Abstract
Wheat is consumed worldwide because of its high nutritional content and convenience to form different products. Whole wheat is an important source of dietary fiber and its consumption is known to lower the risk of colon cancer, diabetes mellitus and cardiovascular disease. Germination of wheat results in better availability of nutrients and offers many health benefits. In this study, germinated wheat flour (GWF) was prepared and analyzed for its proximate composition, functional properties, antioxidant activity and microbial count along with whole wheat flour (WWF). The GWF was having 9.2% higher protein content than that of WWF. No significant change was observed in the ash, fat and crude fiber content after germination. GWF showed higher oil absorption capacity and water solubility index. Falling number of GWF was found to be lower. The total phenolic content increased more than two folds after germination and antioxidant activity increased from an initial of 12.35% in WWF to 33.28% in GWF. The microbial counts of GWF were within acceptable range for processing. Breads were prepared by replacing WWF with GWF at 0–100% levels and were analyzed for their proximate composition and acceptance on Hedonic Scale. The 100% GWF bread was having 8.7% higher protein content than 100% WWF bread. The overall acceptability score for all breads were high (> 7.3) whereas the bread prepared with 50% GWF got the highest overall acceptability score of 8.4. The results of this study indicate that bread with improved nutrition and acceptable quality can be prepared from GWF.
Keywords: Germinated wheat flour, Bread, Sensory, Antioxidant activity, Total phenolic count
Introduction
Wheat (Triticum aestivum L.) is widely consumed in various forms like breads, biscuits, cookies, cakes, pasta, noodles, chapatti and it is a convenient source of carbohydrates, proteins, essential amino acids, minerals, vitamins and health beneficial phytochemicals (Steve 2012).
Whole wheat is an important source of dietary fiber, the consumption of which is known to lower the risk of colon cancer, diabetes mellitus and cardiovascular disease (Koehler et al. 2007). But whole wheat also has some anti-nutrients, which are substances that can prevent the absorption of nutrients and inhibit digestive enzymes (Raes et al. 2014). Germination is one of the processes which could increase the content of bio-accessible minerals and nutrients in plant foods for absorption in human body and thus improve the nutritive quality of whole wheat flour (van Hunget al. 2012). Germination also reduces anti-nutrients like phytic acid thus possible improving the bioavailability of wheat nutrients (Azekeet al. 2011).
Germination of grains causes increased activities of hydrolytic enzymes thus causing improvements in the contents of total proteins, total sugars, Vitamin C, B-group vitamins and folate (Hefni and Witthoft 2011). In germinated wheat flour the carbohydrates are modified to contain more resistant starch (Van Hung et al. 2012) along with increased proteins and fibers as compared with non-germinated wheat potentially affecting glycemic response and is beneficial for diabetic patients (Nelson et al. 2013). In vivo studies indicate blood glucose effects are improved following germinated-wheat consumption compared with non-germinated wheat, in healthy adults (Andersen et al. 2008). Germinated wheat is being promoted for traditional use as a fresh sprouted grain and also as flour to be used in different foods including breakfast items, salads, soups, casseroles, pasta, and baked products. The flour can also be supplemented with normal flour in the preparation of unleavened pancake like chapattis and specialty breads (Lemar and Swanson 1976).
Bread is one of the several wheat based products largely consumed worldwide. Bread is convenient source of energy and other nutrients. However the content of nutrients and their bioavailability is low in traditional breads (Swieca et al. 2017).
This study was aimed to evaluate the effect of germination on physiochemical and antioxidant properties of whole wheat, and to formulate and analyze breads prepared with replacement of whole wheat flour with germinated wheat flour at different levels.
Materials and methods
This study was conducted in the laboratories of Department of Food Science and Technology, Guru Nanak Dev University, Amritsar.
Sample procurement
The certified organic wheat (variety Sharbati) and other materials were purchased from local market of Amritsar.
Germination of wheat and flour extraction
Wheat kernels were washed thoroughly in the running water. The kernels were steeped in distilled water for 8 h at 25 °C then steeped water was drained off using sieve. The grains were then spread on a moistened muslin cloth and allowed to germinate for 3 days at 30 ± 2 °C in a BOD incubator (Model No. KI-216, Khera Instruments Pvt. Ltd., India). The germinated kernels were dried in hot air cabinet drier at 50 °C for approximately 4 h. The whole and germinated wheat were milled using Newport Scientific Super Mill (Newport, Australia) to obtain flourswhich could pass through 0.2 mm mesh. The whole wheat flour (WWF) and germinated wheat flour (GWF) were then packaged in air tight, glass, containers and stored under refrigerated conditions (5–7 °C) till further use.
Proximate composition
The whole wheat flour and germinated wheat flour along with prepared breads were analyzed for moisture, protein, ash, crude fiber and crude fat contents using standard methods of analysis (AOAC 2000). The carbohydrate content was determined using difference method.
