Dear Editor,
Coronavirus disease-19 (COVID-19) is now a global pandemic. Italy was the first European country to be hit by the infection, which has currently been diffused everywhere across Europe. Fever, cough, muscle aches, dyspnea, and fatigue can be presenting symptoms, as well as smell and taste dysfunction, usually in presence of a patent nasopharyngeal airway. Therefore, the American Academy of Otolaryngology-Head and Neck Surgery and the British Association of Otorhinolaryngology advised patients with sudden-onset loss of smell and/or taste to self-isolate [1].
Additionally, Sungnak et al. [2] recently demonstrated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry factors are highly expressed in nasal epithelial cells together with innate immune genes, evidencing the central role of the nose in transmitting the infection to the rest of the body. Another feature of SARS-CoV-2 infection is that it seems to produce absent or minimal symptoms of nasal congestion or rhinorrhea, while other viral rhinitis (i.e., adenovirus, rhinovirus, influenza virus, etc.) are characterized by typical nasal symptoms, including sneezing, rhinorrhea, and nasal congestion [1]; additionally, the nasal mucosa usually appears swollen, edematous, and with seromucous exudate. Pathological changes depend on the virus type. Microscopic observation by means of nasal cytology reveals mucosal disarrangement. Cellular infiltration usually starts within a day since the onset of the infection: neutrophils, lymphocytes, and exfoliated epithelial cells characterize its cytopathological feature [3]. “Ciliocytophthoria” is the main sign of cell injury and is characterized by condensed nuclear chromatin, marginalization of nucleoli, inclusion bodies, intranuclear halo, and cytoplasmic vacuoles [4]. Conversely, the “hyperchromatic supranuclear stria” (SNS) [5], corresponding to the Golgi apparatus, constitutes a marker for the anatomical and functional integrity of the ciliated cells; its rarefaction or disappearance during viral infections is a sign of cellular distress [6].
Given these premises, we performed nasal cytology in 18 COVID-19 patients (5 males, 13 females; median age 50 years [range 26–63]) enrolled in a Hospital close to the first northern-Italian cluster in order to evidence analogies with and differences to the patterns of the other viral upper respiratory infections.
All patients showed very few neutrophils and reduction of the SNS. None of the specimens had ciliocytophthoria or other signs of cell injury (Fig. 1). The slight marks of cell damage were consistent with the mild nasal symptoms experienced by the 18 patients. In fact, only 33.3% (n = 6) of them had rhinorrhea and another 33.3% (n = 6) showed nasal obstruction, while 66.6% (n = 12) reported a smell impairment, which slowly improved over time. Recently, it was evidenced that the neuroinvasive and neurotropic potential of SARS-CoV-2 seems to be site-specific for the olfactory cells [1, 7]. Therefore, a possible explanation is that SARS-CoV-2 causes a mild nasal inflammation, but it has a transient neurotoxic effect on the olfactory system. The lack of clear cytopathological damage evidenced by nasal cytology could support this hypothesis.
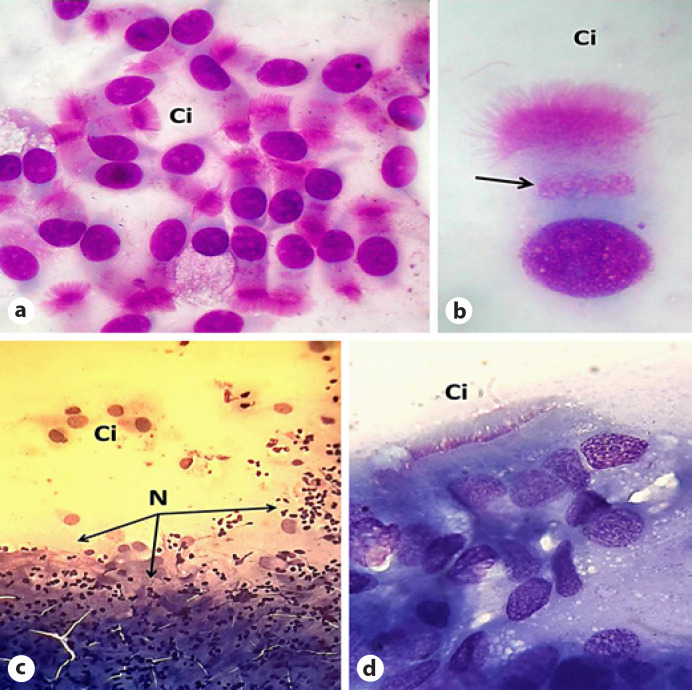
Fig. 1.
a Nasal cytology within normal limits (May-Grünwald-Giemsa, ×800× magnification). b Normal ciliated cell. The arrow indicates the “hyperchromatic supranuclear stria” (SNS; May-Grünwald-Giemsa, ×2,000 magnification). c Nasal cytology in a COVID-19 patient (May-Grünwald-Giemsa, ×400 magnification). d Nasal cytology in a COVID-19 patient evidencing absence of the hyperchromatic SNS (May-Grünwald-Giemsa, ×1,000 magnification). Ci, ciliated cell; N, neutrophils.
In conclusion, although the rarefaction of the SNS is a non-specific marker of cell injury, it represents the only evident sign of cellular distress in the context of COVID-19. Larger studies have to confirm these preliminary results. To our knowledge, this is the first report in the current literature that used nasal cytology to investigate SARS-CoV-2 infection from a rhinologic point of view, and we believe that this noninvasive technique can be useful to study COVID-19 in the future for clinical and research purposes [8].
Statement of Ethics
This research was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, and written informed consent was obtained from all subjects.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
M.G. and M.N. designed the study, participated in data collection and analyses, drafted and approved the final version of this paper. E.M.C.T. participated in data analysis and manuscript preparation, M.C. and G.C. provided critical comments, and approved the final version of this paper.
References
- 1.Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020 Apr;S1473-3099((20)):30293–0. doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 May;26((5)):681–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle WJ, Gentile DA, Skoner DP. Viral and bacterial rhinitis. Clin Allergy Immunol. 2007;19:177–95. [PubMed] [Google Scholar]
- 4.Gelardi M, Ciprandi G. Ciliocytophthoria of nasal epithelial cells after viral infection: a sign of suffering cell. Acta Biomed. 2019 Jan;90(2-S):7–9. doi: 10.23750/abm.v90i2-S.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslani FS, Khademi B, Yeganeh F, Kumar PV, Bedayat GR. Nasal cytobrush cytology: evaluation of the hyperchromatic supranuclear stria. Acta Cytol. 2006 Jul-Aug;50((4)):430–4. doi: 10.1159/000325987. [DOI] [PubMed] [Google Scholar]
- 6.Gelardi M, Cassano P, Cassano M, Fiorella ML. Nasal cytology: description of a hyperchromatic supranuclear stria as a possible marker for the anatomical and functional integrity of the ciliated cell. Am J Rhinol. 2003 Sep-Oct;17((5)):263–8. [PubMed] [Google Scholar]
- 7.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 Feb;92((6)):552–5. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heffler E, Landi M, Caruso C, Fichera S, Gani F, Guida G, et al. Nasal cytology: methodology with application to clinical practice and research. Clin Exp Allergy. 2018 Sep;48((9)):1092–106. doi: 10.1111/cea.13207. [DOI] [PubMed] [Google Scholar]