Abstract
Background
Adenine phosphoribosyltransferase (APRT) deficiency is a rare, hereditary cause of kidney stones and chronic kidney disease (CKD), characterized by 2,8-dihydroxyadenine (DHA) renal parenchymal crystal deposition. The aim of this study was to examine outcomes of kidney transplantation in APRT deficiency patients.
Methods
Included were 13 patients in the APRT Deficiency Registry of the Rare Kidney Stone Consortium, 2 from Westmead Hospital in Sydney, Australia, and 2 from Necker Hospital in Paris, France. The CKD-EPI and CKiD equations were used to calculate glomerular filtration rate estimates (eGFR). Allograft survival was analyzed employing the Kaplan-Meier method. The Wilcoxon-Mann-Whitney test was used to compare alllograft outcomes by xanthine oxidoreductase (XOR) inhibitor treatment status at transplantation.
Results
Seventeen patients (9 females) received 22 kidney transplants. Age at first transplantation was 47.2 (14.9–67.0) years. Ten patients received XOR inhhibitor therapy pretransplant (11 allografts), while 8 patients did not receive such treatment prior to transplantation (11 allografts). Two-year allograft survival was 91% and 55% in the 2 groups, respectively (p=0.16). The median (range) eGFR at 2 years posttransplant was 61.3 (24.0–90.0) mL/min/1.73 m2 when XOR inhibitor therapy was initiated before transplantation and 16.2 (10.0–39.0) mL/min/1.73 m2 (p=0.009) when such treatment was not administered pretransplant.
Conclusions
Kidney allograft outcomes are good in APRT deficiency patients beginning XOR inhibitor therapy pretransplant. Delay in such treatment is a major cause of premature graft loss in these patients. Increased awareness among clinicians is imperative, promoting early diagnosis of APRT deficiency and pharmacotherapy initiation before kidney transplantation.
Introduction
Adenine phophoribosyltransferase (APRT) deficiency (OMIM 102600) is a rare autosomal recessive disorder of purine metabolism. Absence of functional APRT enzyme results in conversion of adenine to 2,8-dihydroxyadenine (DHA), catalyzed by xanthine oxidoreductase (XOR; also known as xanthine oxidase/dehydrogenase). DHA is poorly soluble in the urine at physiological pH, leading to precipitation and formation of recurrent radiolucent kidney stones and/or crystal nephropathy. DHA nephropathy is characterized by widespread DHA crystal deposits, predominantly in the form of large aggregates in the tubular lumen, and smaller crystals in tubular epithelial cells and the interstitium, accompanied by tubulointerstitial inflammation and fibrosis.1,2
Chronic kidney disease (CKD), sometimes without a history of nephrolithiasis is a common presenting feature of APRT deficiency in adults, and 15–20% of patients have already reached end-stage kidney disease (ESKD) at the time of diagnosis.3–5 Furthermore, the disorder is often first recognized in the setting of disease recurrence following kidney transplantation. Three case series2,6,7 and several single-patient reports8–13 of kidney transplantation in patients with APRT deficiency have previously been published, commonly describing disease recurrence and unfavourable outcomes.
Treatment with the XOR inhibitors, allopurinol or febuxostat, reduces DHA production, and in turn its renal excretion and can prevent or halt the progression of crystal nephropathy,14 preserving and even improving kidney function.3 Allopurinol is frequently prescribed in the daily dose of 200–300 mg/day although higher doses (400–600 mg) are likely needed to minimize or prevent recurrent kidney stone formation and renal parenchymal DHA crystal deposition in the native kidney.2,3 Early disease recurrence in transplanted kidneys and accelerated allograft loss has been noted in both untreated and treated patients, suggesting that higher doses may be needed to preserve graft function.6,8
Limited data exist on the outcome of kidney transplantation in patients with APRT deficiency and the role of XOR inhibitor treatment in preserving kidney allograft function. The aim of this study was to examine the outcome of kidney transplantation in patients with APRT deficiency, in particular the effect of XOR inhibitor treatment status at the time of transplantation
Materials and Methods
Ethics
The study was approved by the National Bioethics Committee of Iceland (NBC 09–072) and the Icelandic Data Protection Authority. All living patients consented to participation in the study. The clinical and research activities reported herein are consistent with the principles of the Declaration of Helsinki and Istanbul.
Study Population
Thirteen (21%) of the 61 patients enrolled in the APRT Deficiency Registry of the Rare Kidney Stone Consortium (RKSC, http://www.rarekidneystones.org/) who had undergone kidney transplantation were included in the study. These patients were from Austria (n=1), Iceland (n=2), India (n=1), Italy (n=2) and the United States (n=7). In addition, 2 patients from Australia and 2 patients from France who had received a kidney transplant were included. Transplant outcome data on 8 of these 17 patients have previously been reported.2,6,7,11,15 The diagnosis of APRT deficiency was confirmed by the identification of biallelic pathogenic APRT mutations or completely abolished APRT enzyme function.
Clinical data
Sources of data included the APRT Deficiency Registry of the RKSC and individual patient records at participating hospitals through June 2019. In addition to basic demographic information, the following data were collected: age and clinical characteristics at presentation and diagnosis of APRT deficiency; age at onset of renal replacement therapy; dialysis and modality prior to kidney transplantation; age at transplantation, number of kidney allografts and donor type; date and cause of graft loss; date and cause of death; immunosuppressive therapy; treatment with allopurinol or febuxostat, including dose before and after transplantation; laboratory studies including serum creatinine (SCr) measurements, results of urine microscopy, including assessment of DHA crystals, APRT genotype, APRT enzyme activity measurement results, results of urological imaging; kidney allograft biopsy findings.