Functional properties of flours
The whole wheat flour and germinated wheat flour were analyzed for their functional properties.
Bulk density
Sample (10 g) of wheat was weighed and gently added to a 25 ml graduated cylinder. The bottom of the cylinder was gently tapped on a laboratory bench, several times, until there was no further diminution of the sample level. The final volume of the test flour was measured. Bulk density was calculated as weight of sample per unit volume of sample (Mohsenin 1986).
Water absorption capacity (WAC)
Water absorption capacity of flour was measured by centrifugation method of Sosulski and Cadden (1982). Flour (500 mg) was dispersed in 10 ml of distilled water and placed in pre-weighted centrifuge tubes. The dispersion was stirred for 10 min followed by centrifugation for 30–45 min at 5000 rpm. The supernatant was drained off by allowing the tube to stand inverted for 10 min and then weighed. The results were reported in g of gel obtained per g of original sample.
Water solubility index (WSI)
The supernatant obtained from determination of WAC was collected in pre-weighed and dry petriplates, which was then dried in hot air oven for 24 h at 105 °C temperature and the remaining solid was weighed. The results were expressed as dry solids obtained as a percentage of original weight of the sample (Moorthy et al. 1996).
Oil absorption capacity (OAC)
The oil absorption was determined according to method of Lin et al. (1974). Flour (500 mg) was mixed with 10 ml of sunflower oil in pre-weighed centrifuge tube and centrifuged for 45 min at 5000 rpm. The oil was drained off by inverting the tubes for 10 min and centrifuge tubes were weighed. Gain in weight was noticed and results were reported as g of solids obtained per g of original sample.
Falling number (FN)
Falling number was measured using Perten Falling Number instrument (FN 1500, Perten Instruments, Sweden) as described by Hagberg (1960). Falling number is measure of the strength of α-amylase enzyme to liquefy a starch gel. Falling number is time in sec required to stir and allow the stirrer to fall a measured distance through a hot aqueous flour gel undergoing liquefaction. Flour (7 g) was weighed into dry falling number tube and tip to 45° angle then 25 ml water was added. Rubber stopper was placed and tube shaken in upright position 10 times (up and down). The upper part of the tube was scraped down with viscometer-stirrer. Then the tube was placed in bath and locked into position, taking not more than 5 s and automatic stirring cycle started. At the end of test the time was recorded in seconds.
Antioxidant properties
Sample extraction
Samples were extracted using the methodology of Singh et al. (2002). Briefly, 100 mg sample was mixed with 10 ml of 80% methanol and shaken for 2 h using an orbital shaker at 200 rpm (Remi, India). It was followed by centrifugation at 6000 rpm for 10 min at 25 °C. Extraction was repeated twice and supernatants were pooled. The supernatants, referred as sample extracts, were then filtered using 0.45 µm acrodisc filters for phenolic (TPC) and antioxidant assays (DPPH) and analyzed immediately or stored at − 20 °C until further analysis.
Total phenolic content (TPC)
Total phenolic content (TPC) of flour samples was determined following the method of Singh et al. (2002). Briefly, 100 µl of the sample extract or standard was diluted to 4.8 ml by distilled water, and 300 µl of Folin-Ciocalteu reagent was added. After 8 min, 900 µl of 20% sodium carbonate was added with mixing and the solution was left at 40 °C for 30 min before taking the absorbance at 765 nm on a spectrophotometer (Perkin-Elmer, Waltham, MA, USA). Gallic acid was used as a standard, and the results were reported as µg GAE (Gallic acid equivalents) per g flour sample.
Free radical scavenging assays (DPPH)
The total antioxidant activity of flours was determined using DPPH (1,1 Diphenyl 2 picrylhydrazyl) and ABTS free radical scavenging assays was determined by using methodology of Singh et al. (2002) where 100 µl of the sample extract or standard was reacted with 3.9 ml of 6 × 10−9 mol/l of DPPH solution. The absorbance (Abs) at 515 nm was taken at 0 and 30 min after the reaction (Brand-Williams et al. 1995). Antioxidant activity (AOA) was calculated in percent.
The experiments were repeated three times for each sample and the average of the readings was reported. Formula used is:
Microbiological studies
The whole wheat flour and germinated wheat flour were analyzed for their microbial content viz. total plate count (TPC), and yeast and mold count (YMC) to access the microbial safety of flours before processing. The TPC and YMC of flours were carried out according to standard methods (AOAC 2000). Flour sample (10 g) was aseptically weighed in a flask containing 90 ml sterilized distilled water to make 10−1 dilution. The sample was then rotated on a rotary shaker at 30 °C for 10 min to suspend microbes into water. Then serial dilutions up to 10−6 were prepared from this suspension. For TPC, 1 ml aliquot of all dilutions were added to sterile petri dishes (triplicate for each dilution) to which, 15 ml of sterile, cooled (45 °C) nutrient agar media was poured. Upon solidification, the plates were incubated, in inverted position for 48 h at 35 ± 2 °C.
