Abstract
Retinal functional, biochemical, and anatomical changes have been previously reported in long-term experimental permanent bilateral common carotid artery occlusion (BCCAO). The purpose of the current study was to investigate progressive reductions in retinal oxygen metabolism (MO2) due to inadequate compensation by oxygen delivery (DO2) and extraction fraction (OEF) after BCCAO. Twenty-nine rats were subjected to BCCAO and were imaged after 3 hours, 3 days, 7 days, or 14 days. Six rats underwent a sham procedure. Phosphorescence lifetime and blood flow imaging were performed in both eyes to measure retinal oxygen contents and total retinal blood flow, respectively. DO2, MO2, and OEF were calculated from these measurements. Compared to the sham group, DO2 and MO2 were reduced after all BCCAO durations. OEF was increased after 3 hours and 3 days of BCCAO, but was not different from the sham group after 7 and 14 days. Between 3 and 7 days of BCCAO, DO2 increased, OEF decreased, and there was no significant difference in MO2. These findings may be useful to understand the pathophysiology of retinal ischemia.
Subject terms: Experimental models of disease, Retina
Introduction
Retinal ischemia is implicated in many ocular diseases, including ophthalmic artery occlusions, retinal vascular occlusions, ocular ischemic syndrome, diabetic retinopathy, and glaucoma1,2. Permanent bilateral common carotid artery occlusion (BCCAO) is an established experimental method in animals that reduces, but does not eliminate, blood flow to the retina, as well as the brain3. It has been used to investigate retinal ischemia, and previous studies have shown that long-term BCCAO results in a suppression of b-wave amplitude of the electroretinogram (ERG)4–6 and permanent loss of pupillary light reflex (PLR)7–10. Furthermore, optic nerve degeneration8 as well as reductions of retinal ganglion cell (RGC) layer thickness7,8,11 and inner plexiform layer (IPL) thickness7 have been demonstrated after long-term BCCAO. However, one study4 conducted after 7 days of BCCAO reported no retinal thinning and an increase in thickness of the outer plexiform layer (OPL) presumably due to edema. Although vascular compensation has been shown to normalize cerebral blood flow (CBF) 3 weeks12 and 4 weeks13 after BCCAO, the effect of vascular compensation on total retinal blood flow (TRBF) has not been reported.
We have previously demonstrated that complete occlusion of the ophthalmic vessels followed by reperfusion results in reductions of total retinal blood flow (TRBF), oxygen delivery (DO2), and oxygen metabolism (MO2)14. Furthermore, we reported the effect of reductions in TRBF on DO2, MO2, and oxygen extraction fraction (OEF) immediately and after a few days of BCCAO15–17. However, there is lack of knowledge about alterations in DO2, MO2, and OEF due to long-term, incomplete reduction in TRBF (in our case long-term BCCAO), which is more relevant to clinical ischemic conditions than assessment of changes in these parameters immediately after complete loss of blood flow. With longer durations of ischemia, it is expected that more cells demise due to lack of sufficient oxygen, resulting in reduced MO2. It is not known to what extent compensation by increased DO2 or OEF can maintain MO2. The purpose of the current study was to test the hypothesis that long-term incomplete retinal ischemia by BCCAO causes progressive reductions of MO2 due to inadequate compensation by DO2 and OEF.
Results
Figure 1 shows examples of automatically detected vessel boundaries overlaid on red-free retinal images from eyes in the sham and 3 days BCCAO groups. For the same eyes, projection images generated by superimposing two images of one circulating fluorescent microsphere at 2 time points, 37 msec apart, depicts blood velocity. A larger distance between the positions of the microsphere indicated higher blood velocity (sham) compared to a smaller distance (BCCAO). Examples of retinal vascular oxygen partial pressure (PO2) measurements displayed in pseudo-color in the same eyes from the sham and 3 days BCAAO groups are shown in Fig. 1. Retinal arterial and venous PO2 in the eye from the sham group were higher than those in the eye from the 3 days BCCAO group.
Figure 1.
