Abstract
Purpose
The purpose of this study was to investigate the levels of cytokines in the vitreous, and their correlation with the density of inflammatory cells in fibrovascular membranes (FVMs) in patients with proliferative diabetic retinopathy (PDR) to evaluate intraocular inflammatory conditions with regard to disease activity.
Methods
Thirty-three patients (33 eyes) with PDR requiring vitreoretinal surgery because of FVMs and tractional detachment were enrolled in the study, and compared with 20 patients (20 eyes) with macular hole (MH; control group). All patients underwent complete ophthalmological examinations before surgery. The activity of the disease was noted in patients with PDR. Samples of vitreous and blood were taken, and cytokine (MCP-1, IL-8, IL-6, VEGF, IL-1β, TNF-α, MIP-1α, MIP-1β, IL-10, and IL-12) levels were measured using cytometric bead array (CBA). Samples of FVMs were analyzed with immunohistochemical methods for the presence of inflammatory cells (CD45+, CD14+, CD3+, CD4+, CD8+, and CD19+ cells), and the numerical areal density was calculated (NA). Spearman’s correlation was used to assess the association between variables. The Mann–Whitney test was used to assess the differences between independent groups. The Wilcoxon signed-rank test was used for assessing differences between two related groups. A p value of less than 0.05 was considered statistically significant.
Results
Patients with active PDR had statistically significantly higher levels of MCP-1 (p = 0.003), VEGF (p = 0.009), and IL-8 (p = 0.02) in the vitreous in comparison with those with inactive PDR. CD45+, CD14+, CD3+, CD4+, CD8+, and CD19+ cells were identified in FVMs for patients with PDR. Statistically significantly higher numerical areal density of T lymphocytes (CD3+, CD4+, and CD8+) was demonstrated in patients with active PDR in comparison with patients with inactive PDR. Moderate to strong correlations were found between either MCP-1 or IL-8 in the vitreous, and the numerical areal density of cells (CD45+, CD3+, CD4+, and CD8+) in the FVMs, and weaker between either MCP-1 or IL-8 in the vitreous and the numerical areal density of CD14+ cells in the FVMs.
Conclusions
The correlation of cytokine (MCP-1 and IL-8) vitreous levels with the density of inflammatory cells in FVMs, and differences in cytokine levels in the vitreous between patients with active and inactive PDR, and between the vitreous and serum in PDR indicate the importance of local intraocular inflammation in patients with PDR.
Introduction
Many studies have shown increased levels of vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and interleukin-8 (IL-8) in vitreous samples from patients with proliferative diabetic retinopathy (PDR) [1-4]. Levels of MCP-1, IL-6, and IL-8 correlated with VEGF levels in vitreous samples from patients with PDR [1]. Higher levels of VEGF and IL-6 correlated with disease activity [5]. Higher levels of IL-8 were found to be associated with pronounced ischemia and worse visual prognosis [6]. Some studies have shown higher levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and macrophage inflammatory protein-1β (MIP-1β) in vitreous samples from patients with PDR compared to the control group [2,7,8]. Changes in cytokine levels have also been observed in serum samples from patients with DR [8-11].
Leukocytes, especially lymphocytes, have been shown to have an important role in the development of DR. Increased leukocyte adhesion and leukostasis lead to capillary occlusions and breakdown of the blood–retinal barrier [12]. New blood vessels start to grow in the process of angiogenesis which is induced by progressive ischemia. Angiogenesis is followed by fibrogenesis, and newly formed vessels are progressively transformed into fibrovascular membranes (FVMs). The neovascular tissue in the FVM contracts with time, and consequently, can lead to vitreous hemorrhage or tractional retinal detachment with serious vision loss. T lymphocytes and macrophages are involved in angiogenesis and fibrogenesis. T lymphocytes and B lymphocytes have been found in the vitreous and in FVMs of patients with PDR [13-17]. Kase and coworkers demonstrated that T lymphocytes in the FVM correlated with the progression of PDR and were associated with poor visual prognosis [14]. In a previous study, we found no association between lymphocytes in the FVM and visual prognosis [17]. Monocytes/macrophages have also been found in the neovascular tissue of the FVM [12,14].
Proinflammatory cytokines cause the activation of leukocytes, chemokines promote the migration of responsive cells, and growth factors like VEGF promote angiogenesis. The mechanisms of regulation and mutual influences of various cytokines are complex and not completely elucidated yet. The purpose of this study was to investigate the levels of cytokines in the vitreous and their correlation with the density of inflammatory cells in the FVM of patients with PDR to get a better understanding of the interactions between cytokines and inflammatory cells at different stages of disease activity.
Methods
Patients
Thirty-three consecutively operated patients with PDR and tractional macular detachment requiring vitrectomy (mean age 63.27 ± 11.90 years; 16 men and 17 women) and 20 patients with macular hole (mean age 71.2 ± 10.2 years; eight men and 12 women) were enrolled in the study. Exclusion criteria were recent retinal photocoagulation (less than 6 months), previous vitrectomy, glycated hemoglobin (HbA1c) higher than 10%, and any other ocular disease or known systemic inflammatory or hematologic disease. Written informed consent was obtained from all patients. The study was approved by the National Ethical Committee (National Ethical Committee number 118/12/2011), and was performed in compliance with the Declaration of Helsinki. The study adhered to the ARVO statement on human subjects. All patients underwent a complete ophthalmologic evaluation before surgery with best-corrected visual acuity (BCVA) determination, slit-lamp examination, fundus examination, intraocular pressure measurement, gonioscopy, and optical coherence tomography. The activity of disease was defined based on ophthalmologic evaluation (preoperative and intraoperative). A clinical preoperative and intraoperative assessment of disease activity was performed by two experienced retina specialists (MU, MGP). The activity of the disease was categorized as active PDR when there were perfused capillaries in neovascular membranes, or as inactive or quiescent PDR when no vessels could be discerned in fibrotic membranes or when no blood could be seen in gliotic vessels in fibrotic membranes [18]. There was no disagreement between the surgeons regarding the categorization of any of the studied eyes. Data regarding each patient’s general condition and diabetes control were obtained from the patient and from the patient’s general practitioner or diabetologist. The HbA1c level was measured 1 day before surgery. It was defined as a percentage value, which is related to the percentage of hemoglobin that is glycated, and is an index of blood glucose control over the previous 6 to 8 weeks. Arterial hypertension was defined as systolic blood pressure of 140 mmHg or more or diastolic blood pressure of 85 mmHg or more, or both, or as a condition treated with antihypertensive medications. Hyperlipidemia was defined as total cholesterol higher than 5 mM or triglycerides higher than 2 mM, or both, or as a condition treated with hypolipemic medications.