For YMC, 1 ml aliquot of dilutions (up to 10−5) were added to sterile petri dishes (triplicate for each dilution) to which, 15 ml of sterile, cool (45 °C) potato dextrose agar media was poured. Upon solidification, the plates were incubated, in inverted position for 5 days at 25 ± 2 °C. The upper limit to count TPC and YMC colonies were 300 and 150, respectively. The results were reported as log10 colony forming units (cfu)/g.
Bread formulations and preparation
The breads were prepared by replacing the whole wheat flour with germinated wheat flour at the level of 0% (control), 50%, 75% and 100%. The quantity of other ingredients viz. sugar, salt, butter, dry milk, dry yeast and water, per 250 g of flour was kept constant. The process was initiated by mixing sugar and dry yeast in 30 ml water and let it stand for 5 min for yeast activation. Then the remaining dry ingredients were placed in a bowl and yeast solution added along with remaining water. The batter was mixed until the dough comes together and formed a rough ball. The dough was removed from the bowl and kneaded for about 8 min until it is smooth and elastic. A bowl was greased and dough was placed in it. It was covered with a dry dish towel and let rise at 35 °C until double in approximately 90 min. Then the dough was punched down, formed into a loaf, and placed in a greased loaf pan. Let rise again, until double. Oven was preheated at 190 °C and dough was baked for about 25–30 min. Bread was removed from pan and cooled on a wire rack.
Sensory evaluation
Sensory evaluation of the bread samples was carried out using 13 member panel (untrained) selected randomly from the university community. The panelists were instructed to rate the samples in terms of aroma, texture, taste, color, appearance and overall acceptability based on 9-point hedonic scale ranging from 9 (like extremely) to 1 (dislike extremely). The panelists worked under controlled condition to avoid biased results. After that evaluation results were tabulated and averaged.
Statistical analysis
The readings of proximate composition, functional properties, antioxidant properties and microbial analysis were taken in triplicate and were analyzed using analysis of variance (ANOVA) at (P < 0.05) along with Duncan’s multiple range test using Minitab Statistical Software (Minitab Inc., USA).
Results and discussion
Proximate composition of flours
The proximate composition of whole wheat and germinated wheat flour samples is shown in Table 1. The moisture content of germinated wheat flour (GWF) was 12.15% which was not significantly (P < 0.05) different than that of whole wheat flour (WWF) which was 11.63%. Steve (2012) observed the moisture content of germinated wheat flour to be 13.23%. The ash content of WWF was 1.61% which reduced to 1.11% after germination. The reduction in ash content with germination may be attributed to the loss of minerals during soaking (David et al. 2015). Lemar and Swanson (1976) also observed similar ash content (1.38%) of germinated wheat flour. The fat content didn’t change significantly (P < 0.05) after germination which was 2.39% for WWF and 2.25% for GWF. In a study conducted by Steve (2012) the fat content of germinated wheat flour was observed to be 1.53%. The crude fiber content of WWF was 2.45% which reduced to 2.03% after germination. Lemar and Swanson (1976) reported that the crude fiber content of germinated wheat was 2.07%, which did not change significantly during germination. Crude fiber is the insoluble residue of an acid hydrolysis followed by an alkaline one. Fiber fractions include highly insoluble structural fibers like cellulose, lignin, and hemicellulose that are structural carbohydrates and are important components of the cell wall (Chaudhary and Vyas 2014). The protein content of WWF was 12.18% which increased to 13.30% in the germinated wheat flour. This observed increase in the protein content is in agreement with other scientific findings that processing techniques such as germination improved the nutritional quality of the food products, particularly in terms of protein content (Enujiugha et al. 2003). The apparent differences in protein concentration may reflect a loss of carbohydrate material during respiration or an alteration of the nitrogenous substances present, rather than an actual increase of protein (Lemar and Swanson 1976). The calculated carbohydrate content of GWF was lower than that of WWF sample. This observation could be due to the utilisation of fat and carbohydrate for biochemical activities of the germinating seeds.
Table 1.
