Abstract
Although schizophrenia is defined by waking phenomena, a growing literature documents a deficit in sleep spindles, a defining oscillation of stage 2 non-rapid eye movement sleep. Compelling evidence supports an important role for spindles in cognition, and particularly memory. In schizophrenia, although the spindle deficit correlates with impaired sleep-dependent memory consolidation, recent clinical trials find that increasing spindles does not improve memory. This may reflect that sleep-dependent memory consolidation relies not on spindles alone, but also on their precise temporal coordination with cortical slow oscillations and hippocampal sharp-wave ripples. Consequently, interventions to improve memory in schizophrenia must not only increase spindles, but also preserve or enhance slow oscillations, hippocampal ripples and their temporal relations. Because hippocampal ripples and the activity of the thalamic spindle generator are difficult to measure noninvasively, screening potential interventions requires complementary animal and human studies. In this review we (i) propose that sleep oscillations are novel pathophysiological targets for therapy to improve cognition in schizophrenia; (ii) summarize our understanding of how these oscillations interact to consolidate memory; (iii) suggest that a systems neuroscience strategy is essential to selecting and evaluating effective treatments, and illustrate this with findings from clinical trials; and (iv) selectively review the interventional literature relevant to sleep and cognition, covering both pharmacological and noninvasive brain stimulation approaches. We conclude that coordinated sleep oscillations are promising targets for improving cognition in schizophrenia and that effective therapies will need to preserve or enhance sleep oscillatory dynamics and restore function at the network level.
Keywords: schizophrenia, sleep spindles, slow oscillations, hippocampal ripples, memory, brain stimulation
Cognitive deficits are integral to schizophrenia.
Schizophrenia generally strikes in late adolescence or early adulthood and affects ~1% of the population worldwide. In addition to its psychosocial costs, its economic costs are staggering, with the largest share resulting from unemployment (Cloutier et al., 2016). Cognitive deficits contribute to poor functional outcome (Green et al., 2000) and the vast majority of individuals with schizophrenia are unable to work (Insel, 2009). Cognitive deficits often predate the onset of symptoms and persist throughout the course of schizophrenia, even after the florid psychotic symptoms have been effectively controlled with antipsychotic drugs. The persistence of cognitive deficits in patients, and their presence in some unaffected family members, has led some investigators to call for a reconceptualization of schizophrenia as a cognitive disorder, with psychosis as a late and potentially preventable consequence (Cohen and Insel, 2008). Ameliorating cognitive deficits is a priority of the schizophrenia research community and has been the focus of large-scale studies (e.g., Buchanan et al., 2007; Marder et al., 2004), yet effective treatment is lacking. Clearly, novel approaches to understanding the pathophysiology of cognitive deficits are needed to identify therapeutic targets and treatment biomarkers that will guide the development of novel interventions to improve function and possibly even prevent onset. In this review we (i) propose that sleep oscillations are novel pathophysiological targets for therapy to improve cognition in schizophrenia; (ii) summarize our understanding of how these oscillations interact to consolidate memory; (iii) suggest that a systems neuroscience strategy is essential to selecting and evaluating effective treatments, and illustrate this with findings from a recent clinical trial; and (iv) selectively review the interventional literature relevant to sleep and cognition, covering both pharmacological and noninvasive brain stimulation approaches. We conclude that coordinated non-rapid eye movement (NREM) sleep oscillations are promising targets for improving cognition in schizophrenia and that effective therapies will need to preserve or enhance sleep oscillatory dynamics and restore function at the network level.
Reduced sleep spindles in schizophrenia may impair cognition.
Although schizophrenia is defined by waking phenomena, a growing literature documents sleep spindle deficits both in patients and their first-degree relatives (Manoach and Stickgold, 2019). Sleep spindles are a defining oscillation of Stage 2 NREM sleep (N2) that are seen in the EEG as brief (~1s) powerful bursts of 11-16Hz activity organized in a waxing/waning envelope (Iber et al., 2007). Sleep spindles are generated in the thalamic reticular nucleus (TRN), which is abnormal in schizophrenia (Court et al., 1999; Smith et al., 2001; Steullet et al., 2018). Compelling evidence supports an important role for spindles in cognition, and particularly memory. In experimental models, spindle-like activity induces massive influxes of calcium ions into cortical pyramidal cells and triggers the intracellular mechanisms that are involved in long-term potentiation, a neural mechanism of memory (Sejnowski and Destexhe, 2000). In humans, spindles correlate with IQ, measures of learning ability and the sleep-dependent consolidation of both procedural and declarative memory (for review, see Fogel and Smith, 2011). EEG, magnetoencephalography, intracortical electrocorticography and simultaneous functional MRI and EEG studies converge in showing that learning a task leads to increased sleep spindle activity in the regions that were involved in learning, and that this activity correlates with memory consolidation (Bang et al., 2014; Clemens et al., 2006; Johnson et al., 2012; Tamaki et al., 2013). These findings suggest that spindle activity in brain networks involved in learning mediates the reactivation, transformation and strengthening of memory traces acquired during the day (i.e., consolidation). Although most data linking sleep spindles to cognition are correlational, there is a burgeoning literature in humans suggesting a causal role. Increasing spindles or sigma power (12-15 Hz), which corresponds to the spindle frequency band, either pharmacologically via zolpidem (Kaestner et al., 2013; Mednick et al., 2013) or via transcranial stimulation (Lustenberger et al., 2016; Marshall et al., 2006) improves sleep-dependent memory consolidation, while stimulation that decreases sigma power impairs memory (Marshall et al., 2011). Consistent with this basic literature, in schizophrenia, spindle deficits have been associated with impaired sleep-dependent consolidation of both procedural and declarative memory and predict lower IQ and worse executive function (for review see, Manoach et al., 2016). Studies showing that enhancing spindles improves memory in healthy individuals provide an impetus to target spindle physiology to ameliorate cognitive deficits in schizophrenia.
Increasing spindles alone is insufficient to improve memory.
