In recent years, compelling evidence has emerged that chronic activation of inflammatory physiology is implicated in physical and mental health conditions, such as cardiovascular disease, diabetes, depression, and schizophrenia. Since the refinement of more sensitive immunoassays in the 1990s, many studies have used C-reactive protein (CRP) as a reliable and sensitive index of both the acute phase reaction and more sustained inflammatory activation. CRP concentrations typically are below 3 mg/L, but can rise above 500 mg/L during acute illness. When investigating the role of chronic inflammation in human health, it is critically important to differentiate chronic inflammation from an acute inflammatory challenge (e.g., infection/tissue damage) because we typically would expect CRP levels to decrease once an acute challenge resolves. To accurately exclude acutely sick participants, current best practice is to systematically remove all observations when CRP values exceed 10 mg/L (95.2 nmol/L). However, this criterion may inadvertently exclude individuals of interest to studies of human health.
According to a recent meta-analysis, 42% of 33 studies removed observations with CRP values >10 mg/L, while 12% applied a more idiosyncratic approach to identify extreme values (e.g., 3 SDs above the mean) (1). Despite this appearance of consensus, providing a clear justification for this criterion was rare. Designation of 10 mg/L as a cut-off may emanate from a 1981 paper (2) that reported that 90% of CRP values fell below the lower limit of detection (3 mg/L) and 99% were <10 mg/L in 468 volunteer blood donors. It was solely on the basis that the likelihood of observing CRP values >10 mg/L was small – using outdated assay methods – that the authors concluded: “values greater than 10 mg/L are strongly suggestive of an on-going pathological process.” This cut-off has persisted despite evidence from older (3) and more recent publications (4) that CRP values >10 mg/L are associated with factors other than acute infection. There also is considerable variability of CRP both within and across disease states. In 370 consecutive hospitalized adult patients, median CRP values differed significantly between bacterial infections (120 mg/L), inflammatory diseases (65 mg/L), solid tumor (46 mg/L), non-bacterial infection (32 mg/L), and cardiovascular disease: (6 mg/L). Yet, 33% of patients had CRP values less than 10 mg/L (5). It is now clear that many factors influence circulating CRP values that are unrelated to “pathological” processes (e.g., age, sex, socioeconomic status, race, body mass index, exercise, diet, sleep, medication use) and potential guidelines are available for researchers when deciding to include, exclude, and control for these factors (4). Thus, it may not always be advisable to routinely exclude participants solely on the basis of one high CRP value. For example, Palousa et al. (1986) found that whereas 40% of CRP values >10 mg/L likely were related to acute respiratory infections, 20% seemed to be associated with smoking behavior. Moreover, the distribution of CRP values currently measured in the US population differs profoundly from the 468 volunteer blood donors in the 1981 study. In a nationally representative non-institutionalized sample of US adults assessed from 1999–2010, 30–40% exhibited CRP levels >3 mg/L (6) compared to the 10% of the distribution referenced above. These differences may be due to sample characteristics, such as inclusion of other racial/ethnic groups in more recent samples who tend to have higher CRP than participants from European backgrounds. Another potential explanation is the dietary and lifestyle changes that have occurred over the last 40 years, which affect CRP levels, such as the steady increase in obesity among both children and adults (7).
Regardless of the specific factors that have resulted in higher levels of CRP, excluding participants from studies may affect the generalizability of findings. Truncated samples will reduce statistical power with unintended ramifications for replication and reproducibility. Could discrepant results arise from strategic decisions about how to handle higher CRP values? Equally important, by excluding CRP values >10 mg/L, are individuals of potential interest excluded? For instance, the smokers identified by Palosuo et al. (1986) likely would be of central interest to many studies of human health. Heritable factors also can influence circulating levels of CRP (8), as does assay method, kit manufacturer, and operator skill/experience (9). Perhaps of greatest concern, when the factor under study (e.g., depression) correlates with high CRP values, then eliminating individuals with such values may decrease the likelihood of observing an important relationship that actually exists in nature. Thus, it is important to consider whether removing participants with CRP values >10 mg/L unintentionally excludes individuals of interest to researchers, either because participants engage in more unhealthy behaviors (e.g., smoking, drinking, sedentary), have a heritable propensity for high CRP, or were assessed using a specific methodological approach.
