Abstract
As the coronavirus disease 2019 (COVID-19) continues to spread across the globe, the knowledge of its epidemiology, clinical features, and management is rapidly evolving. Nevertheless, the data on optimal fluid management strategies for those who develop critical illness remain sparse. Adding to the challenge, the fluid volume status of these patients has been found to be dynamic. Some present with several days of malaise, gastrointestinal symptoms, and consequent hypovolemia requiring aggressive fluid resuscitation, while a subset develop acute respiratory distress syndrome with renal dysfunction and lingering congestion necessitating restrictive fluid management. Accurate objective assessment of volume status allows physicians to tailor the fluid management goals throughout this wide spectrum of critical illness. Conventional point-of-care ultrasonography (POCUS) enables the reliable assessment of fluid status and reducing the staff exposure. However, due to specific characteristics of COVID-19 (e.g., rapidly expanding lung lesions), a single imaging method such as lung POCUS will have significant limitations. Herein, we suggest a Tri-POCUS approach that represents concurrent bedside assessment of the lungs, heart, and the venous system. This combinational approach is likely to overcome the limitations of the individual methods and provide a more precise evaluation of the volume status in critically ill patients with COVID-19.
Keywords: Point of care ultrasound, POCUS, Fluid volume status, Nephrology, COVID-19, Lung ultrasound
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly spreading across the globe. While most infected patients have only a mild disease, about 5% develop critical illness requiring intensive care unit (ICU) admission, which is substantial in terms of absolute numbers [1]. Acute hypoxemic respiratory failure from acute respiratory distress syndrome (ARDS) is the most common complication in these patients, and acute kidney injury (AKI) is a frequent accompaniment with an incidence ranging from 0.5 to 27% [2, 3]. Variability in disease manifestations coupled with limited availability of direct evidence on optimal management strategies pose unique challenges to the healthcare providers including nephrologists taking care of these patients. Based on the indirect evidence from studies pertaining to sepsis and ARDS, point-of-care ultrasonography (POCUS) could have a distinctive role in guiding management in this setting.
Why Is Volume Status Assessment Complicated?
The volume status of patients with COVID-19 is dynamic and can range from severe hypovolemia to overt hypervolemia [4]. A subset of patients with COVID-19 present with hypovolemia due to anorexia, vomiting, and diarrhea requiring fluid resuscitation [5]. On the other hand, later in the course of the disease they can develop ARDS and distributive shock, in which case continued fluid expansion may have deleterious consequences exacerbating the pre-existing gas exchange abnormalities, particularly in the setting of oliguric AKI [6]. There is an abundance of data demonstrating the negative effects of volume overload and tissue edema on multiple organ systems as well as survival [7]. Not surprisingly, a recent autopsy study in patients deceased from COVID-19 reported cardiomegaly with right ventricular dilatation, which could be a result of ARDS itself compounded by fluid overload and the effects of positive pressure ventilation [8]. Paradoxically, overzealous attempts at keeping such patients “dry” may not be an optimal strategy as reduction in cardiac output and pulmonary blood flow would lead to an increase in alveolar and physiological dead space, ultimately worsening the gas exchange [9]. Further complicating the picture, cytokine storm is a known feature of COVID-19, which can lead to increased vascular permeability, worsening pulmonary edema, third-space fluid loss, intra-abdominal hypertension, and multiorgan failure [10]. Therefore, achieving an “optimal” intravascular volume is crucial to maintain adequate tissue perfusion while minimizing the alveolar flooding and third-spacing. These factors should be carefully taken into consideration when assessing the fluid status of these patients and synthesizing a treatment plan.
What Is the Problem with “Conventional” Assessment?
Conventional physical examination has limited diagnostic utility to predict the fluid status and to guide vasoactive therapy in hemodynamically unstable patients [11]. Of particular note, the diagnostic accuracy of auscultation for detection of alveolar-pulmonary edema is only about 55–67% [12], which is likely to be even lower when using disposable stethoscopes in the noisy ICU environment. Adding to this, several of our clinical assumptions are either inaccurate or have limited value in the case of critically ill patients. For example, the presence of peripheral edema and/or positive fluid balance do not necessarily indicate fluid overload, as these patients can still be intravascularly depleted or euvolemic. Similarly, not all hypotension is hypovolemia. Interestingly enough, atypical presentations such as heart failure with acute myopericarditis have been reported in patients with COVID-19, which can be associated with hypotension but not volume depletion [13]. Even laboratory parameters commonly associated with volume overload such as an elevated B-type natriuretic peptide level are not reliable owing to their non-specificity [14].