Several studies have attempted to improve cognition in schizophrenia by manipulating sleep. In a small sample of patients, compared to sham stimulation, transcranial direct current stimulation during N2 improved verbal declarative memory (word-list recall), but not motor procedural memory (mirror tracing) and had no significant effects on (unspecified) sleep parameters, which presumably included spindles (Goder et al., 2013). In a placebo-controlled trial that did not include polysomnography, eszopiclone (Lunesta), a non-benzodiazepine sedative hypnotic, improved working memory in schizophrenia, but not symptoms (Tek et al., 2014). Eszopiclone prolongs inhibitory postsynaptic currents in GABAA receptors on neurons in the thalamic spindle generator, the TRN, thereby increasing the burst firing that initiates spindles (Jia et al., 2009). This activity profile of eszopiclone motivated us to examine its effects on spindles and sleep-dependent memory consolidation in schizophrenia.
In a randomized placebo-controlled pilot study, eszopiclone significantly increased spindles in schizophrenia, which correlated with overnight memory improvement, but its effect on memory was not significant (Wamsley et al., 2013). This might have reflected inadequate power due to the small sample size (n=11 placebo, n=10 eszopiclone) and that memory is a less direct manifestation of drug effects than spindles. In a subsequent larger randomized placebo-controlled crossover-design clinical trial, eszopiclone again significantly increased spindles and spindles correlated with memory, but disappointingly, eszopiclone again failed to improve memory, this time in healthy controls as well as patients with schizophrenia (Baran et al., 2017). This raises an obvious question, if spindles are a mechanism of memory consolidation, why does increasing them with eszopiclone not improve memory?
Coordinated NREM sleep oscillations are targets for improving memory.
To understand the basis of this failure, we turned to an emerging literature showing that sleep-dependent memory consolidation relies not on spindles alone, but on their interactions with hippocampal sharp-wave ripples (SWRs; ~100-200Hz) and cortically-generated slow oscillations (SOs; .5 – 1.25 Hz). SOs are large amplitude oscillations that arise from the rhythmic depolarization and hyperpolarization of large populations of cortical pyramidal neurons, which represent periods of neuronal excitability (upstates) and inhibition (downstates), respectively (Steriade et al., 1993). SOs group sleep spindles, which preferentially peak during their excitable upstates (Cox et al., 2018; Demanuele et al., 2017), and hippocampal SWRs, which nest in spindle troughs (Clemens et al., 2007; Clemens et al., 2011; Molle and Born, 2011; Siapas and Wilson, 1998; Staresina et al., 2015) a triple phase-locking that has been demonstrated to improve memory (Latchoumane et al., 2017). Hippocampal SWRs are associated with highly synchronous neural firing in the hippocampus and correspond to the reactivation of specific memories (Ji and Wilson, 2007; Wilson and McNaughton, 1994). The precisely timed orchestration of these three cardinal NREM oscillations is thought to mediate the transformation of memories from dependence on temporary indexing in the hippocampus to more permanent representation in the cortex (Siapas and Wilson, 1998) (Figure 1).
Figure 1:
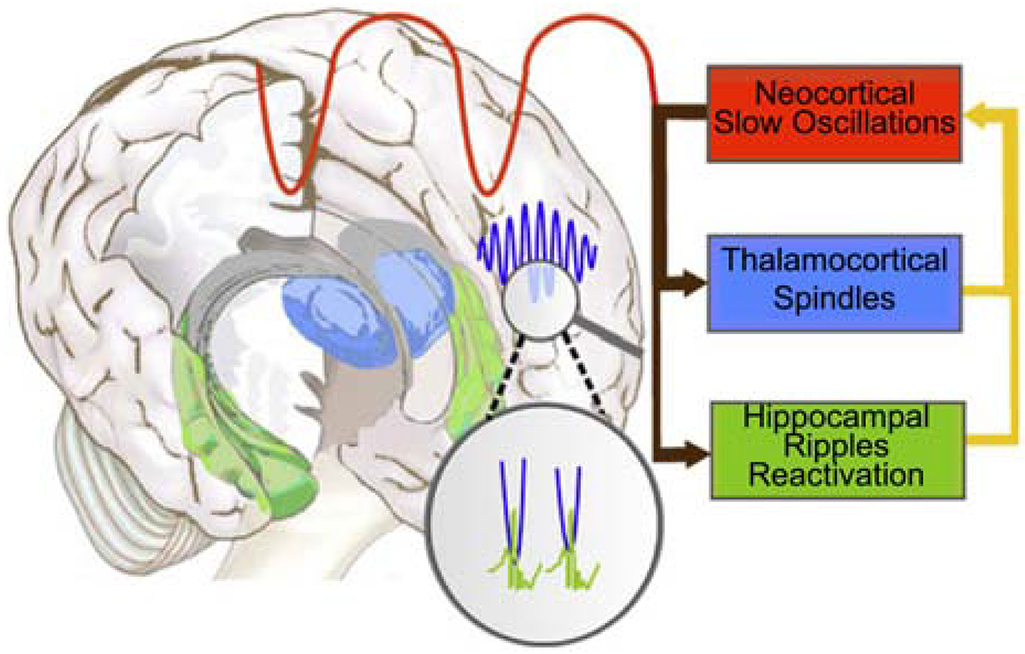
The coordination of sleep spindles with hippocampal ripples and neocortical slow oscillations in the service of consolidating new memories during sleep (adapted from Born and Wilhelm, 2012). During NREM sleep, neocortical slow oscillations drive the reactivation of hippocampal memory representations during sharp wave ripples (green) in the hippocampus together with spindles (blue) in the TRN. Hippocampal ripples nest in the troughs of spindles, which occur during the up states of slow oscillations. This dialogue between slow oscillations, spindles and hippocampal ripples is thought to mediate the transfer of selected new memories from temporary dependence on the hippocampus to longer-term representation in the neocortex (Siapas and Wilson, 1998).
The importance of this coordination for memory is supported by recent findings of both human and rodent studies. In mice, optogenetic induction of spindles on the rising phase of SOs leads to enhanced memory consolidation while suppression of spindles at this phase impairs consolidation (Latchoumane et al., 2017). In humans, the coupling of SOs with spindles degrades in older adults, and is associated with increased forgetting (Helfrich et al., 2018). Finally, in schizophrenia, although the timing of spindle-SO coupling appears to be intact, both the density of spindles (number per minute) and their temporal coordination with SOs predict overnight memory improvement and together the prediction is significantly better than either variable alone (Demanuele et al., 2017). This suggests that both the density of spindles and their timing in relation to SOs are important for memory.