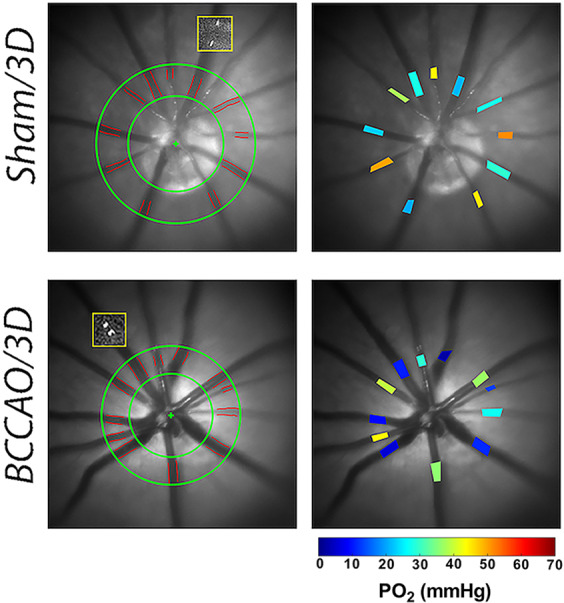
Technique of retinal vascular oxygen partial pressure (PO2) and blood flow imaging performed in sham and 3 days (3D) bilateral common carotid artery occlusion (BCCAO) groups. Red-free fundus images show the automatically detected retinal vessel boundaries outlined in red between green circles. Yellow boxes overlaid on the red-free fundus images show the intravenous microspheres at two time points. Reduced blood velocity can be observed in the rat from the 3 days BCCAO group compared to the rat from the sham group by the smaller distance the microsphere moved during the same time period. Retinal vascular PO2 measurements are presented in pseudo-color. Color bar shows PO2 values in mmHg.
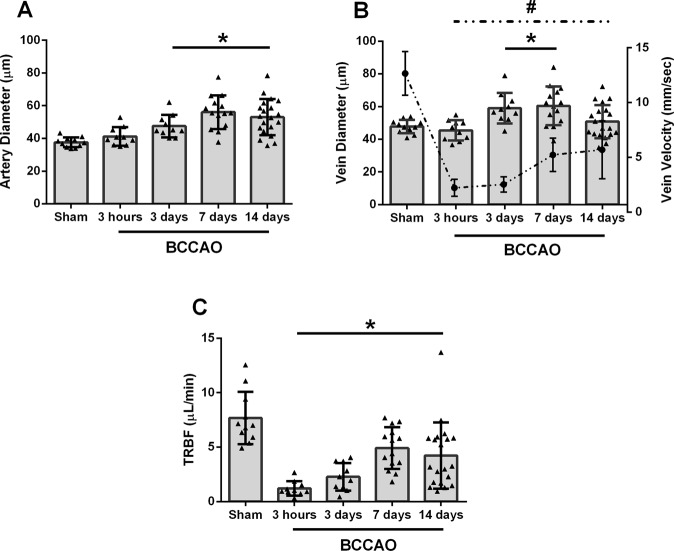
Diameter, velocity, and total retinal blood flow
The mean and standard deviation of arterial diameter (DA), venous diameter (DV), venous velocity (VV), and TRBF for each group (sham, 3 hours, 3 days, 7 days, and 14 days BCCAO) are displayed in Fig. 2. DA, DV, VV, and TRBF of the sham group were 38 ± 3 µm, 48 ± 4 µm, 12.7 ± 2.0 mm/sec, and 7.7 ± 2.4 µL/min, respectively.
Figure 2.
Comparison of arterial diameter (DA), venous diameter (DV), venous velocity (VV), and total retinal blood flow (TRBF) measured 3 hours, 3 days, 7 days, and 14 days after bilateral common carotid artery occlusion (BCCAO) as well as in sham group. Error bars indicate standard deviations. Asterisk and # (in panel B) indicate significantly different from sham group (P ≤ 0.05). (A) DA was increased in 3 days, 7 days, and 14 days groups compared to sham group. (B) DV was increased in 3 days and 7 days groups compared to sham group. VV (interrupted line) was significantly lower in 3 hours, 3 days, 7 days, and 14 days groups compared to sham group. (C) TRBF was decreased at 3 hours, 3 days, 7 days and 14 days groups compared to sham group.
Compared to the sham group, DA and DV were not significantly different after 3 hours of BCAAO (P ≥ 0.36). Differences between groups as estimated by the statistical model are presented by the symbol β. DA and DV were higher after 3 days (β = +10 µm and +12 µm, respectively) and 7 days (β = +19 µm and +13 µm, respectively) (P ≤ 0.02). DA was also higher (β = +15 µm) (P < 0.001), while DV was not significantly different (P = 0.46) after 14 days of BCCAO.
Compared to the sham group, VV was significantly lower after 3 hours (β = −10.5 mm/sec), 3 days (β = −10.1 mm/sec), 7 days (β = −7.4 mm/sec), and 14 days of BCCAO (β = −7.0 mm/sec) (P < 0.001).
TRBF was decreased after 3 hours (β = −6.4 µL/min), 3 days (β = −5.3 µL/min), 7 days (β = −2.6 µL/min), and 14 days of BCCAO (β = −3.4 µL/min) compared to the sham group (P ≤ 0.02). There was no significant difference in TRBF between 3 hours and 3 days of BCCAO (P = 0.07) or between 7 days and 14 days of BCCAO (P = 0.49). However, TRBF was increased between 3 days and 7 days of BCCAO (P = 0.01).
