Abstract
Background.
Most studies of immune dysregulation in perinatal mood and anxiety disorders have focused on peripheral cytokines, but literature from non-perinatal mood disorders also implicates T-cell defects. We sought to characterize proportions of T-cell subtypes in women with postpartum depression.
Materials and Methods.
We enrolled 21 women with postpartum depression (PPD), 39 healthy postpartum controls, and 114 healthy non-postpartum women. Blood was collected in sodium-heparin EDTA tubes and was analyzed using flow cytometry. We conducted statistical tests including linear regression analysis that were aimed at determining differences in proportions of T cell populations among groups.
Results.
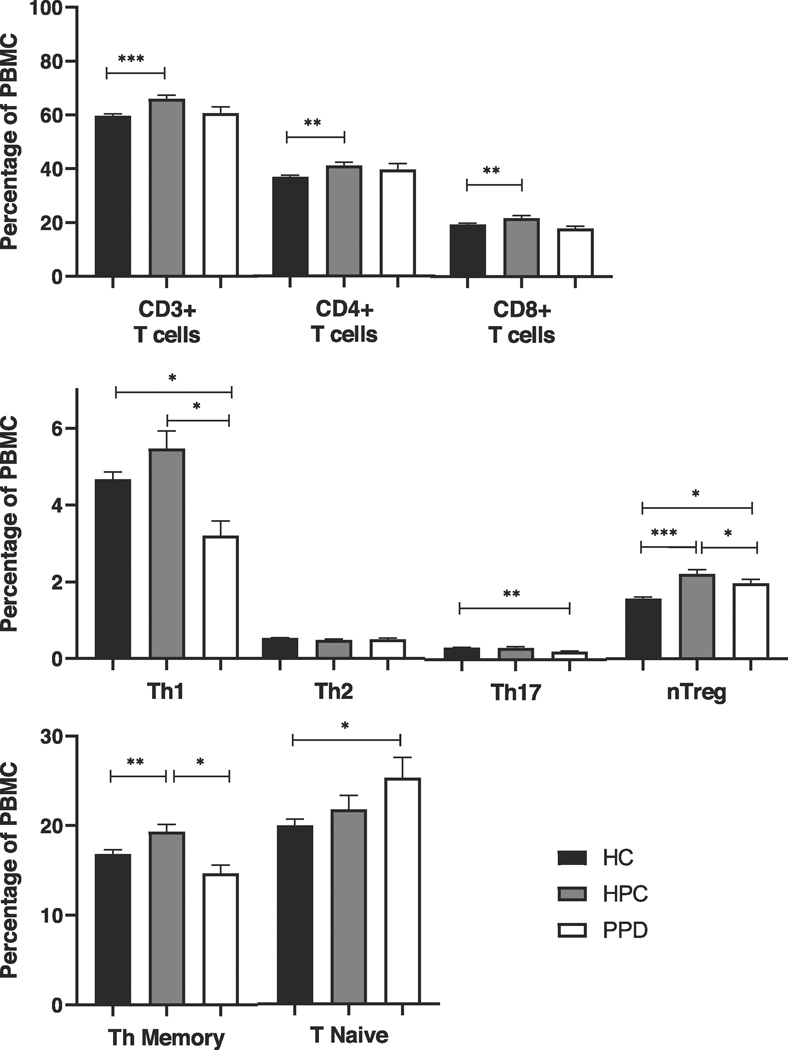
Mean counts of T-cells (all CD3+ T cells), T-helper cells, (CD3+CD4+ T cells), and T-cytotoxic cells (CD3+CD8+ T cells) were significantly increased in healthy postpartum women compared to healthy non-postpartum controls (p < 0.001, p = 0.007, and p = 0.002, respectively), but not in women with PPD. The increases in healthy postpartum women were driven by increases in TH1 cells and T regulatory cells, increases that were nonexistent or attenuated in women with postpartum depression. Mean counts of CD4+ T-helper memory cells were also increased in healthy postpartum women (p = 0.009), but slightly decreased in women with PPD (p = 0.066), when compared to healthy non-postpartum controls.
Conclusions.
Our study confirms that the postpartum period in healthy women is a time of enhanced T cell activity. Women with postpartum depression failed to show physiological enhanced T-cell activity postpartum, and future research is needed to elucidate etiological mechanisms and consequences.
Keywords: T cells, immune, pregnancy, postpartum, depression, mood
INTRODUCTION
It is now well established that immune system dysregulation plays a role in major depressive disorder, with numerous studies linking peripheral cytokine alterations to depressed mood (Dowlati et al., 2010; Howren et al., 2009) and others showing that anti-inflammatory treatment can help some depressed patients (those with elevated inflammatory markers at baseline) (Haroon et al., 2018; Raison et al., 2013). Because immune dysregulation appears to play a role only for certain subsets of depressed people, it has been a logical progression to investigate the role of the immune system in psychiatric illness during the perinatal period. It is a time of known immune dysregulation and one of the few periods in life when there is an obvious biological trigger (parturition) that can be linked to psychiatric symptoms – specifically, to symptoms of perinatal depression, including the diagnosed depressive disorders that occur in up to 15–20% of women (Gaynes et al., 2005), with potentially devastating effects on women and families.
