat injury. Future research is needed to better characterize the potential effects of KP abnormalities on both the acute and cumulative effects of SRC.
Supplementary Material
Highlights.
Athletes with concussion had acute elevations in mood symptoms
Serum kynurenic acid to 3-hydroxykynurenine ratio increased acutely following concussion
Greater serum KynA/3HK predicted fewer mood symptoms at later visits following concussion
Athletes with both acute and prior concussion had elevated serum quinolinic acid levels
Acknowledgements
The authors thank Ashlee Taylor and Brenda Davis from University of Oklahoma Integrative Immunology Center for analysis of c-reactive protein; Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination; Dr. Aniko Szabo from the Department of Biostatistics at the Medical College of Wisconsin for statistical consultation; and Dr. Julien Roeser from Charles River Laboratories for quantification of kynurenine metabolites.
Funding
This work was supported by the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH-14-1-0561. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Support for this work was also provided by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R21NS099789. TM acknowledges additional support from the National Institute of Neurological Disorders And Stroke (R01NS102225) and through a project funded through the Research and Education Program, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. JS acknowledges support from the National Institute of General Medical Sciences (P20GM121312) and the National Institute of Mental Health (R21MH113871). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The REDCap electronic database and the Adult Translational Research Unit used for this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors report no competing interests.
References
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014; 159: 33–45. [DOI] [PubMed] [Google Scholar]
- Amaral M, Outeiro TF, Scrutton NS, Giorgini F. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. J Mol Med (Berl) 2013; 91: 705–713. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun 2015; 43: 110–117. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Heyes MP, Wisniewski SR, Sinz EH, Clark RS, et al. Quinolinic acid in the cerebrospinal fluid of children after traumatic brain injury. Crit Care Med 1999; 27: 493–497. [DOI] [PubMed] [Google Scholar]
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010; 303: 1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, Huber DL, Wild A, Nelson LD, McCrea MA. Age of First Exposure to American Football and Behavioral, Cognitive, Psychological, and Physical Outcomes in High School and Collegiate Football Players. Sports Health 2019; 11: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Kontos AP, Levin H, Schneider K, Wilde EA, Cantu RC, et al. National Institute of Neurological Disorders and Stroke and Department of Defense Sport-Related Concussion Common Data Elements Version 1.0 Recommendations. J Neurotrauma 2018; 35: 2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci 2001; 13: 2141–2147. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, et al. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst 2005; 21: 185–93; discussion 194. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res 2017; 99: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colín-González AL, Maldonado PD, Santamaría A. 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology 2013; 34: 189–204. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett 2008; 441: 29–34. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 2011; 36: 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI) 18: Administration, Scoring, and Procedures Manual. Minneapolis, Minn: NCS Pearson; 2000 [Google Scholar]
- Derogatis LR, Fitzpatrick M. The SCL-90-R, the Brief Symptom Inventory (BSI), and the BSI-18. 2004
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 2008; 84: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolin K, Allers KA, Pleiner S, Liesener A, Farrell C, Tozzi L, et al. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology 2018; 95: 8–17. [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Ritchie LJ, Koltek M, Hosain S, Cordingley D, Chu S, et al. Psychiatric outcomes after pediatric sports-related concussion. J Neurosurg Pediatr 2015; 16: 709–718. [DOI] [PubMed] [Google Scholar]
- Espey MG, Chernyshev ON, Reinhard JFJ, Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport 1997; 8: 431–434. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989; 244: 798–800. [DOI] [PubMed] [Google Scholar]
- Foster AC, Miller LP, Oldendorf WH, Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp Neurol 1984; 84: 428–440. [DOI] [PubMed] [Google Scholar]
- Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett 1984; 48: 273–278. [DOI] [PubMed] [Google Scholar]
- Gill J, Merchant-Borna K, Lee H, Livingston WS, Olivera A, Cashion A, et al. Sports-Related Concussion Results in Differential Expression of Nuclear Factor-kappaB Pathway Genes in Peripheral Blood During the Acute and Subacute Periods. J Head Trauma Rehabil 2016; 31: 269–276. [DOI] [PubMed] [Google Scholar]
- Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, et al. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj 2018; 32: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014; 75 Suppl 4: S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J 2012; 279: 1356–1365. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005; 49: 15–23. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005; 57: 719–726. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HPJ, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 2007; 39: 903–909. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, et al. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol 1992; 40: 71–80. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Morrison PF. Quantification of local de novo synthesis versus blood contributions to quinolinic acid concentrations in brain and systemic tissues. J Neurochem 1997; 68: 280–288. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci 2001; 21: 7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017; 95: 1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalabalikis P, Papazoglou K, Gouriotis D, Papadopoulos N, Kardara M, Papageorgiou F, et al. Correlation between serum IL-6 and CRP levels and severity of head injury in children. Intensive Care Med 1999; 25: 288–292. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 1990; 73: 889–900. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 1989; 52: 1319–1328. [DOI] [PubMed] [Google Scholar]