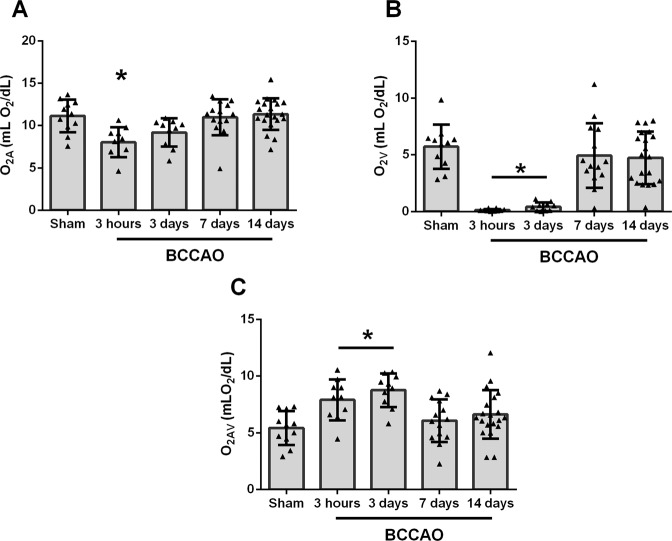
Vascular oxygen content
The mean and standard deviation of arterial oxygen content (O2A), venous oxygen content (O2V), and arteriovenous oxygen difference (O2AV) for each group are presented in Fig. 3. O2A, O2V, and O2AV of the sham group were 11.2 ± 1.9 mLO2/dL, 5.7 ± 1.9 mLO2/dL, and 5.4 ± 1.5 mLO2/dL, respectively. O2A was lower after 3 hours of BCCAO (β = −3.1 mLO2/dL) compared to the sham group (P = 0.005). Likewise, O2V was lower after 3 hours (β = −5.7 mLO2/dL) and 3 days of BCCAO (β = −5.4 mLO2/dL) (P < 0.001). Accordingly, O2AV was higher after 3 hours (β = +2.5 mLO2/dL) and 3 days of BCCAO (β = + 3.4 mLO2/dL) compared to the sham group (P ≤ 0.01).
Figure 3.
Comparison of arterial oxygen content (O2A), venous oxygen content (O2V), and arterio-venous oxygen content difference (O2AV) measured 3 hours, 3 days, 7 days, and 14 days after bilateral common carotid artery occlusion (BCCAO) as well as in sham group. Error bars indicate standard deviations. Asterisk indicates significantly different than sham group (P ≤ 0.05). (A) O2A was significantly lower in 3 hours compared to sham group. (B) O2V was significantly lower in 3 hours and 3 days groups compared to sham group. (C) O2AV was significantly higher in 3 hours and 3 days groups compared to sham group.
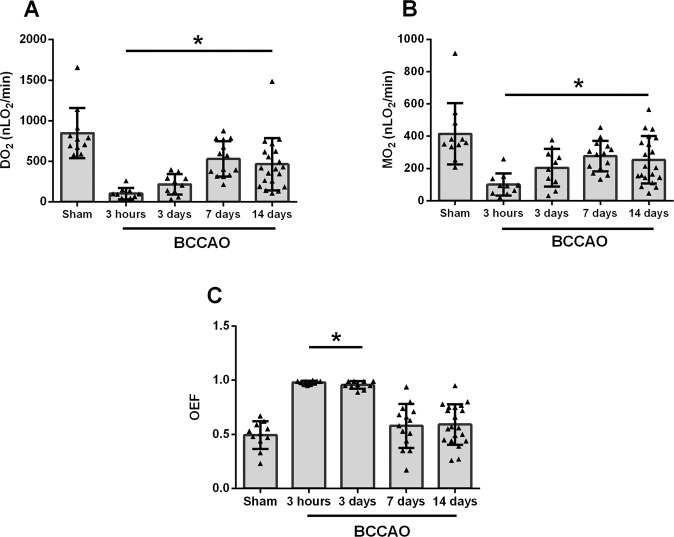
Oxygen metabolism, oxygen delivery, and oxygen extraction fraction
The mean and standard deviation of oxygen metrics (DO2, MO2, and OEF) for each group are presented in Fig. 4. OEF was calculated as the ratio of MO2 to DO2 or alternatively as the ratio of O2AV to O2A18.
Figure 4.
