Abstract
There are numerous species of bacteria resides in the lumen of human colon. The word ‘colon’, resembles colony or the colonization of microbiota of which plays an important role in the fermentation of prebiotics. The standpoint of prebiotic nowadays is well reported for attenuating gut dysbiosis in many clinical studies tested on animals and human. However, because of the huge amount of gut microbiome, the attempt to connect the dots between bacterial population and the host are not plainly discernible. Thus, a need to analyse recent research on the pathways of prebiotic metabolism adopted by commonly studied probiotics i.e. Bifidobacteria and Lactobacillus. Several different substrate-dependent gene expressions are induced to break down oligosaccharide molecules shown by those probiotics. The hydrolysis can occur either by membrane bound (extracellular) or cytoplasmic (intracellular) enzyme of the enteric bacteria. Therefore, this review narrates several prebiotic metabolisms occur during gut fermentation, and metabolite production i.e. organic acids conversion.
Keywords: Colonic fermentation, Oligosaccharide, Prebiotic, Short chain fatty acids, Probiotic
Introduction
The term prebiotic defines as a substrate that is selectively utilized by host microorganisms conferring health benefits (Gibson et al. 2017). The definition dynamically change and redefine through the years of development and scientific studies on prebiotics. The scientists agree on the prebiotic effects includes the health benefit not only in the gut but also can be administered onto the skin and other distant sites of the human body. The usage also allowed in the application of animal practices. The consensus also open up to widen the candidature of prebiotic to non-carbohydrate based constituent as long as the modulation of health is mediated by native gut bacteria or probiotic. Also important to address common misconception of the term prebiotic and fermented products are the same thing. Although fermented products were fermented by bacteria or probiotics, it does not necessarily made them prebiotics. While prebiotics can come from various source of food, both type of food may contain probiotics. Despite the known benefits of fermented food and beverages towards gut well-being, their recommended consumptions have not been extensively express for the worldwide guidelines, (Bell et al. 2017) as these so called fermented food is a traditional heritage, to some part of the world are delicacies belong to a certain group of ethnics. Dominant microorganisms inhabit the colon generally from the phyla; Firmicutes and Bacteroidetes (Walker et al. 2011). We have known that the microbial within the colon expressed no straightforward interactions with host metabolic pathway (Tremaroli and Bäckhed 2012). At the most basic level, the relationship is highly mutualistic as the host provides shelter plus the food supply while the bacteria in return assist in digestion complex food components apart from supply of vitamin B and K to the host. But many advance research confirmed the link between diet we consumed and changes on gut microbiota occurred upon substrate intervention (Ríos-Covián et al. 2016; David et al. 2014). At least 2 decades past of gut research, lots of the unknown before to so many of us become clear. The realisation of that is the relationship is far more complex and becoming ambiguous of what does really means by the gut environment of having mutualists, commensals and pathogens. Dysbiosis in the gut can reflect changes to different host systems, particularly metabolic and immune processes. Bacteria that recorded as harmless or used to known as beneficial in the gut, now turns out to contribute to chronic symptoms of atherosclerosis and obesity (Postler and Ghosh 2017) as intestinal microbiota metabolism of l-carnitine, a nutrient in red meat and central to fat metabolism, promotes atherosclerosis (Koeth et al. 2013). The findings on obese to lean mice gut microbiota transfer clearly showed changes in microbiome does gives effect in weight management (Turnbaugh et al. 2006, 2008). Another finding also supporting the outcome, where obese or lean human faecal microbiota was transferred to germ-free mice (Ridaura et al. 2013; Tremaroli et al. 2015). Rigorous study of faecal transplants involving long term commitment will be necessary to really understand the mechanisms to regulates if not lessen the severity of disorders related with metabolic syndrome.
Scientific research on the gut microbiota has dramatically expanded over the past 2 decades, with the advent of new sequencing technologies and advances in data analysis. However, researchers are still only beginning to understand the importance of our microbiome to our health in areas that go far beyond digestion. For example, emerging evidence suggests that our mood, and even our brain structure, may be associated with the bacterial composition of our microbiome. Years ago, health wellness awareness arose from the concern of weight management, now started move to digestive health. If we look at our gut environment the same as our ecosystem, very much of what is happening inside our gut will really making sense. Put it as the gut bacteria would be the animal kingdoms and prebiotics is the food (producer). If you think of it as a food chain in an ecosystem, there will definitely a collection of food chains becoming the food web. Now, this different unpredicted relationship linkage will explain their cross feeding situation, where its open up not just a competition but also for cooperation that can be achieved by the intermediate fermentation products by one or more bacterial species to other populations (Sarbini and Rastall 2011). Now you understand that, in animal kingdoms are hierarchised by the prey and the predators, whilst the prey normally consists of primary to tertiary consumers. Even with a quick guess we would sorts the bacteria out one that is pathogenic is the predators and vice versa. Then, what would have happen if there is an ecological imbalance in the ecosystem? Or do we mean a gut dysbiosis? Thus to understand further it would be more accurate if we can establish a similar ecological pyramids for our gut ecosystem. We do know that the imbalance was affected by the numbers of every tiers since they are species coexisting with other species in the environment. This also means, an introduction of probiotics or prebiotics in the natural gut environment can be considered as disruptions in the so-called balance ecosystem. Thus we suggests a way of classifying these different species to their designated role in the gut ecosystem. This may explain the finding by Arumugam et al. (2011); according to their metagenomic study people fall into three categories of gut microbiota namely bacteroide, prevotella and ruminicoccus. A concern of using specific probiotic strains with concern of resistance towards bioavailability or susceptibility Any application of probiotics need to consider specific properties against responds to antibiotic, bile acids and pancreatic juice. Recent evidence indicates that the effects of probiotics are functionality of the gut microbiota. Although different strains exhibit specific level of tolerance (Parker et al. 2018), selecting the best strain for particular treatments might be tough, while the Lactobacillus casei strain top most of the characteristics needed, one might need to consider exact disease to treatments method when incorporate together with prebiotics product (Bubnov et al. 2018). Yissachar et al. (2017) reported to develop a novel system that are able to reproduce previous observations made in vivo. This, if made available sooner for research applications will taking us close to clinical studies equivalent and further to interpret more complex relation between host and gut microbiota and able to give insights on treating ailments. Therefore, a current review on the knowledge of prebiotic, specifically on the mechanisms that possibly explained how these intestinal bacteria attenuate the dysbiosis in a host is needed. This review will deliberate on; major established prebiotics (inulin, fructo-oligosaccharides and galacto-oligosaccharides) and how it could modulate gut microbiota composition in general; the structure and function relationships of these carbohydrate based prebiotic, their fermentation pathways by gut microbiota; and exploring different metabolisms occurred upon short chain fatty acids (SCFA) biosynthesis.
Carbohydrates prebiotic
Prebiotic potential varies among carbohydrates depends on many characteristics of their particular structure. Inulin, inulin derived fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS) and lactulose have been extensively studied as prebiotics since 2 decades ago (Kothari et al. 2014). Carbohydrates microbial fermentation yields mainly short chain fatty acids (SCFA) (acetate, propionate, and butyrate), which at normal physiological concentrations, SCFA provide beneficial improvements for enterocytes proliferation and host overall intestinal epithelial cells immune respond (Lee and Hase 2014). The summary of all the metabolism by the bacteria strains is arranged side by side in Table 1 for better comparison.
Table 1.
