Abstract
In vitro antifungal activity of the essential oil from Monarda citriodora (MCEO) with possible mode of action was evaluated against A. flavus (AF-LHP-SH1) and 15 other storage molds for controlling postharvest deterioration of stored functional food samples. The chemical profiling of MCEO as done through GC–MS analysis revealed caryophyllene (19.15%) as the major component. The MCEO showed broad spectrum fungitoxicity and completely inhibited the growth of all tested molds and aflatoxin B1 (AFB1) production by AF-LHP-SH1 at 1.40 and 1.20 µL/mL, respectively. Plasma membrane damage and methylglyoxal inhibition was confirmed as the possible antifungal and antiaflatoxigenic mode of action of MCEO. MCEO exhibited remarkable antioxidant activity with IC50 value 2.24 μL/mL as determined through DPPH assay and did not cause adverse effect on seed germination. In addition, the MCEO was encapsulated into chitosan nanoparticle, characterized (SEM, FTIR, XRD) and assessed for their potential against inhibition of growth and AFB1 production. MCEO after encapsulation exhibited enhanced efficacy inhibiting fungal growth and AFB1 production by AF-LHP-SH1 at 0.6 and 0.5 µL/mL, respectively. Encapsulated MCEO may be recommended as novel preservative to extend the shelf life of stored functional food samples.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04318-4) contains supplementary material, which is available to authorized users.
Keywords: Monarda citriodora essential oil, Functional foods, Aflatoxin B1, Methylglyoxal, Chitosan, Nanoencapsulation
Introduction
Functional foods have the potential to minimize the chances of disease occurrence and thereby improve the health are susceptible to deterioration due to fungal and mycotoxin contaminations (Rawat 2015). Many chemical based additives are frequently applied by food and pharma industries to control these food contaminants however; their negative concerns like residual toxicity, resistance development among fungal pests and environmental pollution have forced the food sectors to incline towards the use of plant based products as safer and eco-friendly alternatives (Ferreira et al. 2018). In this sense, essential oils (EOs) have currently attracted great deal of attention due to their potential antifungal, antimycotoxigenic and antioxidant properties (Burt 2004; Kumar et al. 2018). Some EOs have been fully approved as safe due to non-toxic profile and placed under Generally Recognized as Safe (GRAS) category by US-FDA and EPA (USEPA 1993; USFDA 2013). However, in spite of the proved potential of EOs as preservative against fungal and aflatoxin contamination, their direct incorporation into food systems remains limited as they are volatile, poorly soluble in water, undergo degradation in high light, moisture, oxygen and temperature and exert negative impact on organoleptic profile of the food system due to intense aroma (da Silva Gündel et al. 2018). Such limitations could be overcome by encapsulating these EOs into stable polymeric matrices (Maryam et al. 2015). Among different polymers, chitosan has attracted scientific attention due to their abundance, cationic charge, non-toxic nature, (these can also be allergens to people with seafood allergy) high encapsulation efficiency and antimicrobial properties (Gago et al. 2019).
Monarda citriodora Cerv. ex Lag., also known as lemon bee balm, is an aromatic herb belonging to the family lamiaceae has been explored since antiquity as flavoring agent. EO isolated from its different parts possesses wide range of antibacterial and antioxidant activity against food borne pathogens (Lu et al. 2011). However, as per our knowledge, no experimental studies are available in the literature reporting its antifungal and antiaflatoxigenic activity as well as in its nanoencapsulated formulation.
The present study deals with efficacy of chemically characterized MCEO against fungi contaminating selected functional food samples, aflatoxin B1 (AFB1) secretion and antioxidant activity with their possible mode of action. Further, MCEO was encapsulated into a chitosan nanosystem and tested against fungal growth and AFB1 inhibition and compared with the unencapsulated form as a novel preservative.
Materials and methods
Chemicals
Major chemicals viz. chitosan, dichloromethane, 1,2-diaminobenzene, dimethyl sulphoxide, chloroform, tween-80, tween-20, toluene, isoamyl alcohol, potassium hydroxide, n-heptane, 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and culture media such as potato dextrose agar (PDA) and SMKY (Sucrose = 200 g; MgSO4.7H2O = 0.5 g; KNO3 = 0.3 g; Yeast extract powder = 7 g maintained to 1 L by adding distilled water) were purchased from SRL (Pvt. Ltd, Mumbai, India). The EO of Monarda citriodora was supplied by MRK Natural (New Delhi, India).
Functional food samples collection
Seven different functional food samples (seeds) viz. Linum usitatissimum L. (flaxseed), Hordeum vulgare L. (barley), Salvia hispanica L. (chia), Fagopyrum esculentum Moench. (buckwheat), Sesamum indicum L. (sesame), Macrotyloma uniflorum (Lam.) Verdc. (horse gram) and Chenopodium quinoa Willd. (quinoa) were collected from different regions of Uttar Pradesh, India and were stored in autoclaved plastic bags at laboratory temperature until analysis.