Proximate composition of whole and germinated wheat flours
| Parameters (%) | Whole wheat flour | Germinated wheat flour |
|---|---|---|
| Moisture | 11.63 ± 0.38a | 12.15 ± 0.19a |
| Ash | 1.61 ± 0.06a | 1.11 ± 0.20b |
| Fat | 2.39 ± 0.44a | 2.25 ± 0.29a |
| Protein | 12.18 ± 0.23a | 13.3 ± 0.19b |
| Crude fiber | 2.45 ± 0.26a | 2.03 ± 0.09b |
| Carbohydrate | 69.74 ± 0.44a | 69.17 ± 0.09b |
Values in a row followed by same superscript are not significantly (P < 0.05) different than each other
Functional properties of flours
The functional properties of whole and germinated wheat flour samples are presented in Table 2. Functional properties of flour have an important role in the formulation of bakery products. The bulk density of whole wheat flour was 0.92 g/ml and that of germinated wheat was 0.94 g/ml, however, the increase was not significant (P < 0.05). The value of bulk density obtained for germinated wheat flour sample in this study was higher than the bulk density (0.71 g/ml) reported by Akubor and Badifu (2004) for wheat flour. The bulk density value is of importance in packaging. The higher bulk density implies that more quantity of the food samples would be packaged in constant volume thereby ensuring an economical packaging. Nutritionally, lower bulk density promotes easy digestibility of food products, particularly among children with the immature digestive system (Gopaldas and John1992).
Table 2.
Functional, antioxidant and microbial properties of whole and germinated wheat flours
| Parameters | Whole wheat flour | Germinated wheat flour |
|---|---|---|
| Bulk density (g/ml) | 0.92 ± 0.02a | 0.94 ± 0.03a |
| Water absorption capacity (g/g flour) | 2.12 ± 0.19a | 2.75 ± 0.23b |
| Water solubility index (%) | 4.78 ± 0.30a | 18.45 ± 0.23b |
| Oil absorption capacity (g/g flour) | 2.32 ± 0.21a | 3.78 ± 0.43b |
| Falling number (s) | 420 ± 1b | 62 ± 0a |
| pH | 6.81 ± 0.06b | 6.12 ± 0.11a |
| Total phenolic content (g GAE/g flour) | 1002.5 ± 26.16a | 2214.2 ± 53.52b |
| Antioxidant activity (%) | 12.35 ± 0.41a | 33.28 ± 0.28b |
| Bacterial plate count (log10cfu/g) | 2.9 ± 0.01a | 5.4 ± 0.07b |
| Yeast and mold count (log10cfu/g) | 2.5 ± 0.03a | 3.9 ± 0.05b |
Values in a row followed by same superscript are not significantly (P < 0.05) different than each other
pH
The pH of germinated wheat flour was observed to be 6.12 which was significantly (P < 0.05) lower than that of whole wheat flour (6.81). In a study, pH of germinated maize flour was reported 6.28. With the increase in germination time, pH reduced due to secretion of enzymes which results in hydrolysis of complex molecules into simpler acidic compounds (Adedeji et al. 2014).
Water absorption capacity (WAC)
Water absorption capacity is an indication of the maximum amount of water that a food product would absorb and hold. The value of water absorption capacity of whole wheat flour was 2.12 g/g which after germination significantly (P < 0.05) increased to 2.75 g/g. Hussain and Uddin (2012) observed that water absorption capacity of wheat flour germinated for different periods at different temperatures varied from 2.0 to 2.4 g/g. The increase in water absorption capacity may be due to the increase in protein content as the hydrophilic protein may easily take up water molecules. The breakdown of starch also helps in hydration of flour (Elkhalifa and Bernhardt 2010). Studies have shown that the microbial activity is less for food products with low water absorption capacity (Giami and Bekebain 1992). Hence the shelf-life of germinated flour could be extended by product development.
Water solubility index (WSI)
The value for water solubility index of flour increased with germination. The water solubility index of whole wheat flour was 4.78% which after germination increased to 18.45%. The increase in water solubility index of flour due to germination was also reported by Lorenz and D’ Appolonia (1980). The researchers concluded that the increase in WSI during germination can be due to the formation of lower molecular weight compounds, by the activity of amylases and proteases, which are more water soluble.
Oil absorption capacity (OAC)
The value for oil absorption capacity of flour increased with germination. The OAC of whole wheat flour was 2.32 g/g which after germination significantly (P < 0.05) increased to 3.78 g/g. The oil absorption capacity depends upon the availability of non-polar sites of protein to combine with a hydrocarbon chain of oil (Hussain and Uddin 2012). Higher oil absorption capacity is desirable as it imparts better sensory attributes by enhancing flavor and improving mouth feel which are characteristics of fat (Hung and Zayas 1992).
Falling number
The falling number decreased with germination from 420 s for whole wheat flour to 62 s for germinated wheat flour. Similar findings were reported by Poudel et al. (2019). In their study falling number decreased from an initial value of 310 s of whole wheat flour to 62 s for flour blended with 10% germinated flour. Falling number measures the strength of α-amylase enzyme to liquefy a starch gel. The α-amylase attacks the 1–4 α-glycosidic links at random along the starch chains. Thus, this enzyme degrades the starch to a mixture of sugars and dextrins. The falling number decreased in the germinated samples because the viscosity of the cooked flour slurry was lowered by the α-amylase produced during germination.