Transporters involve during substrates uptake with their respective bacteria species prior fermentation
| Substrates | Strains | Enzymes | Transporters | Location | Operon | References |
|---|---|---|---|---|---|---|
| Inulin | Lactobacillus casei | Cell wall-anchored fructan β-fructosidase | – | Extracellular | levH1 | Kuzuwa et al. (2012) |
| Sucrose inulin-type fructan | L. acidophilus NCFM | β-fructosidase | ATP-dependent binding cassette (ABC) transporter (msmEFGK) | Intracellular |
Multiple sugar metabolism msm operon |
Barrangou et al. (2003) |
| Fructo-oligosaccharides |
L. paracasei 1195 L. pentosus Streptococcus mutans Streptomyces exfoliates |
β-fructosidase precursor(FosE) β-fructofuranosidase |
Fructose/mannose PEP-dependent PTS | Extracellular | fosABCDXE | Goh et al. (2007) |
| Short chain fructo-oligosaccharides | L. plantarum WCSF1 | β-fructofuranosidase | Sucrose phosphoenolpyruvate (PEP)-dependent Phosphotransferase system (PTS) | Intracellular | Pts1BCA | Saulnier et al. (2007) |
| Shorter fructo-oligosaccharides chain (GFn type) | Bifidobacterium | β-FFase | Permeases or ABC transporters | Intracellular | cscA gene | Rossi et al. (2005) |
| Galacto-oligosaccharides, lactose, galactosides (lactulose) | L. acidophilus NCFM |
β-galactosidases (GH42 LacA and GH2 LacLM) (Glycolysis and Leloir pathway) |
Lactose permease (LacS) | Intracellular |
gal-lac operon lac operon |
Andersen et al. (2013) |
| Galacto-oligosaccharides | L. ruminus ATCC 26544 | β-galactosidases (LacZ) | Lactose permeases (LacY) | Intracellular | lacZY | Motherway et al. (2013) |
| Galacto-oligosaccharides | B. lactis Bl-04 |
GH2 β-galactosidase GalZ β-galactosidase GalG |
MFS lactose permease ABC transporter |
Intracellular | lacSZ and gosRDEGC | Andersen et al. (2013) |
| Plant derived galactan | B. longum NCC2705 | GH53 cell membrane–bound Endogalactanases (GalA) | Galactan partially hydrolysed externally Liberates galactotriose | Intracellular | Hinz et al. (2005) | |
| Galacto-oligosaccharides, DP > 3 | B. breve UCC2003 |
Endogalactanase (GalA) GH42 β-galactosidase (GalG) |
ABC transporter (GalCDE) | Intracellular | lacSZ and gosRDEGC | Goh and Klaenhammer (2015) and Motherway et al. (2013) |
Inulin and fructo-oligosaccharides
Inulin and FOS are extensively studied since the early era of prebiotic. Generally, the structure of inulin and FOS differs on its polymer chain length, thus an approach to discuss both prebiotics together is fairly relevant. Built on linear fructo-polysaccharides comprised of glucose-fructose subunits, inulin is extracted from roots of chicory plant (Buclaw 2016). Structurally, inulin and FOS are linked by β-d (2 → 1) fructosyl units having a glucose moiety at the terminal reducing end joined by α-d (1 → 2) linkage (Bartolomeo et al. 2013). Inulin has a unique structure among polysaccharides as no bond of the furanose ring is part of the macromolecular backbone (Singh et al. 2016). Enzymatic synthesis of the chicory derived inulin can be achieved by endoinulinases to produce prebiotic FOS known as FFn type FOS where F stands for fructose and ‘n’ is the number of fructosyl units (Goh and Klaenhammer 2015). Inulin-type FOS also are produced from sucrose via transfructosylation using microbial fructosyltransferases (EC 2.4.1.9) of Aspergillus as well as β-fructofuranosidases (EC 3.2.1.26) or β-d-fructosyltransferases reacted under transfer favouring conditions (Niness 1999). Fructo-oligosaccharides molecules contain 1-ketose (GF2), nystose (GF3) and 1-F-β fructofuranosylnystose (GF4) (Oku et al. 1984). They are naturally found in various tuber or root of plants such as raw Jerusalem artichoke which shown to exert beneficial effects on immunity, blood metabolites, intestinal morphometry and hindgut fermentation of rats (Samal et al. 2015). Generally, FOS made up of shorter fructosyl units than inulin ranging from DP of 2 to 10 and 2 to 60 units, respectively (Niness 1999). The chain length or degree of polymerization (DP) plays an important role in the gut fermentation of these prebiotics.
Though most of dietary fibres were fermented in the proximal colon, study also confirmed that short chain FOS are fermented by the bacteria present in the proximal colon, while the long chain FOS are fermented in distal colon (Meyer and Stasse-Wolthuis 2009). Relationship of FOS chain length and bowel transit time may possibly suggest the outcome stated. Intervention of 16 days inulin administration to healthy volunteers showed significant increase of Bifidobacterium adolescentis and B. bifidum (Ramirez-Farias et al. 2008) The β-fructofuranosidases present in bifidobacteria are responsible for inulin degradation (Pokusaeva et al. 2011). This process involves the mode of glycanases and cellular transport system to allow products movement. Multiple studies reported the extracellular hydrolysis of FOS and inulin by Lactobacillus paracasei and L. pentosus (Ryan et al. 2005). The Bacteroides strains tested showed some growth on peptides available in basal medium in the presence of carbohydrates. The butyrate producing strains Firmicutes families, Lachnospiraceae and Ruminococcaceae exhibited different growth profiles on the substrates, which includes starch, inulin, fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS) and xylo-oligosaccharides (XOS). As the fructan chain length increases, the ability of some other strain to utilize it is decreased. Long chain inulin was utilised by Roseburia inulinivorans, but none of the Bifidobacterium species examined are able to utilise it. There are only six Firmicutes strains from 11 tested strains that were able to grow in XOS, showing more selective growth than FOS. These results demonstrate the selectivity of different prebiotics, thus explaining why some are butyrogenic (Scott et al. 2014). Lactobacillus plantarum isolated from fermented fish were able to utilize inulin by the action of inulin degrading enzyme, β-fructosidase, contained in both supernatant and cell wall extracts of the strains. Since there are no presence of this enzyme in the cytoplasmic region, thus indicating it was either from the membrane bound or secreted out. The proteogenomic data revealed inulin degrading and taking up routes widely expressed among L. plantarum strains, which related to fosRABCDXE operon and pstBCA genes (Buntin et al. 2016).
Galacto-oligosaccharides
Galacto-oligosaccharides (GOS) are derived from milk lactose of bovine by β-galactosidase to produce several different lengths of oligomers chain (Prenosil et al. 1987). Commercial GOS products usually derived from lactose in whey production (Yanahira et al. 1995). Transgalactosylation activities of β-galactosidase results in the formation of 4′ or 6′ galactosylactose, longer oligosaccharides, transgalactosylated disaccharides and nonreducing oligosaccharides usually with 2 to 10 DP chain with a terminal glucose (Angus et al. 2005). Formation of that various glycosidic linkages of GOS products contributed from different enzymes origin and hydrolysis conditions which may be one of the reasons to resist acid digestion at different level (Tomomatsu 1994).
Emerging prebiotics
The candidates of prebiotics can goes beyond dietary fibres or polysaccharides, or synthetically derived involving human-made process. A lot of emerging prebiotics were studied are a result of endowed for a new alternative or novel candidates. Several that known interest are cow’s milk, sugar alcohol, plant cell walls, polyphenols, pectin, resistant starch, and glycopeptide-based prebiotics.
In cow’s milk the active components being considered as prebiotic is from the fraction of glycome which also similar to the human milk oligosaccharides glycome (Zivkovic and Barile 2011). One of the component of glycome is made up of epilactose, present in small quantities in heat rated milk (Mu et al. 2013). As cow’s milk is one of the most consumed dairy products, this could potentially add value to many dairy product. Epilactose is obtained with the enzymatic conversion of lactose by cellobiose-2-epimerase from Flavobacterium johnsoniae (Kuschel et al. 2017). Study of epilactose supplementation in Wistar-ST rats showed increase cecal bulk and decrease its pH, support lactobacilli, and bifidobacteria growth, suppress clostridia or bacteroides groups and increase all cecal SCFA (Watanabe et al. 2008; Murakami et al. 2015). The most explore origin of prebiotic always been from plant parts. The plant derivatives from fruits and vegetables particularly can easily contained oligosaccharides.
The most widely accepted definition of dietary fibre refers to polysaccharides, oligosaccharides, and other edible plant substances that resist digestion and absorption in the human gastrointestinal tract. Plant cell walls from common vegetables, fruits, whole grain cereals, and legumes are major sources of such dietary fibres, being a complex polysaccharide matrix, which is formed by extensive interactions between cellulose, hemicelluloses, and pectin. Although the consumption of plant fibres has been associated with the improvement of gastrointestinal wellbeing and the prevention of health threatening diseases e.g. colon cancer, IBD, and Crohn’s disease, the prebiotic potential of the individual plant cell wall polysaccharides has been largely overlooked in the light of more promising and better studied prebiotics, such as inulin and fructo-oligosaccharides.