In conclusion, CRP values >10 mg/L are not always indicative of acute infection/injury. Instead, a more thoughtful approach that recognizes the influence of demographic, behavioral, and technical factors is needed. The question then becomes how best to limit the impact of inherent bias while not inadvertently including sick participants? First, additional steps could be taken to increase confidence that CRP values >10 mg/L are indicative of an acute immune challenge, either through using a clinical index of the acute phase response/infection, such as interferon gammainduced protein-10 (10), and/or by taking participants’ temperature and screening for infection/injury symptoms – it is very likely that this simple screening would identify the suspected cause of very high CRP values (i.e., CRP >50). Second, one could rule out a competing explanation of elevated CRP by simultaneously evaluating hepatic health with a routine blood biochemistry panel and examining certain liver enzymes (i.e., AST/ALT), which could indicate fatty liver disease. Third, when including extreme values in analyses, researchers may wish to perform a statistical Winsorization, which preserves their rank position in the distribution while lessening skewness. Finally, and potentially most importantly, reporting analyses with extreme values included and then excluded would enable researchers to more thoroughly understand the data.
We hope this commentary will stimulate more discussion and transparency on best practices for employing biomarkers related to inflammation in psychoneuroimmunology research.
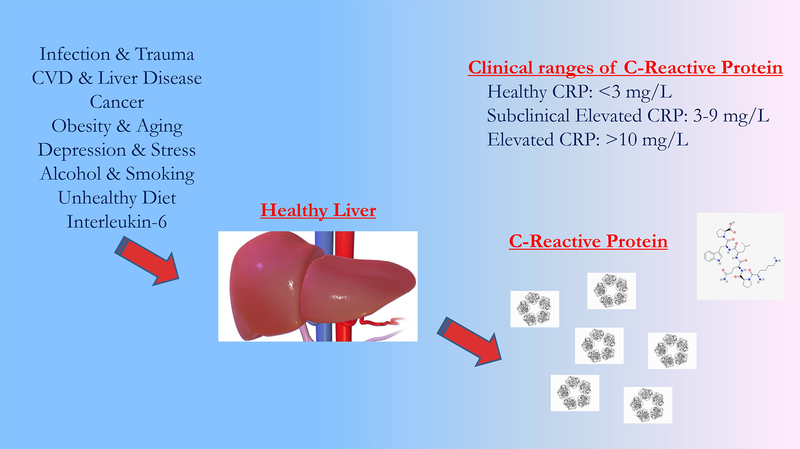
Figure 1.
Potential pathways leading to elevations in C-Reactive Protein.
Acknowledgments
This research was supported by National Institute of Mental Health Grants MH079369 and MH101168 awarded to Lauren Alloy, National Institute of Mental Health Grant MH096478 awarded to Lauren Ellman and National Research Service Awards F31MH118808 to Naoise Mac Giollabhui.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Naoise Mac Giollabhui, Temple University.
Lauren M. Ellman, Temple University
Christopher L. Coe, University of Wisconsin-Madison
Michelle L. Byrne, University of Oregon
Lyn Y. Abramson, University of Wisconsin-Madison
Lauren B. Alloy, Temple University
References
- 1.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis and meta-regression. Unpublished Manuscript [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shine B, de Beer FC, Pepys MB (1981): Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta. 117:13–23. [DOI] [PubMed] [Google Scholar]
- 3.Palosuo T, Husman T, Koistinen J, Aho K (1986): C-reactive protein in population samples. Acta Med Scand. 220:175–179. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. (2009): To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 23:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshet R, Boursi B, Maoz R, Shnell M, Guzner-Gur H (2009): Diagnostic and prognostic significance of serum C-reactive protein levels in patients admitted to the department of medicine. The American journal of the medical sciences. 337:248–255. [DOI] [PubMed] [Google Scholar]
- 6.Ong KL, Allison MA, Cheung BM, Wu BJ, Barter PJ, Rye KA (2013): Trends in C-reactive protein levels in US adults from 1999 to 2010. Am J Epidemiol. 177:1430–1442. [DOI] [PubMed] [Google Scholar]
- 7.Kushner I, Rzewnicki D, Samols D (2006): What does minor elevation of C-reactive protein signify? The American journal of medicine. 119:166. e117–166. e128. [DOI] [PubMed] [Google Scholar]
- 8.Retterstol L, Eikvar L, Berg K (2003): A twin study of C-Reactive Protein compared to other risk factors for coronary heart disease. Atherosclerosis. 169:279–282. [DOI] [PubMed] [Google Scholar]
- 9.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA (2008): ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. The journals of gerontology Series A, Biological sciences and medical sciences. 63:879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. (2011): CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 22:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]