Methods
Samples of peripheral venous blood, undiluted vitreous, and FVMs were obtained during pars plana vitrectomy. Samples of peripheral blood were taken from the cubital vein, stored at room temperature in dark place for 30-45 min and then centrifuged for 10 min at 3000 ×g. Serum was then carefully transfered with a pipette into an Eppendorf tube. Undiluted vitreous samples were obtained at the beginning of vitrectomy by aspiration into a syringe attached to a vitreous cutter before the infusion line with balanced salt solution was opened. Vitreous samples and serum samples were stored in Eppendorf tubes at -80°C until analysis was performed.
The BD Cytometric Bead Array (CBA) Human Soluble Protein Flex Set system (BD Biosciences, San Jose, CA) was used for the detection of IL-1β, TNF-α, MIP-1α (MIP-1α), MIP-1β, MCP-1, IL-6, IL-8, IL-10, IL-12, and VEGF concentrations. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). Data were acquired and analyzed with Becton Dickinson CBA software and CellQuest software.
Fibrovascular membranes were obtained during pars plana vitrectomy, gently removed from the eye, and immediately put into a vial with buffered 10% paraformaldehyde for prefixation. The modified agar sandwich method was then used. The agar sandwich consisted of a solid agar disc and agar in liquid state poured over the disc. The solid and the liquid phases were prepared in advance under sterile circumstances in a laminated flow chamber. The 2.25% granulated agar was first melted by heating to almost boiling point. Each agar disc was 8 mm in diameter and approximately 1 mm thick. The agar discs were made by a trephine. The liquid agar was poured into a shallow dish to a depth of 1 mm, left to cool and harden, and cut into discs. Discs were stored in 70% ethanol at 0 °C to 5 °C. For the liquid phase of the agar sandwich, the melted agar was poured into a 10-ml syringe with a cap and stored cold at 0 °C to 5 °C. When a membrane was obtained, an agar disc was removed from the alcohol, rinsed with normal saline, and put onto a slide. A syringe of liquid phase with the cap was put into a water bath until agar melting point (90 °C) and then left to cool but not harden (50 °C). With the aid of a laboratory microscope, the prefixated membrane was gently straightened on the agar disc. Forceps were used only to grasp the edges of the sample. Eosin 0.1% was used to contrast the membrane as needed. The disc was dried with Surgicel (Eytec, Ripon, North Yorkshire, UK). The membrane was then attached to the disc by drops of liquid agar slowly poured from the syringe in a 0.5-mm layer to form the agar sandwich. The agar sandwich was allowed to cool and harden for 2 to 3 min and placed in fixative (buffered 10% paraformaldehyde) [17]. Samples of FVMs were analyzed with immunohistochemical methods for the presence of inflammatory cells, and density was calculated (numerical areal density, NA). Numerical areal density was calculated for CD45 (leukocyte common antigen), CD3 (T lymphocyte marker), CD4 (T helper lymphocyte marker), CD8 (T cytotoxic lymphocyte marker), CD19 (B lymphocyte marker), and CD14 (monocyte/macrophage marker) positive cells [17,19].
Statistical analysis
The measurement data collected in the study did not meet the normality assumption, so the median and the range between the minimum and maximum values were used for description. Spearman’s correlation was used to assess the association between variables. The Mann–Whitney test was used to assess the differences between independent groups. The Wilcoxon signed-rank test was used for assessing differences between two related samples. A p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 21 (SPSS IBM, New York, NY).
Results
Thirty-three patients (33 eyes) with PDR requiring vitreoretinal surgery because of the FVM and tractional detachment were enrolled in the study, and compared with 20 patients (20 eyes) with macular hole (MH; control group). Clinical data of patients with PDR and control subjects are shown in Table 1. Clinical data of patients with active PDR and patients with inactive PDR are shown in Table 2.
Table 1. Clinical data of patients.
| Clinical data | PDR (n=33) | MH (n=20) | P value |
|---|---|---|---|
| age, y±SD |
63.27±11.9 |
71.2±10.2 |
0.02 |
| sex |
16 men (48.5%)
17 women (51.5%) |
8 men (40.0%)
12 women (60.0%) |
0.32 |
| duration of diabetes, y±SD |
13.85±9.07 |
0 |
|
| incidence of insulin therapy, n (%) |
24 (72.7%) |
0 |
|
| HbA1c, %±SD |
7.93±0.98 |
0 |
|
| incidence of arterial hypertension, n (%) |
31 (93.9%) |
7 (31.8%) |
0.0001 |
| incidence of hyperlipidemia, n (%) |
19 (57.6%) |
6 (27.3%) |
0.0001 |
| BMI, kg/m2±SD |
30.84±4.66 |
24.0±6.3 |
0.0001 |
| incidence of NVI, n |
2 (6.1%) |
0 |
|
| activity of disease | active 25 (75.8%) inactive 8 (24.2%) | 0 |
Legend: PDR – proliferative diabetic retinopathy; MH – macular hole; BMI – body mass index; NVI – iris neovascularization
Table 2. Clinical data of patients with active and inactive PDR.