Early research on the physiological immune dysregulation of the peripartum focused on immune suppression during pregnancy, then on a supposed shift away from T-helper type 1 (TH1) activity and toward T-helper type 2 (TH2) activity (Larocca et al., 2008). More recent work has focused on a more complex model, with enhancement of innate immune barriers but reduced effectiveness of some elements of adaptive immunity across pregnancy (Chen et al., 2012; Holtan et al., 2015; Kraus et al., 2010; Pazos et al., 2012). In the postpartum, healthy women appear to have a rebound of adaptive immunity, in particular a rebound in T-cell activity, that has been identified in both animal and human literature (Bergink et al., 2013; Calcagni and Elenkov, 2006; Wegienka et al., 2011). Moreover, research on T-cell activity in depressive and anxiety disorders outside of pregnancy indicates deficiencies of T regulatory cells as well as dysregulation of TH17 cells (Grosse et al., 2016; Osborne et al., 2019). T regulatory cells have also been shown to decrease in response to acute stress (Freier et al., 2010).
In light of this work on immune dysregulation in healthy pregnancy and in mood and anxiety disorders, numerous researchers have attempted to link immune dysfunction to both antenatal and postpartum depression, with mixed success. Most of these studies have focused on a small number of peripheral cytokines as markers of immune function (Osborne and Monk, 2013). A few recent studies have measured large numbers of peripheral markers and attempted to come up with summary variables (Brann et al., 2017; Edvinsson et al., 2017) – an improvement in technique that has nevertheless not yet yielded a useful measurement tool. In addition, many studies in the perinatal period have conflated antenatal and postpartum depression, therefore making it difficult to draw conclusions about new-onset depression in the postpartum, a type of illness that may carry its own unique genetic signature representing distinct biological pathways (McEvoy et al., 2017).
Despite the relatively large number of studies – including our own (Osborne et al., 2018) – that have focused on peripheral cytokines, this may not be the ideal way to measure the relationship between immune function and psychopathology. It is unclear whether there is a correlation between levels of cytokines in the periphery and those in the central nervous system. One recent study, in perinatal depression, found no correlation between cytokines in the periphery and those measured in cerebrospinal fluid (Miller et al., 2019). Relatively few studies, by contrast, have examined either antenatal or postpartum depression in relationship to shifts among classes of immune cells. One early study found a negative association between T-cell count and dysphoria, but did not examine shifts among different types of cells within the T-cell compartment (Hucklebridge et al., 1994). Examining such shifts may give us important information about the biological mechanisms of perinatal depression, and may also yield novel therapeutic targets.
When first released from the thymus, T cells are “naïve”; upon presentation with antigen, they proliferate and differentiate into effector cells. Once the antigen has been cleared, 95% of the effector cells die, and the remainder take up long-term residence as memory cells (Mahnke et al., 2013). The effector subgroups are identifiable by the panel of cytokines they secrete. Cytotoxic T cells are characterized by the surface marker CD8+, and directly attack damaged cells. Helper T cells (CD4+) coordinate the immune response, and are further subdivided into several groups. T-helper 1 cells (TH1) and T-helper 17 cells (TH17) are involved in the activation of macrophages and secrete IFN-γ, among others, and IL-17, respectively. T-helper 2 cells (TH2) cells secrete IL-4 and IL-5, among others, and are involved in the activation of B cells. The regulator subgroup is formed by the natural T-regulatory cells, which dampen the activity of TH1, TH2, and TH17 cells (Mousset et al., 2019; Osborne et al., 2019; Piccinni, 2011; Saito et al., 2010). Our own group and one other have examined shifts among T cell classes in postpartum psychosis (PPP), another devastating but rare postpartum psychiatric illness (Bergink et al., 2013; Kumar et al., 2017). Our study showed that women with PPP failed to show the T-cell elevation characteristic of healthy postpartum women. Kumar’s group found that women with PPP failed to show an elevation in naïve T-helper cells that was characteristic of healthy postpartum women, but also showed higher levels of both cytotoxic T cells and T-regulatory cells. In addition, T-cell dysregulation has also been shown in numerous studies of mood disorders outside the perinatal period (Grosse et al., 2016; Snijders et al., 2019; Snijders et al., 2016).
Given this paucity of information, we therefore sought to expand the available evidence concerning immune cells and particularly T cell populations in postpartum depression by comparing women with severe PPD (with postpartum onset only) to both healthy postpartum controls and healthy women who were neither pregnant nor postpartum.
MATERIALS AND METHODS
Participants
This study protocol was approved by the institutional review board of the Erasmus Medical Center, Rotterdam (original protocol number MEC-2005226). After receiving a complete description of the study, all subjects provided written informed consent. Twenty-one (n=21) women with an acute postpartum onset of severe depression (PPD) were recruited from the Mother-Baby Inpatient Unit of the Department of Psychiatry of the Erasmus University Medical Center in Rotterdam, the Netherlands, between April 2007 and February 2012. All subjects were diagnosed according to DSMIV-TR (First, 1996) using the Structural Clinical Interview for DSM-IV (SCID – 1/P research version). Symptoms were additionally tracked using the Edinburgh Postnatal Depression Scale (EPDS). Those subjects diagnosed with PPD vi the SCID had a mean EPDS score of 18 (SEM 1.3). The relevant DSM-IV-TR diagnoses included both major depressive disorder alone (n=13) and major depressive disorder comorbid with anxiety disorders (n=8). Recent research in postpartum depression has indicated that there are distinct clinical phenotypes (Putnam et al., 2017), most of which include a significant anxiety component, and we therefore deemed it important to include both of these populations.