Cellulose is a tightly packed aggregate of linear polymers of 1,4 linked β-d-glycopyranose. The polymer aggregates form long microfibrils (of 7000–12,000 glucose molecules/units) varying in length, width, and in the degree of order, depending on the plant source (Heinze and Liebert 2012). Hemicelluloses or also denoted as polyose are a collective term for plant cell wall polysaccharides that are neither cellulose nor pectin, characterized by having β-1,4 linked backbones of glucose, mannose, and xylose (Scheller and Ulvskov 2010). The structural class of different cell-wall polysaccharides in hemicelluloses include xyloglucans, xylans, mannans, glucomannans, β-1,3 linked glucans, and β-1,4 linked glucans. Xyloglucan, the major sugar-type portion in hemicelluloses, consisting of a backbone of 1,4 linked β-glucose residues with short side chains containing xylose, galactose, and terminal fucose. Unlike cellulose, hemicelluloses are non-linear polymers that are shorter in length (about 200 sugar molecules/unit) (Heinze and Liebert 2012). Emerging evidence suggests that the polysaccharide components of the plant cell wall, particularly pectin, demonstrate significant prebiotic effects, hinting that these polysaccharides may potentially provide prebiotic benefits. An interesting in vitro study of aquatic origin polysaccharides of red seaweed showed to exhibit bifidogenic properties (Bajury et al. 2017). The repetitive high molecular disaccharides sequence of β (1-3) and α (1-4)-d-galactopyranose, κ-carrageenan forming the polysaccharide structure of red seaweed may contributed to the effects. This seaweed extracts usually can be found in foods act as thickening and gelling agents.
The two major classes of dietary polyphenols are the phenolic acids and the flavonoids (Manach et al. 2004). The most common polyphenols conjugates are the glycosides comprising about 80% of all polyphenolics in plant tissues, while aglycones are less common in nature (Pérez-Jiménez et al. 2010). While most glycosides are carried to the colon for microbial fermentation, and aglycones may be absorbed through the small intestinal membrane by diffusion or active transport proteins (Manach et al. 2005). Flavonoids naringenin and quercetin showed to suppress a group of gut bacteria such as L. rhamnosus, E. coli, Staphylococcus aureus, Salmonella enterica sv. Typhimurium at physiologically relevant colonic concentrations of 125 to 250 μg/ml. The Gram positive Staphylococcus was found to be more sensitive to the polyphenols than Lactobacillus as other key factors could be involve thereafter flavonoids intervention (Parkar et al. 2008). The antimicrobial effects of three flavonoids were later studied with intestinal bacteria from species of Bacteroides, Lactobacillus, Enterococcus, Bifidobacterium, Ruminococcus and Escherichia and similar inhibitory effects were noted. The aglycone flavonoids, naringenin, hesperetin and quercetin were showed more inhibitory effect than the glycosylated forms, naringin, hesperidin, and rutin respectively (Duda-Chodak 2012). While no clear evidence of how much of the tested concentration aglycones can reach until the colon if not completely absorbed, whereas in glycosylated forms, considered to be more tolerable to bacterial metabolism as the microorganism has a vast repertoire of glycosidases to adapt to the undigested foods reaching the colon (Ley et al. 2008).
Dietary catechol in tea for example; caffeic acid, epicatechin, catechin and 3-Omethylgallic acid are largely antimicrobial, especially towards potential clostridial pathogens, with different effects on the rate of growth of other gut bacteria such as Bacteroides, Bifidobacterium and Lactobacillus species (Lee et al. 2006). Caffeic acid esters may also have a protective effect on cultured intestinal cells implying anticancer benefits (Bordonaro 2014). Apple polyphenol when metabolised in gut were found to inhibit histone deacetylases in intestinal cell systems and thus could work synergistically with butyrate to inhibit the growth of cancer cells (Bordonaro 2014). Blackcurrant anthocyanins such as cyanidin and delphinidin or their glycosides were found to inhibit S. enterica sv. Typhimurium in micromolar amounts and promoting on Lactobacillus rhamnosus, and these findings implying an average consumption of blackcurrant juice would prepare enough supply for the gut (Parkar et al. 2014; Ávila et al. 2009). Strains of lactobacilli and bifidobacteria were also found to metabolize and grow on grape seed polyphenols rich in catechins and oligomeric polyphenols (Tabasco et al. 2011). Besides, berry phenolics such as cyanidin glucoside and gallic acid were found to inhibit E. coli with weakening of the outer membrane (Lacombe et al. 2010). As reviewed by Gupta et al. (2019), grape seed extracts rich in proanthocyanidin provides benefits towards gut inflammation and inhibit the growth of Faecalibacterium prausnitzii within the intestinal lumen.
Antioxidant rich fruits such as blueberry, pear, nectarine and watermelon and bulb type vegetables garlic, spring onion, white onion, leek and scallion are rich in oligosaccharides (Jovanovic-Malinovska et al. 2014). The consumption of plant based diets generates an increase in overall populations of gut Firmicutes, increasing production levels of short chain fatty acids, positively affecting health (David et al. 2014). Heteropolysaccharides from plant cell walls such as xylans and pectins would appear to be ideal substrates to generate di and oligosaccharides with prebiotic potential (Yoo et al. 2012). Pectins are considered as a soluble dietary fibre with several beneficial gastrointestinal physiological effects including the delay of colon emptying while increase faecal transit time and improve glucose tolerance (Schwartz et al. 1988). A one-step approach of enzymatic hydrolysis of sugar beet pulp can yield pectic oligosaccharides (Babbar et al. 2017). Pectic components derived from citrus-peel of orange and lemon shown to have prebiotic properties (Li et al. 2016; Gómez et al. 2016). Although it is not common to find pectin-degrading Firmicutes, but one that found to have the ability is Eubacterium eligens which also promoting IL-10 production by epithelial cells that can confer anti-inflammatory effects. (Chung et al. 2017). Comparative prebiotic study by Islamova et al. (2017) on the activity of pectinic substances derived from different fruit parts, concluding that the origin of pectin have a significant effect on the prebiotic effect tested of different probiotic strains, compared to lactulose prebiotic.
While resistant starch (RS) is not a new food, its use as a prebiotic has gained momentum only recently. Resistant starch is defined as sum of remnants of starch from small intestine digestive degradation that resist it and reach the human colon (Englyst et al. 1996). High amylose maize starch, a form of resistant starch (Type 2) may function as prebiotic (Wang et al. 2019). It is resistant to amylase digestion in the small intestine and this is influenced by its encapsulation within the cell wall, by microarchitecture of the starch polymer, by the amylose to amylopectin ratio that formed the crystalline structure due to retrogradation treatment and by lipid content (Zaman and Sarbini 2015). A recent study based on infant faecal microbiota appeared to positively increase the abundance of Bifidobacterium and Bacteroides (Gopalsamy et al. 2019). Resistant starch arrives in the large bowel either encapsulated within broken down grains and seeds, as a soluble jelly-like matrix or as an insoluble polysaccharides. In vitro fermentation of several types of starch showed a positive correlation between total resistant starch content and the most preferable fermentation substrate by Lactobacillus acidophilus and Bifidobacterium animalis (Arshad et al. 2018). Another study on antibiotic shifting of gut bacteria diversity showed that resistant starch is bifidogenic and the only tested fibres that promote butyrate-producing bacteria (Tsitko et al. 2019). The solid entities provide primary contact necessary for gut bacteria anchored to before utilize the starch via the action of glycosidases (Flint et al. 2008). This also as reviewed by Tiwari et al. (2019) that the granule dimension and surface area influence the availability of resistant starch to gut microbiota in the proximal and distal guts of pigs.