| Clinical data | active PDR (n=25) | inactive PDR (n=8) | P value |
|---|---|---|---|
| age, y±SD |
61.56±12.73 |
68.63±6.98 |
0.15 |
| sex |
14 men (56%)
11 women (44%) |
2 men (25%)
6 women (75%) |
0.13 |
| duration of diabetes, y±SD |
11.68±8.42 |
20.63±8.03 |
0.013 |
| incidence of insulin therapy, n (%) |
17 (68%) |
7 (87.5%) |
0.29 |
| HbA1c, %±SD |
7.86±0.97 |
8.16±1.05 |
0.45 |
| incidence of arterial hypertension, n (%) |
23 (92%) |
8 (100%) |
0.43 |
| incidence of hyperlipidemia, n (%) |
14 (56%) |
5 (62.5%) |
0.75 |
| BMI, kg/m2±SD |
31.15±4.52 |
29.88±5.3 |
0.51 |
| incidence of NVI, n (%) | 2 (8%) | 0 | 0.42 |
Legend: PDR – proliferative diabetic retinopathy; MH – macular hole; BMI – body mass index; NVI – iris neovascularization
Patients with PDR had statistically significantly higher vitreous levels of MCP-1 (p = 0.0001), VEGF (p = 0.0001), IL-8 (p = 0.0001), and IL-6 (p = 0.0001) in comparison with the vitreous levels of the control group (MH; Table 3). In patients with PDR, higher levels of cytokines (MCP-1, IL-6, IL-8, VEGF, IL-1β, and TNF-α) were found in the vitreous in comparison with serum (Table 4). Patients with active PDR had statistically significantly higher levels of MCP-1 (p = 0.003), VEGF (p = 0.009), and IL-8 (p = 0.02) in vitreous in comparison with those with inactive PDR (Table 5).
Table 3. Comparison of samples from patients with PDR and patients with MH – median (minimum-maximum); Mann–Whitney test.
| Cytokine | vitreous |
serum |
||||
|---|---|---|---|---|---|---|
| PDR (n=33) | MH (n=20) | P value | PDR (n=33) | MH (n=20) | P value | |
| MCP-1 (pg/ml) |
1614.98 (403.05–3990.74) |
374.17 (295.01–881.87) |
0.0001 |
72.6 (25.06–169.65) |
43.4 (30.24–76.64) |
0.02 |
| IL-8 (pg/ml) |
93.29 (20.04–295.71) |
14.49 (13.12–16.64) |
0.0001 |
11.99 (10.12–31.69) |
12.71 (11.21–13.33) |
0.84 |
| IL-6 (pg/ml) |
60.82 (13.64–798.23) |
6 (5.28–8.1) |
0.0001 |
5.46 (2.41–24.76) |
9.14 (5.81–298.4) |
0.02 |
| VEGF (pg/ml) |
304.64 (26.41–1439.3) |
4.58 (3.5–31.79) |
0.0001 |
38.66 (6.17–105.56) |
25.73 (17.62–63.61) |
0.55 |
| IL-1β (pg/ml) |
9.15 (7.51–67.36) |
10.5 (7.7–35.99) |
0.51 |
7.6 (6.91–15.16) |
7.7 (7.11–95.55) |
0.55 |
| TNF-α (pg/ml) |
13.41 (3.26–69.09) |
21.5 (5.16–38.4) |
0.25 |
5.7 (2.9–23.35) |
4.79 (4,0–98.76) |
0.82 |
| MIP-1α (pg/ml) |
9.55 (6.9–68.26) |
14.25 (9.76–47.34) |
0.004 |
15.07 (7.78–91.74) |
20.38 (11.83–91.07) |
0.27 |
| MIP-1β (pg/ml) |
12.09 (2.9–38.02) |
13.44 (0.9–14.4) |
0.31 |
18.76 (6.89–48.83) |
17.72 (15.51–60.5) |
0.80 |
| IL-10 (pg/ml) |
5.67 (4.9–8.12) |
6.05 (5.84–6.08) |
0.02 |
7.4 (5.3–15.28) |
6.63 (5.7–58.43) |
0.67 |
| IL-12 (pg/ml) | 3.17 (0.68–22.26) | 3.99 (2.56–11.18) | 0.04 | 17.7 (2.49–42.91) | 18.84 (7.09–71.71) | 0.25 |
Legend: PDR – proliferative diabetic retinopathy; MH – macular hole; IL-1β - interleukin-1β; TNF-α - tumor necrosis factor-α; MIP-1α - macrophage inflammatory protein-1α; MIP-1β - macrophage inflammatory protein-1β; MCP-1 - monocyte chemoattractant protein-1; IL-6 - interleukin-6; IL-8 - interleukin-8; IL-10 - interleukin-10; IL-12 - interleukin-12; VEGF - vascular endothelial growth factor
Table 4. Paired comparison of vitreous and serum samples from patients with PDR – median (minimum-maximum); Wilcoxon signed rank test.
| Cytokine | patients with PDR (n=33) |
||
|---|---|---|---|
| vitreous | serum | P value | |
| MCP-1 (pg/ml) |
1614.98 (403.05–3990.74) |
72.6 (25.06–169.65) |
0.0001 |
| IL-8 (pg/ml) |
93.29 (20.04–295.71) |
11.99 (10.12–31.69) |
0.0001 |
| IL-6 (pg/ml) |
60.82 (13.64–798.23) |
5.46 (2.41–24.76) |
0.0001 |
| VEGF (pg/ml) |
304.64 (26.41–1439.3) |
38.66 (6.17–105.56) |
0.0001 |
| IL-1β (pg/ml) |
9.15 (7.51–67.36) |
7.6 (6.91–15.16) |
0.002 |
| TNF-α (pg/ml) |
13.41 (3.26–69.09) |
5.7 (2.9–23.35) |
0.005 |
| MIP-1α (pg/ml) |
9.55 (6.9–68.26) |
15.07 (7.78–91.74) |
0.002 |
| MIP-1β (pg/ml) |
12.09 (2.9–38.02) |
18.76 (6.89–48.83) |
0.009 |
| IL-10 (pg/ml) |
5.67 (4.9–8.12) |
7.4 (5.3–15.28) |
0.001 |
| IL-12 (pg/ml) | 3.17 (0.68–22.26) | 17.7 (2.49–42.91) | 0.0001 |
Legend: PDR – proliferative diabetic retinopathy; IL-1β - interleukin-1β; TNF-α - tumor necrosis factor-α; MIP-1α - macrophage inflammatory protein-1α; MIP-1β - macrophage inflammatory protein-1β; MCP-1 - monocyte chemoattractant protein-1; IL-6 - interleukin-6; IL-8 - interleukin-8; IL-10 - interleukin-10; IL-12 - interleukin-12; VEGF - vascular endothelial growth factor
Table 5. Comparison of vitreous samples from patients with active PDR and inactive PDR – median (minimum-maximum); Mann–Whitney test.