Guidelines issued by professional organizations for the management of critically ill patients with COVID-19 recommend adopting a “conservative” fluid administration strategy and measuring dynamic parameters to assess fluid responsiveness [15]. POCUS is a bedside ultrasound examination performed by the clinician to answer focused question(s) and guide management. Studies have consistently confirmed that it can improve the diagnostic accuracy of physical examination in various clinical backdrops [12, 16]. However, isolated use of lung or inferior vena cava (IVC) ultrasound is subject to limitations in this context. For example, lung ultrasound (LUS) findings in ARDS may be difficult to distinguish from that of cardiogenic pulmonary edema and, sometimes, patient positioning may limit the scan zones available for evaluation. Similarly, IVC ultrasound may not be reliable owing to its dependence on intra-abdominal pressure, changes in tidal volume, local mechanical factors (e.g., thrombosis, IVC filter, venous cannulae), and patient-triggered inspiratory efforts.
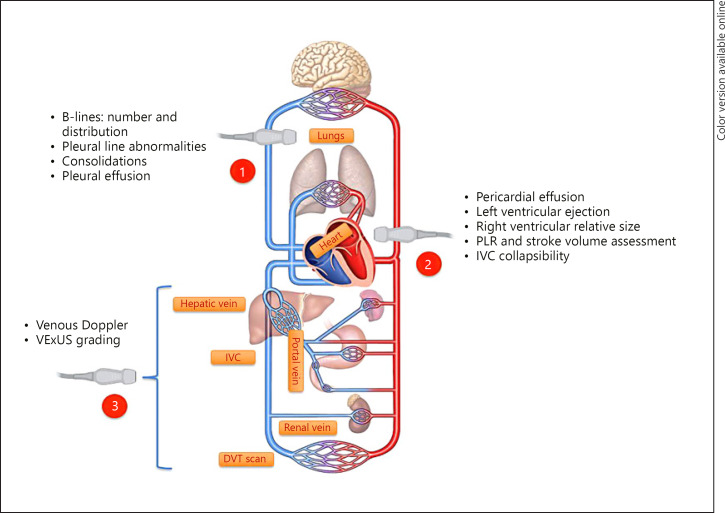
We believe that a “Tri-POCUS” approach (Fig. 1) combining LUS, focused cardiac ultrasound (FoCUS), and venous Doppler ultrasound can overcome the shortcomings of individual techniques and enhance the reliability by providing valuable insights into patients' hemodynamics when interpreted in the clinical context.
Fig. 1.
The Tri-POCUS approach for volume status assessment in critically ill patients. Common abnormalities/assessments pertinent to each of these sonographic applications are listed. PLR, passive leg-raise maneuver to assess fluid responsiveness; VExUS, venous excess ultrasound grading; IVC, inferior vena cava; DVT, deep vein thrombosis.
Lung Ultrasound
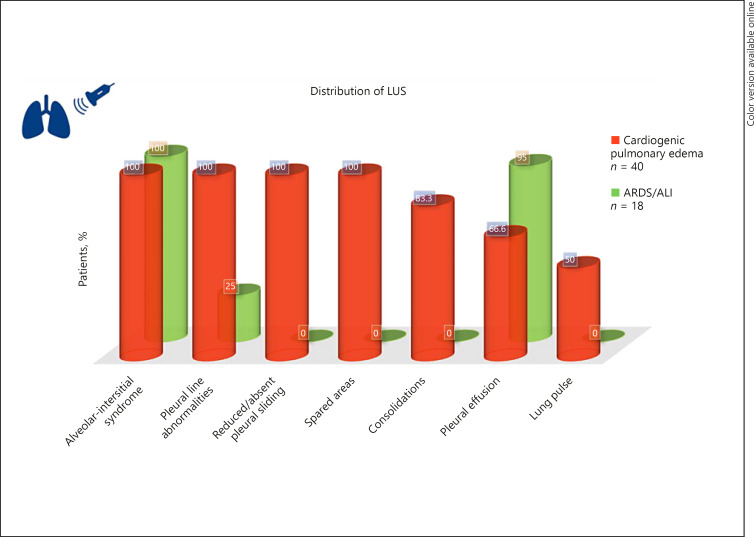
Though relatively non-specific, the characteristic findings of COVID-19 disease on LUS include a thickened or irregular pleural line, subpleural consolidations, and multifocal or confluent B-lines [17]. Patients who develop ARDS demonstrate similar findings, though more widespread and bilateral with confluent B-lines, indicating loss of aeration and increased extra-vascular lung water. As mentioned before, it might be difficult to distinguish this appearance from that of cardiogenic pulmonary edema that can also be seen in patients with COVID-19. However, certain features can help differentiate these two entities, as depicted in Figure 2. Notably, reduced or absent pleural sliding as well as the presence of lung pulse and spared areas are highly suggestive of ARDS, while pleural effusions are more commonly seen in cardiogenic pulmonary edema [18]. In addition, LUS-derived aeration scores can be used to monitor response to therapy and for prognostication in COVID-19 patients with ARDS [19]. Furthermore, LUS is a valuable tool to detect complications of positive pressure ventilation such as pneumothorax and guide appropriate intervention.