Successful interventions will have to preserve or enhance network interactions.
This literature implies that interventions to improve sleep-dependent memory consolidation in schizophrenia must not only increase thalamocortical spindles, but must also preserve or enhance cortical SOs, hippocampal SWRs and their temporal relations. If eszopiclone, or any other intervention that increases spindles, does not preserve all three oscillations and their precise temporal coordination, it may fail to improve memory. This is illustrated by our recent randomized placebo-controlled clinical trial of eszopiclone in patients with schizophrenia and healthy controls (Baran et al., 2017). Importantly, regardless of drug or group, spindle, spindle-SO coordination and SO parameters were highly stable within individuals across nights allowing us to attribute any changes seen to eszopiclone rather than night to night variation. This stability, also observed in other studies, suggests NREM oscillations as traits of the sleep EEG and has led to their description as electrophysiological “fingerprints” (Bodizs et al., 2009; Cox et al., 2018; De Gennaro et al., 2005; Finelli et al., 2001).
Regardless of whether participants were taking placebo or eszopiclone, spindles peaked in SO upstates (Figure 2a, Mylonas et al., 2019). Spindle density at a central electrode (Cz) predicted overnight improvement on a motor procedural memory task in schizophrenia, replicating previous findings (Demanuele et al., 2017; Wamsley et al., 2012). But there was a significantly stronger effect for the density of coupled spindle-SO events, which predicted memory in a cluster of predominantly right frontal and central electrodes that included Cz and did not differ by group (Mylonas et al., 2019). These electrodes overlay the right motor cortex, contralateral to the hand that trained on the motor memory task (Figure 2b). The location of the correlated electrode cluster suggests that coupled spindle-SO events in motor regions involved in learning the task mediated its sleep-dependent consolidation. This interpretation is consistent with studies showing that regionally specific increases in sleep spindle activity after learning correlate with enhanced memory performance after awakening (Bang et al., 2014; Clemens et al., 2006; Johnson et al., 2012; Tamaki et al., 2013). The findings further suggest that the density of coupled spindle-SO events is a better biomarker of memory consolidation than spindle density alone.
Figure 2:
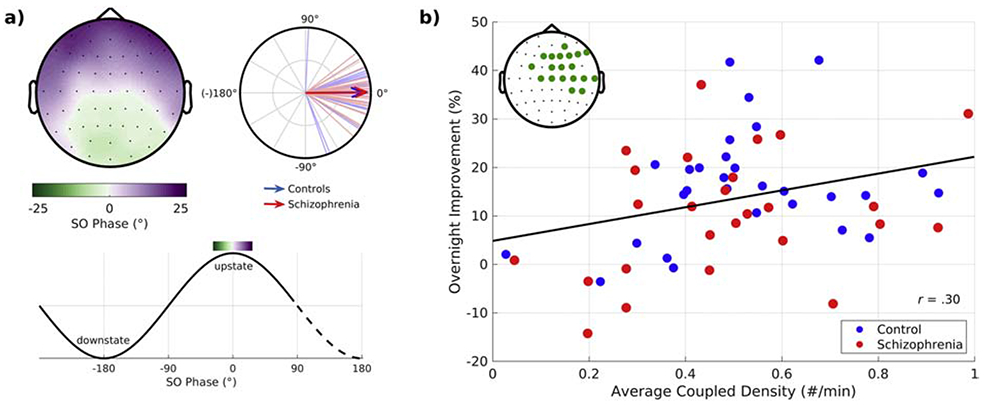
The temporal coordination of spindles with slow oscillations (SOs) in relation to sleep-dependent memory consolidation. a) SO phase at spindle peak. Top row: Topographical maps show mean SO phase at the peak of the spindle averaged across schizophrenia and healthy control participants. The circular plot shows the SO phase distributions across participants at Cz. Lines represent the mean SO phase for each participant. Arrows represent group means and their length represents the consistency of the SO phase across participants. Bottom row: Mapping of SO phase to the topographical map. b) Overnight improvement on a procedural motor memory task it is plotted against the density of coupled spindle-SO events in the electrode cluster showing a significant correlation (inset shows the electrodes comprising the significant cluster). The regression line for the combined groups is shown.
In addition to increasing spindle density, eszopiclone increased the density of spindle-SO coupled events. Since both the density of spindles and spindle-SO coupled events correlated with overnight memory improvement, why did eszopiclone fail to improve memory? The answer may be that eszopiclone also disrupted the timing of spindle-SO coupling by rendering it less consistent (i.e., the phase of the SO upstate at which spindles peaked was significantly more variable on eszopiclone). The precise timing of the spindle peak in the excitable SO upstate is thought to facilitate the cortical plasticity necessary for memory formation, and SOs are thought to orchestrate these phase relationships (Molle and Born, 2011). Eszopiclone also changed the morphology of SOs, decreasing their amplitude across all electrodes and increasing their duration in a large, primarily frontocentral, cluster of electrodes. Decreased SO amplitude, which could reflect that fewer neurons were recruited in SO expression, correlated with less consistent spindle-SO coupling. This suggests that lower amplitude SOs were less able to consistently couple spindles. These findings suggest that eszopiclone disrupts the fidelity of the thalamocortical dialogue necessary for the consistent, precisely timed coupling of spindles with SOs that mediates memory. Collectively, this body of work suggests that to improve memory in schizophrenia, an intervention needs to not only increase spindles, but also to preserve or enhance SOs and their temporal coordination with spindles.
Complementary animal and human studies are necessary to evaluate candidate interventions.
During NREM sleep, cortical SOs coordinate spindle generation in the TRN and the reactivation of memory representations during hippocampal SWRs (Ji and Wilson, 2007; Wilson and McNaughton, 1994). Most human studies are limited to measuring spindles and SOs as the measurement of hippocampal SWRs requires invasive recordings. A recent study of amnestic patients with hippocampal damage, however, demonstrates the importance of the hippocampus to sleep-dependent memory consolidation. While an intact hippocampus was not necessary for learning a procedural memory task, it was necessary for its consolidation over a night of sleep (Schapiro et al., 2019). In schizophrenia, the hippocampus is abnormal (Heckers and Konradi, 2010) and postmortem studies show a selective loss of hippocampal interneurons (Konradi et al., 2011), which are thought to generate SWRs (Donoso et al., 2018). This leads to the question of whether a loss of hippocampal interneurons in schizophrenia could disrupt ripple generation and contribute to reduced sleep-dependent memory consolidation, a possibility that would be difficult to test directly.