| Patients with PDR (n=33) |
|||
|---|---|---|---|
| Cytokine | active PDR (n=25) | inactive PDR (n=8) | P value |
| MCP-1 (pg/ml) |
1625.84 (441.3–3990.74) |
929.35 (403.05–1625.84) |
0.003 |
| IL-8 (pg/ml) |
114.15 (37.57–295.71) |
52.53 (20.04–147.13) |
0.02 |
| IL-6 (pg/ml) |
65.08 (23.7–798.23)-295.71) |
27.43 (13.64–220.31) |
0.09 |
| VEGF (pg/ml) |
500.89 (26.41–1439.3) |
147.97 (29.86–304.64) |
0.009 |
| IL-1β (pg/ml) |
8.13 (7.51–67.36) |
19.51 (7.71–33.93) |
0.55 |
| TNF-α (pg/ml) |
13.41 (3.26–69.09) |
13.62 (3.51–31.52) |
0.91 |
| MIP-1α (pg/ml) |
6.9 (6.9–68.26) |
11.31 (6.9–28.16) |
0.49 |
| MIP-1β (pg/ml) |
12.02 (2.9–30.67) |
14.46 (4.56–38.02) |
0.52 |
| IL-10 (pg/ml) |
5.61 (4.9–6.9) |
5.78 (5.37–8.12) |
0.20 |
| IL-12 (pg/ml) | 2.77 (0.68–14.99) | 3.48 (1.07–22.26) | 0.55 |
Legend: PDR – proliferative diabetic retinopathy; MH – macular hole; IL-1β - interleukin-1β; TNF-α - tumor necrosis factor-α; MIP-1α - macrophage inflammatory protein-1α; MIP-1β - macrophage inflammatory protein-1β; MCP-1 - monocyte chemoattractant protein-1; IL-6 - interleukin-6; IL-8 - interleukin-8; IL-10 - interleukin-10; IL-12 - interleukin-12; VEGF - vascular endothelial growth factor
Strong correlations were found between VEGF and IL-8 (Spearman’s coefficient 0.74; p = 0.0001), between VEGF and MCP-1 (Spearman's coefficient 0.64; p = 0.0001), between VEGF and IL-6 (Spearman’s coefficient 0.59; p = 0.0001), between MCP-1 and IL-8 (Spearman’s coefficient 0.76; p = 0.0001), between MCP-1 and IL-6 (Spearman’s coefficient 0.81; p = 0.0001), and between IL-8 and IL-6 (Spearman’s coefficient 0.69; p = 0.0001). There were weak correlations between VEGF and MIP-1α (Spearman’s coefficient 0.33; p = 0.03), between IL-8 and IL-1β (Spearman’s coefficient 0.3; p = 0.048), between IL-8 and IL-12 (Spearman’s coefficient 0.3; p = 0.04), between IL-10 and IL-12 (Spearman’s coefficient 0.4; p = 0.007), between IL-10 and MIP-1α (Spearman’s coefficient 0.38; p = 0.01), between IL-6 and MIP-1α (Spearman’s coefficient 0.33; p = 0.03), and between IL-1β and MIP-1β (Spearman’s coefficient 0.33; p = 0.03).
Capillary-like structures in a fibrous matrix containing several mononuclear cells were observed in all hematoxyilin-eosin-stained sections of FVMs. CD45+ (leukocyte common antigen), CD14+ (monocyte/macrophage), CD3+ (T lymphocyte marker), CD4+ (T helper lymphocyte marker), CD8+ (T cytotoxic lymphocyte marker), and CD19+ (B lymphocyte marker) cells were identified in FVMs of patients with PDR. Statistically significantly higher numerical areal density of the T lymphocytes (CD3+, CD4+, and CD8+) was demonstrated in patients with active PDR in comparison with patients with inactive PDR (Table 6).
Table 6. Comparison of numerical areal densities (NA) of inflammatory cells in FVMs of patients with acitve PDR and patients with inactive PDR – median (minimum-maximum); Mann–Whitney test.
| Numerical areal density (NA) | PDR (n=33) | active PDR (n=25) | inactive PDR (n=8) | P value |
|---|---|---|---|---|
| NA of CD45+ cells per mm2 |
237.31 (0–1253.99) |
237.31 (38.80–1253.99) |
205.03 (0–442.07) |
0.27 |
| NA of CD3+ cells per mm2 |
22.86 (0–178.57) |
45.45 (10.85–178.57) |
12.17 (0–22.86) |
0.008 |
| NA of CD4+ cells per mm2 |
39.37 (0–535.71) |
90.85 (6.51–535.71) |
21.51 (0–31.25) |
0.003 |
| NA of CD8+ cells per mm2 |
35.24 (0–408.65) |
35.24 (0–408.65) |
30.27 (0–64.58) |
0.16 |
| NA of CD19+ cells per mm2 |
4.34 (0–115.27) |
11.16 (0–115.27) |
0 (0–0) |
0.001 |
| NA of CD14+ cells per mm2 | 216.2 (21.48–1529.02) | 207.13 (21.48–1529.02) | 261.36 (99.81–525.00) | 0.95 |
Legend: PDR – proliferative diabetic retinopathy; NA- numerical areal density; CD45+ cells – leukocytes; CD3+ cells - T lymphocytes; CD4+ cells -(T helper lymphocytes; CD8+ cells - T cytotoxic lymphocytes; CD19+ cells - B lymphocytes; CD14+ cells - monocytes/macrophages
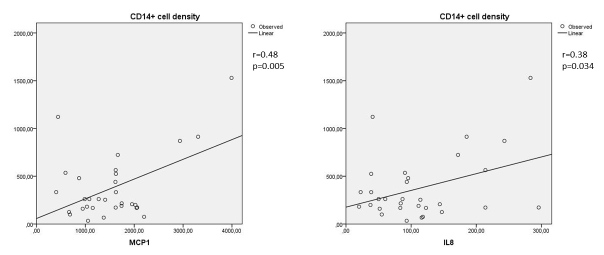
Moderate to strong correlations were found between either MCP-1 or IL-8 in the vitreous and the numerical areal density of cells (CD45+, CD3+, CD4+, and CD8+) in the FVMs (Figure 1 and Figure 2). A moderate correlation was found between MCP-1 in the vitreous and the numerical areal density of CD14+ cells in the FVMs. There was a weak correlation between IL-8 in the vitreous and the numerical areal density of CD14+ cells in the FVMs (Figure 3).