Determination of pH and moisture content
To measure pH, each sample was milled to prepare the powder and subsequently added into distilled water to achieve the ratio of 1:10 (w/v). The pH of sample was measured using electronic pH meter (Systronics µpH system 361, Delhi, India). The moisture content (MC) of the sample (10 g) was measured by weighing the sample followed by drying at 121 °C in an oven for 24 h until constant weight of sample was obtained and the MC was calculated using Eq. 1.
| 1 |
Mycobiota analysis
Fungal diversity associated with selected functional foods was analyzed using direct plating and serial dilution (Kedia et al. 2014). For direct plating, the seed sample was surface sterilized with 0.1% sodium hypochlorite solution, rinsed with distilled water and placed in Petri plates (9 × 9 cm) containing PDA medium (10 mL). For serial dilution, 1 g of well milled sample was homogenized in distilled water (10 mL) and placed on mechanical shaker for 1 min. Four-fold serial dilution was prepared for each sample. Aliquot of 1 mL from 10−3 dilution was then added into each of five Petri plates containing PDA medium. The plates of both direct plating and serial dilution were sealed with parafilm wax and incubated for 7 days. The developed fungal genera and respective species were identified based on color and morphological characteristics following the manual of soil fungi (Gilman and Joseph 1998). Percent relative density (PRD) and occurrence frequency (POF) of the isolated fungi were calculated using Eqs. 2 and 3.
| 2 |
| 3 |
Maintenance of fungal isolates
Fifteen different fungal isolates (Aspergillus luchuensis, Aspergillus niger, Aspergillus sydowii, Aspergillus repens, Aspergillus fumigatus, Aspergillus chevalieri, Aspergillus terreus, Alternaria grisea, Alternaria humicola, Aspergillus versicolor, Fusarium oxysporum, Penicillium italicum, Penicillium spinolosum, Alternaria alternata and mycelia sterilia) along with toxigenic strain of A. flavus were cultured in Petri plates and maintained on PDA slants at 4 °C.
Detection of the most toxigenic A. flavus isolate
Different A. flavus isolates obtained during mycobiota analysis of functional food samples were randomly selected and assessed for their AFB1 producing potential. For this, A. flavus isolates were inoculated into conical flask containing 25 mL SMKY medium and incubated at 27 ± 2 °C. After 10 days of incubation in BOD incubator (Delux automatic, New Delhi, India), the content (medium) of each flask (25 mL) was extracted using chloroform in a separating funnel followed by evaporation on water bath at 85 °C. The residues left after evaporation were then dissolved in methanol (1 mL) and 50 μL of it was spotted on silica gel G plate and developed in toluene: isoamyl alcohol: methanol, 90:32:2 (v/v/v) for AFB1 separation. The developed plate was observed on UV-transilluminator (360 nm) (Hitachi-2900, Japan) and the presence of AFB1 was indicated by blue color fluorescence, which was quantified by dissolving the samples into 5 mL cold methanol followed by centrifugation and measurement of absorbance at 360 nm. The AFB1 content was calculated by using Eq. 4.
| 4 |
where 312 = molecular weight of AFB1, 21,800 = molar extinction coefficient and 1 = path length.
Chemical characterization of MCEO
The volatile constituents of the MCEO were analyzed using gas chromatography-mass spectrophotometry (GC–MS) equipped with TG-5 MS capillary column (30 m × 0.25 mm × 0.25 µm) fitted to TSQ Duo triple quadruple mass spectrophotometer (MS). The EO was diluted in hexane (100 times) and analyzed under following conditions: injection volume 1 µL, initial column temperature was set to 60–240 °C at a rate of 3 °C/min and increased up to 270 °C at 5 °C /min, split less injector temperature was 250 °C with a split ratio of 1:50. Helium was used as a carrier gas with flow of 1 mL/min, temperature of transfer line and ion source were 250 and 220 °C, respectively, scan range of mass analyzer was set from 40 to 450 amu for 0.2 s. The components obtained were identified on the basis of retention time, area percent and spectral peaks available in published data (Adams 2007).
Antifungal investigation of MCEO: Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
Poisoned food technique was used to determine the MIC and MFC of MCEO against AF-LHP-SH1 in PDA medium. A fungal disc (5 mm) of AF-LHP-SH1 was placed at the centre of Petri plates containing PDA medium (10 mL) dispersed with different concentrations (0.20–1.40 μL/mL) of MCEO. Petri plates without MCEO served as control. Then, the plates were sealed with parafilm wax and incubated at 27 ± 2 °C in BOD incubator for 7 days followed by measurement of growth (in cm). The inhibition of growth (%) was calculated from the following Eq. 5.
| 5 |
where Dc is the average colony diameter in control and; Dt is the average colony diameter in MCEO treated sets.
The lowest concentration of MCEO completely inhibiting the growth of AF-LHP-SH1 was considered as MIC. The MFC was determined by inoculating the agar disc from the above MIC experiment into the Petri plates containing PDA medium without amendments and incubated at 27 ± 2 °C for seven days. The concentration of MCEO preventing restoration of fungal growth was considered as MFC (Kohiyama et al. 2015).
Fungitoxic spectrum of MCEO
The broad range toxicity of MCEO against 15 additional molds (A. luchuensis, A. niger, A. sydowii, A. repens, A. fumigatus, A. chevalieri, A. terreus, A. grisea, A. humicola, A. versicolor, F. oxysporum, P. italicum, P. spinolosum, A. alternata and mycelia sterilia) contaminating stored functional food samples were examined using poisoned food technique. For this, a 5 mm disc of each fungus was aseptically inoculated into Petri plates amended with MCEO at 1.40 µL/mL (MIC) dose. The plates without MCEO were considered as control. The plates were then sealed with parafilm wax, kept in BOD and percent inhibition of growth was calculated similar to the abovementioned equation.
Effect of MCEO in suppression of AFB1 in AF-LHP-SH1
AFB1 inhibitory potential of MCEO was determined in SMKY medium as suggested by Dwivedy et al. (2018) with some modifications. An aliquot containing 25 μL spore suspension of test fungus was inoculated into conical flasks receiving different concentrations (0.20–1.40 μL/mL) of MCEO. Control sets were prepared without MCEO. After 10 days of incubation in BOD incubator at 27 ± 2 °C, the content of each conical flask was extracted using chloroform and the amount of AFB1 in the sample was quantified based on previous procedure described in materials and methods section dealing with detection of the most toxigenic A. flavus.