In the wake of our clinical trial, we tested eszopiclone in rats and found that it suppresses hippocampal ripples (Becker et al., 2019) and this may have contributed to its failure to improve memory. This obviously would have been important information to consider prior to embarking on an expensive and lengthy clinical trial. More generally, the mission to find effective treatments for cognitive deficits would benefit greatly from having information on their effects on all three oscillations (spindles, SOs, SWRs), their coordination and memory. Because hippocampal SWRs are difficult to measure noninvasively, and TRN activity has seldom been examined in vivo in humans since its size and location make it difficult to identify with neuroimaging (Viviano and Schneider, 2015), this endeavor requires complementary animal and human studies. An increase in spindles with preserved or enhanced coupling with cortical SOs and hippocampal SWRs, and improved memory in the animal studies would indicate candidacy for human studies and then clinical trials. In addition to facilitating the identification of the most promising interventions for future clinical trials, such complementary research can elucidate how coordinated sleep oscillations act in concert to mediate memory. This understanding will facilitate the identification and treatment of circuit dysfunction and sleep-related cognitive deficits in neurodevelopmental, neuropsychiatric and neurodegenerative disorders characterized by spindle deficits and cognitive impairment (e.g., autism, Farmer et al., 2018; Limoges et al., 2005; Tessier et al., 2015), Parkinson’s Disease with dementia (Latreille et al., 2015), Mild cognitive Impairment; Alzheimer’s Disease (Gorgoni et al., 2016).
One promising candidate for complementary research is zolpidem, a nonbenzodiazepine sedative hypnotic. Both eszopiclone and zolpidem bind to GABAA receptors in the thalamus. While eszopiclone preferentially acts on receptor subunits expressed in the TRN, zolpidem acts to prolong the decay time of inhibitory postsynaptic currents of thalamocortical relay neurons, which propagate spindles to the cortex (Jia et al., 2009). In addition to having different mechanisms of action, there is evidence that eszopiclone and zolpidem also have different effects on spindle-SO coupling, memory consolidation and hippocampal SWRs. Both eszopiclone and zolpidem boost spindle activity in humans (Kaestner et al., 2013; Mednick et al., 2013; Niknazar et al., 2015; Wamsley et al., 2013), but only zolpidem is associated with increased coupling of spindles with SOs (in humans, Niknazar et al., 2015), increased hippocampal SWR complexes (in rodents, Koniaris et al., 2011) and improved sleep-dependent memory (in humans, Kaestner et al., 2013; Mednick et al., 2013; Niknazar et al., 2015). The memory enhancing effect of zolpidem may depend on these effects on spindles, spindle-SO coupling and hippocampal SWRs and it would be important to test all three oscillations, their coupling and memory in a single study, prior to any clinical trial in schizophrenia. This is possible using in vivo rodent preparations that can record from the cortex, thalamus and hippocampus simultaneously (Varela et al., 2011).
Genetic studies, by illuminating spindle mechanisms, can provide specific targets for treatment.
Genetic studies are providing unprecedented opportunities to advance our understanding of the pathophysiology of schizophrenia, and particularly of spindle deficits, and can guide the development of treatments targeted to specific spindle mechanisms. Complementary animal and human research will facilitate the efficient translation of these advances to the clinic by screening potential treatments and selecting the most promising candidates for clinical trials. For example, CACNA1I, a risk gene for schizophrenia that is implicated in both common (Schizophrenia Working Group of the Psychiatric Genomics, 2014) and rare (Gulsuner et al., 2013) variation, encodes a calcium channel (Cav3.3) that is expressed only in the brain and preferentially in the TRN and hippocampus (Allen Mouse Brain Atlas, GTex, mouse.brainmap.org, www.gtexportal.org). Cav3.3 is the major spindle pacemaker in the thalamus and mouse CACNA1I knockouts have a specific spindle deficit (Astori et al., 2011). One de novo CACNA1I mutation found in schizophrenia disrupts Cav3.3 channel activity in the TRN. In an experimental model, this disruption reduced the rebound burst firing necessary for spindle generation (Andrade et al., 2016). These findings suggest the possibility that variation in CACNA1I contributes to spindle deficits and, by extension, cognitive deficits in schizophrenia. These discoveries have motivated a search for chemical compounds that modulate Cav3.3 function (Scolnick and Pan, 2017).
Similarly, PTCHD1, which is mutated in ~1% of individuals with autism spectrum disorder and intellectual disability, is selectively expressed in the TRN early in postnatal development. Deletion of PTCHD1 in mouse TRN causes spindle deficits and learning impairment. Importantly these deficits can be rescued by a drug that corrects signaling in the affected channel, identifying another potential treatment target for ameliorating spindle deficits to improve cognition (Wells et al., 2016). These studies show that genetic variation associated with neurodevelopmental disorders affects TRN function, results in physiological and behavioral phenotypes reminiscent of those seen in humans with these disorders, and are potentially treatable.
Thus, risk genes for neurodevelopmental disorders, both schizophrenia and autism, affect TRN function and spindle expression suggesting the possibility of a pathogenic role (Andrade et al., 2016; Astori et al., 2011; Krol et al., 2018; Wells et al., 2016). During gestation, axons that connect the cortex and the thalamus pass through the TRN, which helps guide them to their terminations (Mitrofanis and Guillery, 1993). As early as the first postnatal week in rodents, spindle bursts, a precursor to adult sleep spindles that are similar in shape, frequency and origin (Lindemann et al., 2016), refine these reciprocal thalamocortical connections (Cirelli and Tononi, 2015; Evrard and Ropert, 2009; Pinault, 2011). These findings suggest mechanisms by which risk genes that affect the TRN early in neurodevelopment could disrupt the establishment of thalamocortical circuitry and contribute to vulnerability to neurodevelopmental disorders. This is congruent with converging evidence that implicates abnormal communication of the thalamus with the cortex in the pathophysiology of schizophrenia. This evidence includes abnormally increased thalamocortical functional connectivity with sensory and motor cortices (Anticevic et al., 2015), which correlates with spindle deficits suggesting TRN dysfunction as a plausible a common mechanism (Baran et al., 2019). While causal experiments are needed to examine the effects of perturbing migration and synaptic refinement on the establishment of thalamocortical circuitry, one might speculate that if genetically-mediated TRN abnormalities, by disrupting the development of this circuitry, sets the stage for schizophrenia, then normalizing circuit function is a goal for preventative therapy.