Comparison of oxygen delivery (DO2), oxygen metabolism (MO2), and oxygen extraction fraction (OEF) measured 3 hours, 3 days, 7 days, and 14 days after bilateral common carotid artery occlusion (BCCAO) as well as in sham group. Error bars indicate standard deviations. Asterisk indicates significantly different than sham group (P ≤ 0.05). (A) DO2 was significantly lower in 3 hours, 3 days, 7 days, and 14 days groups compared to sham group (B) MO2 was significantly lower in 3 hours, 3 days, 7 days, and 14 days groups compared to sham group. (C) OEF was significantly higher in 3 hours and 3 days groups compared to sham group.
DO2 was 848 ± 311 nLO2/min in the sham group. Compared to the sham group, DO2 was decreased after 3 hours (β = −745 nLO2/min), 3 days (β = −624 nLO2/min), 7 days (β = −312 nLO2/min), and 14 days of BCCAO (β = −383 nLO2/min) (P ≤ 0.01). There was no significant difference in DO2 between 3 hours and 3 days of BCCAO (P = 0.06) or between 7 days and 14 days of BCCAO (P = 0.57). However, DO2 was increased between 3 days and 7 days of BCCAO (P = 0.005).
MO2 was 415 ± 190 nLO2/min in the sham group. Compared to the sham group, MO2 was decreased after 3 hours (β = −307 nLO2/min), 3 days (β = −196 nLO2/min), 7 days (β = −135 nLO2/min), and 14 days of BCCAO (β = −155 nLO2/min) (P ≤ 0.04). There was no significant difference in MO2 between 3 hours and 3 days of BCCAO (P = 0.07), between 3 days and 7 days of BCCAO (P = 0.36), or between 7 days and 14 days of BCCAO (P = 0.77).
OEF was 0.49 ± 0.13 in the sham group. Compared to the sham group, OEF was increased after 3 hours (β = +0.50) and 3 days (β = +0.47) (P < 0.001) but was not significantly different than the sham group after 7 days or 14 days of BCCAO (P ≥ 0.14). There was no significant difference in OEF between 3 hours and 3 days of BCCAO (P = 0.09) or between 7 days and 14 days of BCCAO (P = 0.83). However, OEF was decreased between 3 days and 7 days of BCCAO (P = 0.002).
Discussion
For the first time alterations in the ability of the retinal vasculature to deliver oxygen and the retinal tissue to utilize oxygen were shown by the evaluation of oxygen metrics at several time points over long durations of BCCAO. We demonstrated reduced DO2 and MO2 up to 14 days after BCCAO. However, there was no progressive decrease in MO2, leading us to reject our hypothesis. This is likely because the partial reduction of blood flow allowed continued survival of some cells following the initial insult and irreversible injury to other cells. Furthermore, by 7 to 14 days MO2 had stabilized at a reduced level and DO2 had reached a corresponding reduced value such that OEF approximated the normal value.
Both DA and DV increased after 3 days and 7 days of BCCAO, indicating vasodilation of major retinal vessels in response to BCCAO. Consistent with findings of the current study, a previous study found increased retinal arterial diameter following elevation of intraocular pressure (IOP) in humans19 due to adaptation of the vessels to the momentary metabolic requirements of cells causing vasodilation to compensate for reduction in perfusion pressure as a form a vascular autoregulation. However, in contrast to the finding of the current study, increased IOP also resulted in decreased venous diameter19. After 14 days of BCCAO, DA remained elevated, while DV was not different than the sham group. Normalization of DV suggests that the retinal vasculature and tissue may have reached a new, reduced metabolic steady state, likely because of an increase in the number of metabolically inactive or lost cells after long-term hypoperfusion due to BCCAO.
In the current study, both TRBF and DO2 were reduced up to 14 days after BCCAO, similar to findings of our previous study performed immediately following BCCAO16. The BCCAO model causes an abrupt and permanent decrease of blood flow in both the retinal and choroidal circulations and thus resembles human ophthalmic artery occlusion and ocular ischemic syndrome. However, it differs from other human conditions in which only the retinal circulation is involved, such as retinal artery occlusions and diabetic retinopathy. The presence of blood flow during BCCAO may be possible by retrograde flow through the distal internal carotid artery from the Circle of Willis and then orthograde via the pterygopalatine artery (Blair et al., unpublished data). The observed compensatory dilation of major retinal vessels accounts for the measured increase in both TRBF and DO2 from 3 days to 7 days. The vascular compensatory response is presumably due to enlargement of vertebral and basilar arteries, which feed the circle of Willis20.