Antioxidant properties
The percentage antioxidant activity and the total phenolic content of WWF and GWF were determined and reported in Table 2. A significant (P < 0.05) increase in the antioxidant activity was observed in germinated wheat flour in comparison to whole wheat flour sample. The antioxidant activity increased by 169% after germination, it was 12.35% for WWF and 33.28% for GWF. A study by Zilic et al. (2014) reported that the sprouting of whole wheat grain increased its antioxidant capacity from an initial value of 19.11 to 22.70 mmol Trolox Eq/kg. Yang et al. (2001) reported that the antioxidant compounds such as vitamin C and tocopherols increased with the length of germination, which resulted in an increase in the antioxidant activity of the germinated wheat flour. The increase in antioxidant activity may also be attributed to various factors like the release of bound phenolic compounds from the breakdown of cellular constituents and cell walls (Oboh and Akindahunsi 2004), as a result of polymerization, glycosylation and/or oxidation of the phenolics during germination (Nsimba et al. 2008).
The total phenolic content (TPC) of germinated wheat flour was higher than the whole wheat flour. Upon germination, the total phenolic content of wheat flour increased from 1002.5 µg GAE/g flour to 2214.16 µg GAE/g flour. The phenolic compounds depend on the type of grain, the seed preparation, and the procedure used (Boatenget al. 2008). Earlier research in cereals indicated that a major portion of phenolics was present as soluble conjugated or insoluble bound forms (Sosulski and Cadden 1982). The increase in TPC upon germination could be attributed to the bound phenolic compounds becoming free by the action of enhanced hydrolytic activity (Maillardet al. 1996).
Microbiological studies
The results of microbial studies are shown in Table 2. The initial bacterial plate count of whole wheat flour was 2.9 log10cfu/g of flour sample. After germination for 3 days, the count increased more than 2 log cycles (5.4 log10cfu/g of flour sample). In earlier study, germination resulted in 2.3 logcycle increase in the microbial populationsin wheat (Weiss et al. 2007). The yeast and mold count in whole wheat flour was 2.5 log10cfu/g of flour sample. This microbial population increased more than 1 log cycle (3.9 log10cfu/g of flour sample) after 3 days of germination. Similar findings were reported by Peles et al. (2012), that the bacterial plate count increased from 4.9 to 6.2 log10cfu/g after sprouting of wheat whereas the yeast and mold count increased from 3.5 to 4.0 log10cfu/g. However, a typical range of bacterial plate count, and yeast and mold count found in wheat used for processing is 3 to 8 log10cfu/g and 2 to 7 log10cfu/g, respectively (Manthey et al. 2004). Thus the prepared GWF, although having higher bacterial plate count, and yeast and mold count than the WWF, is fit for processing into breads.
Proximate composition of the prepared breads
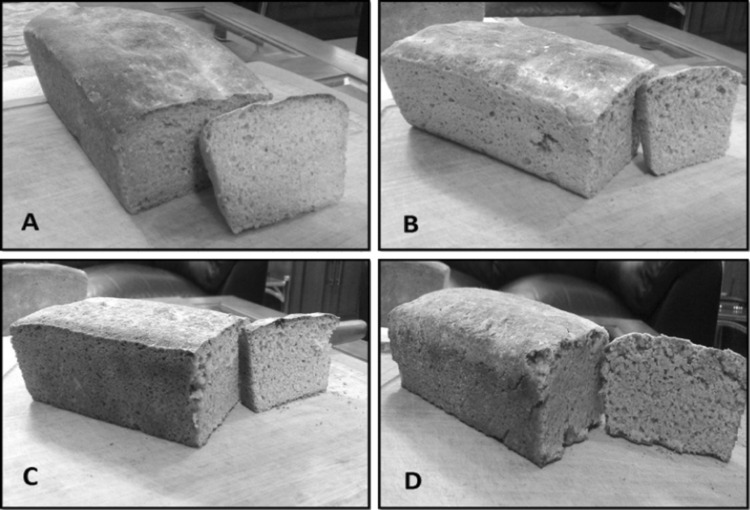
The breads were prepared with the incorporation of the germinated wheat flour at the level of 50%, 75% and 100% whereas the bread with 100% whole wheat flour was considered as control (Fig. 1). The proximate composition analysis of the prepared breads was performed (Table 3). The moisture content values of breads with varying levels of germinated wheat flour were significantly (P < 0.05) different than each other. Bread formulated with 100% germinated wheat flour showed the highest value for moisture i.e. 34.14% which was not significantly (P < 0.05) different than that of bread prepared with 50% GWF (33.94%). Bread formulated with 75% GWF had the lowest moisture content (32.46%). Whole wheat flour bread (control) showed moisture content of 33.12%. High moisture content of 100% GWF bread may be due to more water absorption capacity of germinated wheat flour. There is no legal standard for the moisture content of bread, but generally moisture content of bread must not exceed 38% (Kent and Evers 1994). The ash content of breads did not change significantly (P < 0.05) with the addition of GWF. The ash content ranged from 1.96 to 2.71% in the prepared breads. The fat content of the prepared breads ranged from 2.66 to 2.97%. However, no significant (P < 0.05) difference was observed in the fat content of breads with the addition of GWF.The increased fat content of breads (as compared to flours) may be attributed to the addition of butter in the preparation process. The protein content of 100% germinated wheat flour bread was the highest (14%) followed by 75% GWF bread (13.49%), then 50% GWF bread (13.07%). Whereas, control bread with 100% whole wheat flour had the least protein content (12.88%). The protein content of wheat bread, as reported by Tiimub (2013), was observed to be 11.88%. Crude fiber content did not change significantly among the four different types of bread. Fiber content of different formulated germinated wheat bread ranged from 1.82 to 2.11%. The carbohydrate content for 100% germinated wheat bread was the least (45.28%), whereas bread with 75% GWF showed the maximum amount of carbohydrates (46.7%). Germination induces substantial alterations in grain carbohydrates that have been reported in wheat, therefore a decrease in carbohydrate content of the germinated wheat bread was observed. Singh et al. (2005) also reported a decrease in carbohydrate content which they attributed to the increase in protein content.