Can we increase spindles and preserve coordinated network activity through noninvasive brain stimulation during sleep?
Based on a single published study (Demanuele et al., 2017) and the findings of our recent clinical trial, the timing of spindle-SO coupling appears to be intact in schizophrenia. There is no information about whether hippocampal SWRs and their coupling with spindles and SOs are also intact. As illustrated by our eszopiclone studies, some drugs that increase spindles may disrupt spindle-SO timing, suppress hippocampal SWRs and, consequently, fail to improve sleep-dependent memory consolidation. Other drugs that enhance spindles, such as zolpidem, may also enhance their coupling with SOs and improve memory (Niknazar et al., 2015). The advent of noninvasive brain stimulation as a treatment modality in psychiatry (for review see, Philip et al., 2017) offers novel alternative approaches to modify oscillatory dynamics during sleep to improve memory consolidation.
Electrical stimulation during NREM sleep, primarily transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS), have been used to enhance sleep-dependent memory consolidation. These methods deliver low levels of current through scalp electrodes. (For a technical review, see Woods et al., 2016). Compared with sham stimulation, electrical stimulation applied at SO frequency improves sleep-dependent declarative memory consolidation in some studies (Ladenbauer et al., 2016; Ladenbauer et al., 2017; Marshall et al., 2006; Marshall et al., 2004; Westerberg et al., 2015) but not others (Bueno-Lopez et al., 2019; Eggert et al., 2013; Passmann et al., 2016; Sahlem et al., 2015). Disrupting SOs using theta frequency stimulation (Marshall et al., 2011) or cross-hemispheric stimulation at SO frequency (Garside et al., 2015) impairs declarative memory. Studies that have used phase-amplitude coupling (PAC) to examine the effects of stimulation on the temporal coordination of spindles with SOs have found increased PAC to be associated with improved declarative memory (Ketz et al., 2018; Ladenbauer et al., 2017), consistent with a pharmacological study showing that increasing spindle-SO PAC with zolpidem improves declarative memory (Niknazar et al., 2015). A meta-analysis of electrical stimulation during sleep reported that it can modulate (improve or disrupt) sleep-dependent declarative memory but has no significant effect on procedural memory (Barham et al., 2016). In contrast, stimulation at spindle frequency (12 Hz) time-locked to spindle detection, improved motor procedural memory, but not declarative memory (Lustenberger et al., 2016). These studies are consistent with a growing literature in suggesting that different sleep stages and oscillations mediate the consolidation of declarative and procedural memory and that spindle-SO coordination is an important ingredient.
At present, it is difficult to identify the optimal electrical stimulation parameters for modulating oscillations and improving memory by comparing studies since the methods vary in important ways. These include sample composition (e.g., young or elderly adults, mild cognitive impairment); whether stimulation occurred during a daytime nap or nocturnal sleep; the length, frequency and location of stimulation; the memory task employed; and whether the stimulation was open or closed-loop (i.e., time-locked to the detection of an endogenous oscillation). These differences likely contribute to heterogeneous outcomes. In addition, precisely how transcranial electrical stimulation modulates sleep oscillations is unknown. An impediment to a mechanistic understanding is that the large EEG artifacts produced by electrical stimulation obscure its effects on brain oscillations both during stimulation and immediately after. A study that bypassed this problem by using intracranial EEG to measure oscillations during electrical stimulation on the scalp found that stimulation at SO frequency during sleep did not reliably increase spindle-SO PAC (Lafon et al., 2017). This contrasts with PAC findings from scalp EEG (Ketz et al., 2018; Ladenbauer et al., 2017). The authors attributed the null finding to an attenuation of the electrical field reaching the cortical surface (Lafon et al., 2017). In contrast, in the same participants, auditory stimulation, consisting of short bursts of noise played during NREM sleep, evoked SOs and increased spindle-SO PAC.
Auditory stimulation during sleep has also been employed to improve memory. This method has the advantages of evoking endogenous oscillations and allowing recording during stimulation (Figure 3 shows an example paradigm). Compared with sham, in multiple studies, closed-loop auditory stimulation during SO upstates was associated with increased SO and fast-sigma power during SO upstates and improved sleep-dependent declarative memory consolidation (Leminen et al., 2017; Ngo et al., 2013; Ong et al., 2016; Ong et al., 2018; Papalambros et al., 2017; Papalambros et al., 2019). Auditory evoked SOs (Papalambros et al., 2019) and fast-sigma power (Ngo et al., 2013) correlated with improved memory performance. Both the memory and physiological effects of auditory stimulation were relatively robust to methodological differences including sample composition and timing of sleep (overnight vs. daytime nap), but the timing of stimulation mattered. Open-loop stimulation, stimulation during the SO downstate and stimulation at spindle frequency did not increase spindle activity or improve memory consolidation (Ngo et al., 2013; Ngo et al., 2019; Weigenand et al., 2016). How upstate auditory stimulation leads to SO and spindle enhancement is an open question (for a working hypothesis see, Bellesi et al., 2014), but K-complexes, a form of slow oscillation, can be elicited by noise (Loomis et al., 1938) and spindles often coincide with K-complexes (Kokkinos and Kostopoulos, 2011) and are thought to gate sensory input to prevent arousals (Dang-Vu et al., 2010).