Also known as polyols, sugar alcohols have wide range of digestibility resistance, some that are very susceptible to human digestive enzyme to some like isomalt can reach the colon and are fermented by the gut microbiota (Gostner et al. 2005). This natural sweeteners originated from fruits and vegetables. Polyols, especially in patients who suffer from inflammatory bowel discomfort can induce flatulence in high dosage. Alas in control manner, several polyols like isomalt and maltitol, increase bifidobacteria counts in subjects who do not suffer from bowel disease and also shown to promote butyrate production in vitro (Gostner et al. 2006; Beards et al. 2010). On the other hand, different human clinical trials showed that lactitol decreases the populations of Bacteroides, Clostridium, coliforms, and Eubacterium, overall decrease in faecal pH (Ackerman et al. 2017; Ballongue et al. 1997). In addition, in vitro feline faecal culture, lactitol reduces the abundance of Enterobacteriaceae, increases the production of butyrate, thus may exerts prebiotic effect on feline members (Pinna et al. 2014; Peuranen et al. 2004). Another study of synbioitc products of L. acidophilus NCFM and lactitol decrease the abundance of the Blautia coccoides–Eubacterium rectale bacterial group and Clostridium cluster XIVab (Björklund et al. 2012). Low doses of (10 g) lactitol were tested in adults subject, reported to promote significant increase in bifidobacteria, propionate and butyrate despite also having minimal bowel discomfort (Finney et al. 2007). Xylitol reduces the abundance of faecal Bacteroidetes and the genus Barnesiella, increases Firmicutes and the genus Prevotella, and affects C. difficile in mice fed a high-fat diet with medium-dose dietary xylitol (Uebanso et al. 2017). A study by Tamura et al. (2013) compared the faecal microbiome of mice after being fed either a 0.05% isoflavone and 5% xylitol diet or a 0.05% isoflavone-only diet as a control. The Bacteroides concentration was lower in the xylitol-rich diet than the latter. In vitro human faecal fermentation of xylitol, l-Sorbose and xylitol cause prebiotic stimulation of the growth and metabolic activity of Anaerostipes spp. in the human colon (Sato et al. 2017).
Glycopeptide shown to have prebiotic effect as shown in the study of edible bird’s nest (EBN) glycopeptide fractions (Daud et al. 2019a). Several different components of glycopeptide fraction shown to improve beneficial gut bacteria and total production of short chain fatty acids after 24 h of in vitro monitoring. The study employed hydrolysis utilizing bacterial enzymes help to produce glycopeptide carrying specific bioactivity with improved physicochemical properties can be generated especially for the usage as prebiotics (Daud et al. 2019b). For example, under controlled hydrolysis of EBN by pepsin-trypsin managed to produce bioactive peptides tested to offer antioxidant properties possible for application as functional food (Ghassem et al. 2017). These may have selective proliferation to bifidobacteria if delivered in intended dosage amount. More pilot studies have still to be demonstrated that these molecules in correct doses have prebiotic efficacy (in vivo) before it can be bring forward to prebiotic markets.
Utilisation of fructan-type oligosaccharides by Lactobacillus
The capacity to utilize carbohydrates depends on the presence of a functional transport system and intracellular metabolic pathways of gut microbiota (Buntin et al. 2016). The ability to hydrolysed the prebiotics come from the βfructosidase and βfructofuranosidase, belongs to glycosyl hydrolase family 32, and conserved amino acid residues essential for its catalytic activity (Pons et al. 1998). In L. acidophilus and Bifidobacterium breve, ABC transporters have been observed to be involved in FOS uptake (Ventura et al. 2007). The inulin degrading enzyme by the bacteria is quite rare among lactobacilli, which mainly limited to some strains of L. paracasei, L. casei, L. acidophilus and L. delbrueckii (Takemura et al. 2010, Tsujikawa et al. 2013, Velikova et al. 2014, Barrangou et al. 2003). Three pathways have been documented to be associated including fos operon (Goh et al. 2006, 2007) (Table 1), msm operon (Barrangou et al. 2003), and Pts1BCA operon to degrade the prebiotics in Lactobacillus (Saulnier et al. 2007). One thing to note about the fos operon is that the hydrolysis is carried out by cell wall-anchored βfructosidase precursor (FosE), suggesting extracellular degradation before uptake through PTS transporters, unlike any other operon that involve cytoplasmic hydrolysis. The msm operon encoding the pathways by allowing ATP dependent binding cassette ABC transporters to carry the FOS molecules inside before being hydrolysed by β intracellular fructosidase. Whereas, for Pts1BCA operon, it is mediated by phosphoenolpyruvate dependant phosphotransferase system (PTS), followed by cytoplasmic hydrolysis of FOS (Buntin et al. 2016).
Utilisation of FOS in Bifidobacterium
Fructo-oligosaccharides fermentation of bifidobacteria relies on cytoplasmic β-FFase and take up by permeases or ABC transporters. Generally, most Bifidobacterium species are not capable to utilise longer chain inulin but prefer shorter chain FOS substrates. Activation of operon will not occur in the presence of the FFn type instead, where GFn type do. Meaning that CscA hydrolysed at the β-2,1 linkage between the glucose (G) and fructose (F) moieties, but chains of fructose (FF) molecules left untouched as residual hydrolytic products. While, different observation in mixed faecal cultures as other faecal bacterial species first degrade inulin to oligomers providing preferable chain length for bifidobacterial (Rossi et al. 2005) (Table 1).
Utilisation of GOS in Lactobacillus
There is only one study of L. acidophilus NCFM described the lactose permease (LacS) involved in GOS degradation despite the established status of GOS as prebiotic. In the presence of GOS, the gallac operon is activated that encodes LacS, a galactosidepentosehexuronide (GPH) family permease, two cytoplasmic βgalactosidases (GH42 LacA and GH2 LacLM), and enzymes of the Leloir pathway for galactose metabolism (Andersen et al. 2013). Galactooligosaccharide is intracellularly hydrolysed at glucose and galactose linkage before further metabolized via glycolysis for glucose production. The β-d-galactose is converted to glucose 1phosphate in four steps of Leloir pathways enzymatic reaction. In the first step, β-d-galactose is epimerized to α-d-galactose by galactose mutarotase. Next is the phosphorylation of α-d-galactose by galactokinase to galactose-1-phosphate. Then, galactose-1-phosphate uridylyltransferase, catalyses conversion of galactose-1-phosphate to UDP-galactose using UDP-glucose as the uridine phosphate source. The last step is UDP-galactose is recycled to UDP-glucose by UDP-galactose-4-epimerase for the transferase reaction (Holden et al. 2003). In another study using lactose as the substrate, lac operon was also induced in L. acidophilus, indicating possible interaction with other galactosides component too. The MFS-type transporter also gives advantage in terms of channelling substrates efficiently especially during nutrient scarce conditions.
Utilisation of GOS in Bifidobacterium
The bacteria, Bifidobacterium longum NCC2705 and B. breve UCC2003 possess analogous extracellular GH53 cell membrane–bound endogalactanases (GalA) capable of liberating galactotriose from galactan polymers with β-1,4 and β-1,3 linkages as well as GOS in an exotype fashion toward the reducing end of the polymers (Hinz et al. 2005). The endogalactanase specifically targets GOS with DP > 3. The partially degraded GOS were primarily imported via the aforementioned GalCDE ABC transporter and hydrolysed by GalG. On the other hand, LacS in L. acidophilus is the sole transporter for GOS, lactose, and lactulose (Andersen et al. 2013), whereas in B. breve, LacS is the sole uptake system for only lactose and lactulose (Motherway et al. 2013).