Antifungal mode of action of MCEO
Ergosterol measurement in the plasma membrane of treated AF-LHP-SH1
Effect of MCEO on ergosterol content was determined following Tian et al. (2012a) with few modifications. For this, AF-LHP-SH1 was aseptically inoculated into SMKY medium containing different concentrations (0.20–1.00 µL/mL) of MCEO. Control sets were prepared without EO. Thereafter, the flasks were kept at 27 ± 2 °C in BOD and after 4 days of incubation, the fungal mycelium was filtered and washed with distilled water followed by measurement of the wet weight. An aliquot containing 5 mL of 25% alcoholic KOH solution was then added into each mycelial pellet in a culture tube and vortexed for 2 min followed by incubation on water bath (85 °C) for 4 h. The ergosterol was extracted by adding 2 mL of distilled water and 5 mL of n-heptane and subsequent vortex for 2 min. The transparent n-heptane layer was used to analyze ergosterol using UV visible spectrophotometry between 230 to 300 nm. The amount of ergosterol was calculated by following formula:
where 290 and 518 are the E values (in percentage/cm) determined for crystalline ergosterol and 24(28) dehydroergosterol, respectively.
Determination of cellular ions and 260 and 280 nm absorbing materials
To determine the cellular ions and loss of 260 and 280 nm absorbing materials, 5 days old mycelia of AF-LHP-SH1 grown in SMKY medium was harvested, washed and treated with MCEO at 0.70 µL/mL (1/2 MIC), 1.40 µL/mL (MIC) and 2.80 µL/mL (2 MIC) concentrations. After overnight incubation in BOD, the samples were filtered using Whatman no. 1 filter paper and the resulting supernatants were analyzed for Ca2+, Mg2+, K+ ions using Atomic Absorption Spectrophotometry (AAS) and 260 and 280 nm absorbing materials through UV visible spectrophotometry (Hitachi U 2900, Japan).
Flow cytometry based assay to determine the lesion in plasma membrane
The investigation on membrane lesions was performed following the modified method of Tian et al. (2012a) with minor changes. For this, briefly, the spore suspension of AF-LHP-SH1 was obtained from the fresh culture using phosphate buffered saline (PBS) solution (5%) containing d-glucose (2%). The density of spore was adjusted to 4.98 × 107 spore/mL using a hemocytometer and transferred to culture tube. To each tube, MCEO was added to achieve the final concentrations of 0.70 (1/2 MIC), 1.40 (MIC) and 2.80 μL/mL (2 MIC). Culture tube without EO was served as control. The culture tubes were then incubated overnight at 27 ± 2 °C on mechanical shaker. The fungal spores were washed and re-suspended in 1 μL of PBS containing 1 μg/mL propidium iodide (PI) dye and kept for 30 min at room temperature. Spores without stain were used as autofluorescence control and the stained PI+ spores was analyzed using flow cytometer (BD FACSCalibur, New Delhi, India).
Effect of MCEO on cellular methylglyoxal (MG) level of AF-LHP-SH1
The effect of MCEO treatment on MG level in AF-LHP-SH1 was determined following Upadhyay et al. (2018). Briefly, 0.3 g of mycelial biomass was fumigated with different concentrations of MCEO ranging from 0.20 to 2.80 µL/mL. Media with and without Tween-20 were served as positive and negative control, respectively. After overnight incubation, 0.3 g fungal mass was crushed in 0.5 M chilled perchloric acid (3 mL) and centrifuged at 11,000×g for 10 min at 4 °C. The supernatant obtained were then neutralized with saturated solution of potassium carbonate and an additional round of centrifugation at same speed was performed. The supernatant obtained was used for MG estimation. An aliquot containing 250 µL of 7.2 mM 1, 2-diaminobenzene was added into a test tube and mixed with 100 µL 5 M perchloric acid and 650 µL supernatant followed by measurement of absorbance at 341 nm after 30 min. The exact amount of MG was calculated from the calibration curve prepared using different concentrations of pure MG.
Antioxidant activity of MCEO through DPPH free radical assay
Antioxidant activity of MCEO was measured by recording the extent of bleaching of purple colored DPPH radical to straw colored non radical solution following Dwivedy et al. (2017). For determination of antioxidant potency of MCEO, desired concentrations (0.20–2.00 μL/mL) of MCEO was added separately into test tubes containing 0.004% methanolic DPPH solution (2 mL). The reaction mixture was shaken and after 30 min of incubation, the absorbance was measured at 517 nm. The IC50 value was calculated from the graph plotted between the concentration of MCEO and radical scavenging activity. The per cent inhibition (I) was calculated using Eq. 6.
| 6 |
Measurement of total phenolic content
Total phenolic content of MCEO was assessed using Folin-Ciocalteu’s reagent following Gholivand et al. (2010) with slight modifications. 1000 μL MCEO was added into the conical flask containing 100 uL DMSO followed by addition of 25 mL distilled water. After that, 1 mL Folin-Ciocalteu reagent and 3 mL of 2% sodium carbonate was added into the solution and after 2 h of reaction, the absorbance was measured at 760 nm. Total phenolic content was calculated using Eq. 7.
| 7 |
Effect of MCEO on seed germination
Definite number (20) of S. hispanica seeds were fumigated with MCEO at a concentration equivalent to MIC and 2 MIC and stored in sealed plastic container for 6 months. Fresh seeds were considered as control. Thereafter, the seeds were sown into the moist soil under natural environmental conditions. The analysis was performed at a regular interval of 7 days up to 5 weeks for determination of seed germination (%) by measuring the length of radicles and plumules.