Fig. 2.
LUS patterns in ARDS versus cardiogenic pulmonary edema based on the data from Copetti et al. [18]. Alveolar-interstitial syndrome was defined as the presence of more than 3 B-lines or “white lung” appearance for each examined area. Spared areas were defined as the areas of normal lung pattern in at least one intercostal space surrounded by areas of alveolar-interstitial syndrome. Lung pulse was defined as the absence of lung sliding with the perception of heart activity at the pleural line. LUS, lung ultrasound; ARDS, acute respiratory distress syndrome; ALI, acute lung injury.
Focused Cardiac Ultrasound
FoCUS allows for rapid evaluation of the “five Es,” as described by Kennedy Hall et al. [20], which include pericardial effusion, qualitative assessment of left ventricular ejection, right and left ventricular equality (e.g., right ventricle enlarges due to pulmonary hypertension or embolism), exit (aortic root diameter), and the entrance (IVC). In the context of fluid status assessment, exit can be used to indicate stroke volume/cardiac output measurement at the left ventricular outflow tract. Compared to static measures of preload, such as the central venous pressure, dynamic calculation of stroke volume reliably predicts “volume responsiveness” or patient's position on the Frank-Starling curve. Volume responsiveness is usually defined as a measurable increase of 15% in cardiac output (or stroke volume in the absence of significant variation in the heart rate) in response to fluid challenge. Instead of exogenous administration of fluids, a “passive leg-raise” (PLR) maneuver is typically performed, which redistributes approximately 300–400 mL of blood from the lower extremities to the heart and stroke volume is measured 1–2 min later using cardiac ultrasound [21]. Assessment of the response to PLR virtually eliminates the need for “empiric” intravenous fluid administration, which can be detrimental in patients with ARDS. This test can be performed in both mechanically ventilated and spontaneously breathing patients and is fairly reliable even in the presence of arrhythmias. Moreover, FoCUS can be performed in COVID-19 patients undergoing prone ventilation, albeit with a minor modification in the image acquisition technique [22].
Variations in the diameter of IVC may be used to predict fluid responsiveness, particularly when using a handheld ultrasound device without spectral Doppler capability or in case of unobtainable cardiac windows. In a study including 39 mechanically ventilated patients with septic shock, 12% respiratory variation calculated as the difference between the maximum and the minimum diameter, normalized by the mean of the two values was associated with an increase of cardiac output after fluid infusion [23]. Nevertheless, in view of the previously stated limitations, the findings should be interpreted with caution.
Venous Doppler
Evaluation of blood flow pattern in the hepatic, portal, and intrarenal veins using bedside Doppler ultrasound to assess venous congestion is another attractive means to gauge fluid status in critically ill patients, though not specifically studied in those with ARDS. Recently, a venous excess ultrasound (VExUS) grading system has been developed using these waveforms to quantify systemic congestion [24]. In brief, when the IVC diameter is 2 cm or more, three grades of congestion were defined based on the severity of abnormalities on hepatic, portal, and intrarenal vein Doppler ultrasound. Hepatic vein Doppler is considered mildly abnormal when the systolic (S) component is smaller than the diastolic (D) component, but still below the baseline while it is considered severely abnormal when the S component is reversed, that is above the baseline. Portal vein Doppler is considered mildly abnormal when the variation in velocities during the cardiac cycle ranges between 30 and 50%, while ≥50% pulsatility is severely abnormal. Intrarenal venous Doppler is considered mildly abnormal when it is pulsatile with an S and D phase, and severely abnormal when it is discontinuous with only a D phase.