Synthesis of organic acids
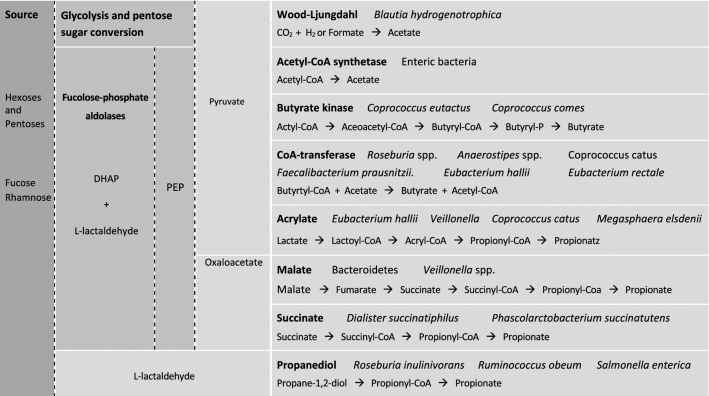
Postler and Ghosh (2017) classified metabolites into distinct categories of three types: (1) metabolites that are transformed by gut microbiota from drugs or dietary components; (2) metabolites that are secreted by host and modified by gut microbiota; (3) metabolites that are synthesized by gut microbiota de novo. Those metabolites that falls in each group are SCFA, bile acids and ATP, respectively. The major metabolites produced by the gut microbiota are the so called short chain fatty acids (SCFA). Short chain fatty acids are important metabolites from the carbohydrate fermentation by gut bacteria. Three SCFA that are mainly produced namely acetate, propionate, and butyrate (Besten et al. 2013). They are produced primarily from carbohydrate fermentation, although protein fermentation does contribute to SCFA production as well. Acetate most predominantly accumulated in the colon at least half of total SCFA produced. Acetate is also gaining attention. As a central metabolite in human metabolism, it is involved in many biochemical pathways, including fatty acid and glucose metabolism. Its role in obesity, insulin resistance and type 2 diabetes is currently under investigation (Diamant et al. 2011). The production of acetate is described in two pathways, through catabolism of hydrogen and carbon dioxide in Wood–Ljungdahl pathway (Louis et al. 2014), and fermentation by enteric bacteria as shown in Fig. 1. However, more studies are required to investigate these pathways to determine the variation of individual since the study only from two subjects of which methane gas production is not included. If larger methane formation in the colon competing the same carbon hydroxide molecule then lower acetate production from the Wood–Ljungdahl pathway will takes place.
Fig. 1.
Pathways that are recognised for short chain fatty acids formation by representative bacterial genera and species from the human colon bacterial cross-feeding. Substrate is non-digestable carbohydrates that are converted to pyruvate before continuing to different pathways. As shown in dotted arrow, some bacteria reverse utilise lactate to produce propionate (Veilonella spp.) and butyrate (Anaerostipes spp and E. hallii) via CoA transferase pathway (Besten et al. 2013). The bacterial species listed are not exhaustive, only as reviewed from studies in culture isolates of dominant species and metagenomic analyses. PEP phosphoenolpyruvate, DHAP dihydroxyacetonephospate
Three types of pathways for colonic bacteria producing propionate; succinate, acrylate, and propanodiol pathways (Reichardt et al. 2014). The succinate pathway presents in several Firmicutes and Bacteroidetes synthesising propionate from the intermediate, succinate and methylmalonylCoA is converted to propionylCoA. Succinate pathway is common considering the dominant population of Bacteriodetes suggesting higher number of reactions may takes place Salonen et al. 2014). In acrylate pathway, production of lactate by lactic acid bacteria, bifidobacteria, and proteobacteria starts to accumulate in the colon. Lactate will then be converted to propionate by lactoylCoA dehydratase and downstream enzymatic reaction. Lactate although is not SCFA, is an important precursor for bacteria such as Eubacterium hallii converting it to propionate, which reduce the accumulation of lactate in the colon (Duncan et al. 2004). In the propanodiol pathway, acetaldehyde from deoxysugars is converted by the CoAdependent propionaldehyde dehydrogenase that converts propionaldehyde to propionylCoA. This pathway was observed in proteobacteria and members of the Lachnospiraceae family (Louis et al. 2014; Reichardt et al. 2014).
The SCFA that has received the most attention so far is butyrate. Butyrate is the major fuel for colonocytes and as such considered to be very important for the health of the gut epithelium (Hamer et al. 2008; Havenaar 2011). One of the proposed beneficial effects of butyrate on human intestinal health is the prevention and inhibition of colon carcinogenesis, due to induction of differentiation and apoptosis of transformed cells (Hamer et al. 2008, 2012). In addition, it has been shown to have anti-inflammatory properties (Hamer et al. 2008, 2012). Even though there are other fuels that can be used by colonic epithelial cells, lack of butyrate has been considered to be detrimental for health. For example, butyrate concentrations are lower in inflammatory bowel disease (IBD) (Hamer et al. 2008). Moreover, butyrate known to has anti-inflammatory effects by the inhibition of histone deacetylase activity, resulting in hyperacetylation of histones, and as a consequence suppression of NFκB activation. In addition, it has been proposed that butyrate reinforces the colonic barrier function, by increasing production of mucins and antimicrobial peptides, such as β-defensin or trefoil factors. It has also been shown that butyrate increase the epithelial integrity by increasing the expression of tight junction proteins (Van Immerseel et al. 2010). This is why in the past there has been a great focus on butyrate production by the gut microbiota. Substrates have been selected that lead to an increase in the amount and ratio of butyrate using a healthy microbiota (Fässler et al. 2006; Rose et al. 2010) or a microbiota originating from IBD patients (Rose et al. 2010). Members of the microbiota involved in the production of butyrate have attracted attention too. Examples are members of the Lachnospiraceae and Ruminococcaceae, such as E. rectale, Roseburia intestinalis, and F. prausnitzii (Louis and Flint 2009). They are considered potential therapeutic probiotics for IBD (Van Immerseel et al. 2010). The prevalence of F. prausnitzii is often decreased also in other conditions of intestinal dysbiosis (Miquel et al. 2013, 2014). These include, besides IBD, irritable bowel syndrome (IBS) and coeliac disease. Two independent pathways were described in butyrate productions. The less common pathway, which only limited to some Coprococcus species (Louis and Flint 2017), is the butyrate kinase pathway which employs phosphotransbutyrylase and butyrate kinase enzymes to convert butyryl-CoA into butyrate (Louis et al. 2004). Coprococcus may also compete with other enteric bacteria in acetate production. Second major pathway is the butyrylCoA: acetate CoAtransferase pathway, in which butyrylCoA is converted to butyrate in a single step enzymatic reaction, used in most butyrate producing gut bacteria (Louis et al. 2010). To date, it is worth to note that only R. inulinivorans and Coprococcus catus are known to be able to produce both propionate and butyrate using the mentioned pathways (Louis et al. 2004). Short chain fatty acids mostly alter bit of every intestinal immune system to exhibit anti-inflammatory properties as part of improving cells integrity. One way is through the enhance of regulatory T cells (Tregs). Acetate and butyrate only helps to expand pre-existing Tregs and propionate and butyrate only stimulate the de novo differentiation of naive T cells into Tregs (Arpaia et al. 2013; Furusawa et al. 2014; Singh et al. 2014; Smith et al. 2013). In the in vivo model of colitis induced mice, SCFA were shown to weaken disease severity by the action of secreting cytokine interleukin (IL-)18 from intestinal epithelial cells that is attained when the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome through FFAR2 and HCAR2 were activated (Kalina et al. 2002; Macia et al. 2015; Singh et al. 2014). Another study showed increased mucin secretion by goblet cells also indicate was induced by SCFA mechanism (Fukuda et al. 2011; Gaudier et al. 2004; Willemsen 2003; Wrzosek et al. 2013).
Other end-products
Beside SCFA, gut microbiota also produce other important metabolites from food fermentation. Another major substrate that is fermented by the gut microbiota is protein. Most protein that makes it to the colon is incorporated into bacterial biomass, since most members of the microbiota prefer to ferment carbohydrate. However, in the distal gut, where fermentable carbohydrate is depleted, the microbiota will switch to protein fermentation. This leads to the production of all kind of putrefactive microbial metabolites, such as ammonia, branched chain fatty acids (BCFA), phenolic compounds, H2S and methanethiol, and nitrosamines and other biogenic amines, which are all thought to be harmful for the host (Cummings et al. 1979; Hughes et al. 2000; Russell et al. 2011). In addition to host proteins, significant amounts of dietary protein (~ 6 g) reach the large intestine per day. When high amounts of dietary protein is consumed, more of its portion will end up in the colon resulting an increased fermentation and production of toxic microbial metabolites (Magee et al. 2000). These occur then in concentrations that have been implicated in increased colon cancer risk. Overall, high protein intake, in particular of red meat, may increase risk of developing colorectal cancer (Gill and Rowland 2002; Norat and Riboli 2001). In a recent study, this was confirmed in which high protein diets resulted in increased proportions of BCFA and concentrations of phenylacetic acid and N-nitroso compounds (Russell et al. 2011). A high protein, low carbohydrate diet also decreased the proportion of butyrate, which was shown to be concomitant with a reduction in the butyrate producing Roseburia/E. rectale group of bacteria. In addition, this diet also greatly reduced concentrations of fibre derived, antioxidant phenolic acids such as ferulic acid and its derivatives. In line with previous research, a long term adherence to such high protein diets may increase the risk of colonic disease. Polyamines can also exert direct influence on innate immune cells. Arginine another metabolite, is also known to has immunomodulatory effect (Miller-Fleming et al. 2015). Spermine administration demonstrated on mouse models showed anti-inflammatory effects (Steege et al. 1999; Zhang et al. 1997; Zhu et al. 2009). Choline is an essential vitamin, provide important functions in the body. It acts as a methyl donor in biochemical reactions, is a precursor for the biosynthesis of phospholipids, acetylcholine and of lipoproteins, and is involved in homocysteine reduction. It is also make up the phosphatidylcholine, components that helps improve brain cognitive function.
