Abstract
Importance
The novel coronavirus 2019 (SARS-CoV-2) has been well described in adults. Further, the impact on older children and during the perinatal time is becoming better studied. As community spread increases, it is important to recognize that neonates are vulnerable to community spread as well. The impact that community-acquired SARS-CoV-2 has in the neonatal time period is unclear, as this population has unique immunity considerations.
Objective
To report on a case series of SARS-CoV-2 in neonates through community acquisition in the USA.
Design
This is an early retrospective study of patients admitted to the Neonatal Intensive Care Unit (NICU) identified as having SAR-CoV-2 through positive real-time polymerase chain reaction assay of nasopharyngeal swabs.
Findings
Three patients who required admission to the NICU between the ages of 17 and 33 days old were identified. All 3 had ill contacts in the home or had been to the pediatrician and presented with mild to moderate symptoms including fever, rhinorrhea, and hypoxia, requiring supplemental oxygen during their hospital stay. One patient was admitted with neutropenia, and the other 2 patients became neutropenic during hospitalization. None of the patients had meningitis or multiorgan failure.
Conclusions and Relevance
Infants with community-acquired SARS-CoV-2 may require hospitalization due to rule-out sepsis guidelines if found to have fever and/or hypoxia. Caregivers of neonates should exercise recommended guidelines before contact with neonates to limit community spread of SARS-CoV-2 to this potentially vulnerable population, including isolation, particularly as asymptomatic cases become prevalent.
Keywords: COVID-19, SARS-CoV-2, Neonate, Neutropenia
Introduction
The global pandemic of novel coronavirus disease 2019 caused by SARS-CoV-2 has demanded the world's attention. Community-acquired SARS-CoV-2 (CA-SARS-CoV-2) infection is well described in adults [1], and the epidemiology of the disease suggests that it is more likely to cause significant morbidity and mortality in adults, particularly those with chronic comorbidities [2]. However, children under 1 year of age, have higher hospitalization rates than adults, very similar to influenza [3]. Reports indicate that SARS-CoV-2 infects children and that there is a potential perinatal vertical transmission [4, 5, 6], though, to our knowledge, reports of CA-SARS-CoV-2 infection in infants aged less than 2 months are limited to single case reports [7, 8, 9]. Here we describe a cohort of patients found to be SARS-CoV-2 positive and describe their demographic, epidemiologic, and clinical features.
Methods
In this early retrospective study, patients who were admitted to the Neonatal Intensive Care Unit (NICU) at the Children's Hospital Colorado (CHCO) and tested positive for SARS-CoV-2 were identified. Demographic, epidemiologic, and clinical data were obtained from the medical record system and confirmed by the families of the patients where necessary.
The diagnosis was confirmed by real-time polymerase chain reaction (RT-PCR) assay of nasopharyngeal swabs for SARS-CoV-2 (Simplexa®COVID-19; DiaSorin, Stillwater, MN, USA). Patients were also tested for other viruses and bacteria by RT-PCR in nasopharyngeal specimens using the Respiratory 2 (RP2) Panel (FilmArray®; BioFire Diagnostics, Salt Lake City, UT, USA). At CHCO, all neonates with respiratory symptoms or fever who are admitted to the hospital are tested for these pathogens during the current SARS-CoV-2 outbreak.
Results
Between March 28, 2020, and April 1, 2020, three young infants with confirmed SARS-CoV-2 infection were identified. All 3 were tested upon presentation to the emergency room and no repeat testing was performed. Two of the 3 patients were male. All were healthy term infants born at 39–40 weeks of gestation. One infant was born via cesarean section due to a prior cesarean section in the mother. Two of the 3 infants were exclusively receiving maternal breast milk at the time of admission (Table 1).
Table 1.