MO2 was reduced at all time points between 3 hours and 14 days after BCCAO, consistent with previously reported findings of functional impairments shown by ERG4–6 and PLR7–10. Threshold values for rates of MO2 have been reported that correlate well with brain tissue survival, and they appear to be superior to OEF and other parameters for predicting outcome21–24. Future longitudinal studies are needed to establish MO2 thresholds for retinal tissue survival under ischemic conditions.
Under conditions of reduced blood flow up to 3 days after BCCAO, OEF essentially approximated its maximum value of 1, along with extremely low values of O2V, which indicates inadequate oxygen availability to meet the tissue’s demand. In the brain, experimental studies have shown that elevation of OEF is associated with threatened tissue25. It has been proposed that with misery perfusion, in which blood flow is reduced relative to the regional metabolic demand for oxygen26, along with maximized OEF, cellular dysfunction or injury can occur at 2 levels of severity: first, ischemic hypoxia, in which cells adapt to low tissue oxygenation and maintain structural integrity, and second, ischemic anoxia, in which metabolism stops and complex metabolic cascades leading to cell death have been initiated27. In hypoxic neural tissue, electrical activity and function may be restored through prompt restoration of blood flow27–29. This salvageable, hypoxic tissue at risk for irreversible cell death is classified as penumbra27. The retina is considered a part of the central nervous system (CNS), and although morphologies of RGCs and CNS neurons differ to some extent, they have similar properties30. Therefore, it is possible that during BCCAO some retinal cells may have existed in a state of penumbra, such that their function may be potentially recovered with timely restoration of blood flow.
Normalization of OEF coupled with reduced DO2 and MO2 after 7 days and 14 days of BCCAO suggests that the oxygen metabolic demand of the tissue had diminished due to anoxic conditions resulting in cell death. Since cells located farthest from the capillaries will have the least oxygen supply28,29, they likely will die first, while cells closer to capillaries may be able to survive. The reduced MO2 measured after one week of BCCAO represents the net metabolism of the remaining living cells, and DO2 had reached a corresponding reduced value such that OEF normalized.
The current study had some limitations. First, the systemic physiology of the animals was not monitored during imaging. Although the same anesthesia protocol was used for all animals, there may have been some inter-animal variations conditions. Second, the measured responses to ischemia may be dependent on the duration of anesthesia and age. The observed increase in DO2 between 3 days and 7 days after BCCAO was not detected in related studies conducted under a longer duration of anesthesia in rats of different ages17. Third, the effect of BCCAO on choroidal circulation and recovery of retinal function which depends on changes in both retinal and choroidal hemodynamics were not evaluated in the current study. Fourth, reduced MO2 may have been in part caused by reduced oxygen extraction from the retinal blood supply, which can occur by an increase in oxygen delivery from the choroidal circulation due to lower consumption or death of photoreceptors induced by the ischemic insult. Accordingly, the findings may not be generalizable to other groups with different strains, species, age, sex, or anesthesia durations. Fifth, anesthesia may have caused systemic hypoxia in the sham group, resulting in vasodilation and increased blood flow compared to non-anesthetized condition, and hence TRBF recovery in the study groups may have been underestimated. However, since all groups of rats were under similar physiological conditions, this factor minimally affected the direction of reported changes. Finally, the findings were based on a cross sectional study and future longitudinal studies are needed to characterize the time course of changes in oxygen metrics in the same animal.
In conclusion, sustained impairments of DO2 and MO2 were demonstrated up to 14 days after BCCAO. Additionally, OEF was increased initially after BCCAO, but with longer durations of ischemia, DO2 stabilized at a value such that the ratio between MO2 and DO2, that is, OEF, approximated the normal value. These findings contribute to better understanding of the pathophysiology of retinal ischemic injury that may be necessary for development and testing of therapeutic interventions for retinal ischemia.
Materials and Methods
Animals
All procedures were approved by the University of Southern California Institutional Animal Care and Use Committee and adhered to the articles of the statement of Use of Animals in Ophthalmic and Vision research by the Association for Research in Vision and Ophthalmology. The experiments have been reported following the Animal Research: Reporting in Vivo Experiments guidelines. The study was performed in 35 adult (age: 12–20 weeks) male Long-Evans rats (weight: 240–520 g) (Charles River, San Diego, CA). Twenty-nine rats were subjected to permanent BCCAO and imaged after 3 hours (N = 5), 3 days (N = 6), 7 days (N = 8), or 14 days (N = 10). Six rats underwent a sham procedure and were imaged after 3 days (N = 3) or 14 days (N = 3). Five rats died before images could be acquired and were not included in the experimental data.