Conclusion
At least a decade has passed on exploration of the genetic and enzymatic basis of carbohydrates fermentation by probiotic. More or less help to design extensive studies to fill the gap between what we have known about the important metabolites produced in gut fermentation. More ground can be covered in the field of pre- and probiotics by exploiting accumulated knowledge underlying the physiology of bacterial gene expression. In general, two stages of metabolism present during microbial fermentation of indigestible carbohydrates, one is the uptake of the whole longer chain prebiotic components, or enzymatically hydrolysed into shorter polymers extracellularly before transporting the derivatives into cytoplasmic region, and the other is the biosynthesis of short chain fatty acids. Recently, focus on interaction among microbiota in cross feeding are gaining interest since multiple research have found that some single probiotic strain does not grow as expected as in co-culture and mixed faecal inoculum with prebiotic substrate. By this knowledge, researchers will be able to further understand how gut bacteria respond towards different prebiotic. Advancement in evaluation technique i.e. in vitro model and cultured isolates of gut bacteria assessment helps to determine bacteria that are responsible for different metabolic activities. In addition, it is also crucial to consider other factors such as gut transit time, absorption through intestinal villi and individual diet habits which may change the metabolite profiles. Integrated bioinformatic framework built by Manor and Borenstein (2017) said to enable such taxonomic, functional information and contributions of individual species to be analysed within the microbiota. Through this research, significant insights on our understanding of gut microbiota will become apparent. Another interesting question to be explored is how much does genetic inheritance of the host associated in the composition of the gut environment, does it determinant for specific individual fingerprint or likely can be classified as in our blood group type, thus new findings will start to arise with every advance framework developed.
Acknowledgements
Funding was provided by Malaysia Ministry of Education (Grant No. FRGS/2/2013/SG05/UPM/02/23).
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ackerman DL, Craft KM, Townsend SD. Infant food applications of complex carbohydrates: structure, synthesis, and function. Carbohydr Res. 2017;437:16–27. doi: 10.1016/j.carres.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JM, Barrangou R, Hachem MA, et al. Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genomics. 2013;14:312. doi: 10.1186/1471-2164-14-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus F, Smart S, Shortt C. Prebiotic ingredients with emphasis on galacto-oligosaccharides and fructo-oligosaccharides. Probiotic Dairy Prod. 2005;18:120–137. doi: 10.1002/9780470995785.ch6. [DOI] [Google Scholar]
- Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad NH, Zaman SA, Rawi MH, Sarbini SR. Resistant starch evaluation and in vitro fermentation of lemantak (native sago starch), for prebiotic assessment. Int Food Res J. 2018;25:951–957. [Google Scholar]
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila M, Hidalgo M, Sánchez-Moreno C, et al. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res Int. 2009;42:1453–1461. doi: 10.1016/j.foodres.2009.07.026. [DOI] [Google Scholar]
- Babbar N, Dejonghe W, Sforza S, Elst K. Enzymatic pectic oligosaccharides (POS) production from sugar beet pulp using response surface methodology. J Food Sci Technol. 2017;54:3707–3715. doi: 10.1007/s13197-017-2835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajury DM, Rawi MH, Sazali IH, et al. Prebiotic evaluation of red seaweed (Kappaphycus alvarezii) using in vitro colon model. Int J Food Sci Nutr. 2017;68:821–828. doi: 10.1080/09637486.2017.1309522. [DOI] [PubMed] [Google Scholar]
- Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol. 1997;32:41–44. doi: 10.1080/00365521.1997.11720716. [DOI] [PubMed] [Google Scholar]
- Barrangou R, Altermann E, Hutkins R, et al. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc Natl Acad Sci. 2003;100:8957–8962. doi: 10.1073/pnas.1332765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo FD, Startek J, Ende WVD. Prebiotics to fight diseases: reality or fiction? Phytother Res. 2013 doi: 10.1002/ptr.4901. [DOI] [PubMed] [Google Scholar]
- Beards E, Tuohy K, Gibson G. A human volunteer study to assess the impact of confectionery sweeteners on the gut microbiota composition. Br J Nutr. 2010;104:701–708. doi: 10.1017/s0007114510001078. [DOI] [PubMed] [Google Scholar]
- Bell V, Ferrão J, Fernandes T. Nutritional guidelines and fermented food frameworks. Foods. 2017;6:65. doi: 10.3390/foods6080065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besten GD, Eunen KV, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.r036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund M, Ouwehand AC, Forssten SD, et al. Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age. 2012;34:987–999. doi: 10.1007/s11357-011-9294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordonaro M. Approaches that ascertain the role of dietary compounds in colonic cancer cells. World J Gastrointest Oncol. 2014;6:1. doi: 10.4251/wjgo.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnov RV, Babenko LP, Lazarenko LM, et al. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J. 2018;9:205–223. doi: 10.1007/s13167-018-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucław M. The use of inulin in poultry feeding: a review. J Anim Physiol Anim Nutr. 2016;100:1015–1022. doi: 10.1111/jpn.12484. [DOI] [PubMed] [Google Scholar]
- Buntin N, Hongpattarakere T, Ritari J, et al. An inducible operon is involved in inulin utilization in Lactobacillus plantarum strains, as revealed by comparative proteogenomics and metabolic profiling. Appl Environ Microbiol. 2016 doi: 10.1128/aem.02402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WSF, Meijerink M, Zeuner B, et al. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol. 2017 doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Hill MJ, Jivraj T, et al. The effect of meat protein and dietary fiber on colonic function and metabolism I. Changes in bowel habit, bile acid excretion, and calcium absorption. Am J Clin Nutr. 1979;32:2086–2093. doi: 10.1093/ajcn/32.10.2086. [DOI] [PubMed] [Google Scholar]
- Daud N’A, Sarbini SR, Babji AS, et al. Characterization of edible swiftlet’s nest as a prebiotic ingredient using a simulated colon model. Ann Microbiol. 2019;69:1235–1246. doi: 10.1007/s13213-019-01507-1. [DOI] [Google Scholar]
- Daud NA, Yusop SM, Babji AS, et al. Edible bird’s nest: physicochemical properties, production, and application of bioactive extracts and glycopeptides. Food Rev Int. 2019 doi: 10.1080/87559129.2019.1696359. [DOI] [Google Scholar]
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant M, Blaak EE, Vos WMD. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–281. doi: 10.1111/j.1467-789x.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- Duda-Chodak A. The inhibitory effect of polyphenols on human gut microbiota. J Physiol Pharmacol. 2012;63:497–503. [PubMed] [Google Scholar]
- Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/aem.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englyst HN, Kingman SM, Hudson GJ, Cummings JH. Measurement of resistant starch in vitro and in vivo. Br J Nutr. 1996;75:749–755. doi: 10.1079/bjn19960178. [DOI] [PubMed] [Google Scholar]
- Fässler AM, Arrigoni D, Venema D, et al. In vitro fermentability of differently digested resistant starch preparations. Mol Nutr Food Res. 2006;50:1220–1228. doi: 10.1002/mnfr.200600106. [DOI] [PubMed] [Google Scholar]
- Finney M, Smullen J, Foster HA, et al. Effects of low doses of lactitol on faecal microflora, pH, short chain fatty acids and gastrointestinal symptomology. Eur J Nutr. 2007;46:307–314. doi: 10.1007/s00394-007-0666-7. [DOI] [PubMed] [Google Scholar]
- Flint HJ, Bayer EA, Rincon MT, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, et al. Erratum: commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2014;506:254. doi: 10.1038/nature13041. [DOI] [PubMed] [Google Scholar]