Fig. 1.
Images of breads prepared from germinated wheat flour (GWF) blended with whole wheat flour: a 0% GWF; b 50% GWF; c 75% GWF; and d 100% GWF
Table 3.
Proximate composition of breads prepared from germinated wheat flour (GWF) blended with whole wheat flour
| Bread type | Moisture (%) | Ash (%) | Crude fat (%) | Protein (%) | Crude fiber (%) | Carbohydrate (%) |
|---|---|---|---|---|---|---|
| 0% GWF | 33.12 ± 0.13ab | 2.71 ± 0.30a | 2.92 ± 0.10a | 12.88 ± 0.06a | 2.11 ± 0.17a | 46.26 ± 0.46bc |
| 50% GWF | 33.94 ± 0.29bc | 2.49 ± 0.49a | 2.66 ± 0.21a | 13.07 ± 0.14ab | 1.99 ± 0.07a | 45.84 ± 0.16ab |
| 75% GWF | 32.46 ± 0.42a | 2.56 ± 0.23a | 2.97 ± 0.24a | 13.49 ± 0.37bc | 1.82 ± 0.08a | 46.70 ± 0.31c |
| 100% GWF | 34.14 ± 0.39c | 1.96 ± 0.24a | 2.75 ± 0.18a | 14.00 ± 0.05c | 1.86 ± 0.24a | 45.28 ± 0.28a |
Values in a column followed by same superscript are not significantly (P < 0.05) different than each other
Sensory evaluation
Sensory analysis of bread is shown in Table 4. Thirteen semi-trained panelists participated in order to assess the sensory attributes of the bread samples. They were asked to evaluate slice from each loaf for appearance, color, flavor and aroma, taste, texture, and overall acceptability using a 9-point hedonic scale ranging from 1 (dislike extremely) to 9 (like extremely). According to sensory evaluation, it was found that in terms of appearance and color, 100% whole wheat bread was highly preferred with sensory scores of 7.7, and 8.3, respectively. While in terms of texture, flavor and aroma, taste and overall acceptability, bread with 50% GWF formulation was preferred with sensory scores 8.5, 8.1, 8.8 and 8.4, respectively. Hence bread with formulation 50% GWF is more acceptable than any other formulation. To launch a product in the market, usually a sensory score of at least 7 (like moderately) is required (Hobbs et al. 2014).
Table 4.