Rats were acclimated for 3 days before being subjected to random grouping of cohorts. They were kept under environmentally controlled conditions with a 12-hour/12-hour light/dark cycle at 20–22 °C, were fed a standard rat chow diet, and had free access to food and water. For BCCAO procedure, anesthesia was administered with 1.5–2.5% isoflurane, balance oxygen. The common carotid arteries were accessed via a midline prelaryngeal incision, and cleanly dissected from the sympathetic and vagus nerves. Silk sutures (5–0 gauge) were used to completely ligate both common carotid arteries, leaving blood flow to the eye from other pathways, likely retrograde through the distal internal carotid artery from the Circle of Willis and then orthograde via the pterygopalatine artery (Blair et al., unpublished data). Sham groups underwent the same procedure, but without ligation of the carotid arteries. For imaging, rats were anesthetized with intraperitoneal injections of Ketamine (90 mg/kg) and Xylazine (5 mg/kg). Additional doses were given as needed. Prior to imaging, a catheter was placed in the femoral artery for delivery of 2-µm polystyrene fluorescent microspheres (Life Technologies, Eugene, OR) at a concentration of 107 particles/mL and Pd-Porphine (Frontier Science, Boston, MA) at a dosage of 20 mg/kg. Pupils were dilated with 2.5% Phenylephrine (Paragon, Portland, OR) and 1% Tropicamide (Bausch and Lomb, Tampa, FL). Rats were placed on a water circulating heated holder for imaging. A glass cover slip with 2.5% hypromellose ophthalmic demulcent solution (HUB Pharmaceuticals, Plymouth, MI) was applied to the cornea to maintain hydration and eliminate its refractive power. Imaging was performed in both eyes. Personnel who conducted the experiments were knowledgeable of the group allocation during the imaging sessions.
Blood flow imaging
Venous blood velocity (V) and diameter (D) were measured by our previously described imaging system31,32. For D measurements, the light illumination of a slit lamp biomicroscope coupled with a green filter (540 ± 5 nm) was used to capture red-free retinal images. Registered mean images were analyzed to determine the vessel boundaries based on the full width at half maximum of intensity profiles perpendicular to the vessel centerline at several consecutive locations along each vessel31,32. Measurements in individual vessels were averaged to obtain mean DA and DV per eye. For V measurements, a 488-nm diode excitation laser and an emission filter (560 ± 60 nm) were used to acquire 520 fluorescence images at 108 Hz. Image sequences were analyzed to determine the displacement of microspheres along each vein segment over time31,32. Measurements in individual veins were averaged to calculate a mean VV per eye. Blood flow was calculated in each vein as VπD2/4 and then summed over all the veins to calculate a total retinal blood flow (TRBF) per eye.
Vascular PO2 Imaging
Retinal vascular PO2 was measured using our established optical section phosphorescence lifetime imaging system33. A vertical laser line (532 nm) was projected on the retina at an angle and an infrared filter with a cutoff wavelength of 650 nm was placed in the imaging path. Phosphorescence lifetimes of Pd-Porphine within all major retinal arteries and veins were determined using a frequency-domain approach and converted to PO2 measurements using the Stern-Volmer equation34,35. Three PO2 measurements were averaged for each vein and artery.
Oxygen delivery, metabolism, extraction fraction
The oxygen (O2) content of the retinal blood vessels was determined as the sum of oxygen bound to hemoglobin and dissolved in blood36: O2 content = SO2 × HgB × C + k × PO2, where SO2 is the oxygen saturation calculated from the rat hemoglobin dissociation curve using measured PO2 and blood pH values from literature, HgB is the rat hemoglobin concentration value (13.8 g/dL)37, C is the maximum oxygen-carrying capacity of hemoglobin (1.39 mL O2/g)38, and k is the solubility of oxygen in blood (0.0032 mL O2/dL mmHg)39. Mean O2A and O2V were calculated by averaging values in all arteries and veins, respectively. O2AV was computed as the difference between O2A and O2V. In each eye, DO2, MO2, and OEF were calculated as: TRBF × O2A, TRBF × O2AV, and MO2/DO2, respectively. Alternatively, OEF can by calculated as the ratio of O2AV to O2A, without measurements of TRBF, as demonstrated previously18.
Data analysis
Statistical analyses were performed using SSPS Statistics, Version 24 (IBM Armonk, New York). Since there was no statistically significant difference in oxygen metrics (DO2, MO2, OEF, and TRBF) among the sham groups (3 days and 14 days) by mixed linear model analysis, data in both sham groups were combined to generate a single sham group. Data in both eyes were classified into 5 groups according to duration of BCCAO (3 hours, 3 days, 7 days, 14 days) or sham. Compiled O2A, O2V and TRBF data were evaluated by group and 5 outliers (values beyond 3 times the interquartile range) were removed, leaving data in a total of 65 eyes: 3 hours (N = 10 eyes), 3 days (N = 10 eyes), 7 days (N = 14 eyes), 14 days (N = 20 eyes) BCCAO groups and sham group (N = 11 eyes). Given an effect size of 0.5, to detect DO2 differences among groups with 80% power and alpha = 0.05, a sample size of 11 is needed. Oxygen metrics were compared among groups by mixed linear models with group and eye as fixed effects and animal as a random effect. The models generated estimated differences (β) between groups. Statistical significance was accepted at P ≤ 0.05.