- Gaudier E, Jarry A, Blottière HM, et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol-Gastrointest Liver Physiol. 2004 doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- Ghassem M, Arihara K, Mohammadi S, et al. Identification of two novel antioxidant peptides from edible birds nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017;8:2046–2052. doi: 10.1039/c6fo01615d. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gill CIR, Rowland IR. Diet and cancer: assessing the risk. Br J Nutr. 2002 doi: 10.1079/bjn2002632. [DOI] [PubMed] [Google Scholar]
- Goh YJ, Klaenhammer TR. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu Rev Food Sci Technol. 2015;6:137–156. doi: 10.1146/annurev-food-022814-015706. [DOI] [PubMed] [Google Scholar]
- Goh YJ, Zhang C, Benson AK, et al. Identification of a putative operon involved in fructooligosaccharide utilization by Lactobacillus paracasei. Appl Environ Microbiol. 2006;72:7518–7530. doi: 10.1128/aem.00877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YJ, Lee J-H, Hutkins RW. Functional analysis of the fructooligosaccharide utilization 0peron in Lactobacillus paracasei 1195. Appl Environ Microbiol. 2007;73:5716–5724. doi: 10.1128/aem.00805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez B, Gullón B, Yáñez R, et al. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: a comparative evaluation. J Funct Foods. 2016;20:108–121. doi: 10.1016/j.jff.2015.10.029. [DOI] [Google Scholar]
- Gopalsamy G, Mortimer E, Greenfield P, et al. Resistant starch is actively fermented by infant faecal microbiota and increases microbial diversity. Nutrients. 2019;11:1345. doi: 10.3390/nu11061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostner A, Schäffer V, Theis S, et al. Effects of isomalt consumption on gastrointestinal and metabolic parameters in healthy volunteers. Br J Nutr. 2005;94:575–581. doi: 10.1079/bjn20051510. [DOI] [PubMed] [Google Scholar]
- Gostner A, Blaut M, Schäffer V, et al. Effect of isomalt consumption on faecal microflora and colonic metabolism in healthy volunteers. Br J Nutr. 2006;95:40–50. doi: 10.1079/bjn20051589. [DOI] [PubMed] [Google Scholar]
- Gupta M, Dey S, Marbaniang D, et al. Grape seed extract: having a potential health benefits. J Food Sci Technol. 2019 doi: 10.1007/s13197-019-04113-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Preter VD, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol-Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenaar R. Intestinal health functions of colonic microbial metabolites: a review. Benefic Microbes. 2011;2:103–114. doi: 10.3920/bm2011.0003. [DOI] [PubMed] [Google Scholar]
- Heinze T, Liebert T. Celluloses and polyoses/hemicelluloses. Polym Sci Compr Ref. 2012 doi: 10.1016/b978-0-444-53349-4.00255-7. [DOI] [Google Scholar]
- Hinz SWA, Pastink MI, Van Den Broek LAM, et al. Bifidobacterium longum endogalactanase liberates galactotriose from Type I Galactans. Appl Environ Microbiol. 2005;71:5501–5510. doi: 10.1128/aem.71.9.5501-5510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885–43888. doi: 10.1074/jbc.r300025200. [DOI] [PubMed] [Google Scholar]
- Hughes R, Magee EA, Bingham S. Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intest Microbiol. 2000;2:51–58. [PubMed] [Google Scholar]
- Islamova ZI, Ogai DK, Abramenko OI, et al. Comparative assessment of the prebiotic activity of some pectin polysaccharides. Pharm Chem J. 2017;51:288–291. doi: 10.1007/s11094-017-1600-9. [DOI] [Google Scholar]
- Jara Pérez-Jiménez, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem. 2010;58:4959–4969. doi: 10.1021/jf100128b. [DOI] [PubMed] [Google Scholar]
- Jovanovic-Malinovska R, Kuzmanova S, Winkelhausen E. Oligosaccharide profile in fruits and vegetables as sources of prebiotics and functional foods. Int J Food Prop. 2014;17:949–965. doi: 10.1080/10942912.2012.680221. [DOI] [Google Scholar]
- Kalina U, Koyama N, Hosoda T, et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur J Immunol. 2002;32:2635–2643. doi: 10.1002/1521-4141(200209)32:9<2635::aid-immu2635>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari D, Patel S, Goyal A. Therapeutic spectrum of nondigestible oligosaccharides: overview of current state and prospect. J Food Sci. 2014 doi: 10.1111/1750-3841.12536. [DOI] [PubMed] [Google Scholar]
- Kuschel B, Riemer F, Pfost D, et al. Large-scale purification of epilactose using a semi-preparative HPLC system. Eur Food Res Technol. 2017;243:391–402. doi: 10.1007/s00217-016-2752-7. [DOI] [Google Scholar]
- Lacombe A, Wu VC, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int J Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Lee W-J, Hase K. Gut microbiota–generated metabolites in animal health and disease. Nat Chem Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic faecal bacterial metabolites on intestinal microbiota. Res Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P-J, Xia J-L, Nie Z-Y, Shan Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT Food Sci Technol. 2016;69:203–210. doi: 10.1016/j.lwt.2016.01.042. [DOI] [Google Scholar]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Louis P, Duncan SH, Mccrae SI, et al. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/jb.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015 doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000;72:1488–1494. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005 doi: 10.1093/ajcn/81.1.230s. [DOI] [PubMed] [Google Scholar]
- Manor O, Borenstein E. Systematic characterization and analysis of the taxonomic drivers of functional shifts in the human microbiome. Cell Host Microbe. 2017;2:254–267. doi: 10.1016/j.chom.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr. 2009;63:1277–1289. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427:3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Miquel S, Martín R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Miquel S, Martín R, Bridonneau C, et al. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5:146–151. doi: 10.4161/gmic.27651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motherway MO, Kinsella M, Fitzgerald GF, Sinderen DV. Transcriptional and functional characterization of genetic elements involved in galacto-oligosaccharide utilization by Bifidobacterium breve UCC2003. Microb Biotechnol. 2013;6:67–79. doi: 10.1111/1751-7915.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Li Q, Fan C, et al. Recent advances on physiological functions and biotechnological production of epilactose. Appl Microbiol Biotechnol. 2013;97:1821–1827. doi: 10.1007/s00253-013-4687-2. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ojima-Kato T, Saburi W, et al. Supplemental epilactose prevents metabolic disorders through uncoupling protein-1 induction in the skeletal muscle of mice fed high-fat diets. Br J Nutr. 2015;114:1774–1783. doi: 10.1017/s0007114515003505. [DOI] [PubMed] [Google Scholar]
- Niness KR. Inulin and oligofructose: What are they? J Nutr. 1999 doi: 10.1093/jn/129.7.1402s. [DOI] [PubMed] [Google Scholar]
- Norat T, Riboli E. Meat consumption and colorectal cancer: a review of epidemiologic evidence. Nutr Rev. 2001;59:37–47. doi: 10.1111/j.1753-4887.2001.tb06974.x. [DOI] [PubMed] [Google Scholar]
- Oku T, Tokunaga T, Hosoya N. Nondigestibility of a new sweetener, “Neosugar”, in the rat. J Nutr. 1984;114:1574–1581. doi: 10.1093/jn/114.9.1574. [DOI] [PubMed] [Google Scholar]
- Parkar SG, Stevenson DE, Skinner MA. Erratum to ‘The potential influence of fruit polyphenols on colonic microflora and human gut health’ [Int. J. of Food Microbiol. 124 (2008) 295–298] Int J Food Microbiol. 2008;128:416. doi: 10.1016/j.ijfoodmicro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Parkar SG, Redgate EL, Mcghie TK, Hurst RD. In vitro studies of modulation of pathogenic and probiotic bacterial proliferation and adhesion to intestinal cells by blackcurrant juices. J Funct Foods. 2014;8:35–44. doi: 10.1016/j.jff.2014.02.021. [DOI] [Google Scholar]