Sensory characteristics of breads prepared from germinated wheat flour (GWF) blended with whole wheat flour
| Parameters | 0% GWF | 50% GWF | 75% GWF | 100% GWF |
|---|---|---|---|---|
| Appearance | 7.7 ± 0.48b | 7.4 ± 0.78ab | 6.8 ± 0.60a | 6.9 ± 0.82ab |
| Color | 8.3 ± 0.63c | 7.9 ± 0.49bc | 6.4 ± 0.65a | 7.5 ± 0.52b |
| Texture | 7.5 ± 0.78a | 8.5 ± 0.52b | 8.1 ± 0.80ab | 7.8 ± 0.90ab |
| Flavor and aroma | 7.8 ± 0.60a | 8.0 ± 0.49a | 8.0 ± 0.41a | 7.8 ± 0.69a |
| Taste | 7.8 ± 0.60b | 8.8 ± 0.44c | 8.5 ± 0.66c | 7.1 ± 0.64a |
| Overall acceptability | 7.8 ± 0.60a | 8.4 ± 0.65b | 7.6 ± 0.51a | 7.4 ± 0.51a |
Values in a row followed by same superscript are not significantly (P < 0.05) different than each other
Conclusion
Germinated wheat flour was prepared and was analyzed for its proximate composition, total plate count, and yeast and mold counts. It was also analyzed for its functional properties and antioxidant activities. Germinated wheat flour was found to be high in protein content as well as moisture content, however, no significant change was observed in the ash, fat and crude fiber content. Germinated wheat flour showed high oil absorption capacity and water solubility index. Falling number of germinated wheat flour was found to be low due to higher α-amylase activity in it. Concentration of antioxidants increased with germination and hence germinated wheat flour is reported to be high in antioxidant activity and total phenolic content. The total plate count along with yeast and mould count were found to be within acceptable range for processing. Breads were prepared from germinated wheat flour with and without the inclusion of whole wheat flour. The formulated breads were analyzed for their proximate composition and acceptance. Germinated wheat flour bread exhibited high protein content and moisture content. Panelists evaluated all the breads, and gave a satisfactory overall acceptability score (> 7.3) for all supplemented bread, suggesting that germinated wheat flour can be incorporated in bread with good consumer acceptance. Germinated wheat flour is a viable option towards improving nutritional value of bread. Germinated wheat flour possesses a good nutritional and functional profile thus can be incorporated in various food products for value addition.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adedeji OE, Oyinloye OD, Ocheme OB. Effects of germination time on the functional properties of maize flour and the degree of gelatinization of its cookies. Afr J Food Sci. 2014;8:42–47. [Google Scholar]
- Akubor PI, Badifu GI. Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. Int J Food Sci Technol. 2004;39:223–229. [Google Scholar]
- Andersen G, Koehler P, Somoza V. Postprandial glucose and free fatty acid response is improved by wheat bread fortified with germinated wheat seedlings. Curr Top Nutraceutical Res. 2008;6:15–22. [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersburg: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- Azeke MA, Egielewa SJ, Eigbogbo MU, Ihimire IG. Effect of germination on the phytase activity, phytate and total phosphorous content of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (sorghum bicolor) and wheat (Triticum aestivum) J Food Sci Technol. 2011;48:724–729. doi: 10.1007/s13197-010-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng J, Verghese M, Walker LT, Ogutu S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.) LWT Food Sci Technol. 2008;41:1541–1547. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Chaudhary N, Vyas S. Effect of germination on proximate composition and anti nutritional factor of millet (ragi) based premixes. Int J Food Nutr Sci. 2014;3:72–77. [Google Scholar]
- David O, Arthur E, Kwadwo SO, Badu E, Sakyi P. Proximate composition and some functional properties of soft wheat flour. Int J Innov Res Sci Eng Technol. 2015;4:166–172. [Google Scholar]
- Elkhalifa AEO, Bernhardt R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010;121:387–392. [Google Scholar]
- Enujiugha VN, Badejo AA, Iyiola SO, Oluwamukomi MO. Effect of germination on the nutritional and functional properties of African oil bean (Pentaclethra macrophylla) seed flour. J Food Agric Environ. 2003;1:72–75. [Google Scholar]
- Giami SY, Bekebain DA. Proximate composition and functional properties of raw and processed full-fat fluted pumpkin (Telfairia occidentalis) seed flour. J Sci Food Agric. 1992;59:321–325. [Google Scholar]
- Gopaldas T, John C. Evaluation of a controlled 6 months feeding trial on intake by infants and toddlers, fed high energy–low bulk gruel versus ahigh energy–high bulk gruel in addition to their habitual home diet. J Trop Pediatr. 1992;38:278–283. doi: 10.1093/tropej/38.6.278. [DOI] [PubMed] [Google Scholar]
- Hagberg S. A rapid method for determining alpha-amylase activity. Cereal Chem. 1960;37:218–222. [Google Scholar]
- Hefni M, Witthoft CM. Increasing the folate content in Egyptian baladi bread using germinated wheat flour. LWT Food Sci Technol. 2011;44:706–712. [Google Scholar]