Glomerular filtration rate estimates (eGFR) were calculated from SCr using the modified Schwartz (CKiD) equation16 in children and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in adults.17 As previously described, nonstandardized SCr values were reduced by 5% before eGFR was calculated.18 Staging of CKD was according to the KDIGO (Kidney Disease: Improving Global Outcomes) classification system.19 Graft loss was defined as initiation of dialysis, retransplantation or death with a functioning graft. Delayed graft function was defined as the need for dialysis in the first posttransplant week. Posttransplant acute kidney injury (AKI) was defined according to the KDIGO AKI criteria.20
Analytical considerations
Data are presented as number, percentage and median (range). In the analysis, allografts were grouped according to whether the patients were receiving XOR inhibitor treatment prior to kidney transplantation and Wilcoxon-Mann-Whitney test and Fisher’s exact test were used to compare the group of patients who initiated XOR inhibitor treatment pretransplant to those who first started such treatment following transplant surgery. Patients receiving dialysis were assigned an eGFR of 10 mL/min/1.73 m2. Kaplan-Meier analysis was used to estimate death-censored allograft survival and groups were compared using the log-rank test. Statistical analyses were performed using SPSS (IBM SPSS Statistics version 21.0, 2012, Armonk, NY, USA).
Results
Clinical characteristics of patients at diagnosis of APRT deficiency
Seventeen patients had undergone kidney transplantation, of whom 9 (53%) were females.
The clinical characteristics of the patients at the time of diagnosis of APRT deficiency are presented in Table 1. The median age at diagnosis was 44.5 (11.9–67.9) years, by which time 13 of the 17 patients (76%) had initiated renal replacement therapy for ESKD. Of the 17 patients, 15 had a diagnostic delay of 7.8 (1.1–47.9) years following presentation of the underlying disease. Eleven (65%) patients were diagnosed with APRT deficiency prior to the first kidney transplantation; 1 of the 11 patients had CKD stage 3a, 3 had reached CKD stages 4–5 and 7 were already on hemodialysis. The diagnosis of APRT deficency was suggested by detection of DHA crystals on urine microscopy in 2 of these 11 cases, kidney stone analysis in 1 case and renal histological findings of crystal nephropathy in 5 individuals. Three patients were diagnosed through family screening of index cases, 2 of whom had a personal history of kidney stone disease.
Table 1.
Clinical characteristics at the time of diagnosis of APRT deficiency
| Patient | Sex | History of kidney stones | Age at diagnosis (yrs) | Diagnostic delay (yrs) | Kidney function (eGFR, mL/min/1.73m2) | Age at kidney biopsy (yrs) | Original native kidney biopsy findings |
|---|---|---|---|---|---|---|---|
| 1 | M | No | 62 | 1.1 | ESKD | 62 | DHA crystal nephropathy, global sclerosis (21 of 45 glomeruli); severe, interstitial fibrosis and arteriosclerosis |
| 2 | F | No | 43 | 5.1 | ESKD | 38 | Crystals, thought to be consistent with primary hyperoxaluria |
| 3 | M | Yes | 43 | 11.1 | ESKD | NA | |
| 4 | F | Yes | 68 | 47.9 | ESKD | NA | |
| 5 | F | No | 52 | 7.5 | ESKD | NA | |
| 6 | F | No | 52 | 6.0 | ESKD | 45 | Interstitial inflammation with inflammatory infiltrate; refractory golden-brown crystalline material seen |
| 7 | F | Yes | 59 | 24.0 | ESKD | NA | |
| 8 | M | Yes | 12 | 10.4 | ESKD | NA | |
| 9 | F | Yes | 49 | 7.8 | 9 | 42 | Tubulointerstitial fibrosis; presumed calcium oxalate crystal deposits |
| 10 | M | Yes | 42 | 39.2 | 17 | 42 | DHA crystals; interstitial inflammation |
| 11 | F | Yes | 45 | 1.4 | ESKD | NA | |
| 12 | F | No | 21 | 0 | ESKD | 21 | Tubulointerstitial nephritis with extensive calcium oxalate deposits |
| 13 | M | No | 40 | 4.7 | ESKD | 35 | Chronic interstitial nephritis; crystals seen but not identified |
| 14 | F | Yes | 24 | 4.0 | 54 | NA | |
| 15 | M | Yes | 50 | 20.0 | ESKD | 50 | Small number of scattered tubules contain intraluminal polarizable crystals believed to be calcium oxalate |
| 16 | M | No | 43 | 0.2 | ESKD | 42 | 2,8-DHA crystals with advanced glomerulosclerosis, tubular atrophy and interstitial fibrosis |
| 17 | M | Yes | 52 | 20.0 | 6 | NA |
Abbreviations: NA, not available; ESKD, end-stage kidney disease; DHA, 2,8-dihydroxyadenine.
The diagnosis of APRT deficiency was not made until after kidney transplantation in 6 patients. In 5 of the patients the diagnosis was made following their first transplantation and in 1 individual after the failure of 2 kidney allografts. The presumed cause of ESKD in these 6 patients was primary hyperoxaluria (n=2), chronic interstitial nephritis (n=2), kidney stone disease (n=1) and CKD of unknown causes (n=1).