Preparation of MCEO loaded chitosan nanoparticle
Method of Yoksan et al. (2010) was adopted to prepare MCEO loaded chitosan nanoparticle. Firstly, 1.5% chitosan powder (w/v) was dispersed in 1% acetic acid (v/v) and kept on magnetic stirrer for overnight. Tween 80 was then added as an emulsifier into the solution and agitated again on magnetic stirrer at 45 °C for 2 h. Different volumes of MCEO (0.06, 0.12, 0.18, 0.24 and 0.30 mL) dissolved in dichloromethane (DCM) were added into the chitosan solution followed by homogenization (13,000×g for 10 min under ice bath condition) using high speed homogenizer (T18 Digital Ultra- Turrax, IKA, Germany) to achieve an oil-in-water emulsion. Tripolyphosphate (0.4% (w/v)) was then added as cross linking agent to the emulsion with constant stirring over magnetic stirrer. The prepared nanoparticle was then separated by centrifugation (REMI compufuge CPR-4) at 10,000×g for 10 min at 4 °C and the pellet obtained was dissolved with deionised water followed by sonication to get the homogenous suspension. The suspension was lyophilized at − 52 °C for 72 h and stored at freezing temperature until characterization.
Physico-chemical characterization of MCEO loaded chitosan nanoparticle
Determination of encapsulation efficiency (EE) and loading capacity (LC)
The amount of MCEO loaded into chitosan nanoparticle was assessed by measuring the absorbance using UV–Vis spectrophotometry. For this, 3 mL ethanol was properly mixed with 100 µL of prepared nanoparticle and subsequently centrifuged at 10,000×g for 10 min. A blank was also prepared in the same way using chitosan nanoparticle. The obtained supernatant was used to measure the absorbance at 237 nm and the percent EE and LC was calculated by Eqs. 8 and 9, respectively using standard curve of MCEO (Hosseini et al. 2013).
| 8 |
| 9 |
Morphology of MCEO loaded chitosan nananoparticle
Scanning Electron Microscopy (SEM) was performed to determine the morphological features of chitosan and MCEO loaded chitosan nanoparticle. Briefly, 5 mg of freeze-dried nanoparticle was dissolved in 10 mL deionised water and sonicated for 8 min under ice bath condition. 10 µL of it was then dropped on glass slide to make a thin film. After drying at room temperature, the sample was coated with gold using sputter and visualized under SEM (Evo-18 researcher, Zeiss) at an accelerating voltage of 20 kv (Shojaee-Aliabadi et al. 2014).
Fourier transform infrared spectroscopy (FTIR) and X ray diffraction (XRD) analysis
FTIR was performed for chitosan powder, chitosan nanoparticle, MCEO and MCEO loaded chitosan nanoparticle at wave number 400–5000 cm−1. Samples were prepared in form of disc by crushing in KBr. 16 scans at a resolution of 4 cm−1 were assessed for each spectrum (Xu et al. 2019).
XRD pattern was performed for chitosan powder, chitosan nanoparticle and MCEO loaded chitosan nanoparticle over a 2θ range of 10–80° using X ray diffractometer (Bruker D8 Advance) with a step angle of 0.02° min−1 and scan speed of 5° min−1 (Zhang et al. 2014).
Antifungal and antiaflatoxigenic efficacy of MCEO loaded chitosan nanoparticle
To determine antifungal and antiaflatoxigenic activity of MCEO after encapsulation, requisite amount of MCEO loaded chitosan nanoparticle was added into conical flasks containing SMKY medium to achieve the desired concentrations ranging from 0.10–0.60 μL/mL. Flasks with only chitosan nanoparticle were served as control. Thereafter, 25 μL spore suspension of AF-LHP-SH1 was inoculated in each flask and incubated at 27 ± 2 °C in BOD for 10 days. The AFB1 was quantified following method earlier described in materials and methods section concerned with detection of the most toxigenic A. flavus isolate (Dwivedy et al. 2018).
Statistical analysis
All the experiments were performed in triplicate and data are presented as mean ± standard error. One way ANOVA using Tukey’s B multiple range tests was performed to determine the level of significance (p < 0.05) within means using SPSS (IBM SPSS Statistics, IL, USA).
Results and discussion
Mycobiota analysis and detection of the most toxigenic A. flavus isolate
Results obtained during mycobiota analysis of selected functional food samples revealed the presence of 629 fungal isolates belonging to 5 different genera and 16 species. A. flavus was recorded as the dominant species with maximum PRD (46.74%) followed by A. fumigatus (17.48%) and A. luchuensis (6.99%) (Table S1). The moisture content (MC) and pH of selected samples was found suitable for fungal growth and aflatoxin production. A total of 48 A. flavus isolates were screened for AFB1 production and 38 were recorded to be toxigenic with the AFB1 content ranging from 0.200 to 5.610 µg/mL. The isolate i.e. AF-LHP-SH1 was shown to produce highest AFB1 content (5.610 µg/mL), which was far beyond the limits set by European commission (EU) and United States-Food and Drug analysis (US-FDA) (Table S2). Therefore, this isolate was selected as the test fungus for further investigations. It is well known that different environmental factors such as moisture, oxygen, temperature, pH, and functional food composition are the key determinants responsible for infestation of food products by different fungi and subsequent contamination with different mycotoxins (Singh et al. 2008). Thus, prior to mycobiota analysis, we determined MC and pH of the samples in order to analyze their relationship with fungal and mycotoxin contamination. S. hispanica with higher MC and pH favored maximum fungal contamination and hence maximum AFB1.