All women had an onset of symptoms within six months following delivery, and 14 had an onset within 4 weeks postpartum (67%). Those with a history of bipolar disorder, non-puerperal psychotic episodes, substance abuse, or psychiatric symptoms during pregnancy were excluded from the study. The median onset of symptoms occurred at day 7 postpartum (IQR 0.5–40.0). Mean time of blood collection occurred at day 61 postpartum. Of these 21 subjects, at the time of blood collection, nine were using benzodiazepines (median 2 days), two were using antipsychotics (seven and nine days), and one was using antidepressant medication (one day). Women admitted to our ward with depressive symptoms have an antidepressant-free observation period, which enabled us to enroll the majority of subjects before the start of antidepressant treatment. Eleven subjects had a previous history of non-puerperal depressive or anxiety symptoms. Physical examination and routine laboratory screening were performed at the time of study enrollment to confirm the absence of infection or other hematological abnormalities. All subjects were in an acute disease state at the moment of blood collection.
The healthy postpartum control group (HPC) consisted of 39 age-matched healthy postpartum women recruited between January 2009 and March 2012 (Erasmus MC, Rotterdam), with an EPDS score ≤10 (mean 3.8; SEM 0.4 ) at the time of postpartum blood sampling at mean 31 days postpartum.
One hundred twenty-four age-matched healthy non-postpartum women were included as an additional control group (HC). Inclusion criteria for both healthy postpartum and healthy non-postpartum women included the absence of any medical, neurologic, psychiatric, or autoimmune disorders, as well as having no current or recent clinical evidence of acute infection. All blood draws, from cases and controls, occurred in the morning, allowing us to minimize diurnal variations in immune factors.
Blood collection and preparation
Blood was collected in sodium-heparin tubes (30 ml) in the morning and transported to the laboratory at room temperature. Peripheral blood mononuclear cell (PBMC) suspensions were isolated using low-density gradient centrifugation by Ficoll (GE Healthcare, Uppsala, Sweden) within 8 hours. PBMCs were counted and frozen in medium (RPMI-1640 containing 25mM Hepes and UltraGlutamine (Lonza, Verviers, Belgium), with the addition of 10% fetal calf serum (Lonza), 10% dimethylsulfoxide (Merck, Hohenbrunn, Germany) and 1% Penicillin/Streptomycin) and stored in liquid nitrogen to enable testing case and control immune cells in the same experiment.
Flow cytometric analysis
PBMCs were defrosted and washed once with medium. Average recovery of cells after thawing was 82% and viability 97%, as determined by Trypan blue staining. Differences between different groups were not observed. Two different staining procedures were used: staining A determined percentages of different types of T cells, and staining B determined T helper subsets. (Details of the staining methods are included in supplemental information.)
All specific staining antibodies used are routinely tested for effectiveness by the manufacturer and titrated for optimal concentrations in our laboratory. Specificity of the staining antibodies was controlled using five isotype controls provided by the manufacturer (BD) and background positivity was negligible (between 0.2% and 1% of the specific staining depending on the isotype control, both in patient samples and controls).
Stained samples were analyzed by 8-color flow cytometry on a FACS Canto II (BD biosciences) and analyzed by FlowJo software (Tree Star, Ashland, OR, USA). Gating strategy for staining B is given in supplemental figure 1. T cell subsets of staining B were expressed as percentages of total lymphocytes, which could reliably be detected as a clear population in forward sideward scatter after the 4-hr culture.
Data exploration of flow cytometry data also revealed 10 outlier HC women (>3 SD). In accord with our statistician we decided to exclude all data of these healthy controls from further analysis including sociodemographic characteristics, leaving an N=114 in our HC group.
Statistical Analysis
Statistical analysis was performed using SPSS version 24.0. Sample characteristics were evaluated using Chi2 tests or Fisher’s exact test (if cell sizes were <5), and independent samples t-tests. Immune cell data were mean and standard error of the mean (SEM). To compare immune cell data between PPD women, HPC, women, and HC women, we used separate linear regression analyses (e.g. HC vs HPC and PPD; and HPC vs PPD). Comparisons with HC women were adjusted for body mass index (BMI). Comparisons between HPC and PPD women were adjusted for BMI, postpartum day of blood draw, and educational level. Confounders (e.g. BMI, postpartum day of blood draw, and educational level) were selected based on the existence of a significant associations with both predictor (sample) and outcome variable (immune cell data, see Supplemental Table 1). We report Cohen’s delta alongside p-values to represent the size of the difference. Normality of the data was explored visually using histograms and Q-Q plots, and tested statistically using Shapiro-Wilk tests. Normality of the error distribution was checked in the context of the regression analyses. Analyses were performed using untransformed immune cell data.
RESULTS
Sample Characteristics
We analyzed 21 PPD subjects, 39 HPC, and 114 HC. There were no differences in age, weight, ethnicity, marital status, gravidity, parity, delivery by Caesarean section, and delivery by vacuum extraction between women with PPD and HPC women (Table 1). Women with PPD had a higher BMI compared to HPC (p=.029). The HPC women were more likely to have education beyond high school (p=.020). Blood draw took place later after partus in PPD women than in HPC women (p<.001). The majority of HPC women were breastfeeding (71.8%), while very few PPD subjects were (4.8%, p<0.001). Demographic characteristics for HC women included only weight and BMI, hence we were unable to compare other demographic characteristics with the HC women.
Table 1.