Acknowledgements
The authors thank James Burford for performing all animal procedures, Selin Auvazian and Nathanael Matei for assisting in imaging and image analysis. This study was supported by the National Eye Institute, Bethesda, MD [grants EY017918 and EY029220] and an unrestricted departmental award from Research to Prevent Blindness, New York, NY.
Author contributions
M.S. designed the study. S.L. and S.F. performed imaging and image analysis. S.L. and M.S. analyzed the data. S.L. wrote and edited the manuscript. S.L., N.B. and M.S. read and revised the manuscript. M.S. approved manuscript for submission.
Data availability
Data are available upon request to the corresponding author.
Competing interests
M.S. holds a patent on the imaging system. S.L., S.F., and N.B. have no conflicting interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moran EP, et al. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H738–749. doi: 10.1152/ajpheart.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YH, Sung MS, Park SW. Clinical Features of Ocular Ischemic Syndrome and Risk Factors for Neovascular Glaucoma. Korean J Ophthalmol. 2017;31:343–350. doi: 10.3341/kjo.2016.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slakter JS, Spertus AD, Weissman SS, Henkind P. An experimental model of carotid artery occlusive disease. Am. J. Ophthalmol. 1984;97:168–172. doi: 10.1016/S0002-9394(14)76086-6. [DOI] [PubMed] [Google Scholar]
- 4.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp. Eye Res. 1995;61:83–90. doi: 10.1016/S0014-4835(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 5.Danyadi B, et al. PACAP Application Improves Functional Outcome of Chronic Retinal Ischemic Injury in Rats-Evidence From Electroretinographic Measurements. J Mol Neurosci. 2014;54:293–299. doi: 10.1007/s12031-014-0296-5. [DOI] [PubMed] [Google Scholar]
- 6.Crespo-Garcia, S. et al. Individual and temporal variability of the retina after chronic bilateral common carotid artery occlusion (BCCAO). PLoS One13, 10.1371/journal.pone.0193961 (2018). [DOI] [PMC free article] [PubMed]
- 7.Guo XJ, et al. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp. Eye Res. 2014;125:156–163. doi: 10.1016/j.exer.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Stevens WD, Fortin T, Pappas BA. Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke. 2002;33:1107–1112. doi: 10.1161/01.STR.0000014204.05597.0C. [DOI] [PubMed] [Google Scholar]
- 9.Lavinsky D, Arterni NS, Achaval M, Netto CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:199–204. doi: 10.1007/s00417-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Fan S, Li J, Wang Y-L. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuroophthalmology. 2014;38:180–188. doi: 10.3109/01658107.2014.908928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Yamamoto H, Parel J-M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006;82:767–779. doi: 10.1016/j.exer.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Jing Z, et al. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J. Cereb. Blood Flow Metab. 2015;35:1249–1259. doi: 10.1038/jcbfm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otori T, et al. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clinical and Experimental Pharmacology and Physiology. 2003;30:266–272. doi: 10.1046/j.1440-1681.2003.03825.x. [DOI] [PubMed] [Google Scholar]
- 14.Blair NP, Tan MR, Felder AE, Shahidi M. Retinal Oxygen Delivery, Metabolism and Extraction Fraction and Retinal Thickness Immediately Following an Interval of Ophthalmic Vessel Occlusion in Rats. Sci Rep. 2019;9:8092. doi: 10.1038/s41598-019-44250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair NP, Felder AE, Tan MR, Shahidi M. A Model for Graded Retinal Ischemia in Rats. Transl Vis Sci Technol. 2018;7:10. doi: 10.1167/tvst.7.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamian P, Burford J, Farzad S, Blair NP, Shahidi M. Alterations in Retinal Oxygen Delivery, Metabolism, and Extraction Fraction During Bilateral Common Carotid Artery Occlusion in Rats. Invest Ophthalmol Vis Sci. 2019;60:3247–3253. doi: 10.1167/iovs.19-27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matei, N. et al. Relation of Retinal Oxygen Measures to Electrophysiology and Survival Indicators after Permanent, Incomplete Ischemia in Rats. Translational Stroke Research, 10.1007/s12975-020-00799-9 (2020). [DOI] [PMC free article] [PubMed]