Chemical characterization of MCEO
GC–MS analysis showed the presence of 40 different components constituting up to 96.89% of the total EO. Among them, Caryophyllene was found as the most abundant (19.15%) component followed by Citral (13.27%), D-Limonene (11.8%) and cis-Verbenol (11.37%). The retention time (RT) and percent area of the analyzed components are presented in Table 1. The major component of the EO found in this study was found different from the earlier investigation of Dorman and Deans (2004), where they reported Thymol as the major component of M. citriodora EO isolated from flowers and leaves. The considerable differences in antifungal activity of MCEO might be attributed to different components present in dill EO and synergistic action between them (Tian et al. 2012a). Such changes in chemical composition might be attributed to different geographical condition, genetic makeup, time of harvesting and techniques of extraction (Burt 2004). Hence, before suggesting any EO for its formulation in food system, it is desirable to check its volatile composition with proper standardization.
Table 1.
GC-MS analysis of Monarda citriodora essential oil (MCEO)
| S. No. | Compounds | RT (min) | % Area |
|---|---|---|---|
| 1. | 5,7-Dimethyl-1,6-octadiene, (R)- | 4.02 | 0.06 |
| 2. | Tricyclene | 4.18 | 0.15 |
| 3. | a-Pinene | 4.38 | 1.14 |
| 4. | 3,7-Dimethyl-1,6-octadiene, (R)- | 4.45 | 0.06 |
| 5. | Camphene | 4.7 | 0.74 |
| 6. | 1-(1,2-Dimethyl-2-cyclopenten-1-yl)ethanone | 5.16 | 0.3 |
| 7. | 1-Isopropenyl-2-methylcyclohexane | 5.68 | 0.02 |
| 8. | 1,7,7-Trimethylbicyclo[2.2.1]heptane-2,5-diol | 5.78 | 0.08 |
| 9. | β-Thujene | 5.94 | 0.13 |
| 10. | 3-Carene | 6.09 | 0.56 |
| 11. | Terpinolene | 6.24 | 2.92 |
| 12. | 1,4-Cineol | 6.33 | 0.64 |
| 13. | D-Limonene | 6.53 | 11.8 |
| 14. | Eucalyptol | 6.81 | 1.01 |
| 15. | c-Terpinene | 7.45 | 0.39 |
| 16. | Linalool | 8.91 | 1.52 |
| 17. | trans-3,3-Dimethylcyclohexylideneethanal | 9.28 | 0.1 |
| 18. | Fenchol, exo- | 9.48 | 0.23 |
| 19. | 1-Terpinenol | 10.28 | 0.32 |
| 20. | Citronellal | 10.64 | 5.62 |
| 21. | Neoisomenthol | 11.22 | 0.95 |
| 22. | 1-p-Menthene | 11.52 | 2.63 |
| 23. | 4-Thujanol | 11.79 | 0.12 |
| 24. | cis-p-mentha-1(7),8-dien-2-ol | 11.95 | 0.29 |
| 25. | a-Terpineol | 12.45 | 0.09 |
| 26. | Methyl 2,5-octadecadiynoate | 12.66 | 0.06 |
| 27. | cis-p-mentha-2,8-dien-1-ol | 12.86 | 0.05 |
| 28. | Carveol | 13.52 | 0.12 |
| 29. | cis-Geraniol | 13.8 | 7.57 |
| 30. | Isogeraniol | 14.05 | 0.06 |
| 31. | cis-Verbenol | 14.19 | 11.37 |
| 32. | Isogeraniol | 14.51 | 7.65 |
| 33. | Citral | 15.38 | 13.27 |
| 34. | trans-Carane | 16.26 | 0.08 |
| 35. | a-Copaene | 18.13 | 1.40 |
| 36. | Geranyl acetate | 19.78 | 2.93 |
| 37. | Caryophyllene | 20.43 | 19.15 |
| 38. | Humulene | 22.24 | 0.43 |
| 39. | Caryophyllene oxide | 27.37 | 0.27 |
| 40. | Isocembrol | 41.19 | 0.42 |
| Total | 96.89 | ||
RT = retention time; Compounds in bold represent the major components
Antifungal and antiaflatoxigenic activity of MCEO against AF-LHP-SH1
The results of antifungal activity of MCEO tested against AF-LHP-SH1 and 15 other functional food contaminating molds are presented in Table 2 and Fig. S1a. From the results, it is clearly evident that the MCEO at 1.40 µL/mL concentration completely inhibited the growth of all tested molds (A. luchuensis, A. niger, A. sydowii, A. repens, A. fumigatus, A. chevalieri, A. terreus, A. grisea, A. humicola, A. versicolor, F. oxysporum, P. italicum, P. spinolosum, A. alternata and mycelia sterilia) including AF-LHP-SH1, which was considered as their MIC. In addition, MCEO caused dose dependent inhibition of AFB1 and completely inhibited its production at 1.20 µL/mL (Table 2). The reduction in AFB1 content is directly correlated with the biomass of test fungus. Moreover, the MCEO showed fungistatic nature rather than fungicidal as the revival of fungal growth was observed for all the tested molds even at > 5.0 µL/mL during MFC assessment. The concentration of MCEO required for growth and AFB1 inhibition by A. flavus was found lower than some of the previously reported EOs by different workers (Prakash et al. 2016; Dwivedy et al. 2017). Based on broad spectrum toxicity, MCEO may be recommended as a promising antifungal agent for the preservation of stored functional food samples, however, their application at large scale remains restricted due to flavor consideration and susceptibility to degradation upon exposure to natural environment. Therefore, it is recommended to encapsulate the test EO into stable nanomatrix using food grade polymer to overcome the existing challenges during large scale application directly into food system.