General and obstetric characteristics of subject with first-onset postpartum depression (PPD), healthy postpartum controls (HPC), and healthy non-postpartum controls (HC)
| HC (n=114) | HPC (n=39) | PPD (n=21) | Difference between HPC and PPD | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | P | |
| Age (years) | 30.25 | (.61) | 33.00 | (.67) | 32.09 | (1.12) | 0.492 |
| Weight | 67.88 | (.92) | 71.55 | (1.67) | 74.66 | (2.81) | 0.322 |
| BMI | 23.12 | (.27) | 23.88 | (0.50) | 26.97 | (0.96) | 0.029 |
| Blood withdrawal, days postpartum | 30.97 | (2.84) | 61.00 | (8.50) | <0.001 | ||
| n | % | n | % | ||||
| Dutch ethnicity | 35/39 | 89.7% | 17/20 | 85.0% | 0.594 | ||
| Education beyond high school | 37/39 | 94.9% | 14/19 | 73.7% | 0.020 | ||
| Married/ cohabiting | 36/39 | 92.3% | 18/20 | 90.0% | 0.763 | ||
| Primiparity | 24/39 | 61.5% | 16/21 | 76.2% | 0.251 | ||
| Primigravidity | 22/39 | 56.4% | 13/21 | 61.9% | 0.681 | ||
| Caesarean section | 5/39 | 12.8% | 5/21 | 23.8% | 0.276 | ||
| Vacuum extraction | 4/39 | 10.3% | 3/21 | 14.3% | 0.687 | ||
| Breastfeeding | 28/39 | 71.8% | 1/21 | 4.8% | <0.001 | ||
| Medication use | 12/21 | 57.1% | |||||
HC = healthy non-postpartum controls, HPC = healthy postpartum controls, PPD = patients with postpartum depression
Percentages of Overall T Cells among Peripheral Blood Mononuclear Cells (PBMCs)
Mean counts of T cells (all CD3+ T cells), T-helper cells, (CD3+CD4+ T cells) and T-cytotoxic cells (CD3+CD8+ T cells) were significantly increased in HPC compared to HC women (p < 0.001, p = 0.007, and p = 0.002, respectively; see Table 2). For PPD women, this was not the case; the mean count of CD8+ cells fell somewhat below that of HC women, while the mean count for CD4+ cells was intermediate between those of HC and HPC women, with no significant differences in either case. Mean counts of CD4+ T-helper memory cells (measured in staining B) were increased in HPC women compared to HC women, (p = 0.009), but somewhat decreased in the PPD women compared to HC women (p = 0.066). The mean count of T-helper naïve cells (calculated) was increased in PPD women as compared to HC (p = 0.045), with HPC women’s levels in between the other two groups.
Table 2.
Proportions of T cell subsets across all three groups.
| Percentage of Peripheral Blood Mononuclear Cells (PBMCs) | HC N=114 | HPC N=39 | PPD N=21 | HC vs. HPC HC | HC vs. PPD | HPC vs. PPD | |||
|---|---|---|---|---|---|---|---|---|---|
| M (SEM) | M (SEM) | M (SEM) | p value1 | Cohen’s d | p value1 | Cohen’s d | p value2 | Cohen’s d | |
| CD3+ T cells | 59.74 (0.62) | 66.02 (1.35) | 60.65 (2.36) | <0.001 | 0.83 | 0.458 | 0.10 | 0.122 | 0.56 |
| CD4+ T cells | 36.87 (0.65) | 41.02 (1.30) | 39.65 (2.20) | 0.007 | 0.55 | 0.431 | 0.33 | 0.260 | 0.15 |
| CD8+ T cells | 19.35 (0.42) | 21.68 (1.06) | 17.85 (0.91) | 0.002 | 0.41 | 0.994 | 0.35 | 0.428 | 0.70 |
| TH1 | 4.67 (0.19) | 5.47 (0.46) | 3.20 (0.38) | 0.032 | 0.33 | 0.031 | 0.81 | 0.069 | 0.99 |
| TH2 | 0.53 (0.01) | 0.48 (0.03) | 0.50 (0.04) | 0.082 | 0.30 | 0.390 | 0.19 | 0.826 | 0.12 |
| TH17 | 0.31 (0.01) | 0.31 (0.03) | 0.21 (0.02) | 0.896 | 0.01 | 0.006 | 0.57 | 0.365 | 0.55 |
| T reg | 1.57 (0.04) | 2.21 (0.11) | 1.96 (0.11) | <0.001 | 1.09 | 0.030 | 0.82 | 0.044 | 0.42 |
| TH memory | 16.83 (0.48) | 19.32 (0.81) | 14.68 (0.94) | 0.009 | 0.49 | 0.066 | 0.46 | 0.017 | 0.99 |
| TH naive (calculated) | 19.91 (0.69) | 21.70 (1.53) | 25.22 (2.27) | 0.333 | 0.21 | 0.045 | 0.60 | 0.356 | 0.36 |
Adjusted for BMI
Adjusted for BMI, postpartum day of blood draw, and educational level.
Percentages of TH1, TH2, TH17, and T regulatory cells
We next sought to separate out T-cell subsets by testing in staining B for the capacity of CD4+ cells to produce the characteristic cytokines of TH1, TH2, and TH17 cells, and for the intracellular presence of the transcription factor FOXP3 (characteristic of regulator cells). We saw substantial differences between HC and HPC and between PPD and HPC (Table 2). In HPC women, the rise in CD4+ T-helper cells was due to a rise in TH1 cells and T regulatory cells (p = 0.032 and p < 0.001, respectively) compared to HC women. Mean counts of TH2 and TH17 cells did not differ between these two groups. In PPD women, by contrast, these rises were nonexistent or attenuated. TH1 cells were even lower than in HC women (p = 0.069 vs. HPC and 0.031 vs. HC), and T regulatory cell counts were intermediate between the other two groups (p = 0.044 vs. HPC and 0.030 vs. HC). TH2 cells again did not differ between groups, but TH17 cells were somewhat lower in PPD women than in both other groups (p = 0.365 vs. HPC and 0.006 vs. HC). While not all differences reached statistical significance, effect sizes in some cases were substantial (see Table 2).