- Parker EA, Roy T, Dadamo CR, Wieland LS. Probiotics and gastrointestinal conditions: an overview of evidence from the Cochrane Collaboration. Nutrition. 2018 doi: 10.1016/j.nut.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuranen S, Tiihonen K, Apajalahti J, et al. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br J Nutr. 2004;91:905–914. doi: 10.1079/bjn20041114. [DOI] [PubMed] [Google Scholar]
- Pinna C, Stefanelli C, Biagi G. In vitro effect of dietary protein level and nondigestible oligosaccharides on feline faecal microbiota1. J Anim Sci. 2014;92:5593–5602. doi: 10.2527/jas.2013-7459. [DOI] [PubMed] [Google Scholar]
- Pokusaeva K, Fitzgerald GF, Sinderen DV. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons T, Olmea O, Chinea G, et al. Structural model for family 32 of glycosyl-hydrolase enzymes. Proteins Struct Funct Genetics. 1998;33:383–395. doi: 10.1002/(sici)1097-0134(19981115)33:3<383::aid-prot7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil JE, Stuker E, Bourne JR. Formation of oligosaccharides during enzymatic lactose: part I: state of art. Biotechnol Bioeng. 1987;30:1019–1025. doi: 10.1002/bit.260300904. [DOI] [PubMed] [Google Scholar]
- Ramirez-Farias C, Slezak K, Fuller Z, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2008;101:541–550. doi: 10.1017/s0007114508019880. [DOI] [PubMed] [Google Scholar]
- Reichardt N, Duncan SH, Young P, et al. Erratum: phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1352. doi: 10.1038/ismej.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D, Ruas-Madiedo P, Margolles A, et al. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DJ, Venema K, Keshavarzian A, Hamaker BR. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br J Nutr. 2010;103:1514–1524. doi: 10.1017/s0007114509993515. [DOI] [PubMed] [Google Scholar]
- Rossi M, Corradini C, Amaretti A, et al. Fermentation of fructo-oligosaccharides and inulin by bifidobacteria: a comparative study of pure and faecal cultures. Appl Environ Microbiol. 2005;71:6150–6158. doi: 10.1128/aem.71.10.6150-6158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93:1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Fitzgerald GF, Sinderen DV. Transcriptional regulation and characterization of a novel fructofuranosidase-encoding gene from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2005;71:3475–3482. doi: 10.1128/aem.71.7.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, Lahti L, Salojärvi J, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal L, Chaturvedi VB, Saikumar G, et al. Prebiotic potential of Jerusalem artichoke (Helianthus tuberosus L.) in Wistar rats: effects of levels of supplementation on hindgut fermentation, intestinal morphology, blood metabolites and immune response. J Sci Food Agric. 2015;95:1689–1696. doi: 10.1002/jsfa.6873. [DOI] [PubMed] [Google Scholar]
- Sarbini SR, Rastall RA. Prebiotics: metabolism, structure, and function. Funct Food Rev. 2011;3:93–106. doi: 10.2310/6180.2011.00004. [DOI] [Google Scholar]
- Sato T, Kusuhara S, Yokoi W, et al. Prebiotic potential of L-sorbose and xylitol in promoting the growth and metabolic activity of specific butyrate-producing bacteria in human faecal culture. FEMS Microbiol Ecol. 2017 doi: 10.1093/femsec/fiw227. [DOI] [PubMed] [Google Scholar]
- Saulnier DMA, Molenaar D, Vos WMD, et al. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol. 2007;73:1753–1765. doi: 10.1128/aem.01151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. Hemicelluloses. Ann Rev Plant Biol. 2010;61:263–289. doi: 10.3945/an.111.000455. [DOI] [PubMed] [Google Scholar]
- Schwartz SE, Levine RA, Weinstock RS, et al. Sustained pectin ingestion: effect on gastric emptying and glucose tolerance in non-insulin-dependent diabetic patients. Am J Clin Nutr. 1988;48:1413–1417. doi: 10.1093/ajcn/48.6.1413. [DOI] [PubMed] [Google Scholar]
- Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RS, Singh RP, Kennedy JF. Recent insights in enzymatic synthesis of fructo-oligosaccharides from inulin. Int J Biol Macromol. 2016;85:565–572. doi: 10.1016/j.ijbiomac.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steege JCT, Forget PP, Buurman WA. Oral spermine administration inhibits nitric oxide-mediated intestinal damage and levels of systemic inflammatory mediators in a mouse endotoxin model. Shock. 1999;11:115–119. doi: 10.1097/00024382-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Tabasco R, Sánchez-Patán F, Monagas M, et al. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: resistance and metabolism. Food Microbiol. 2011;28:1345–1352. doi: 10.1016/j.fm.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Takemura N, Hagio M, Ishizuka S, et al. Inulin prolongs survival of intragastrically administered Lactobacillus plantarum No. 14 in the gut of mice fed a high-fat diet. J Nutr. 2010;140:1963–1969. doi: 10.3945/jn.110.128082. [DOI] [PubMed] [Google Scholar]
- Tamura M, Hoshi C, Hori S. Xylitol affects the intestinal microbiota and metabolism of daidzein in adult male mice. Int J Mol Sci. 2013;14:23993–24007. doi: 10.3390/ijms141223993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari UP, Singh AK, Jha R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: a review. Anim Nutr. 2019;5:217–226. doi: 10.1016/j.aninu.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomatsu H. Health effects of oligosaccharides. Food Technol. 1994;48:61–65. [Google Scholar]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitko I, Wiik-Miettinen F, Mattila O, et al. A small in vitro fermentation model for screening the gut microbiota effects of different fiber preparations. Int J Mol Sci. 2019;20:1925. doi: 10.3390/ijms20081925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa Y, Nomoto R, Osawa R. Difference in degradation patterns on inulin-type fructans among strains of Lactobacillus delbrueckii and Lactobacillus paracasei. Biosci Microbiota Food Health. 2013;32:157–165. doi: 10.12938/bmfh.32.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebanso T, Ohnishi A, Kitayama R, et al. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients. 2017;9:662. doi: 10.3390/nu9060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F, Ducatelle RD, Vos MD. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol. 2010;59:141–143. doi: 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- Velikova PV, Blagoeva GI, Gotcheva VG, Petrova PM. Novel bulgarian Lactobacillus strains ferment prebiotic carbohydrates. J BioSci Biotechnol. 2014;55–60:1314–6246. [Google Scholar]
- Ventura M, Oconnell-Motherway M, Leahy S, et al. From bacterial genome to functionality; case bifidobacteria. Int J Food Microbiol. 2007;120:2–12. doi: 10.1016/j.ijfoodmicro.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mortimer EK, Katundu KGH, et al. The capacity of the fecal microbiota from Malawian infants to ferment resistant starch. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Nishimukai M, Taguchi H, et al. Prebiotic properties of epilactose. J Dairy Sci. 2008;91:4518–4526. doi: 10.3168/jds.2008-1367. [DOI] [PubMed] [Google Scholar]
- Willemsen LEM. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzosek L, Miquel S, Noordine M-L, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanahira S, Kobayashi T, Suguri T, Nakakoshi M, Miura S, Ishikawa H, Nakajima I. Formation of oligosaccharides from lactose by Bacillus circulans β-galactosidase. Biosci Biotechnol Biochem. 1995;59:1021–1026. doi: 10.1271/bbb.59.1021. [DOI] [PubMed] [Google Scholar]
- Yissachar N, Zhou Y, Ung L, et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell. 2017 doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H-D, Kim D-J, Paek S-H, Oh S-E. Plant cell wall polysaccharides as potential resources for the development of novel prebiotics. Biomol Ther. 2012;20:371–379. doi: 10.4062/biomolther.2012.20.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman SA, Sarbini SR. The potential of resistant starch as a prebiotic. Crit Rev Biotechnol. 2015 doi: 10.3109/07388551.2014.993590. [DOI] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Ashok M, Li J, et al. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15:275–282. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic AM, Barile D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv Nutr. 2011;2:284–289. doi: 10.3945/an.111.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]