- 18.Teng P, Wanek J, Blair NP, Shahidi M. Inner Retinal Oxygen Extraction Fraction in Rat. Invest Ophthalmol Vis Sci. 2013;54:647–651. doi: 10.1167/iovs.12-11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel E, Vilser W. Autoregulative behavior of retinal arteries and veins during changes of perfusion pressure: a clinical study. Graefes Arch Clin Exp Ophthalmol. 2004;242:13–17. doi: 10.1007/s00417-003-0663-3.. [DOI] [PubMed] [Google Scholar]
- 20.Oldendorf WH. Trophic changes in the arteries at the base of the rat brain in response to bilateral common carotid ligation. J. Neuropathol. Exp. Neurol. 1989;48:534–547. doi: 10.1097/00005072-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 21.An H, et al. Defining the ischemic penumbra using magnetic resonance oxygen metabolic index. Stroke. 2015;46:982–988. doi: 10.1161/strokeaha.114.008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W, Powers WJ. Oxygen metabolism in acute ischemic stroke. J Cereb Blood Flow Metab. 2018;38:1481–1499. doi: 10.1177/0271678x17722095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers WJ, Grubb RL, Jr., Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5:600–608. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- 24.Touzani O, Young AR, Derlon JM, Baron JC, MacKenzie ET. Progressive impairment of brain oxidative metabolism reversed by reperfusion following middle cerebral artery occlusion in anaesthetized baboons. Brain Res. 1997;767:17–25. doi: 10.1016/s0006-8993(97)00515-5. [DOI] [PubMed] [Google Scholar]
- 25.Young AR, et al. Relationships between high oxygen extraction fraction in the acute stage and final infarction in reversible middle cerebral artery occlusion: an investigation in anesthetized baboons with positron emission tomography. J. Cereb. Blood Flow Metab. 1996;16:1176–1188. doi: 10.1097/00004647-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Maddula M, Sprigg N, Bath PM, Munshi S. Cerebral misery perfusion due to carotid occlusive disease. Stroke Vasc Neurol. 2017;2:88–93. doi: 10.1136/svn-2017-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod D, Beatty S. Evidence for an enduring ischaemic penumbra following central retinal artery occlusion, with implications for fibrinolytic therapy. Prog Retin Eye Res. 2015;49:82–119. doi: 10.1016/j.preteyeres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 28.McLeod D. Krogh cylinders in retinal development, panretinal hypoperfusion and diabetic retinopathy. Acta Ophthalmol. 2010;88:817–835. doi: 10.1111/j.1755-3768.2009.01796.x. [DOI] [PubMed] [Google Scholar]
- 29.McLeod D. Ischemic penumbra in retina endures: vascular neuropathology is reconciled. Neural Regen Res. 2016;11:737–739. doi: 10.4103/1673-5374.181367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 31.Wanek J, Teng P-Y, Albers J, Blair NP, Shahidi M. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomed Opt Express. 2011;2:2562–2568. doi: 10.1364/BOE.2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanek J, Teng P-y, Blair NP, Shahidi M. Inner Retinal Oxygen Delivery and Metabolism Under Normoxia and Hypoxia in Rat. Investigative Ophthalmology & Visual Science. 2013;54:5012–5019. doi: 10.1167/iovs.13-11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felder AE, Wanek J, Teng P-Y, Blair NP, Shahidi M. A method for volumetric retinal tissue oxygen tension imaging. Curr. Eye Res. 2018;43:122–127. doi: 10.1080/02713683.2017.1373823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shonat RD, Kight AC. Oxygen tension imaging in the mouse retina. Ann Biomed Eng. 2003;31:1084–1096. doi: 10.1114/1.1603256. [DOI] [PubMed] [Google Scholar]
- 35.Lakowicz JR, Szmacinski H, Nowaczyk K, Berndt KW, Johnson M. Fluorescence lifetime imaging. Anal. Biochem. 1992;202:316–330. doi: 10.1016/0003-2697(92)90112-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro BA, P. W., Kozlowski-Templin R. Clinical Application of Blood Gases. 427 (Mosby, 1994).
- 37.Crystal, G. Cardiac Anesthesia: Principles and Clinical Practice. 37–57 (Lippincott Williams & Wilkins, 2001).
- 38.West, J. Pulmonary Physiology and Pathophysiology: an Integrated, Case-Based Approach. 2 edn, (Lippincott Williams & Wilkins, 2007).
- 39.Costanzo, L. Physiology. 4 edn, (Lippincott Williams & Wilkins, 2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to the corresponding author.