Pharmacotherapy of APRT deficiency and kidney allograft outcomes
The 17 patients received 22 kidney allografts as outlined in Table 2. The first kidney transplantation was carried out at the median age of 47.2 (14.9–67.0) years, 1.8 (0.7–13.5) years after reaching ESKD in 14 cases while 3 patients underwent preemptive transplantation. The maintenance immunosuppression regimen consisted of a calcineurin inhibitor (18 allografts) or sirolimus (1 allograft) in conjunction with mycophenolate mofetil and steroids; 2 patients received cyclosporine and azathioprine without steroids and azathioprine was the sole immunosuppressive agent in 1 case.
Table 2.
Pharmacotherapy of APRT deficiency and kidney allograft outcomes
| Patient | Treatment before Tx | Age at RRT (yrs) | Graft number | Age at Tx (yrs) | Type of donor | Pharmacotherapy | Outcome | Last follow-up (yrs after Tx) | Latest eGFR (mL/min/1.73 m2) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | 62 | 1 | 65 | DD | Febuxostat 40 mg/day from 3 weeks post-Tx | Recurrence of DHA 3 weeks post-Tx; functioning graft | 1.6 | 25 |
| 2 | No | 38 | 1 | 39 | DD | None | Graft lost due to recurrence of DHA nephropathy 1 month post-Tx | 0.1 | ESKD |
| No | 39 | 2 | 42 | DD | Allopurinol 200 mg/day from 6 months post-Tx | Recurrence of DHA nephropathy 3 days post-Tx and allograft failure at 13 months post-Tx; died while on dialysis | 1.1 | ESKD | |
| 3 | No | 42 | 1 | 43 | DD | Allopurinol 300 mg/day from 4 months post-Tx | Died with a functioning graft | 0.5 | 41 |
| 4 | No | 65 | 1 | 67 | DD | Allopurinol 150 mg/day from 3 weeks post-Tx | Functioning graft | 6.5 | 28 |
| 5 | No | 45 | 1 | 46 | DD | Allopurinol 150 mg/day from 3 years post-Tx | Died with a functioning graft | 5.4 | 29 |
| 6 | No | 50 | 1 | 51 | DD | Allopurinol 300 mg/day from 5 weeks post-Tx, later febuxostat 80 mg/day | Recurrence of DHA nephropathy 24 days post-Tx; lost after 19 months | 1.7 | AKI/dialysis |
| 7 | No | 44 | 1 | 58 | LRD | Allopurinol 300 mg/day from 2 months post-Tx | Functioning graft | 9.7 | 37 |
| 8 | No | 12 | 1 | 15 | DD | None | Graft lost 18 months post-Tx | 1.5 | ESKD |
| No | 16 | 2 | 17 | DD | Allopurinol 300 mg/day from day 28 post-Tx | Recurrence of DHA nephropathy 1 month post-Tx, graft lost after 3.4 years | 3.4 | ESKD | |
| Yes | 20 | 3 | 21 | DD | Allopurinol 300 mg/day | Chronic allograft failure | 7.8 | ESKD | |
| No | 27 | 4 | 35 | DD | Allopurinol 150 mg twice-a-week, from day 36 post-Tx | Recurrence of DHA nephropathy 1 month post-Tx; died with a functioning graft | 1.4 | 15 | |
| 9 | Yes | 54 | 1 | 56 | DD | Allopurinol 300 mg/day for 7 years pre-Tx, subsequently 600 mg/day | Functioning graft | 13.3 | 50 |
| 10 | Yes | 46 | 1 | 46 | LUD | Allopurinol 400 mg/day for 3 years pre-Tx | Functioning graft | 4.4 | 71 |
| 11 | Yes | 43 | 1 | 47 | DD | Allopurinol 300 mg/day for 2 years pre-Tx | Functioning graft | 4.4 | 45 |
| 12 | Yes | 21 | 1 | 22 | LRD | Allopurinol 300 mg/day 1 year pre-Tx, later also febuxostat 120 mg/day | Graft lost due to recurrence of DHA nephropathy 5 years post-Tx | 5.2 | ESKD |
| Yes | 28 | 2 | 29 | LUD | Allopurinol 600 mg/day and febuxostat 120 mg/day | Functioning graft | 1.8 | 69 | |
| 13 | Yes | 36 | 1 | 41 | LRD | Allopurinol 200 mg/day for 1 month pre- Tx, subsequently 400 mg/day | Died with a functioning graft | 0.3 | 39 |
| 14 | Yes | 41 | 1 | 41 | DD | Allopurinol for 15 years pre-Tx, subsequently 600 mg/day | Functioning graft | 3.9 | 80 |
| 15 | Yes | 50 | 1 | 53 | LUD | Allopurinol for 2 years pre-Tx, subsequently 600 mg/day | Functioning graft | 2.8 | 65 |
| 16 | Yes | 42 | 1 | 47 | DD | Allopurinol 150 mg/day for 4 years pre-Tx, subsequently 300 mg/day and febuxostat 40 mg/day | Recurrence of DHA nephropathy 10 days post-Tx; functioning graft | 1.0 | 60 |
| 17 | Yes | 66 | 1 | 66 | DD | Allopurinol 200 mg/day for 12 years pre-Tx, subsequently 300 mg/day | Functioning graft | 2.1 | 82 |
Abbreviations: DD, deceased donor; LRD, living-related donor; LUD, living-unrelated donor; ESKD: end-stage kidney disease; RRT, renal replacement therapy; Tx, kidney transplantation.