Demographic characteristics
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age at initial symptoms, days | 16 | 25 | 31 |
| Age at hospital admission, days | 17 | 27 | 33 |
| Gestational age (birth), weeks | 39 | 39 | 39 |
| Sex | Male | Male | Female |
| Mode of delivery | Induced vaginal | Repeat cesarean section | NSVD |
| Ill contacts | Mother and sister | Mother and father | None known |
| Time between ED admission and diagnosis | 10 h 40 min | 9 h | 12 h 45 min |
| Time between NICU admission and diagnosis | 4 h 43 min | 1 h 12 min | 6 h 19 min |
| Hospital LOS (inpatient), h | 77 | 80 | 81 |
LOS, length of stay; NSVD, normal spontaneous vaginal delivery.
The age at onset of symptoms was 16–31 days and none had previous testing for SARS-CoV-2. The age at hospital admission was 17–33 days. Two of the infants had known ill contacts with upper respiratory infection symptoms; one had no known sick contacts but had recently visited with the pediatrician. All of the infants presented with fever, rhinorrhea, and mild hypoxia with desaturations on room air to 80–90%. The youngest infant also had tachycardia, systemic vasodilation, and bilateral conjunctivitis and required 20 mL/kg normal saline fluid resuscitation in the emergency department (ED) prior to NICU admission. The time between ED admission and diagnosis was 9 h to 12 h 45 m (Table 2).
Table 2.
Clinical characteristics and laboratory studies
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| White blood cells, n × 103/µL | 4.86 | 9.06 | 13.69 |
| Absolute lymphocyte count | 1,070 | 5,130 | 8,290 |
| Absolute neutrophil count (admission nadir) | 2,360 → 920 | 2,170 → 1,170 | 2,530 → 840 |
| Absolute monocyte count | 1,280 | 1,720 | 2,590 |
| CSF WBC/RBC/protein/glucose | 2/1476/42/50 | 2/1/62/50 | N/A |
| CSF meningoencephalitis panel | negative | negative | N/A |
| HSV PCR | negative | negative | N/A |
| Respiratory pathogen panel | negative | negative | negative |
| SARS-CoV-2 PCR | positive | positive | positive |
| C-reactive protein, mg/dL | 1.5 | 1.1 | 1.2 |
| Procalcitonin | 0.1 ng/mL | 0.5 ng/mL | 0.1 ng/mL |
| Chest radiograph | Hypoinflated lungs with increased haziness in the RLL which could represent atelectasis or evolving pneumonia | Mostly clear lungs without focal consolidation; hazy opacities in upper lung zones; no pleural effusion | Low lung volumes with nonspecific hazy opacities in the bilateral upper lung zones; no focal consolidation |
RLL, right lower lobe; N/A, not available.
All 3 infants required supplemental oxygen via a nasal cannula to maintain oxygen saturations >90%. The maximum respiratory support ranged from 0.25 to 1 L/min. Chest radiographs showed low lung volumes with hazy opacities without areas of focal consolidation in all of the patients (Fig. 1). The youngest patient had lymphopenia and a mildly elevated C-reactive protein level on admission, both of which resolved by the time of discharge. The absolute neutrophil count dropped from normal to low (<1,500/μL) during hospitalization. All other laboratory studies for the 3 patients, including hematocrit, platelet count, liver function test, lactate, and blood gas, were within normal limits for age. Bacterial cultures remained negative and antimicrobials were discontinued within 24–36 h based on identified viral infection. None of the infants had serious complications from infection. The length of stay for these patients ranged from 77 to 81 h and the patients were discharged when they had been afebrile for at least 24 h.
Fig. 1.