Table 2.
Effect of different concentrations of MCEO on growth and AFB1 production by AF-LHP-SH1
| Conc. (µL/mL) | Antifungal activity of MCEO | Antiaflatoxigenic activity of MCEO | ||||
|---|---|---|---|---|---|---|
| Colony diameter (cm) | % growth inhibition | MDW (g) | % mycelia inhibition | AFB1 content (µg/mL) | % inhibition of AFB1 content | |
| Control | 6.600 ± 0.058a | 0.000 ± 0.000a | 0.349 ± 0.002a | 0.00 ± 0.00a | 4.927 ± 0.017a | 0.000 ± 0.000a |
| 0.20 | 6.167 ± 0.088b | 6.567 ± 1.011b | 0.343 ± 0.002b | 2.18 ± 0.959a | 3.964 ± 0.044b | 19.547 ± 1.13b |
| 0.40 | 4.700 ± 0.115c | 28.807 ± 1.123c | 0..253 ± 0.001c | 27.48 ± 0.429b | 3.019 ± 0.016c | 38.715 ± 0.44c |
| 0.60 | 4.100 ± 0.058d | 37.873 ± 0.763d | 0.210 ± 0.001d | 39.88 ± 0.487c | 2.824 ± 0.017d | 41.133 ± 1.41c |
| 0.80 | 3.667 ± 0.145e | 44.397 ± 2.679e | 0.132 ± 0.003e | 62.29 ± 1.086d | 1.478 ± 0.028e | 69.985 ± 0.693d |
| 1.00 | 1.867 ± 0.088f | 71.720 ± 1.273f | 0.100 ± 0.002f | 71.38 ± 0.423e | 0.715 ± 0.022f | 85.480 ± 0.423e |
| 1.20 | 1.067 ± 0.120 g | 83.810 ± 1.941 g | 0.028 ± 0.003 g | 91.89 ± 0.815f | 0.00 ± 0.000 g | 100.00 ± 0.000f |
| 1.40 | 0.000 ± 0.000 h | 100.00 ± 0.000 h | 0.00 ± 0.00 g | 100.00 ± 0.00 g | 0.00 ± 0.000 g | 100.00 ± 0.000f |
MDW = Mycelial dry weight, AFB1 = aflatoxin B1
Values are mean (n = 3) ± SE, Mean followed by same letter in the same column are not significantly different according to ANOVA (P < 0.05; Tukey’s B test)
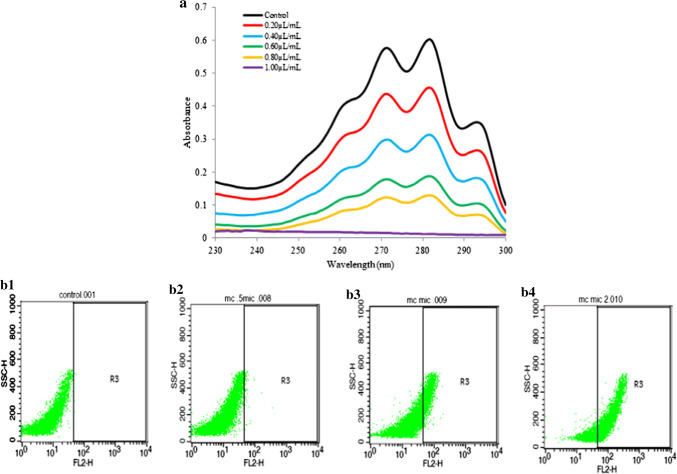
Antifungal mode of action of MCEO
Antifungal mode of action of MCEO was determined by measuring ergosterol content, cellular ions leakage, and release of 260 and 280 nm absorbing materials from fumigated AF-LHP-SH1 cell (Basak and Guha 2017; Kong et al. 2019; Chaudhari et al. 2019). Ergosterol is the major sterol found in fungal cell membrane responsible for maintaining the cellular homeostasis via regulating membrane fluidity (Alcazar-Fuoli and Mellado 2013). A dose dependent inhibition of ergosterol was observed with increasing concentrations of MCEO (Fig. 1a). Similar trend was also observed for cellular ions (Ca2+, Mg2+ and K+) and 260 and 280 nm absorbing materials leakage (Fig. S1b and c). Maximum leakage of ions and 260 and 280 nm absorbing materials was observed at 2 MIC (2.80 µL/mL) followed by MIC (1.40 µL/mL) and least at 1/2 MIC (0.70 µL/mL). Non polar MCEO may interfere with ergosterol biosynthesis leading to changes in membrane permeability and integrity. Such alteration may subsequently lead to enhanced leakage of cellular ions viz. Ca2+, Mg2+ and K+ as well as 260 and 280 nm absorbing materials, resulting into inhibition of fungal growth and eventually cell death, thereby, indicating plasma membrane as an important antifungal target site of action. The findings are similar to those reported by Khan et al. (2010) and Tian et al. (2012b), where they reported inhibition of fungal membrane ergosterol upon treatment with EOs as the possible mechanism of fungal growth inhibition.