DISCUSSION
Our study clearly confirms that the postpartum period in healthy women is a time of altered immune activity, with increases in T cells compared to the non-postpartum period. The postpartum increases in T cells involved both the CD8+ cytotoxic and CD4+ helper T cells and were seen in both the T-helper naïve and memory populations. We also found that both the pro-inflammatory TH1 and the immune suppressive T-regulatory cells were increased. Previous research, though scarce, has also shown that pregnancy and the postpartum condition persistently affect these lymphocyte populations. In the 1990s a Japanese group (Watanabe et al., 1997) showed that T-regulatory cells were increased in early pregnancy, while the number of T-cytotoxic cells decreased. In late pregnancy, T-helper cell numbers decreased. After delivery, T-helper cells, T-cytotoxic cells, and T-suppressor cells increased for a period of up to half a year. The investigators took these observations as indicating that early pregnancy alterations were related to the tolerance of the fetus, late pregnancy alterations to maintenance of pregnancy, and postpartum alterations to the combat of infections. The postpartum alterations could also explain the increased incidence of some autoimmune disorders postpartum (including multiple sclerosis and autoimmune thyroiditis) (Langer-Gould et al., 2010; Shi et al., 2009; Weetman, 2010).
Additional literature has supported a pattern of lymphocyte suppression during pregnancy followed by rebound after delivery in T-helper memory cells in particular. Matthiessen and colleagues found that T-helper memory cells decreased substantially during pregnancy and began to rebound early in the postpartum (at 2–7 days), still remaining lower than pre-pregnancy levels (Matthiesen et al., 1996). Kieffer and colleagues (Kieffer et al., 2017) looked much later in the postpartum (6 months) and found significantly higher proportions of T-helper memory cells in parous compared to nulligravid women, indicating that pregnancy persistently affects the pre-pregnancy CD4+ memory cell pool in human peripheral blood. Collectively, the two studies on T-helper memory cells support our own finding of a clear increase in T cells, including T-helper memory cells, in healthy women in the postpartum period, and it is tempting to speculate that these increases serve a physiological role in healing processes and in combatting infections in this vulnerable period and may also represent a tolerance induction toward paternal antigens (as speculated by (Kieffer et al., 2019)).
Women with postpartum depression, however, displayed a remarkably different pattern. The failure of postpartum depressed subjects to mount a physiological T-cell activation in the postpartum period is consistent with our earlier findings in postpartum psychosis subjects (Bergink et al., 2013). The abnormal apportioning of subsets here is also comparable to that found in our postpartum psychosis subjects: Cells with a TH1 potential (IFN-γ production) were reduced in PPD compared to HPC and HC women controls, as were TH17 cells, while cells with an immune suppressive capability (T regulatory cells) were significantly less activated as compared to the HPC women. It was particularly notable that T-helper memory cells failed to rise and were even reduced when compared to HPC women. Memory T cells, which remember previously encountered antigens through exposure to semen, fetal cells in pregnancy, or microchimerism, are thought to play a key role in fetal-maternal tolerance. Preeclampsia is considered to be a disease of immune maternal-fetal incompatibility, and a recent study showed lower memory T cells not only during pregnancy but also postpartum in women who had preeclampsia during pregnancy compared to healthy controls (Kieffer et al., 2019). We earlier showed high co-occurrence of preeclampsia and postpartum mood disorders (Bergink et al., 2015), and lower memory T cells (and maternal-fetal incompatibility) might be evidence of a relationship in their underlying pathophysiology.
Some of these differences we found were more pronounced than others, and it may be that with a larger sample size these less pronounced differences would become more clear. The one category in which we saw not even a glimmer of difference between the two postpartum groups was in the TH2 cells, indicating that this is primarily a story of cells associated with pro-inflammatory action (i.e., TH1 and TH17) and the cells that suppress that action (T-reg).
This inability of postpartum depressed and postpartum psychotic women to mount a physiological T-cell immune activation in the postpartum period suggests a defect in the T-cell system. Indeed, older research on functional T-cell parameters (such as lymphocyte stimulation assays) delivers evidence for such a defect in the T-cell system intrinsic to those with a major mood disorder (Toben and Baune, 2015). We reported that subjects with a major depressive episode (outside the postpartum period) were characterized by decreased serum levels of the T-cell growth factors IL-7 and sCD25 and by mildly reduced levels of T-helper and T-regulatory cells (Grosse et al., 2016). Snijders and colleagues similarly reported reduced levels of T cells and T-helper cells in children of a bipolar parent (at high risk for a mood disorder) from adolescence to young adulthood (Snijders et al., 2016), and another study from the same group showed that the familial liability to develop bipolar disorder determined the reduced levels of T cells (Snijders et al., 2019). Two other groups also confirmed shifts in the T-helper populations, with T regulatory cells decreased and TH17 and TH2 cells increased in bipolar disorder (Becking et al., 2018; Vogels et al., 2017) in contrast to the decreases in TH17 cells characteristic for unipolar depression (Becking et al., 2018). In sum, there is ample evidence that T-cell defects mark mood disorders, and our data here indicate that this pattern extends to postpartum depression as well.
Of course, merely establishing a connection between T-cell defects and postpartum affective disorders does not significantly advance our science about either the results or the causes of such disorders. If women with PPD have T cell defects, are they in fact more vulnerable to postpartum infections? Is their tolerance to paternal antigens in future pregnancies lest robust than that of healthy women? To our knowledge there are no data supporting a higher infection rate or an increased spontaneous abortion rate in subsequent pregnancies for women suffering from a postpartum depression (though a higher infection rate has been described in major depressed individuals in general) (Kohler-Forsberg et al., 2019). T cell defects might also have substantial effects on brain development and white matter integrity. Poletti and colleagues (Poletti et al., 2017) found the percentage of circulating TH17 cells to correlate positively with white matter integrity, particularly in fiber tracts connecting the forebrain with the limbic system, in both healthy controls and bipolar depressed subjects. The frequency of circulating T-regulatory cells correlated positively with white matter tract disruption in these areas and to lower neuronal responses to negative versus positive morally tuned stimuli in the right dorsolateral prefrontal cortex of bipolar depressed subjects.