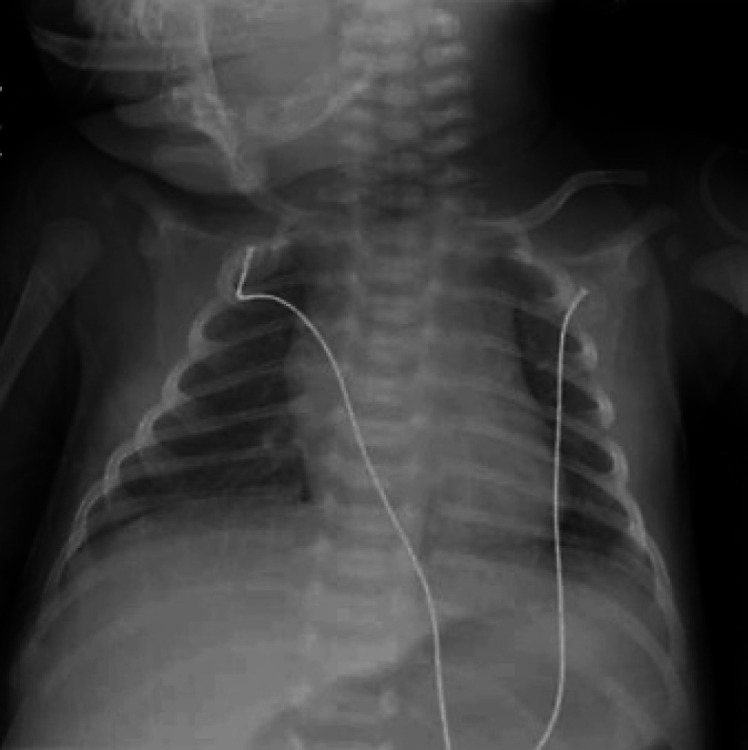
Representative chest radiograph from patient 1 showing hypoinflated lungs with increased haziness in the right lower lobe and patchy consolidation.
Discussion
We describe 3 of the youngest patients diagnosed with CA-SARS-CoV-2 in the USA. Previous reports describe infants with ages ranging from 15 to 56 days [7, 8, 9]. An additional report of 6 neonates found to be SARS-CoV-2 positive in China fails to identify the route of acquisition [10]. Hence, most of the literature regarding SARS-CoV-2 infection in neonates has been regarding the early neonatal period and little is known about CA-SARS-CoV-2 in older neonates. As the outbreak continues to extend, understanding CA-SARS-CoV-2 in the neonatal population will become increasingly important.
Since their original identification in the 1960s, human coronaviruses account for 5–7% of hospitalizations in children in the USA [11] and can be frequently detected in hospitalized neonates with fever and respiratory infection and produce outbreaks [12]. During the previous epidemic of SARS-CoV-1 in 2003, only 1 neonate − a premature infant, 50 days old, presenting with hypothermia, fever, respiratory distress, and cyanosis − was reported infected with symptoms [13].
From the early reports of the SARS-CoV-2 pandemic, while most children experience a very mild or subclinical illness, neonates may experience 2 different modes of disease similar to other RNA viruses, i.e., enteroviruses and parechoviruses. Presentation in the neonatal period may be due to intrapartum transmission around delivery, characterized by pneumonia and sepsis-like syndrome in up to 10% of neonates [14]. This more severe early onset of disease in the first week of life is likely due to the absence of serotype-specific neutralizing antibody given the absence of immunity in the mothers. In the late neonatal period, infants may be more vulnerable and manifest a moderate illness given their immaturity of B-cell and biased Th2 responses [15].
All of our patients met the diagnosis requirements previously published [16], showing: (1) clinical symptoms including fever and the need for respiratory support, (2) abnormal chest radiographs, and (3) being at high risk for SARS-CoV-2 infection due to close contact with a person with symptoms consistent with SARS-CoV-2 or living in an area with widespread CA-SARS-CoV-2. All of our patients were hypoxic and required oxygen support. While the high-altitude of Denver (CO, USA) and CHCO (1,610 m) may pose a risk for transient hypoxia in the neonate [17], they are unlikely to develop pulmonary hypertension, as was demonstrated by the rapid decrease in oxygen requirement in these patients.