Figure 1.
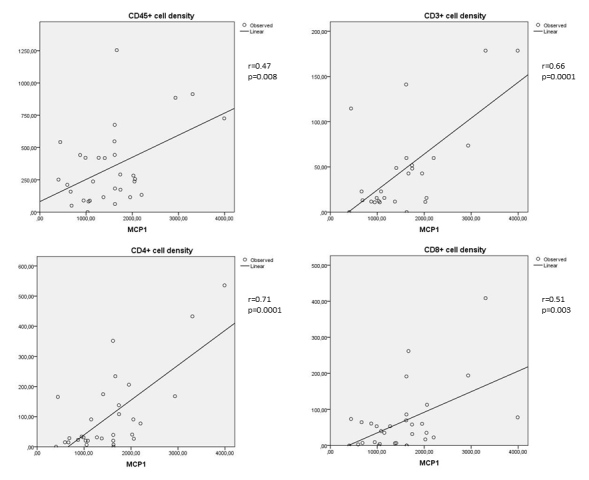
Correlations between MCP-1 and the numerical areal densities of lymphocytes (Spearman's coefficent r). Upper left: correlation between MCP-1 and the numerical areal density of CD45+ cells (r = 0.47; p = 0.008); upper right: correlation between MCP-1 and the numerical areal density of CD3+ cells (r = 0.66; p = 0.0001); lower left: correlation between MCP-1 and the numerical areal density of CD4+ cells (r = 0.71; p = 0.0001); lower right: correlation between MCP-1 and numerical areal density of CD8+ cells (r = 0.51; p = 0.003). Numerical areal density, NA.
Figure 2.
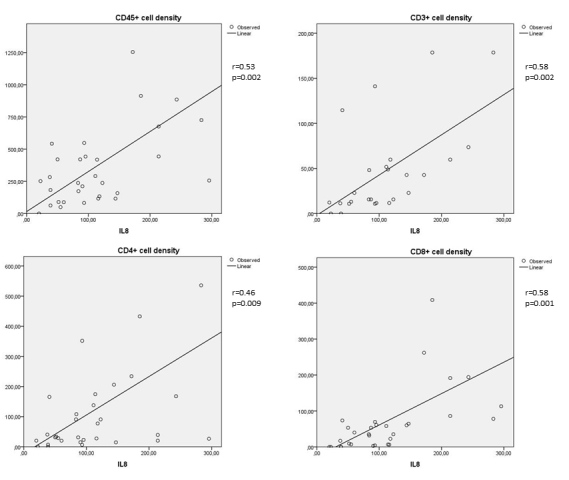
Correlations between IL-8 and the numerical areal densities of lymphocytes (Spearman's coefficent r). Upper left: correlation between IL-8 and the numerical areal density of CD45+ cells (r = 0.53; p = 0.002); upper right: correlation between IL-8 and the numerical areal density of CD3+ cells (r = 0.58; p = 0.002); lower left: correlation between IL-8 and the numerical areal density of CD4+ cells (r = 0.46; p = 0.009); lower right: correlation between IL-8 and the numerical areal density of CD8+ cells (r = 0.58; p = 0.001). Numerical areal density, NA.
Figure 3.
Correlation between MCP-1 and the numerical areal densities of monocytes/macrophages and between IL-8 and the numerical areal densities of monocytes/macrophages (Spearman's coefficent r). Left: correlation between MCP-1 and the numerical areal density of CD14+ cells (r = 0.48; p = 0.005); right: correlation between IL-8 and the numerical areal density of CD14+ cells (r = 0.38; p = 0.034). Numerical areal density, NA.
There were strong correlations between IL-6 and the numerical areal density of either CD8+ cells (Spearman's coefficient 0.62; p = 0.0001) or CD45+ cells (Spearman's coefficient 0.68; p = 0.0001). This study showed a moderate correlation of IL-1β vitreous levels with CD14+ cells (monocytes/macrophages; Spearman’s coefficient 0.43; p = 0.017).
Finally, there was no correlation between VEGF and the numerical areal density of any of the cells in the FVM. Moreover, this study found no correlation between other tested cytokines (TNF-α, MIP-1α, MIP-1β, IL-10, and IL-12) and inflammatory cells in the FVMs of patients with PDR.
Discussion
In this study, we demonstrated that vitreous levels of MCP-1 and IL-8 correlated well with the density of T lymphocytes and monocytes/macrophages in FVMs. There were also correlations between IL-6 and T lymphocytes (CD8+ cells) and between IL-1β and monocytes/macrophages (CD14+ cells). To our knowledge, this is the first study reporting the correlation of vitreous inflammatory chemokines (MCP-1, IL-8, IL-6, and IL-1β) with T lymphocytes and/or monocytes/macrophages in the FVM of patients with PDR.
The mechanisms of immune changes in diabetes and diabetic complications are not completely understood. Macrophages, lymphocytes, and cytokines are involved in low-grade inflammation in diabetes leading to retinal and vascular changes in PDR [20]. Cytokines are involved in leukocyte activation; they modulate cell proliferation and promote angiogenesis [21]. Cytokines that are produced locally in the eye may directly or indirectly influence the development of DR.