Figure 3:
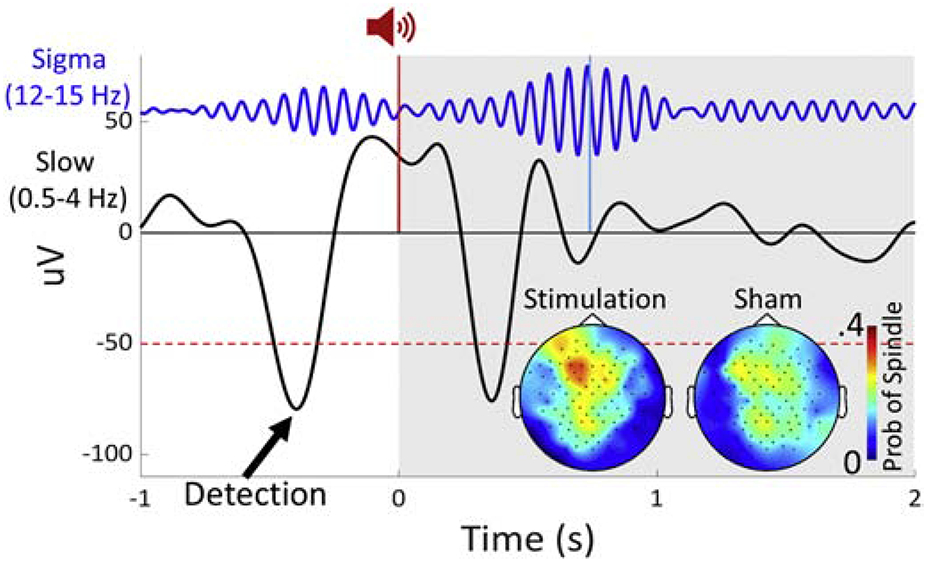
Example of a closed-loop auditory stimulation paradigm and data from a single subject (pilot data courtesy of Dr. Baxter). Closed-loop auditory stimulation during slow oscillation upstates may increase spindle density. EEG trace at Fz filtered in the sigma (12-15 Hz) and slow wave (0.5-4 Hz) bands time-locked to the auditory stimulus. Slow oscillations were detected at Fz in real-time. The auditory stimulus (a 50 ms burst of pink noise) was delivered during the slow oscillation upstate (red vertical line). The light blue line indicates a detected spindle peak. The grey shading indicates the time window after sham/stimulation over which the probability of evoking a spindle was calculated. (Inset) Probability of evoking a spindle following stimulation/sham.
Collectively, these studies demonstrate the potential of noninvasive brain stimulation to modulate sleep oscillations and improve memory in clinical and nonclinical populations. The burgeoning research on these approaches may identify the optimal methods for targeting different types of sleep-dependent memory consolidation and allow interventions to be tailored to particular populations. Each method has advantages and disadvantages. Electrical stimulation allows targeted neuromodulation of specific cortical regions. While presently limited to the superficial structures, newer methods may allow the targeting of deeper structures such as the hippocampus (Grossman et al., 2017). Safety concerns, however, limit the application of electrical stimulation to a fraction of the entire sleep period. Auditory stimulation, while not focal, has fewer safety concerns and evokes endogenous oscillations. While both electrical and auditory stimulation have shown promising results in individuals with mild cognitive impairment (Ladenbauer et al., 2017; Papalambros et al., 2019), resulting in increased SOs and a correlated improvement in sleep-dependent declarative memory consolidation, their mechanisms are unclear and they have seldom been employed in other clinical populations. The relative ease of use, scalability, accessibility and relative safety of these brain stimulation methods should motivate further study. One can imagine a near future in which these interventions are implemented in the home using commercially available devices.
Conclusions:
The evidence reviewed above indicates that (i) individuals with schizophrenia and their first-degree relatives have sleep spindle deficits, (ii) spindle deficits are associated with impaired memory consolidation, (iii) spindles are initiated in the TRN and need to be precisely temporally coordinated with cortical SOs and hippocampal SWRs to support memory consolidation during sleep, (iv) these oscillations are traits that can be manipulated by drugs and noninvasive brain stimulation techniques to enhance memory, (v) genetic studies are implicating specific pathophysiologic mechanisms of spindle deficits, and identifying additional novel targets and treatments, (vi) To translate these advances, we urgently need efficient methods to identify the most promising interventions for use in clinical trials to treat sleep-dependent cognitive deficits in schizophrenia, and this will require complementary animal and human studies, (vii) effective therapies will need to preserve or enhance sleep oscillatory dynamics and restore function at the network level. In summary, cross-disciplinary research can foster a more complete understanding of the role of sleep oscillations in cognition, can identify pathophysiological targets for treatment of cognitive deficits and can select the most promising interventions for clinical trials.
Acknowledgments:
Support from: NHLBI T32HL007901 (BB); R01 MH092638 (DSM, DM); K24MH099421 (DSM); 1S10RR023401, 1S10RR019307, 1S10RR023043; NCRR UL1TR001102-01 to Harvard Clinical and Translational Science Center.
Role of the funding source:
The funding sources played no role in the content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
None of the authors have any conflicts of interest to declare.