- Hobbs DA, Ashouri A, George TW, Lovegrove JA, Methven L. The consumer acceptance of novel vegetable-enriched bread products as a potential vehicle to increase vegetable consumption. Food Res Int. 2014;58:15–22. [Google Scholar]
- Hung SC, Zayas JF. Protein solubility, water solubility and fat binding of corn germ protein flour compared with milk proteins. J Food Sci. 1992;57(372–376):384. [Google Scholar]
- Hussain I, Uddin MB. Optimization effect of germination on functional properties of wheat flour by response surface methodology. Int Res J Plant Sci. 2012;3:31–37. [Google Scholar]
- Kent NL, Evers AD. Technology of cereals. Cambridge: Woodhead Publishing; 1994. [Google Scholar]
- Koehler P, Hartmann G, Wieser H, Rychlik M. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J Agric Food Chem. 2007;55:4678–4683. doi: 10.1021/jf0633037. [DOI] [PubMed] [Google Scholar]
- Lemar LE, Swanson BG. Nutritive value of sprouted wheat flour. J Food Sci. 1976;41:719–720. [Google Scholar]
- Lin MJY, Humbert ES, Sosulski F. Certain functional properties of sunflower meal products. J Food Sci. 1974;39:368–370. [Google Scholar]
- Lorenz K, D'Appolonia B. Cereal sprouts: composition, nutritive value, food applications. Crit Rev Food Sci Nutr. 1980;13:353–385. doi: 10.1080/10408398009527295. [DOI] [PubMed] [Google Scholar]
- Maillard MN, Soum MH, Boivin P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. LWT Food Sci Technol. 1996;29:238–244. [Google Scholar]
- Manthey FA, Wolf-Hall CE, Yalla S, Vijayakumar C, Carlson D. Microbial loads, mycotoxins, and quality of durum wheat from the 2001 harvest of the northern plains region of the United States. J Food Prot. 2004;67:772–780. doi: 10.4315/0362-028x-67.4.772. [DOI] [PubMed] [Google Scholar]
- Mohsenin NN. Physical properties of plant and animal materials: Structure, physical characteristics, and mechanical properties. New York: Gordon and Breach Science Publishers; 1986. [Google Scholar]
- Moorthy SN, Rickard J, Blanshard JMV (1996) Influence of gelatinization characteristics of cassava starch and flour on the textural properties of some food products. Colombia, pp 150–155
- Nelson K, Stojanovska L, Vasiljevic T, Mathai M. Germinated grains: a superior whole grain functional food? Can J Physiol Pharmacol. 2013;91:429–441. doi: 10.1139/cjpp-2012-0351. [DOI] [PubMed] [Google Scholar]
- Nsimba RY, Kikuzaki H, Konishi Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008;106(2):760–766. [Google Scholar]
- Oboh G, Akindahunsi AA. Change in the ascorbic acid, total phenol and antioxidant activity of sun-dried commonly consumed green leafy vegetables in Nigeria. Nutr Health. 2004;18:29–36. doi: 10.1177/026010600401800103. [DOI] [PubMed] [Google Scholar]
- Peles F, Gyori Z, Bacskai T, Szabo Z, Murvai M, Kovacs B. Microbiological quality of organic wheat grains and sprouts. Analele Universitatii din Oradea. 2012;18:53–60. [Google Scholar]
- Poudel R, Finnie S, Rose DJ. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J Cereal Sci. 2019;86:33–40. [Google Scholar]
- Raes K, Knockaert D, Struijs K, Van-Camp J. Role of processing on bioaccessibility of minerals: Influence of localization of minerals and anti-nutritional factors in the plant. Trends Food Sci Technol. 2014;37:32–41. [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Singh S, Raina CS, Bawa AS, Saxena DC. Effect of heat-moisture treatment and acid modification on rheological, textural, and differential scanning calorimetry characteristics of sweetpotato starch. J Food Sci. 2005;70:373–378. [Google Scholar]
- Sosulski FW, Cadden AM. Composition and physiological properties of several sources of dietary fiber. J Food Sci. 1982;47:1472–1477. [Google Scholar]
- Steve IO. Influence of germination and fermentation on chemical composition, protein quality and physical properties of wheat flour (Triticum aestivum) J Cereals Oilseeds. 2012;3:35–47. [Google Scholar]
- Swieca M, Dziki D, Gawlik-Dziki U. Starch and protein analysis of wheat bread enriched with phenolics-rich sprouted wheat flour. Food Chem. 2017;228:643–648. doi: 10.1016/j.foodchem.2017.02.052. [DOI] [PubMed] [Google Scholar]
- Tiimub BM. Proximate analyses of three brands of bread under different storage conditions available on the Ghanaian market. Food Sci Qual Manag. 2013;12:23–29. [Google Scholar]
- Van Hung P, Maeda T, Yamamotto S, Morita N. Effects of germination on nutritional composition of waxy wheat. J Sci Food Agric. 2012;92:667–672. doi: 10.1002/jsfa.4628. [DOI] [PubMed] [Google Scholar]
- Weiss A, Hertel C, Grothe S, Ha D, Hammes WP. Characterization of the cultivable microbiota of sprouts and their potential for application as protective cultures. Syst Appl Microbiol. 2007;30:483–493. doi: 10.1016/j.syapm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Yang TK, Basu B, Ooraikul F. Studies on germination conditions and antioxidant contents of wheat grain. Int J Food Sci Nutr. 2001;52:319–330. doi: 10.1080/09637480120057567. [DOI] [PubMed] [Google Scholar]
- Zilic S, Basic Z, Sukalovic VHT, Maksimovic V, Jankovic M, Filipovic M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int J Food Sci Technol. 2014;49:1040–1047. [Google Scholar]