The shaded area denotes allografts where xanthine oxidoreductase treatment was not initiated prior to kidney transplantation.
Ten patients received treatment with allopurinol 200 (100–300) mg/day for 2.6 (0.1–15.6) years prior to the transplantation of 11 allografts (Table 2). Delayed graft function was noted in the case of 3 kidney allografts, all from deceased donors, and AKI in 4 allografts, occurring within the first posttransplant week in 2 cases. Transplant biopsies were performed in 8 allografts from 7 patients (Table 3). Recurrence of DHA crystal nephropathy (Figure 1) was found in 3 of these allografts, at 10 days, 8 weeks and 4 months after transplantation. At the time of biopsy, 1 of the patients (No. 16) was taking allopurinol in the daily dose of 150 mg/day while the remaining 2 allografts were from the same patient (No. 12) prescribed allopurinol 300–600 mg/day before and following both transplants, though significant medication nonadherence following the first transplant was acknowledged. Renal histopathological findings suggestive of acute rejection were found in 2 cases, immediately posttransplant in 1 case and 1 month following transplant surgery in the other. No crystals were observed. One patient (No. 13) died with a functioning graft 4 months after transplantation from bacterial sepsis associated with peritonitis and 1 graft was lost nearly 8 years posttransplant with an allograft biopsy consistent with chronic allograft nephropathy (patient No. 8). No DHA crystals were detected.
Table 3.
Clinical features at the time of kidney allograft biopsy and renal histopathological findings
| Patient | Allograft | Delayed graft function | Time from Tx, (months) | Treatment with XOR inhibitor | Renal histolopathogical findings | eGFR (mL/min/1.73 m2) |
|---|---|---|---|---|---|---|
| 1 | 1 | No | 0.75 | Febuxostat 40 mg | Numerous Intratubular DHA crystal deposits | 23 |
| 2 | 1 | No | 0.75 | None | Extensive crystal deposits | Dialysis |
| 2 | No | 0.03 | None | Acute tubular necrosis; no crystals | 6 | |
| 0.1 | None | Intratubular crystal deposits | 25 | |||
| 4 | None | Extensive crystal deposits within the interstitium | 29 | |||
| 5 | None | Mild focal interstitial fibrosis and tubular atrophy associated with mild inflammation; polarizable intraluminal crystals in several tubules | 20 | |||
| 12 | Allopurinol 200 mg | Minimal interstitial fibrosis and focal tubular atrophy; occasional intratubular crystals | 17 | |||
| 3 | 1 | No | 3.5 | None | Intratubular crystal deposits | 40 |
| 4 | None | Subjectively more crystals within the tubules | - | |||
| 4 | 1 | No | 0.75 | None | Diffuse intratubular crystals identified as DHA | 8 |
| 5 | 1 | No | 36 | None | Intratubular crystals assumed to be uric acid | 20 |
| 37 | None | Diffuse crystal nephropathy with tubulointerstitial inflammatory infiltrates; crystals thought to be uric acid | 10 | |||
| 39 | Allopurinol 150 mg | Persistence of intratubular brown crystals | 23 | |||
| 6 | 1 | Yes | 0.1 | None | No crystals detected | Dialysis |
| 0.75 | None | Crystal deposits presumed to be oxalate | 24 | |||
| 1.2 | Allopurinol 300 mg | Crystal deposits suspected to be DHA | 26 | |||
| 2.1 | Allopurinol 300 mg | DHA crystals identified | 43 | |||
| 12 | Febuxostat 80 mg | Crystals present (15%); severe tubular atrophy and interstitial fibrosis (>50%) | 48 | |||
| 7 | 1 | No | 2 | Allopurinol 300 mg | DHA crystals present | 52 |
| 12 | Allopurinol 300 mg | No crystals detected | 47 | |||
| 8 | 2 | Yes | 1 | None | Multiple strongly birefringent DHA crystals in tubules | - |
| 12 | Allopurinol 300 mg | Crystal deposits within tubules and interstitium | - | |||
| 19 | Allopurinol 300 mg | No crystals detected | - | |||
| 3 | Yes | 1 | Allopurinol 300 mg | No crystal detected | - | |
| 4 | No | 0.25 | None | No crystals detected | - | |
| 1.2 | None | Intratubular crystal deposits | - | |||
| 2 | Allopurinol 150 mg × 2/week | Multiple deposits of highly birefringent crystals | 11 | |||
| 9 | 1 | Yes | 0.25 | Allopurinol 300 mg | Acute tubular necrosis; no crystals | - |
| 12 | 1 | No | 0.75 | Allopurinol 600 mg* | - | 51 |
| 4 | Allopurinol 300 mg* | Extensive DHA crystal deposits | 20 | |||
| 5 | Allopurinol 450 mg* | 20–30% reduction in DHA crystals | 20 | |||
| 12 | Allopurinol 300 mg, Febuxostat 80 mg* | Some reduction in DHA crystals | 28 | |||
| 24 | Allopurinol 300 mg, Febuxostat 80 mg* | Rare crystals within the interstitium | 24 | |||
| 36 | Allopurinol 300 mg, Febuxostat 80 mg* | >100 crystals within the parenchyma | 23 | |||
| 2 | No | 2 | Allopurinol 300 mg, Febuxostat 80 mg | Rare intratubular DHA crystal deposits | 69 | |
| 13 | 1 | No | 3 | Allopurinol 400 mg | No crystals detected | 39 |
| 15 | 1 | No | 4 | Allopurinol 600 mg | - | 50 |
| 12 | Allopurinol 600 mg | - | 64 | |||
| 16 | 1 | Yes | 0.3 | Allopurinol 150 mg | DHA crystals in ∼30% of tubules | Dialysis |
| 0.6 | Allopurinol 300 mg | DHA crystals in ∼40% of tubules | Dialysis | |||
| 2 | Allopurinol 600 mg | Subjectively fewer DHA crystals | 33 | |||
| 12 | Allopurinol 300 mg, Febuxostat 80 mg | Scant DHA crystals | 45 | |||
| 17 | 1 | No | 3 | Allopurinol 300 mg | No crystals detected | 99 |
Reported nonadherence to XOR inhibitor treatment.