With regard to the origin of the T-cell defects in subjects with mood disorders, particularly in the postpartum, a few putative mechanisms come to mind. Tryptophan is an essential growth factor for T cells, and reduced tryptophan levels are a hallmark of mood disorders. In a previously published paper we showed reduced tryptophan levels in both postpartum depression and postpartum psychosis (Veen et al., 2016), and it is tempting to speculate that these reduced tryptophan levels are related to the T cell defects. Other groups have had similar findings (Duan et al., 2018; Teshigawara et al., 2019). It may also be that non-depressed people have the ability to buffer a decrease in tryptophan that occurs for all women after childbirth, as increases in cortisol spur immune activity that downregulates the metabolism of tryptophan into serotonin (Duan et al., 2018). Substantial work is clearly needed on the connection between these T-cell defects and other findings showing increases in inflammatory activity postpartum, measured primarily in cytokines (Brann et al., 2017; Osborne and Monk, 2013) – we may need to look more at function of different immune cell populations than at number. In this case, the population may prove illuminating, as our sample was limited to women who developed new-onset symptoms in the postpartum and many cytokine studies include women who were or may have been depressed in pregnancy as well.
Another possibility is the interaction with pregnancy hormones. Sex steroids and prolactin are known to influence T-cell growth and differentiation (Recalde et al., 2018), and altered levels of these hormones have been suggested in at least some studies of postpartum mood disorders, though evidence is mixed (McEvoy and Osborne, 2019; Osborne et al., 2017; Schiller et al., 2015). Studies that link endocrine alterations to those of the immune system in postpartum mood disorders do not yet exist.
Our study has a number of limitations. While we were able to include reasonably large control groups, the sample of women with postpartum depression is quite small, given the stringent inclusion criteria (postpartum onset, severe depression, largely antidepressant-free). Because we wanted to restrict to postpartum onset, our group of cases does not match the DSM-V definition, which requires onset during pregnancy or within 4 weeks postpartum. We may, therefore, have missed small differences among groups that would have been evident in a larger population (but thus also avoided the heterogeneity common to studies on PPD that have less strict inclusion criteria). In addition, we did not have sociodemographic details other than weight and BMI for our non-postpartum healthy controls, so it is possible that some of our results reflect differences between groups that are affected by these characteristics. Only one of our subjects was taking antidepressant medication, so we were unable to control for this variable in our analyses. Other limitations may come from possible differences among samples in the amount of time spent in storage (though we are aware of no literature that addresses whether such differences actually exist), or from the timing difference in the blood draw between groups. We are reassured on the latter point, however, because we controlled for this difference in our analyses, and limited previous literature actually supports an increase in T-helper activity across the postpartum period, which would mean that our PPD subjects (who had the later blood draw) should show HIGHER and not LOWER activity than our healthy subjects (Watanabe et al., 1997). In addition, our T-cell data do not represent absolute counts per ml of blood but are instead relative to numbers of PBMCs and lymphocytes; future studies would benefit from measuring the absolute numbers of leukocytes per ml blood at the time of testing.
Despite these limitations, our work adds to the small but growing number of investigations of immune alterations in perinatal mood disorders that attempts to reach beyond measures of peripheral cytokines to look at other immune system defects that may play an etiological role in these devastating illnesses. Our clear results showing that healthy postpartum women show a rebound of T-cell activity, particularly in pro-inflammatory activity (TH1) and compensatory mechanisms (Treg) is consistent with consistent with previous literature on pregnancy. Our finding that postpartum depressed women do not mount this response adds to our previous similar results in a population of women with postpartum psychosis, and adds to the growing body of literature indicating that T-cell dysregulation may be an important feature of mood disorders. Future research characterizing these differences in larger populations, and extending into different classes of immune cells, will be instructive
Supplementary Material
Percentage of T-cells and T-cell subset populations (as percentage of lymphocytes) in three populations: postpartum depression (PPD, N=21, in white); healthy postpartum controls (HPC, N=39, in gray); and healthy non-postpartum controls (HC, N=114, in black).
Highlights.
Little is known about T-cell functioning in postpartum depression (PPD)
We compared women with postpartum depression to two groups of healthy controls
Healthy postpartum women had higher mean T-cell counts than non-perinatal women
Women with PPD failed to show this physiological enhanced T-cell activity
Acknowledgments
We greatly appreciate Annemarie Wijkhuijs for her technical assistance. We thank statistician Andre Wierdsma for pointing out we should move beyond a world governed by “p<0.05” – don’t say “ statistically significant,” but instead remember ATOM – “Accept uncertainty, be Thoughtful, Open, and Modest” (Wasserstein et al., 2019).