Fig. 1.
a Effect of MCEO on ergosterol biosynthesis; b Effect of MCEO on AF-LHP-SH1 plasma membrane lesion through PI binding assay. b1 Represents control without treatment of MCEO, b2 refers to fungal cells treated at ½ MIC (0.70 µL/mL); b3 fungal cells treated at MIC (1.40 µL/mL), and b4 fungal cells treated at 2MIC (2.80 µL/mL)
Antifungal mode of action of MCEO was further demonstrated by flow cytometry based analysis to confirm the lesions produced in fungal plasma membrane. The result revealed a dose dependent increment in the number of PI+ cells with respect to control (Figs. 1b1, 2, 3, 4). PI is one of the most frequently used dye showing ability to bind specifically with the nucleic acid, especially ds DNA and gives off red fluorescence. Non polar MCEO may easily penetrate the fungal plasma membrane, thereby causing membrane disruption and allowing the PI to enter the cells and bind with the DNA resulting into fluorescence (Silva et al. 2019). Thus, the higher fluorescence of cells treated with MCEO as compared to control, denotes plasma membrane as the possible target of EO (Figs. 1b1, 2, 3, 4).
Fig. 2.
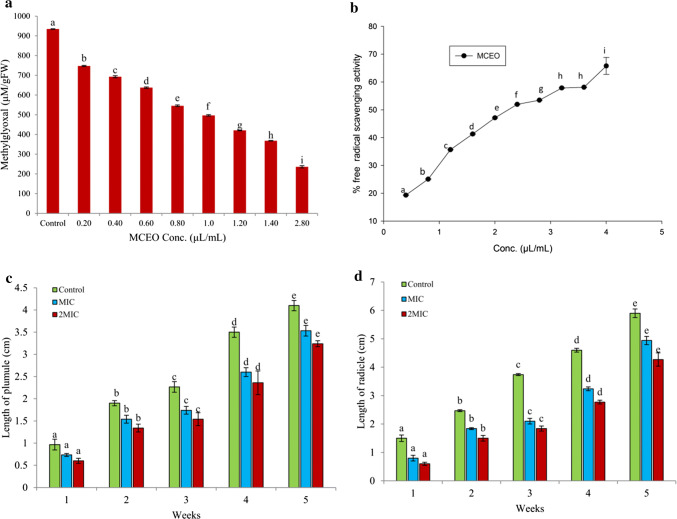
a Effect of MCEO on methylglyoxal inhibition; b Antioxidant activity of MCEO measured through DPPH assay, and c, d effect of MCEO treatment on germination ability of Salvia hispanica seeds
Fig. 3.
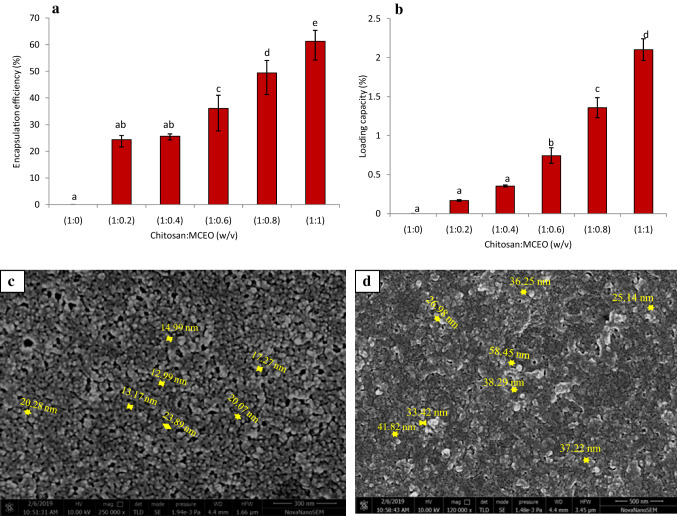
a, b Encapsulation efficiency (EE) and loading capacity (LC) of MCEO loaded chitosan nanoparticle at different chitosan to MCEO ratio (1:0–1:1, w/v); c, d SEM images of chitosan nanoparticle and MCEO loaded chitosan nanoparticle
Fig. 4.
a FTIR spectra of pure chitosan powder, chitosan nanoparticle, MCEO and MCEO loaded chitosan nanoparticle; b XRD patterns of pure chitosan powder, chitosan nanoparticle and MCEO loaded chitosan nanoparticle; c Effect of different concentrations of MCEO loaded chitosan nanoparticle on growth and AFB1 production by AF-LHP-SH1
Antiaflatoxigenic mode of action of MCEO
Methylglyoxal (MG) has been reported to play an important role in enhancing the level of AFB1 by up regulating the gene afl R in A. flavus cells (Chen et al. 2006). It is interesting to examine the concentration of MG upon treatment with different concentrations of MCEO. Result obtained in the present investigation showed a positive relation between essential oil dose and inhibition of MG (Fig. 2a). In control, MG was recorded to be 934.68 µM/gFW which was decreased to 368.25 and 236.08 µM/gFW, respectively at MIC and 2 MIC concentrations. MG, a highly reactive and cyototoxic compound synthesized enzymatically during glycolysis and non-enzymatically during glycation and lipid peroxidation process was also reported to enhance the production of ROS and aflatoxin (Yadav et al. 2005; Kalapos 2008). Thus, the differential inhibition of MG biosynthesis upon treatment with MCEO may be the possible route responsible for AFB1 inhibition and such mechanism can provide a clue for the future development of aflatoxin resistant varieties. This is the first report of inhibition of MG biosynthesis in cells treated with MCEO.
Antioxidant activity of MCEO
In this study, the antioxidant activity of MCEO was assessed through DPPH assay and the IC50 value was found to be 2.24 µL/mL (Fig. 2b). The activity was found greater than some of the previously reported EOs and some synthetic antioxidants by different workers (Prakash et al. 2016; Dwivedy et al. 2017). The total phenolic content of MCEO was also measured and found to be 2.705 ± 0.0147 µg gallic acid equivalent/1000 µL concentration of MCEO. During processing and storage, free radicals are the major threat causing rancidity and degradation of food bioactive components resulting into great economic burden (Lobo et al. 2010). The free radicals generated during storage and processing may inevitably promote the biosynthesis of aflatoxin which was previously reported by Jayashree and Subramanyam (2000). Hence, the MCEO with prominent free radical scavenging activity can effectively prolong the shelf life of stored functional foods by preventing the chances of aflatoxin contamination and can be employed as suitable plant based shelf life enhancer of functional foods.