Abbreviations: DHA, 2,8-dihydroxyadenine; eGFR, estimated glomerular filtration rate; Tx, kidney transplantation.
The shaded area denotes allografts where xanthine oxidoreductase treatment was not initiated prior to kidney transplantation.
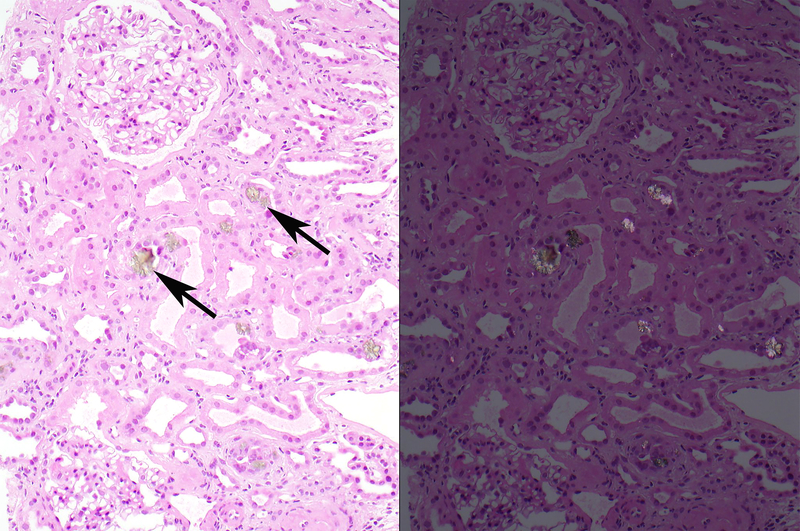
Figure 1.
Recurrent DHA nephropathy in an allograft from 1 of the patients (No 12; Table 2). A. On a hematoxylin and eosin stained section, numerous brown crystals (arrows) are seen within tubular lumens and within the tubular epithelial cytoplasm (left panel). Upon examination under polarized light, these crystals are strongly birefringent (right panel).
B. On high magnification (600x) of the hematoxylin and eosin stained section, small brown crystals (arrows) are often seen within the tubular epithelial cytoplasm.
Eight patients did not receive XOR inhibitor therapy prior to transplantation of 11 kidney allografts (Table 2). In the case of 9 allografts, XOR inhibitor treatment was initiated at a median of 0.1 (0.1–2.9) years posttransplant. Delayed graft function was noted in 2 allografts, both coming from deceased donors, and AKI was observed in 7 transplants. Recurrence of DHA nephropathy was observed in all 10 allograft biopsies that were performed (Table 3), which was significantly more common than in those receiving treatment pretransplant (p=0.004; Table 4). Four allografts belonging to 2 patients were lost due to disease recurrence, 1.3 (0.1–3.4) years posttransplant. Both patients were untreated before and after their first transplant, but initiated treatment with allopurinol 200 mg/day and 300 mg/day at 1 and 7 months following the second transplant, respectively. Five patients who initiated pharmacotherapy posttransplant had persistent biopsy-proven DHA allograft nephropathy, 4 of whom were treated with allopurinol in doses ranging from 150 mg twice a week to 300 mg daily. Three patients died with a functioning graft (Table 2); 1 patient from miliary tuberculosis at 5 months posttransplant (patient No. 3), 1 from breast cancer 5.8 years posttransplant (patient No. 5) and the third one from sepsis 1.4 years following a fourth kidney transplant (patient No. 8). One patient, who had lost 2 allografts due to disease recurrence, expired while on hemodialysis 8 years after the second transplant (patient No. 2). Another patient developed acute liver failure 1.5 years posttransplant, suspected to be drug-induced, though the offending agent was not identified. The patient (No. 6) died while on hemodialysis for AKI 2 months later, in the setting of multiorgan failure.
Table 4.
Allograft outcomes in patients who initiated XOR inhibitor treatment prior to kidney transplantation compared with those who did not receive such treatment until posttransplant, or not at all.
| No XORi therapy pretransplant | XORi therapy pretransplant | p-value | |
|---|---|---|---|
| Number of patients | 8 | 10 | |
| Number of grafts | 11 | 11 | |
| Age at transplant, years | 42.8 (14.9–67.0) | 45.5 (20.7–66.2) | 0.974 |
| Delayed graft function | 2 | 3 | 1.0 |
| Posttransplant acute kidney injury | 7 | 4 | 0.395 |
| eGFR, mL/min/1.73 m2 | |||
| At 6 months | 24.9 (9.6–53.3) [9 grafts] | 61.5 (22.5–93) [10 grafts] | 0.003 |
| At 12 months | 27.5 (10.0–67.5) [9 grafts] | 64.8 (28–93.8) [10 grafts] | 0.035 |
| At 2 years | 16.2 (10.0–39.0) [6 grafts] | 61.3 (24.0–90.0) [8 grafts] | 0.009 |
| Biopsy-proven recurrence of DHA nephropathy | 10 | 3 | 0.004 |
| Graft loss due to recurrence of DHA nephropathy | 4 | 1 | 0.31 |
| Death | 5 | 1 | 0.043 |
| Death with a functioning graft | 3 | 1 | 0.275 |
Data are presented as median (range).