Role of the Funding Source
Dr. Osborne’s work is supported by the NIMH (K23 MH110607–01A1) and the Doris Duke Early Clinician Investigator Award. This work was funded by MOODSTRATIFICATION, European Union (EU) project no: 754740, and by MOODINFLAME, EU project no: 222963 (coordinated by Prof. Drexhage, department of Immunology, ErasmusMC). The funding organizations had no further role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Supplemental information: Supplemental Figure 1; Supplemental Table 1; Supplemental Methods
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becking K, Haarman BCM, Grosse L, Nolen WA, Claes S, Arolt V, Schoevers RA, Drexhage HA, 2018. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. Journal of neuroimmunology 319, 28–36. [DOI] [PubMed] [Google Scholar]
- Bergink V, Burgerhout KM, Weigelt K, Pop VJ, de Wit H, Drexhage RC, Kushner SA, Drexhage HA, 2013. Immune system dysregulation in first-onset postpartum psychosis. Biological psychiatry 73, 1000–1007. [DOI] [PubMed] [Google Scholar]
- Bergink V, Laursen TM, Johannsen BM, Kushner SA, Meltzer-Brody S, Munk-Olsen T, 2015. Pre-eclampsia and first-onset postpartum psychiatric episodes: a Danish population-based cohort study. Psychol Med 45, 3481–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann E, Papadopoulos F, Fransson E, White R, Edvinsson A, Hellgren C, Kamali-Moghaddam M, Bostrom A, Schioth HB, Sundstrom-Poromaa I, Skalkidou A, 2017. Inflammatory markers in late pregnancy in association with postpartum depression-A nested case-control study. Psychoneuroendocrinology 79, 146–159. [DOI] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I, 2006. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences 1069, 62–76. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Liu YL, Sytwu HK, 2012. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clinical & developmental immunology 2012, 258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biological psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Duan KM, Ma JH, Wang SY, Huang Z, Zhou Y, Yu H, 2018. The role of tryptophan metabolism in postpartum depression. Metab Brain Dis 33, 647–660. [DOI] [PubMed] [Google Scholar]
- Edvinsson A, Brann E, Hellgren C, Freyhult E, White R, Kamali-Moghaddam M, Olivier J, Bergquist J, Bostrom AE, Schioth HB, Skalkidou A, Cunningham JL, Sundstrom-Poromaa I, 2017. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 80, 15–25. [DOI] [PubMed] [Google Scholar]
- Freier E, Weber CS, Nowottne U, Horn C, Bartels K, Meyer S, Hildebrandt Y, Luetkens T, Cao Y, Pabst C, Muzzulini J, Schnee B, Brunner-Weinzierl MC, Marangolo M, Bokemeyer C, Deter HC, Atanackovic D, 2010. Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology 35, 663–673. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC, 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence report/technology assessment (Summary), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Hoogenboezem T, Ambree O, Bellingrath S, Jorgens S, de Wit HJ, Wijkhuijs AM, Arolt V, Drexhage HA, 2016. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain, behavior, and immunity 54, 38–44. [DOI] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC, Miller AH, 2018. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95, 4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan SG, Chen Y, Kaimal R, Creedon DJ, Enninga EA, Nevala WK, Markovic SN, 2015. Growth modeling of the maternal cytokine milieu throughout normal pregnancy: macrophage-derived chemokine decreases as inflammation/counterregulation increases. Journal of immunology research 2015, 952571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL1, and IL-6: a meta-analysis. Psychosomatic medicine 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Hucklebridge FH, Smith MD, Clow A, Evans P, Glover V, Taylor A, Adams D, Lydyard PM, 1994. Dysphoria and immune status in postpartum women. Biological psychology 37, 199–206. [DOI] [PubMed] [Google Scholar]
- Kieffer TE, Faas MM, Scherjon SA, Prins JR, 2017. Pregnancy persistently affects memory T cell populations. Journal of reproductive immunology 119, 1–8. [DOI] [PubMed] [Google Scholar]
- Kieffer TEC, Laskewitz A, Scherjon SA, Faas MM, Prins JR, 2019. Memory T Cells in Pregnancy. Frontiers in immunology 10, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, Mors O, Benros ME, 2019. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA psychiatry 76, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, Loubeau M, Ge Y, Garrido JL, Rodriguez-Garcia M, Moran TM, 2010. Peripheral blood cytokine profiling during pregnancy and post-partum periods. American journal of reproductive immunology (New York, N.Y. : 1989) 64, 411–426. [DOI] [PubMed] [Google Scholar]
- Kumar MM, Venkataswamy MM, Sathyanarayanan G, Thippeswamy H, Chandra PS, Mani RS, 2017. Immune system aberrations in postpartum psychosis: An immunophenotyping study from a tertiary care neuropsychiatric hospital in India. Journal of neuroimmunology 310, 8–13. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Gupta R, Huang S, Hagan A, Atkuri K, Leimpeter AD, Albers KB, Greenwood E, Van Den Eeden SK, Steinman L, Nelson LM, 2010. Interferon-gamma-producing T cells, pregnancy, and postpartum relapses of multiple sclerosis. Archives of neurology 67, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca L, Ramhorst R, Roca V, Calafat M, Aisemberg J, Franchi A, Perez Leiros C, 2008. Neuroimmune-endocrine interactions during early pregnancy in an autoimmune context: focus on macrophage activation. Neuroimmunomodulation 15, 84–90. [DOI] [PubMed] [Google Scholar]
- Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E, 2013. The who’s who of T-cell differentiation: human memory T-cell subsets. European journal of immunology 43, 2797–2809. [DOI] [PubMed] [Google Scholar]
- Matthiesen L, Berg G, Ernerudh J, Hakansson L, 1996. Lymphocyte subsets and mitogen stimulation of blood lymphocytes in normal pregnancy. American journal of reproductive immunology (New York, N.Y. : 1989) 35, 70–79. [DOI] [PubMed] [Google Scholar]