Our study describes previously healthy, full-term infants prior to CA-SARS-CoV-2 infection who presented with fever and underwent rule-out-sepsis protocols. All of these patients had mild to moderate courses with short hospital stays, consistent with previous pediatric literature [4, 7]. None of our patients had abnormal procalcitonin despite procalcitoninemia being reported in 64% of childhood SARS-CoV-2 patients [4]. Further, patient 1 (the youngest patient in this series), who exhibited multiple symptoms and the highest amount of respiratory support, was the only patient who showed a mildly elevated C-reactive protein level and leukopenia (lymphocyte count <1.2 × 109 per liter), which has been previously reported in 3.5% of children with SARS-CoV-2 [4]. None of our patients showed clinical signs of liver dysfunction, meningitis, concurrent viral infection, or other organ involvement. Interestingly, patients 2 and 3 showed a decreased absolute neutrophil count on follow-up complete blood counts, possibly related to viral suppression as the infection persisted.
This study was limited by the small sample size, the lack of inclusion of asymptomatic patients, and its description of and experience at a single center. We expect similar patients will be admitted and future comprehensive reports will add greatly to the current knowledge of CA-SAR-CoV-2. In the meantime, caregivers of neonates should be advised to wear masks and wash their hands before coming into contact with neonates to limit community spread of SARS-CoV-2 to this potentially vulnerable population. Additionally, isolating healthy babies from positive family member cases would be prudent.
Statement of Ethics
This research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This study was approved as nonhuman subject research by the Colorado Multiple Institutional Review Board (reference ID: APP001-1).
Disclosure Statement
None.
Funding Sources
None.
References
- 1.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020 Mar;382((10)):970–1. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialek S, Boundy E, Bowen V, Chow N, Cohn A, Dowling N, et al. CDC COVID-19 Response Team Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020 Mar;69((12)):343–6. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. Chinese Pediatric Novel Coronavirus Study Team SARS-CoV-2 Infection in Children. N Engl J Med. 2020 Apr;382((17)):1663–5. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 Mar;395((10226)):809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA. 2020 Mar; doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA. 2020 Feb;323((13)):1313. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020 Jun;52((6)):427–9. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronado Munoz A, Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late-Onset Neonatal Sepsis in a Patient with Covid-19. N Engl J Med. 2020 May;382((19)):e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Zhu J, Du L. Neonatal Management During the Coronavirus Disease (COVID-19) Outbreak: the Chinese Experience. Neoreviews. 2020 May;21((5)):e293–7. doi: 10.1542/neo.21-5-e293. [DOI] [PubMed] [Google Scholar]
- 11.Varghese L, Zachariah P, Vargas C, LaRussa P, Demmer RT, Furuya YE, et al. Epidemiology and Clinical Features of Human Coronaviruses in the Pediatric Population. J Pediatric Infect Dis Soc. 2018 May;7((2)):151–8. doi: 10.1093/jpids/pix027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagneur A, Vallet S, Talbot PJ, Legrand-Quillien MC, Picard B, Payan C, et al. Outbreaks of human coronavirus in a pediatric and neonatal intensive care unit. Eur J Pediatr. 2008 Dec;167((12)):1427–34. doi: 10.1007/s00431-008-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sit SC, Yau EK, Lam YY, Ng DK, Fong NC, Hui YW, et al. A young infant with severe acute respiratory syndrome. Pediatrics. 2003 Oct;112((4)):e257. doi: 10.1542/peds.112.4.e257. [DOI] [PubMed] [Google Scholar]
- 14.Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. 2020. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed]
- 15.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009 Mar;9((3)):185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Qi H, Bao L, Li F, Shi Y, National Clinical Research Center for Child Health and Disorders and Pediatric Committee of Medical Association of Chinese People's Liberation Army A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health. 2020 Apr;4((4)):258–9. doi: 10.1016/S2352-4642(20)30040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niermeyer S, Shaffer EM, Thilo E, Corbin C, Moore LG. Arterial oxygenation and pulmonary arterial pressure in healthy neonates and infants at high altitude. J Pediatr. 1993 Nov;123((5)):767–72. doi: 10.1016/s0022-3476(05)80857-1. [DOI] [PubMed] [Google Scholar]