This study demonstrated increased vitreous cytokine levels (MCP-1, IL-6, IL-8, and VEGF) in PDR in comparison with control subjects (MH). Moreover, patients with active PDR had statistically significantly higher vitreous levels of MCP-1, VEGF, and IL-8 in comparison with those with inactive PDR. Higher levels of cytokines (MCP-1, IL-6, IL-8, and VEGF) were found in the vitreous of patients with PDR in comparison with the serum levels. Similarly, increased numerical areal density of lymphocytes (CD3+, CD4+, and CD19+ cells) was found in patients with active PDR compared to patients with inactive PDR. Finally, vitreous cytokines (MCP-1 and IL-8 levels) statistically significantly correlated with the numerical areal density of inflammatory cells in FVM in patients with PDR, and these data are in agreement with data from various preclinical and clinical studies indicating the importance of local intraocular low-grade inflammation in PDR [2,13,22].
MCP-1 is a chemokine that recruits monocytes, macrophages, and T lymphocytes to sites of tissue injury, infection, and inflammation [23]. Many types of cells produce MCP-1, but monocytes/macrophages have been found to be the major source of this chemokine [24]. Harada and colleagues demonstrated with immunohistochemical analysis that MCP-1 is widely distributed to glial cell components of FVMs, where it is thought to play an important role for the recruitment of inflammatory macrophages and trigger their transmigration through the vascular endothelial cell layer [23]. The present results are in agreement with several studies that showed higher MCP-1 levels in the vitreous of patients with PDR in comparison with the control group, and correlated with the activity of PDR [2,25-27]. Moderate to strong correlations of vitreous MCP-1 with T lymphocytes (CD3+, CD4+, and CD8+ cells) and monocytes/macrophages (CD14+ cells) in FVMs of patients with PDR, found in the present study, are expected to be the result of its chemotactic effect leading to chronic inflammatory or proliferative disorders.
IL-8 is produced by endothelial and glial cells in retinas with ischemic angiogenesis. Yoshida and coworkers showed that vitreous fluid with high concentrations of IL-8 induced intraocular neoangiogenesis [28]. IL-8 is a chemotactic cytokine for neutrophils and T lymphocytes [28]. Higher levels of IL-8 in the vitreous of patients with PDR in comparison with the control group, found in the present study, are in agreement with the results of Hernandez and coworkers [27]. Moderate correlations of vitreous IL-8 with T lymphocytes (CD3+, CD4+, CD8+ cells) and monocytes/macrophages (CD14+ cells) in FVMs of patients with PDR seem consistent with IL-8 chemotactic function. This could also explain the higher levels of IL-8 and greater numerical areal density of inflammatory cells in patients with active PDR.
Although vitreous levels of MCP-1 and IL-8 were statistically significantly higher in patients with active PDR compared to patients with inactive PDR, and correlations were found between either MCP-1 or IL-8 in vitreous and cells in the FVMs, there was no difference between the numerical areal density of CD14+ cells in patients with active or inactive PDR. The reason for this apparent contradiction could be the expression of CD14+ in other types of cells in FVMs. We used the CD14+ marker to differentiate monocytes/macrophages from other CD45+ cells. However, the CD14+ marker is also expressed in fibroblasts and activated microglia [29-31]. We speculate that more monocytes/macrophages and activated microglia were detected in FVMs of patients with active PDR, and more fibroblasts were detected as CD14+ cells in FVMs of patients with inactive PDR. We were not able to differentiate among these different types of CD14+ cells, and this could have affected the findings.
In addition to the role of IL-8 in PDR in this study, IL-6, a pleiotropic cytokine and a primary mediator of the acute phase response, was found to be an important cytokine. Vitreous levels of IL-6 were found to be elevated in PDR, although the difference between active and inactive forms was not statistically significant. Gustavsson et al., however, reported a correlation between vitreous levels of IL-6 and the stage of the disease [32]. We found strong correlations between vitreous levels of IL-6 and CD45+ cells and between vitreous levels of IL-6 and CD8+ cells. This is in agreement with the known effects of IL-6 on immune cell recruitment and vascular changes. IL-6 has many important effects on blood vessels, including endothelial activation, immune cell recruitment, vascular permeability, and vascular fibrosis [33]. It is presumed that IL-6 may cause increased vascular permeability directly by stimulating endothelial expression of adhesion molecules [33], or indirectly by inducing the expression of VEGF [1,34].
Higher vitreous levels of VEGF, a potent proangiogenic cytokine, in patients with PDR in the present study are in agreement with several previous studies [1,2,35]. Moreover, several studies reported a correlation between vitreous VEGF levels and the activity of PDR [1,2,35], and these findings are in agreement with those of the present study. Many cells in the eye can produce VEGF, including RPE cells, pericytes, glial cells, and ganglion cells, but the main player in the secretion of and response to VEGF is endothelial cells [36,37]. VEGF has been shown to be secreted by T lymphocytes on stimulation by specific antigens or by IL-2 and hypoxia. Activated T lymphocytes might enhance angiogenesis [37]. Yoshimura and coworkers showed that VEGF, MCP-1, IL-8, and IL-6 were significantly elevated in the vitreous of patients with PDR, were correlated with each other, and increased synchronizing [1]. Although we found statistically significant correlations between VEGF, MCP-1, IL-8, and IL-6, there was no correlation between VEGF and inflammatory cells in the FVM in patients with PDR. We speculate that other cytokines may have a greater effect on inflammatory cells than VEGF in the microenvironment of FVMs.
IL-1β, a multifunctional proinflammatory cytokine and the main trigger and amplifier of neuroinflammation, failed to demonstrate any statistically significant effect in the present study involving patients with PDR. There were no statistically significant differences in either the vitreous or serum level of IL-1β in PDR in comparison with the control group, and no differences in the vitreous levels of IL-1β between the active and inactive forms of PDR. However, we found a moderate correlation between the vitreous levels of IL-1β and monocytes/macrophages (CD14+ cells). We speculate that this correlation might be caused by the production of IL-1β in activated glial cells that express the CD14+ antigen [30,31]. The detection of IL-1β is difficult because this cytokine has a short half-life. For this reason, there have been only a few studies reported thus far in which higher levels of IL-1β were found in the vitreous samples of patients with DR in comparison with the control group [8,35,38]. On the contrary, IL-1β was not found in the vitreous of patients with PDR in the study by Yoshimura and coworkers because the concentrations were below the limits of detection [1].