Based on our experience in patients with cardiorenal syndrome, we believe venous congestion assessment provides an important piece of the hemodynamic puzzle, when interpreted in the appropriate clinical context. In addition, patients with COVID-19 are at increased risk for thromboembolic phenomena and when the clinical suspicion is high, Doppler evaluation of the lower extremities to exclude deep vein thrombosis is a valuable adjunct [25].
Case Study
Herein, we concisely describe the case of a hypervolemic patient with minimal symptoms, where Tri-POCUS played a key role in the accurate determination of volume status and guiding the management strategy as well as monitoring the response to therapy.
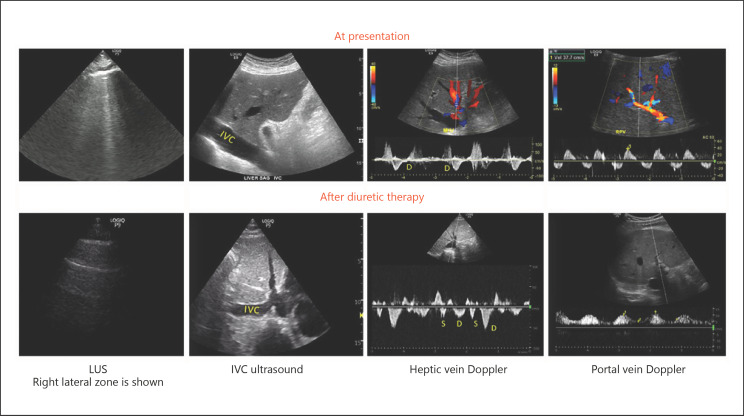
A 55-year-old man with a history of heart failure with reduced ejection fraction of 25% was found to have AKI of uncertain etiology (serum creatinine of 3.5 mg/dL with a baseline of 1.6 mg/dL). His vital signs were stable and he had no symptoms except for his usual dyspnea on exertion. In order to objectively explore his volume status, a quick bedside LUS was performed that revealed an increased extravascular lung water with B-lines particularly in the lateral scan zones. Interestingly, FoCUS showed a dilated IVC of approximately 3 cm that was minimally collapsible with inspiration suggestive of increased right atrial pressure. It also confirmed a low ejection fraction without grossly visible regional wall motion abnormalities and excluded pericardial effusion. Hepatic and portal vein Doppler disclosed stigmata of severe venous congestion with pulsatile portal vein and hepatic vein, with only the D component below the baseline. Based on Tri-POCUS findings, the diagnosis of congestive AKI was made at the bedside and the patient received high-dose intravenous diuretics. Two days later, his serum creatinine improved to 2.2 mg/dL. LUS and venous Doppler studies showed remarkable improvement. There was a reduction in IVC diameter, and both the S and D components were found below the baseline on hepatic vein Doppler. Similarly, portal vein pulsatility reduced to approximately 55% (Fig. 3). Diuretic therapy was titrated further based on these findings.
Fig. 3.
POCUS findings at presentation and after intravenous diuretic therapy in a patient with cardiorenal syndrome demonstrating improvement in hypervolemic status. LUS shows B-lines at presentation that transitioned to A-lines after therapy. IVC shows improvement in the maximal diameter. Hepatic vein waveform shows the appearance of S and D components below the baseline from the initial monophasic pattern. Portal vein waveform shows improvement in pulsatility. Below the baseline tracing at presentation represents flow reversal during the systole, which is a marker of severe congestion.
Why Use Point-of-Care Ultrasound Rather than Traditional Imaging?
First, POCUS is a relatively inexpensive, radiation-free bedside tool that allows rapid diagnosis impacting the management plan, which is vital in critically ill patients. Second, ability to perform multiorgan assessment and use it for procedural guidance during the same encounter improves efficiency and reduces pathogen spread. This is of utmost importance in patients with COVID-19 where the assessment of fluid volume status can be enhanced through multiorgan POCUS due to progressive pulmonary lesions and the dynamic nature of their volume status. In addition, the need for CT scan and repeat chest radiographs is minimized, limiting the staff exposure during patient transport. Moreover, disinfection of the ultrasound machine is easier and not associated with any downtime for the radiology room decontamination, unlike CT scan. Most handheld ultrasound devices can be completely encased in a standard plastic transducer cover that can be discarded after each use.