Abbreviations: eGFR, estimated glomerular filtration rate; DHA, 2,8-dihydroxyadenine; XORi, xanthine oxidoreductase inhibitor; AKI, acute kidney injury.
The median eGFR at 6 months posttransplant was 61.5 (22.5–93.0) mL/min/1.73 m2 in patients who received XOR inhibitor therapy pretransplant, while it was 24.9 (9.6–53.3) mL/min/1.73 m2 in those who did not receive such treatment prior to transplantation (p=0.003; Table 4). Similarly, the graft function was superior in the XOR inhibitor-treated group at 2 years posttransplant, with a median eGFR of 61.3 (24.0–90.0) mL/min/1.73 m2 compared with 16.2 (10.0–39.0) mL/min/1.73 m2 in the untreated group (p=0.009; Table 4). At 2 years posttransplant, the allograft survival was 91% in the group receiving XOR inhibitor treatment pretransplant versus 55% in the untreated group, but the difference did not reach statistical significance (p=0.16; Figure 2). In general, patients receiving higher allopurinol doses appeared to be less likely to experience disease recurrence (Tables 2 and 3).
Figure 2.
Kaplan-Meier curve of death-censored kidney allograft survival from the time of kidney transplantation in patients who received treatment with an XOR inhibitor pretransplant (solid line) and those who did not (broken line).
Discussion
In this study of kidney transplant outcomes in patients with APRT deficiency, allograft function was superior in patients who initiated XOR inhibitor therapy pretransplant compared with those who either first started this therapy posttransplant or did not receive such treatment at all. Timely initiation of pharmacotherapy in adequate doses appears to be a major determinant of kidney allograft function in patients with APRT deficiency.
Most previously reported cases of kidney transplantation in patients with APRT deficiency have demonstrated premature allograft loss or chronic allograft dysfunction in patients who were not on XOR inhibitor treatment at the time of transplantation,6,8,10–12,21,22 usually due to missed diagnosis of this rare disorder. Similar observations were also evident in our study, as all 10 allografts biopsies from untreated patients showed disease recurrence, which lead to the premature loss of 4 grafts in 2 patients. These 2 patients were first placed on treatment with allopurinol at 4 weeks and 6 months following their second transplant in the setting of decreased graft function, and subsequently progressed to allograft failure. By contrast, patients who were receiving treatment with an XOR inhibitor at the time of kidney transplantation demonstrated better long-term allograft function and survival. One patient treated with low dose of allopurinol experienced early biopsy-proven disease recurrence which prompted a dose increase. Poor adherence to pharmacotherapy was reported for 1 patient in the present study due to severe episodic eye symptoms, including blurry vision, burning pain and photophobia, resulting in subsequent allograft loss. Although our small study sample precludes meaningful statistical analysis of allograft outcomes, the patients who initiated XOR inhibitor therapy in adequate doses pretransplant and remained compliant with the treatment appeared to experience graft survival similar to what has been reported for kidney transplantation in general.
Delayed graft function requiring dialysis in the first posttransplant week was reported in roughly a quarter of the cases described herein, both in patients who initiated XOR inhibitor therapy before transplantation and those who did not, all of whom received deceased donor transplants. In patients receiving treatment pretransplant, delayed graft function prompted a large increase in allopurinol dosage in 2 of the 3 cases, with subsequent stabilization of allograft function. One of these 2 patients underwent a kidney biopsy, revealing extensive tubular crystal deposition that diminished with higher doses of allopurinol as observed on a repeat biopsy and improved kidney function. A kidney biopsy performed a month later in the third patient on a stable allopurinol dose of 300 mg/day showed signs of acute cellular rejection but no crystal deposits. Two patients who experienced delayed graft function and did not receive treatment with an XOR inhibitor pretransplant had early biopsy-proven disease recurrence. Treatment with allopurinol was subsequently started in both patients. Interestingly, the diagnosis of APRT deficiency had been made some years prior to kidney transplantation in 1 of these 2 patients but treatment with an XOR inhibitor not initiated for unknown reasons. In addition to ischemic kidney injury, DHA crystal nephropathy likely contributed to the delay in allograft function in the cases where XOR inhibitor treatment was lacking or inadequate.6,21,23
Our data clearly demonstrate superior allograft outcomes among patients on XOR inhibitor therapy at the time of kidney transplantation and through the posttransplant period compared with those who did not initiate treatment until several weeks posttransplant, or not at all. Initiation of XOR inhibitor treatment prior to transplantation may be important as the risk of disease recurrence appears to be particularly high in the early posttransplant period. Most patients in our study who received XOR inhibitor therapy pretransplant, had initiated the treatment more than 12 months prior to the transplant surgery. Interestingly, DHA crystal-induced injury seems to be much more agressive in allografts than in native kidneys.3 As plasma DHA measurements are currently unavailable, it has not been determined if and how effectively dialysis clears DHA from plasma. Hence, it is conceivable that DHA may accumulate before transplantation in patients with kidney failure in the absence of XOR inhibitor therapy, flooding the kidney allograft immediately following the transplant surgery, resulting in early graft dysfunction.6,10,11,22 Furthermore, ischemia-reperfusion injury at the time of transplantation may render the graft more susceptible to crystal deposition.