- McEvoy K, Osborne LM, 2019. Allopregnanolone and reproductive psychiatry: an overview. International review of psychiatry (Abingdon, England) 31, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy K, Osborne LM, Nanavati J, Payne JL, 2017. Reproductive Affective Disorders: a Review of the Genetic Evidence for Premenstrual Dysphoric Disorder and Postpartum Depression. Current psychiatry reports 19, 94. [DOI] [PubMed] [Google Scholar]
- Miller ES, Sakowicz A, Roy A, Yang A, Sullivan JT, Grobman WA, Wisner KL, 2019. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. American journal of obstetrics and gynecology 220, 271.e271–271.e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset CM, Hobo W, Woestenenk R, Preijers F, Dolstra H, van der Waart AB, 2019. Comprehensive Phenotyping of T Cells Using Flow Cytometry. Cytometry. Part A : the journal of the International Society for Analytical Cytology. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Brar A, Klein SL, 2019. The role of Th17 cells in the pathophysiology of pregnancy and perinatal mood and anxiety disorders. Brain, behavior, and immunity 76, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL, 2017. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology 79, 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 38, 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Yenokyan G, Fei K, Kraus T, Moran T, Monk C, Sperling R, 2018. Innate immune activation and depressive and anxious symptoms across the peripartum: An exploratory study. Psychoneuroendocrinology 99, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Sperling RS, Moran TM, Kraus TA, 2012. The influence of pregnancy on systemic immunity. Immunologic research 54, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni MP, 2011. Possible role of Th1, Th2 and Th17 CD4+ T helper subpopulations on human pregnancy development. Advances in Neuroimmune Biology 2, 105–110. [Google Scholar]
- Poletti S, de Wit H, Mazza E, Wijkhuijs AJM, Locatelli C, Aggio V, Colombo C, Benedetti F, Drexhage HA, 2017. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain, behavior, and immunity 61, 317–325. [DOI] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, Deligiannidis KM, Payne J, Altemus M, Newport J, Apter G, Devouche E, Viktorin A, Magnusson P, Penninx B, Buist A, Bilszta J, O’Hara M, Stuart S, Brock R, Roza S, Tiemeier H, Guille C, Epperson CN, Kim D, Schmidt P, Martinez P, Di Florio A, Wisner KL, Stowe Z, Jones I, Sullivan PF, Rubinow D, Wildenhaus K, Meltzer-Brody S, 2017. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry 4, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde G, Moreno-Sosa T, Yudica F, Quintero CA, Sanchez MB, Jahn GA, Kalergis AM, Mackern-Oberti JP, 2018. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmunity reviews 17, 504–512. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M, 2010. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. American Journal of Reproductive Immunology 63, 601–610. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR, 2015. The role of reproductive hormones in postpartum depression. CNS Spectrums 20, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Li C, Li Y, Guan H, Fan C, Teng Y, Ouyang Y, Shan Z, Teng W, 2009. Circulating lymphocyte subsets and regulatory T cells in patients with postpartum thyroiditis during the first postpartum year. Clinical and experimental medicine 9, 263–267. [DOI] [PubMed] [Google Scholar]
- Snijders G, Brouwer R, Kemner S, Bootsman F, Drexhage HA, Hillegers MHJ, 2019. Genetic and environmental influences on circulating NK and T cells and their relation to bipolar disorder. International journal of bipolar disorders 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders G, Schiweck C, Mesman E, Grosse L, De Wit H, Nolen WA, Drexhage HA, Hillegers MHJ, 2016. A dynamic course of T cell defects in individuals at risk for mood disorders. Brain, behavior, and immunity 58, 11–17. [DOI] [PubMed] [Google Scholar]
- Teshigawara T, Mouri A, Kubo H, Nakamura Y, Shiino T, Okada T, Morikawa M, Nabeshima T, Ozaki N, Yamamoto Y, Saito K, 2019. Changes in tryptophan metabolism during pregnancy and postpartum periods: Potential involvement in postpartum depressive symptoms. J Affect Disord 255, 168–176. [DOI] [PubMed] [Google Scholar]
- Toben C, Baune BT, 2015. An Act of Balance Between Adaptive and Maladaptive Immunity in Depression: a Role for T Lymphocytes. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 10, 595–609. [DOI] [PubMed] [Google Scholar]
- Veen C, Myint AM, Burgerhout KM, Schwarz MJ, Schutze G, Kushner SA, Hoogendijk WJ, Drexhage HA, Bergink V, 2016. Tryptophan pathway alterations in the postpartum period and in acute postpartum psychosis and depression. J Affect Disord 189, 298–305. [DOI] [PubMed] [Google Scholar]
- Vogels RJ, Koenders MA, van Rossum EF, Spijker AT, Drexhage HA, 2017. T Cell Deficits and Overexpression of Hepatocyte Growth Factor in Anti-inflammatory Circulating Monocytes of Middle-Aged Patients with Bipolar Disorder Characterized by a High Prevalence of the Metabolic Syndrome. Frontiers in psychiatry 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, Schirm AL, Lazar NA, 2019. Moving to a World Beyond “p < 0.05”. The American Statistician 73, 1–19. [Google Scholar]
- Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, Amino N, 1997. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. American journal of reproductive immunology (New York, N.Y. : 1989) 37, 368–377. [DOI] [PubMed] [Google Scholar]
- Weetman AP, 2010. Immunity, thyroid function and pregnancy: molecular mechanisms. Nature reviews. Endocrinology 6, 311–318. [DOI] [PubMed] [Google Scholar]
- Wegienka G, Havstad S, Bobbitt KR, Woodcroft KJ, Zoratti EM, Ownby DR, Cole Johnson C, 2011. Within-woman change in regulatory T cells from pregnancy to the postpartum period. Journal of reproductive immunology 88, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.