References
- Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ, 2016. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Scientific reports 6, 34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TG, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD, 2015. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry 72(9), 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Luthi A, 2011. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A 108(33), 13823–13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang JW, Khalilzadeh O, Hamalainen M, Watanabe T, Sasaki Y, 2014. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vision Res 99, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Demanuele C, Vuper TC, Seicol B, Fowler R, Correll D, Parr E, Callahan C, Morgan A, Stickgold R, Manoach DS, 2017. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: A double-blind randomized trial, APSS, Boston. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Karahanoglu FI, Mylonas D, Demanuele C, Vangel M, Stickgold R, Anticevic A, Manoach DS, 2019. Increased Thalamocortical Connectivity in Schizophrenia Correlates With Sleep Spindle Deficits: Evidence for a Common Pathophysiology. Biol Psychiatry Cogn Neurosci Neuroimaging 4(8), 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham MP, Enticott PG, Conduit R, Lum JA, 2016. Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: Evidence from a meta-analysis. Neurosci Biobehav Rev 63, 65–77. [DOI] [PubMed] [Google Scholar]
- Becker L, Penagos H, Manoach DS, Wilson MA, Varela C, 2019. Disruption of CA1 Sharp-Wave Ripples by the nonbenzodiazepine hypnotic eszopiclone, Society for Neuroscience, Chicago. [Google Scholar]
- Bellesi M, Riedner BA, Garcia-Molina GN, Cirelli C, Tononi G, 2014. Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front Syst Neurosci 8, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodizs R, Kormendi J, Rigo P, Lazar AS, 2009. The individual adjustment method of sleep spindle analysis: methodological improvements and roots in the fingerprint paradigm. J Neurosci Methods 178(1), 205–213. [DOI] [PubMed] [Google Scholar]
- Born J, Wilhelm I, 2012. System consolidation of memory during sleep. Psychological research 76(2), 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA, 2007. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull 33(5), 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Lopez A, Eggert T, Dorn H, Danker-Hopfe H, 2019. Slow oscillatory transcranial direct current stimulation (so-tDCS) during slow wave sleep has no effects on declarative memory in healthy young subjects. Brain stimulation 12(4), 948–958. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G, 2015. Cortical Development, Electroencephalogram Rhythms, and the Sleep/Wake Cycle. Biol Psychiatry 77(12), 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P, 2006. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett 403(1-2), 52–56. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J, 2007. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130(Pt 11), 2868–2878. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Molle M, Eross L, Jakus R, Rasonyi G, Halasz P, Born J, 2011. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci 33(3), 511–520. [DOI] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, Francois C, Wu E, 2016. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 77(6), 764–771. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Insel TR, 2008. Cognitive neuroscience and schizophrenia: translational research in need of a translator. Biol Psychiatry 64(1), 2–3. [DOI] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry D, 1999. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem 73(4), 1590–1597. [DOI] [PubMed] [Google Scholar]
- Cox R, Mylonas DS, Manoach DS, Stickgold R, 2018. Large-scale structure and individual fingerprints of locally coupled sleep oscillations. Sleep 41(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM, 2010. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol 20(15), R626–627. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M, 2005. An electroencephalographic fingerprint of human sleep. Neuroimage 26(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Demanuele C, Bartsch U, Baran B, Khan S, Vangel MG, Cox R, Hamalainen M, Jones MW, Stickgold R, Manoach DS, 2017. Coordination of Slow Waves with Sleep Spindles Predicts Sleep-Dependent Memory Consolidation in Schizophrenia. Sleep 40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso JR, Schmitz D, Maier N, Kempter R, 2018. Hippocampal Ripple Oscillations and Inhibition-First Network Models: Frequency Dynamics and Response to GABA Modulators. J Neurosci 38(12), 3124–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H, 2013. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain stimulation 6(6), 938–945. [DOI] [PubMed] [Google Scholar]
- Evrard A, Ropert N, 2009. Early development of the thalamic inhibitory feedback loop in the primary somatosensory system of the newborn mice. J Neurosci 29(31), 9930–9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer CA, Chilakamarri P, Thurm AE, Swedo SE, Holmes GL, Buckley AW, 2018. Spindle activity in young children with autism, developmental delay, or typical development. Neurology 91(2), e112–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli LA, Achermann P, Borbely AA, 2001. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology 25(5 Suppl), S57–62. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, 2011. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev 35(5), 1154–1165. [DOI] [PubMed] [Google Scholar]
- Garside P, Arizpe J, Lau CI, Goh C, Walsh V, 2015. Cross-hemispheric Alternating Current Stimulation During a Nap Disrupts Slow Wave Activity and Associated Memory Consolidation. Brain stimulation 8(3), 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, Marshall L, 2013. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res 144(1-3), 153–154. [DOI] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, D’Atri A, Tempesta D, Ferrara M, Marra C, Rossini PM, De Gennaro L, 2016. Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. Neural Plast 2016, 8376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26(1), 119–136. [DOI] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, Cassara AM, Neufeld E, Kuster N, Tsai LH, Pascual-Leone A, Boyden ES, 2017. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169(6), 1029–1041 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Consortium on the Genetics of, S., Group, P.S., Nimgaonkar VL, Go RC, Savage RM, Swerdlow NR, Gur RE, Braff DL, King MC, McClellan JM, 2013. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154(3), 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C, 2010. Hippocampal pathology in schizophrenia. Current topics in behavioral neurosciences 4, 529–553. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP, 2018. Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97(1), 221–230 e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF, 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications American Academy of Sleep Medicine, Westchester, Ill. [Google Scholar]
- Insel TR, 2009. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry 66(2), 128–133. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA, 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10(1), 100–107. [DOI] [PubMed] [Google Scholar]
- Jia F, Goldstein PA, Harrison NL, 2009. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther 328(3), 1000–1006. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG, 2012. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A 109(45), 18583–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner EJ, Wixted JT, Mednick SC, 2013. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci 25(10), 1597–1610. [DOI] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. J Neurosci 38(33), 7314–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos V, Kostopoulos GK, 2011. Human non-rapid eye movement stage II sleep spindles are blocked upon spontaneous K-complex coincidence and resume as higher frequency spindles afterwards. J Sleep Res 20(1 Pt 1), 57–72. [DOI] [PubMed] [Google Scholar]
- Koniaris E, Drimala P, Sotiriou E, Papatheodoropoulos C, 2011. Different effects of zolpidem and diazepam on hippocampal sharp wave-ripple activity in vitro. Neuroscience 175, 224–234. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S, 2011. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131(1-3), 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A, Wimmer RD, Halassa MM, Feng G, 2018. Thalamic Reticular Dysfunction as a Circuit Endophenotype in Neurodevelopmental Disorders. Neuron 98(2), 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenbauer J, Kulzow N, Passmann S, Antonenko D, Grittner U, Tamm S, Floel A, 2016. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. Neuroimage 142, 311–323. [DOI] [PubMed] [Google Scholar]