We noted disease recurrence in all allograft biopsies from patients who either did not receive XOR inhibitor treatment pretransplant or received inadequate doses. By contrast, no signs of DHA crystal nephropathy were noted in the majority of transplants treated with allopurinol in the daily dose of 300 mg or greater. One patient who began treatment with a low dose of allopurinol (150 mg/day) before transplantation had early disease recurrence and required hemodialysis for 3 weeks posttransplant. Recurrence of DHA nephropathy in patients treated with similarly low allopurinol doses has been reported previously,6,22 indicating that higher doses are needed. Even in cases of delayed diagnosis, prompt initiation of XOR inhibitor therapy can have beneficial impact on graft outcomes, as seen in the present study where graft function improved in several cases following institution of allopurinol therapy.
The XOR inhibitor dose and duration of treatment pretransplant required to successfully prevent progressive DHA allograft nephropathy is currently not known and requires further study. However, based on our experience we recommend treatment with allopurinol in the dose of 400 mg/day for a minimum of 3 months before the transplantation. Recent data do not suggest increased risk of adverse effects in individuals with advanced CKD,24 and we do not routinely lower allopurinol doses in APRT deficiency patients with reduced kidney function. In fact, patients with progressive allograft dysfunction may need higher allopurinol doses. In patients who do not tolerate allopurinol, febuxostat should be prescribed in the daily dose of 80 mg. Microscopic assessment of crystalluria is widely used to monitor XOR inhibitor treatment but lacks precision and is associated with significant interobserver variations. Our group recently developed a UPLC-MS/MS assay for quantification of urinary DHA that holds great promise for the future.25 However, additional studies must be performed to determine the level of urine DHA that must be achieved to prevent crystal deposits and kidney allograft injury.
Traditionally, DHA crystal deposition has been believed to cause diffuse tubular obstruction, resulting in progressive CKD. More recent studies suggest that inflammatory mechanisms do play an important role in crystal-induced kidney injury, including NLPR3 inflammasome activation provoked by crystal uptake into intracellular lysosomes, leading to nephron destruction.1 Future studies will provide better understanding of the pathobiological mechanisms in crystal nephropathies, hopefully leading to the discovery of novel therapeutic options.
Misinterpretation of kidney biopsy findings was common in the present study, similar to previously reported cases.6,21,26 Observation of crystal deposits in the renal parenchyma should always prompt further evaluation, in which case it is particularly important to rule out 2,8-DHA nephropathy as effective treatment is available. When kidney sections are viewed under light microscopy, the crystals are brown with hematoxylin and eosin stain, have a needle, rod, or rhomboid shape, and are strongly birefringent. Importantly, caution must be taken not to confuse DHA crystals with oxalate deposits.6 Indeed, primary hyperoxaluria was the original histologic diagnosis that was erroneously made in 4 patients (5 allografts) in the current study significantly delaying the initiation of pharmacotherapy.
Our study has limitations, including the small sample size and retrospective observational design which is hampered by variable level of documentation, testing and duration of follow-up. Nevertheless, APRT deficiency is a rare disease and the series of patients presented in this report includes the largest transplant dataset described to date.
In conclusion, this study demonstrates improved allograft outcomes among patients with APRT deficiency who receive treatment with an XOR inhibitor prior to or at the time of kidney transplantation. Thus, increased awareness of among clinicians is imperative for promoting early diagnosis of APRT deficiency and initiation of XOR inhibitor treatment pretransplant. Notably, large doses of allopurinol may be needed to adequately prevent recurrence of DHA nephropathy, apparently 400 mg/day or greater. Moreover, it may be necessary to initiate the treatment several weeks or even months prior to transplantation to minimize the risk of disease recurrence due to enhanced susceptibility of the kidney allograft. As delay in diagnosis and appropriate pharmacotherapy is a major cause of premature graft loss in patients with APRT deficiency, it is important to increase the awareness of the disorder among physicians caring for patients with CKD.
Acknowledgments
Part of this work was presented in an abstract form at the American Society of Nephrology Kidney Week, November 11–16, 2014, PA. The authors are indebted to the following physicians for their invaluable assistance in clinical data and biosample collection: John Lieske (Mayo Clinic, Rochester, MN, USA), David Goldfarb (New York University, New York, NY, USA), Mirna Vucak-Dzumhur (Westmead Hospital, Australia), Gopala Rangan (Westmead Hospital, Australia), Philipp Eller (Medical University of Graz, Graz, Austria), Varun Agrawal (University of Vermont College of Medicine, Burlington, VT, Claudio Musetti and Piero Stratta (Maggiore della Carità Hospital, Novara, Italy), Bharat V. Shah (Global Hospital, Mumbai, India), Chukwuma Eze (Good Samaritan Hospital, Dayton, OH), Amrik Sahota (Rutgers University, Piscataway, NJ, USA), and Lynette Fairbanks (Guy’s and St. Thomas’ Hospital NHS Foundation Trust, London, UK). We would to like to extend our gratitude to Lynn D. Cornell, MD, Associate Professor of Laboratory Medicine and Pathology and Consultant, Division of Anatomic Pathology, Department of Laboratory Medicine and Pathology, Mayo Clinic Rochester MN, USA, for providing the photomicrographs of the kidney biopsy specimens.