The present study showed lower levels of some other cytokines (MIP-1α, IL-10, and IL-12) in patients with PDR in comparison with control subjects. Similarly, in patients with PDR some cytokine levels (TNF-α) were higher, whereas most cytokine levels were even lower (MIP-1α, MIP-1β, IL-10, and IL-12) in the vitreous compared to serum levels. Finally, there were no statistically significant differences in the tested cytokines (TNF-α, MIP-1α, MIP-1β, IL-10, and IL-12) between the vitreous of patients with active PDR and patients with inactive PDR. We did not find any correlation between TNF-α, MIP-1α, MIP-1β, IL-10, or IL-12 and inflammatory cells in the FVMs of patients with PDR.
TNF-α, a proinflammatory cytokine with short half-life, is difficult to detect. TNF-α levels were found to be higher in the serum of diabetic patients in comparison with the control group, and correlated with the stage of DR [8,10]. El-Asrar and coworkers found TNF-α to be present on endothelial and stromal cells in fibrovascular membranes of patients with PDR [39]. TNF-α was undetectable in the vitreous of patients with PDR in the study by Yoshimura and coworkers [1]. Contrary to Yoshimura et al.’s results, we did not find differences in the vitreous levels of TNF-α in patients with PDR in comparison with the control group.
MIP-1α and MIP-1β, produced by macrophages, activate granulocytes and induce the release of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α [4]. Some studies could not detect MIP-1α and MIP-1β either in the vitreous of patients with PDR or in the vitreous of the control group [4]. However, Yoshimura and coworkers found MIP-1β in the vitreous of some patients with PDR [1]. We were able to prove the presence of MIP-1α in 21 vitreous samples out of 33, and MIP-1β in all vitreous samples of patients with PDR. The vitreous levels of MIP-1α and MIP-1β did not differ statistically significantly between the patients with PDR and the control subjects. Based on the present results, we speculate that these two cytokines probably do not have an important role in the pathogenesis of DR.
Vitreous levels of IL-10 in this study were statistically significantly lower in patients with PDR in comparison with the control group. Furthermore, the findings are not in agreement with the results of Mao and coworkers who found higher levels of the anti-inflammatory cytokine IL-10 in the vitreous of patients with PDR [38]. On the contrary, levels of IL-10 were not found to be higher in the study by Hernandez and coworkers who concluded that higher levels of inflammatory cytokines do not necessarily induce higher expression of cytokines with anti-inflammatory effects [26]. Similarly, Zhou and coworkers found no difference in the vitreous levels of IL-10 between patients with PDR and the control group [7]. Moreover, in the present study there were no statistically significant differences in vitreous levels of IL-10 between patients with active and inactive forms of PDR. Based on the present results, and in accordance with the conclusions of Hernandez and coworkers, IL-10 probably does not have any significant role in the pathogenesis of DR.
IL-12 is an inflammatory cytokine with presumably antiangiogenic effects [40]. In the present study, we found statistically significant differences in the vitreous levels of IL-12 between the cases and controls. IL-12 vitreous levels were lower in patients with PDR. Contrary to these findings, higher levels of IL-12 have been reported in the aqueous humor in the treatment naive patients with DR in comparison with treated patients and the control group [40]. Interestingly, in patients with PDR we found statistically significantly lower levels of IL-12 in the vitreous in comparison with serum levels. Moreover, vitreous IL-12 levels in PDR did not reflect the activity of the disease in the present study, because we did not find any statistically significant difference in vitreous IL-12 levels between cases with the active and inactive forms of PDR. All these findings regarding vitreous IL-12 levels might be in accordance with the lack of a statistically significant effect of IL-12 on the density of inflammatory cells in the FVM of patients with PDR. The findings reported in this study concerning TNF-α, MIP-1α, MIP-1β, IL-10, or IL-12 (lack of correlation of TNF-α, MIP-1α, MIP-1β, IL-10, or IL-12 with inflammatory cells in FVMs of patients with PDR) indicate that these cytokines do not have an important role in the inflammatory FVM microenvironment in patients with PDR.
In conclusion, statistically significant differences were demonstrated in cytokine levels in the vitreous either between active and inactive PDR or between vitreous and serum levels in PDR. To emphasize, this study demonstrated that vitreous levels of some cytokines (MCP-1, IL-8, IL-6, and IL-1β) statistically significantly correlate with the density of inflammatory cells in the FVM, and most probably have a more important role in the development of PDR in comparison with some other cytokines, such as TNF-α, MIP-1α, MIP-1β, IL-10, or IL-12. These findings indicate the importance of local intraocular inflammation in patients with PDR.
Acknowledgments
The authors thank Mrs. Polona Sajovic and Mrs. Petra Nussdorfer, BA for immunohistochemical analysis, Mrs. Visam Bajt, BA, for revising the English. Financial support: supported by a grant from the Slovenian Research agency ARRS (P3-0333).