- Ladenbauer J, Ladenbauer J, Kulzow N, de Boor R, Avramova E, Grittner U, Floel A, 2017. Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J Neurosci 37(30), 7111–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon B, Henin S, Huang Y, Friedman D, Melloni L, Thesen T, Doyle W, Buzsaki G, Devinsky O, Parra LC, A AL, 2017. Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nat Commun 8(1), 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumane CV, Ngo HV, Born J, Shin HS, 2017. Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron 95(2), 424–435 e426. [DOI] [PubMed] [Google Scholar]
- Latreille V, Carrier J, Lafortune M, Postuma RB, Bertrand JA, Panisset M, Chouinard S, Gagnon JF, 2015. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol Aging 36(2), 1083–1090. [DOI] [PubMed] [Google Scholar]
- Leminen MM, Virkkala J, Saure E, Paajanen T, Zee PC, Santostasi G, Hublin C, Muller K, Porkka-Heiskanen T, Huotilainen M, Paunio T, 2017. Enhanced Memory Consolidation Via Automatic Sound Stimulation During Non-REM Sleep. Sleep 40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R, 2005. Atypical sleep architecture and the autism phenotype. Brain 128(Pt 5), 1049–1061. [DOI] [PubMed] [Google Scholar]
- Lindemann C, Ahlbeck J, Bitzenhofer SH, Hanganu-Opatz IL, 2016. Spindle Activity Orchestrates Plasticity during Development and Sleep. Neural Plast 2016, 5787423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart GA III, 1938. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. J Neurophys 1(5), 413–430. [Google Scholar]
- Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Frohlich F, 2016. Feedback-Controlled Transcranial Alternating Current Stimulation Reveals a Functional Role of Sleep Spindles in Motor Memory Consolidation. Curr Biol 26(16), 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R, 2016. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry 80(8), 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Stickgold R, 2019. Abnormal sleep spindles, memory consolidation and schizophrenia. . Annual review of clinical psychology 15, 451–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W, Youens K, 2004. Schizophrenia, IX: Cognition in schizophrenia--the MATRICS initiative. Am J Psychiatry 161(1), 25. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J, 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444(7119), 610–613. [DOI] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Molle M, Born J, 2011. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One 6(2), e16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Molle M, Hallschmid M, Born J, 2004. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci 24(44), 9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP, 2013. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci 33(10), 4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrofanis J, Guillery RW, 1993. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci 16(6), 240–245. [DOI] [PubMed] [Google Scholar]
- Molle M, Born J, 2011. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res 193, 93–110. [DOI] [PubMed] [Google Scholar]
- Mylonas D, Demanuele C, Baran B, Cox R, Stickgold R, Manoach DS, 2019. The effects of eszopiclone on spindles, slow oscillations and their coordination in health and schizophrenia, American Physiological Sleep Society, San Antonio, Texas. [Google Scholar]
- Ngo HV, Martinetz T, Born J, Molle M, 2013. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78(3), 545–553. [DOI] [PubMed] [Google Scholar]
- Ngo HV, Seibold M, Boche DC, Molle M, Born J, 2019. Insights on auditory closed-loop stimulation targeting sleep spindles in slow oscillation up-states. J Neurosci Methods 316, 117–124. [DOI] [PubMed] [Google Scholar]
- Niknazar M, Krishnan GP, Bazhenov M, Mednick SC, 2015. Coupling of Thalamocortical Sleep Oscillations Are Important for Memory Consolidation in Humans. PLoS One 10(12), e0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JL, Lo JC, Chee NI, Santostasi G, Paller KA, Zee PC, Chee MW, 2016. Effects of phase-locked acoustic stimulation during a nap on EEG spectra and declarative memory consolidation. Sleep Med 20, 88–97. [DOI] [PubMed] [Google Scholar]
- Ong JL, Patanaik A, Chee N, Lee XK, Poh JH, Chee MWL, 2018. Auditory stimulation of sleep slow oscillations modulates subsequent memory encoding through altered hippocampal function. Sleep 41(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, Paller KA, Zee PC, 2017. Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Front Hum Neurosci 11, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, Paller KA, Zee PC, Malkani RG, 2019. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann Clin Transl Neurol 6(7), 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmann S, Kulzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Floel A, 2016. Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults. Brain stimulation 9(5), 730–739. [DOI] [PubMed] [Google Scholar]
- Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL, 2017. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am J Psychiatry 174(7), 628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, 2011. Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull 37(2), 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlem GL, Badran BW, Halford JJ, Williams NR, Korte JE, Leslie K, Strachan M, Breedlove JL, Runion J, Bachman DL, Uhde TW, Borckardt JJ, George MS, 2015. Oscillating Square Wave Transcranial Direct Current Stimulation (tDCS) Delivered During Slow Wave Sleep Does Not Improve Declarative Memory More Than Sham: A Randomized Sham Controlled Crossover Study. Brain stimulation 8(3), 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Reid AG, Morgan A, Manoach DS, Verfaellie M, Stickgold R, 2019. The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning. Hippocampus, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C., 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510), 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E, Pan J, 2017. CACNA1I: A Potential Novel Target for Schizophrenia Motivated by Emerging Human genetics. Schizophrenia Bulletin 43(Issue suppl_1), S63. [Google Scholar]
- Sejnowski TJ, Destexhe A, 2000. Why do we sleep? Brain Res 886(1–2), 208–223. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA, 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21(5), 1123–1128. [DOI] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH, 2001. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry 158(9), 1393–1399. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, Fell J, 2015. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci 18(11), 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F, 1993. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13(8), 3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, Salt TE, Cuenod M, Do KQ, Berretta S, 2018. The thalamic reticular nucleus in schizo-phrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol. Psychiatry 23 (10), 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, Nanez JE Sr., Watanabe T, Sasaki Y, 2013. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci 33(34), 13894–13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek C, Palmese LB, Krystal AD, Srihari VH, DeGeorge PC, Reutenauer EL, Guloksuz S, 2014. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res 160(1–3), 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier S, Lambert A, Chicoine M, Scherzer P, Soulieres I, Godbout R, 2015. Intelligence measures and stage 2 sleep in typically-developing and autistic children. Int J Psychophysiol 97(1), 58–65. [DOI] [PubMed] [Google Scholar]
- Varela C, Yang JY, Kumar S, Wilson MA, 2011. Interactions between the midline thalamus, medial prefrontal cortex and dorsal CA1 in the rat, Society for Neuroscience, Washington DC. [Google Scholar]
- Viviano JD, Schneider KA, 2015. Interhemispheric interactions of the human thalamic reticular nucleus. J Neurosci 35(5), 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, Ely AV, Goff DC, Stickgold R, Manoach DS, 2012. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry 71(2), 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS, 2013. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep 36(9), 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigenand A, Molle M, Werner F, Martinetz T, Marshall L, 2016. Timing matters: open-loop stimulation does not improve overnight consolidation of word pairs in humans. Eur J Neurosci 44(6), 2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM, 2016. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/-) mice. Nature 532(7597), 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, Paller KA, 2015. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging 36(9), 2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL, 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265(5172), 676–679. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA, 2016. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127(2), 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]