The Time Is Now
Nephrologists' opinion is often sought for the optimal management of complex fluid and electrolyte disorders. The Tri-POCUS approach allows us to titrate fluid resuscitation strategies according to the phases of critical illness and accompanying organ dysfunction. As such, POCUS-guided objective evaluation of volume status is an invaluable addition to our armamentarium.
Statement of Ethics
Human and animal rights: this article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure Statement
The authors have declared that no conflicts of interest exist.
Funding
No funding was acquired for this work.
Author Contributions
A.Ko. drafted the initial version of the manuscript. C.R. and A.Ka. revised the manuscript critically for important intellectual content. All authors read and approved the manuscript prior to final submission.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb;323((13)):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diap B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazory A, Ronco C, McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc Bayl Univ Med Cent. 2020 Apr;0((0)):1–6. doi: 10.1080/08998280.2020.1754700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Asian Critical Care Clinical Trials Group Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020 May;8((5)):506–17. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Program to Improve Care in Acute Renal Disease (PICARD) Study Group Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009 Aug;76((4)):422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 7.Jaffee W, Hodgins S, McGee WT. Tissue Edema, Fluid Balance, and Patient Outcomes in Severe Sepsis: An Organ Systems Review. J Intensive Care Med. 2018 Sep;33((9)):502–9. doi: 10.1177/0885066617742832. [DOI] [PubMed] [Google Scholar]
- 8.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keddissi JI, Youness HA, Jones KR, Kinasewitz GT. Fluid management in Acute Respiratory Distress Syndrome: A narrative review. Can J Respir Ther. 2018 Dec;55:1–8. doi: 10.29390/cjrt-2018-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020 Apr; doi: 10.1038/s41581-020-0284-7. DOI: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saugel B, Ringmaier S, Holzapfel K, Schuster T, Phillip V, Schmid RM, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care. 2011 Aug;26((4)):402–10. doi: 10.1016/j.jcrc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Cox EG, Koster G, Baron A, Kaufmann T, Eck RJ, Veenstra TC, et al. SICS Study Group Should the ultrasound probe replace your stethoscope? A SICS-I sub-study comparing lung ultrasound and pulmonary auscultation in the critically ill. Crit Care. 2020 Jan;24((1)):14. doi: 10.1186/s13054-019-2719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar; doi: 10.1001/jamacardio.2020.1096. DOI: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koratala A, Kazory A. Natriuretic Peptides as Biomarkers for Congestive States: The Cardiorenal Divergence. Dis Markers. 2017;2017:1454986. doi: 10.1155/2017/1454986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19. JAMA. 2020 Mar; doi: 10.1001/jama.2020.4914. DOI: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 16.Marbach JA, Almufleh A, Di Santo P, Jung R, Simard T, McInnes M, et al. Comparative Accuracy of Focused Cardiac Ultrasonography and Clinical Examination for Left Ventricular Dysfunction and Valvular Heart Disease: A Systematic Review and Meta-analysis. Ann Intern Med. 2019 Aug;171((4)):264–72. doi: 10.7326/M19-1337. [DOI] [PubMed] [Google Scholar]
- 17.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020 May;46((5)):849–50. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008 Apr;6((1)):16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XT, Ding X, Zhang HM, Chen H, Su LX, Liu DW, Chinese Critical Ultrasound Study Group (CCUSG) Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care. 2016 Nov;20((1)):385. doi: 10.1186/s13054-016-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy Hall M, Coffey EC, Herbst M, Liu R, Pare JR, Andrew Taylor R, et al. The “5Es” of emergency physician-performed focused cardiac ultrasound: a protocol for rapid identification of effusion, ejection, equality, exit, and entrance. Acad Emerg Med. 2015 May;22((5)):583–93. doi: 10.1111/acem.12652. [DOI] [PubMed] [Google Scholar]
- 21.Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016 Sep;20((1)):274. doi: 10.1186/s13054-016-1407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugalde D, Medel JN, Romero C, Cornejo R. Transthoracic cardiac ultrasound in prone position: a technique variation description. Intensive Care Med. 2018 Jun;44((6)):986–7. doi: 10.1007/s00134-018-5049-4. [DOI] [PubMed] [Google Scholar]
- 23.Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004 Sep;30((9)):1834–7. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 24.Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020 Apr;12((1)):16. doi: 10.1186/s13089-020-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020 Apr; doi: 10.1111/bjh.16727. DOI:10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]