References
- 1.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, Ueno A, Hata Y, Yoshida H, Ishibashi T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai Y, Wu Z, Wang F, Zhang Z, Yu M. Identification of Chemokines and Growth Factors in Proliferative Diabetic Retinopathy Vitreous. BioMed Res Int. 2014;2014:486386. doi: 10.1155/2014/486386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Asrar AMA, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, Al-Shabrawey M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Maier R, Weger M, Haller-Schober E-M, El-Shabrawi Y, Wedrich A, Theisl A, Aigner R, Barth A, Haas A. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2007;2008:637–43. [PMC free article] [PubMed] [Google Scholar]
- 5.El Asrar AMA, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the Vitreous of Patients With Proliferative Diabetic Retinopathy. Am J Ophthalmol. 1992;114:731–6. doi: 10.1016/s0002-9394(14)74052-8. [DOI] [PubMed] [Google Scholar]
- 6.Petrovič MG, Korošec P, Košnik M, Hawlina M. Association of preoperative vitreous IL-8 and VEGF levels with visual acuity after vitrectomy in proliferative diabetic retinopathy. Acta Ophthalmol. 2010;88:e311–6. doi: 10.1111/j.1755-3768.2010.02030.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Wang S, Xia X. Role of Intravitreal Inflammatory Cytokines and Angiogenic Factors in Proliferative Diabetic Retinopathy. Curr Eye Res. 2012;37:416–20. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 8.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–9. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- 9.Ozturk BT, Bozkurt B, Kerimoglu H, Okka M, Kamis U, Gunduz K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009;15:1906–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, Savli H. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002;16:163–70. doi: 10.1038/sj.eye.6700095. [DOI] [PubMed] [Google Scholar]
- 11.Hang H, Yuan S, Yang Q, Yuan D, Liu Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis. 2014;20:1137–45. [PMC free article] [PubMed] [Google Scholar]
- 12.Noda K, Nakao S, Ishida S, Ishibashi T. Leukocyte adhesion molecules in diabetic retinopathy. J Ophthalmol. 2012;2012:279037. doi: 10.1155/2012/279037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantón A, Martinez-Cáceres EM, Hernández C, Espejo C, García-Arumí J, Simó R. CD4–CD8 and CD28 expression in T cells infiltrating the vitreous fluid in patients with proliferative diabetic retinopathy: a flow cytometric analysis. Arch Ophthalmol. 2004;122:743–9. doi: 10.1001/archopht.122.5.743. [DOI] [PubMed] [Google Scholar]
- 14.Kase S, Saito W, Ohno S, Ishida S. Proliferative diabetic retinopathy with lymphocyte-rich epiretinal membrane associated with poor visual prognosis. Invest Ophthalmol Vis Sci. 2009;50:5909–12. doi: 10.1167/iovs.09-3767. [DOI] [PubMed] [Google Scholar]
- 15.Tang S, Scheiffrath O, Thurau S, Wildner G. Cells of the immune system and their cytokines in epiretinal membranes and in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmic Res. 1993;25:177–85. doi: 10.1159/000267287. [DOI] [PubMed] [Google Scholar]
- 16.Urbančič M, Kloboves Prevodnik V, Petrovič D, Globočnik Petrovič M. A flow cytometric analysis of vitreous inflammatory cells in patients with proliferative diabetic retinopathy. BioMed Res Int. 2013;2013:251528. doi: 10.1155/2013/251528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbančič M, Štunf Š, Živin AM, Petrovič D, Petrovič MG. Epiretinal membrane inflammatory cell density might reflect the activity of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:8576–82. doi: 10.1167/iovs.13-13634. [DOI] [PubMed] [Google Scholar]
- 18.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 19.Weibel E. Practical Methods for Biological Morphometry. London: Academic Press; 1979. [Google Scholar]
- 20.Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, Karasawa Y, Ito M. Elevated Levels of Cytokines Associated with Th2 and Th17 Cells in Vitreous Fluid of Proliferative Diabetic Retinopathy Patients. PLoS One. 2015;10:e0137358–0137358. doi: 10.1371/journal.pone.0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 22.Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in Diabetic Retinopathy. Curr Diabetes Rev. 2007;3:3–14. doi: 10.2174/157339907779802139. [DOI] [PubMed] [Google Scholar]
- 23.Harada C, Okumura A, Namekata K, Nakamura K, Mitamura Y, Ohguro H, Harada T. Role of monocyte chemotactic protein-1 and nuclear factor kappa B in the pathogenesis of proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2006;74:249–56. doi: 10.1016/j.diabres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sassa Y, Yoshida S, Ishikawa K, Asato R, Ishibashi T, Kono T. The kinetics of VEGF and MCP-1 in the second vitrectomy cases with proliferative diabetic retinopathy. Eye (Lond) 2016;30:746–53. doi: 10.1038/eye.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Takeuchi M, Goto H, Iwasaki T. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30:339–44. doi: 10.1097/IAE.0b013e3181bd2f44. [DOI] [PubMed] [Google Scholar]
- 27.Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–22. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida A, Yoshida S, Khalil AK, Tatsuro I, Inomata H. Role of NF-KB-Mediated Interleukin-8 Expression in Intraocular Neovascularization. Invest Ophthalmol Vis Sci. 1998;39:1097–106. [PubMed] [Google Scholar]
- 29.Jersmann HP. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol Cell Biol. 2005;83:462–7. doi: 10.1111/j.1440-1711.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 30.Abcouwer SF. Neural inflammation and the microglial response in diabetic retinopathy. J Ocul Biol Dis Infor. 2012;4:25–33. doi: 10.1007/s12177-012-9086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, Wang CM, Yang WL, Wang P. Microglial CD14 activated by iNOS contributes to neuroinflammation in cerebral ischemia. Brain Res. 2013;1506:105–14. doi: 10.1016/j.brainres.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustavsson C, Agardh C, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2013;91:445–52. [Google Scholar]
- 33.Didion SP. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int J Mol Sci. 2017;18:2563. doi: 10.3390/ijms18122563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi B-Z. Interleukin 6 Induces the Expression of Vascular Endothelial Growth Factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 35.Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye (Lond) 2008;22:223–8. doi: 10.1038/sj.eye.6702584. [DOI] [PubMed] [Google Scholar]
- 36.Aiello LP, Wong J. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. 2000;58(Suppl.77):S-113–9. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- 37.Mor F, Quintana FJ, Cohen IR. Angiogenesis-Inflammation Cross-Talk: Vascular Endothelial Growth Factor Is Secreted by Activated T Cells and Induces Th1 Polarization. J Immunol. 2004;172:4618–23. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 38.Mao C, Yan H. Roles of elevated intravitreal IL-1β and IL-10 levels in proliferative diabetic retinopathy. Indian J Ophthalmol. 2014;62:699–701. doi: 10.4103/0301-4738.136220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Asrar AMA. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. 2012;19:70–4. doi: 10.4103/0974-9233.92118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gverović Antunica A, Karaman K, Znaor L, Sapunar A, Buško V, Puzović V. IL-12 concentrations in the aqueous humor and serum of diabetic retinopathy patients. Graefes Arch Clin Exp Ophthalmol. 2012;250:815–21. doi: 10.1007/s00417-011-1905-4. [DOI] [PubMed] [Google Scholar]