- Klein AP, Tetzlaff JE, Bonis JM, Nelson LD, Mayer AR, Huber DL, et al. Prevalence of Potentially Clinically Significant Magnetic Resonance Imaging Findings in Athletes with and without Sport-Related Concussion. J Neurotrauma 2019; 36: 1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos AP, Covassin T, Elbin RJ, Parker T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil 2012; 93: 1751–1756. [DOI] [PubMed] [Google Scholar]
- Kontos AP, Deitrick JM, Reynolds E. Mental health implications and consequences following sport-related concussion. Br J Sports Med 2016; 50: 139–140. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006; 21: 375–378. [DOI] [PubMed] [Google Scholar]
- Mackay GM, Forrest CM, Stoy N, Christofides J, Egerton M, Stone TW, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol 2006; 13: 30–42. [DOI] [PubMed] [Google Scholar]
- Madsen T, Erlangsen A, Orlovska S, Mofaddy R, Nordentoft M, Benros ME. Association Between Traumatic Brain Injury and Risk of Suicide. JAMA 2018; 320: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuddy WT, Espana LY, Nelson LD, Birn RM, Mayer AR, Meier TB. Association of acute depressive symptoms and functional connectivity of emotional processing regions following sport-related concussion. NeuroImage Clin 2018; 19: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016; 131: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachen S-J, Hanks RA, Millis SR, Rapport LJ. The reliability and validity of the Brief Symptom Inventory− 18 in persons with traumatic brain injury. Arch Phys Med Rehabil 2008; 89: 958–965. [DOI] [PubMed] [Google Scholar]
- Meier T, Giraldo-Chica M, Espana L, Mayer A, Harezlak J, Nencka AS, et al. Resting-state fMRI metrics in acute sport-related concussion and their association with clinical recovery: A study from the NCAA-DOD CARE Consortium. J Neurotrauma 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 2016; 53: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Lancaster MA, Mayer AR, Teague TK, Savitz J. Abnormalities in Functional Connectivity in Collegiate Football Athletes with and without a Concussion History: Implications and Role of Neuroactive Kynurenine Pathway Metabolites. J Neurotrauma 2017; 34: 824–837. [DOI] [PubMed] [Google Scholar]
- Meier TB, Nelson LD, Huber DL, Bazarian JJ, Hayes RL, McCrea MA. Prospective Assessment of Acute Blood Markers of Brain Injury in Sport-Related Concussion. J Neurotrauma 2017; 34: 3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Savitz J, Singh R, Teague TK, Bellgowan PSF. Smaller Dentate Gyrus and CA2 and CA3 Volumes Are Associated with Kynurenine Metabolites in Collegiate Football Athletes. J Neurotrauma 2016; 33: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenigro PH, Alosco ML, Martin BM, Daneshvar DH, Mez J, Chaisson CE, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 2017; 34: 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Semple BD, Hellewell SC, Bye N, Ziebell JM. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol 2019; 137: 731–755. [DOI] [PubMed] [Google Scholar]
- Myint A-M, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98: 143–151. [DOI] [PubMed] [Google Scholar]
- Nitta ME, Savitz J, Nelson LD, Teague TK, Hoelzle JB, McCrea MA, et al. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology 2019; 93: e497–e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009; 14: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478: 197. [DOI] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 2016; 6: e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt KL, Allred D, Cameron KL, Campbell DE, D’Lauro CJ, He X, et al. A cohort study to identify and evaluate concussion risk factors across multiple injury settings: findings from the CARE Consortium. Inj Epidemiol 2019; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016; 124: 511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014; 10: 217–224. [DOI] [PubMed] [Google Scholar]
- Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One 2009; 4: e6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 2010; 15: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie EV, Emery C, Debert CT. Analysis of serum cortisol to predict recovery in paediatric sport-related concussion. Brain Inj 2018; 32: 523–528. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Doran SJ, Barrett JP, Henry RJ, Ma EL, Faden AI, et al. Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury. J Neurotrauma 2018; 35: 1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J. The kynurenine pathway: a finger in every pie [Internet]. Mol Psychiatry 2019. Available from: 10.1038/s41380-019-0414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PSF, et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015; 40: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PSF, Victor TA, et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 2015; 46: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA 2007; 298: 1429–1438. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012; 13: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Savitz J, Teague TK, Polanski DW, Mayer AR, Bellgowan PSF, et al. Mood symptoms correlate with kynurenine pathway metabolites following sports-related concussion. J Neurol Neurosurg Psychiatry 2016; 87: 670–675. [DOI] [PubMed] [Google Scholar]
- Sinz EH, Kochanek PM, Heyes MP, Wisniewski SR, Bell MJ, Clark RS, et al. Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J Cereb Blood Flow Metab 1998; 18: 610–615. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein H-G, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 2011; 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasçı A, Okay Ö, Gezici AR, Ergün R, Ergüngör F. Prognostic value of interleukin-1 beta levels after acute brain injury. Neurol Res 2003; 25: 871–874. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001 [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci 2015; 38: 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan EB, Frugier T, Lim CK, Heng B, Sundaram G, Tan M, et al. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J Neuroinflammation 2015; 12: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W-H, Chen P-C, Wang T-C, Kuo T-Y, Cheng C-Y, Yang Y-H. Endocrine dysfunction following traumatic brain injury: a 5-year follow-up nationwide-based study. Sci Rep 2016; 6: 32987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav Immun 2016; 56: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek R, Barrowman N, Freedman SB, Gravel J, Gagnon I, McGahern C, et al. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children With Acute Concussion in the ED. JAMA 2016; 315: 1014–1025. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Morris HR, Hardy J, Blennow K. Update on fluid biomarkers for concussion. Concussion 2016; 1: CNC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012; 37: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