Financial Disclosure statement: This study was supported by the Rare Kidney Stone Consortium (U54DK083908), a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research. The Rare Kidney Stone Consortium is funded through collaboration between NCATS and National Institute of Diabetes and Digestive and Kidney Diseases. The authors declare no other funding was received for this study.
ABBREVIATIONS
- AKI
acute kidney injury (AKI)
- APRT
adenine phophoribosyltransferase (APRT)
- CKD
chronic kidney disease
- DHA
2,8-dihydroxyadenine
- eGFR
estimated glomerular filtration rate
- ESKD
end-stage kidney disease
- KDIGO
Kidney Disease: Improving Global Outcomes
- SCr
serum creatinine
- XOR
xanthine oxidoreductase
Footnotes
Conflict of Interest (COI) statement:The authors declare no conflicts of interest.
References
- 1.Mulay SR, Evan A, Anders HJ. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant. 2014;29(3):507–514. [DOI] [PubMed] [Google Scholar]
- 2.Zaidan M, Palsson R, Merieau E, et al. Recurrent 2,8-dihydroxyadenine nephropathy: a rare but preventable cause of renal allograft failure. Am J Transplant. 2014;14(11):2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Runolfsdottir HL, Palsson R, Agustsdottir IM, et al. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis. 2016;67(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edvardsson V, Palsson R, Olafsson I, et al. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis. 2001;38(3):473–480. [DOI] [PubMed] [Google Scholar]
- 5.Bollée G, Dollinger C, Boutaud L, et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21(4):679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasr SH, Sethi S, Cornell LD, et al. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25(6):1909–1915. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Shingde M, Nankivell BJ, et al. Adenine phosphoribosyltransferase deficiency: a potentially reversible cause of CKD. Kidney Int Rep. 2019;4(8):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetto B, Madden R, Kurbanov A, et al. Adenine phosphoribosyltransferase deficiency and renal allograft dysfunction. Am J Kidney Dis. 2001;37(5):E37. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy MJ, McCulloch T, Fairbanks LD, et al. Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient. Nephrol Dial Transplant. 2004;19(3):736–738. [DOI] [PubMed] [Google Scholar]
- 10.de Jong DJ, Assmann KJ, De Abreu RA, et al. 2,8-Dihydroxyadenine stone formation in a renal transplant recipient due to adenine phosphoribosyltransferase deficiency. J Urol. 1996;156(5):1754–1755. [DOI] [PubMed] [Google Scholar]
- 11.Eller P, Rosenkranz AR, Mark W, et al. Four consecutive renal transplantations in a patient with adenine phosphoribosyltransferase deficiency. Clin Nephrol. 2004;61(3):217–221. [DOI] [PubMed] [Google Scholar]
- 12.Gagné ER, Deland E, Daudon M, et al. Chronic renal failure secondary to 2,8-dihydroxyadenine deposition: the first report of recurrence in a kidney transplant. Am J Kidney Dis. 1994;24(1):104–107. [DOI] [PubMed] [Google Scholar]
- 13.Sharma SG, Moritz MJ, Markowitz GS. 2,8-dihydroxyadeninuria disease. Kidney Int. 2012;82(9):1036. [DOI] [PubMed] [Google Scholar]
- 14.Edvardsson VO, Runolfsdottir HL, Thorsteinsdottir UA, et al. Comparison of the effect of allopurinol and febuxostat on urinary 2,8-dihydroxyadenine excretion in patients with adenine phosphoribosyltransferase deficiency (APRTd): a clinical trial. Eur J Intern Med. 2018;48:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaglia M, Musetti C, Ghiggeri GM, et al. Unexpectedly high prevalence of rare genetic disorders in kidney transplant recipients with an unknown causal nephropathy. Clin Transplant. 2014;28(9):995–1003. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skali H, Uno H, Levey AS, et al. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162(3):548–554. [DOI] [PubMed] [Google Scholar]
- 19.Levin A, Stevens PE, Bilous RW, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 20.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- 21.Brown HA. Recurrence of 2,8-dihydroxyadenine tubulointerstitial lesions in a kidney transplant recipient with a primary presentation of chronic renal failure. Nephrol Dial Transplant. 1998;13(4):998–1000. [DOI] [PubMed] [Google Scholar]
- 22.Kaartinen K, Hemmilä U, Salmela K, et al. Adenine phosphoribosyltransferase deficiency as a rare cause of renal allograft dysfunction. J Am Soc Nephrol. 2014;25(4):671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertram A, Broecker V, Lehner F, et al. Kidney transplantation in a patient with severe adenine phosphoribosyl transferase deficiency: obstacles and pitfalls. Transpl Int. 2010;23(9):e56–e58. [DOI] [PubMed] [Google Scholar]
- 24.Thurston MM, Phillips BB, Bourg CA. Safety and efficacy of allopurinol in chronic kidney disease. Ann Pharmacother. 2013;47(11):1507–1516. [DOI] [PubMed] [Google Scholar]
- 25.Thorsteinsdottir M, Thorsteinsdottir UA, Eiriksson FF, et al. Quantitative UPLC-MS/MS assay of urinary 2,8-dihydroxyadenine for diagnosis and management of adenine phosphoribosyltransferase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1036–1037:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edvardsson VO, Goldfarb DS, Lieske JC, et al. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol. 2013;28(10):1923–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]