Phytotoxicity investigation of MCEO
Phytotoxicity investigation is the essential parameter before recommending any EO for sowing and other purposes especially for human consumption, therefore in this study, phytotoxicity investigation of MCEO was performed. MCEO caused no adverse effect on length of plumules and radicles of treated S. hispanica seeds and exhibited 100% germination even after 6 months of storage in plastic containers as compared to control sets (Fig. 2c, d). Due to non phytotoxic nature on seed germination, MCEO can be recommended for sowing and consumption purposes.
Preparation and characterization of MCEO loaded chitosan nanoparticle
Encapsulation efficiency (EE) and loading capacity (LC)
Percent EE and LC of MCEO loaded chitosan nanoparticle were calculated at 237 nm. Both EE and LC increased with increasing concentration of chitosan to MCEO (w/v) mixtures and was maximum at the ratio of 1:1 with the value of 61.25 ± 4.07% and 2.10 ± 0.14%, respectively (w/v) (Fig. 3a, b). EE at this level can be considered for successful delivery of EO and the long term preservation of stored food samples.
HR-SEM analysis
HR-SEM analysis of chitosan and MCEO loaded chitosan nanoparticle revealed nearly spherical in shape with the size ranging from 11.27 to 23.89 and 25.14 to 58.45 nm, respectively (Fig. 3c, d). However, the particles were agglomerated. The greater size of MCEO loaded chitosan nanoparticle as compared to chitosan nanoparticle denotes successful encapsulation of MCEO into chitosan matrix. The size of nanoparticle may vary depending on concentration of polymers, cross linkers used, and method of preparation.
FTIR analysis
FTIR spectra of chitosan powder, chitosan nanoparticle, MCEO and MCEO loaded chitosan nanoparticle are presented in Fig. 4a. Pure chitosan showed characteristic peaks at 667 cm−1 (–CH alkenes), 1104 cm−1 (–CO stretching), 1387 cm−1 (methyl–CH), 1645 cm−1 and 2874 cm−1 (–CH stretching). Chitosan nanoparticle exhibited distinct peaks at 711 cm−1 (–CH alkenes), 1090 cm−1 (–CO stretching), 1632 cm−1 (–NH stretching), and 2917 cm−1 (–CH stretching). Two new peaks were recorded at 908 cm−1 and 1224 cm−1 (–P–O–C stretch), which can be due to successful interaction of chitosan with tripolyphosphate (TPP) (Yoksan et al. 2010). Pure MCEO showed comparatively large numbers of peaks at 620 cm−1, 888 cm−1 (−CH bend), 1026 cm−1 (> CH −), 1121 cm−1 (C–C vibration), 1377 cm−1 (O–H), 1450 cm−1 (methyl −CH), and 1676 cm−1 (C=C stretching), suggesting diversity of compounds with diverse range of functional groups. Most of the representative peaks appeared in MCEO loaded chitosan nanoparticle confirming successful entrapment of MCEO into chitosan nanoparticle.
XRD analysis
Change in crystallinity of chitosan before and after the encapsulation of MCEO was recorded through XRD pattern (Fig. 4b). Chitosan powder exhibited a sharp characteristic peak at 2θ ranging from 20°–22° representing high level of crystallinity (Haider et al. 2017). Chitosan nanoparticle displayed sharp decline in peak intensity with gradual peak broadening indicating the loss of crystallinity. Further, more broadening of peak after addition of MCEO was observed suggesting successful encapsulation of MCEO into chitosan nanoparticle. The finding was supported by the earlier work of Jingou et al. (2011) where they observed changes in crystallinity of the chitosan after addition of cross linker and core molecule.
Antifungal and antiaflatoxigenic testing of MCEO loaded chitosan nanoparticle
The antifungal and antiaflatoxigenic activity of MCEO after encapsulation into chitosan nanoparticle was performed in conical flasks using SMKY medium. The results revealed that the MCEO loaded chitosan nanoparticle showed better (twice more) antifungal (0.60 μL/mL) and antiaflatoxigenic (0.50 μL/mL) activity over unencapsulated MCEO (1.40 and 1.20 μL/mL, respectively) (Fig. 4c). The enhanced activity of MCEO loaded chitosan nanoparticle may be attributed to sub cellular size causing easy penetration into cells and stabilization of volatility of MCEO which was able to retain EO sufficiently into chitosan matrices, which in turn resulted into more significant antifungal and antiaflatoxigenic activity (Basak and Guha 2018). Further, the enhanced performance of EO after encapsulation may be due to their sustained release and stability against different environmental factors such as light, temperature, oxygen and moisture. The enhanced antifungal activity of EO after encapsulation into chitosan is also demonstrated by previous workers (Dwivedy et al. 2018). Based on findings, the nanoencapsulated MCEO may be recommended as novel antifungal agent for the preservation of stored functional foods against fungal and AFB1 contamination.
Conclusion
MCEO is being first time reported to have strong efficacy against fungal infestation and AFB1 contamination of stored functional food samples. The inhibition of methylglyoxal by MCEO shed light on mechanism involved in reduction of aflatoxin synthesis and suggested the development of aflatoxin resistant varieties of functional foods through green transgenic approach. The enhanced antifungal and antiaflatoxigenic performance of MCEO loaded chitosan nanoparticle at the concentration lower than unencapsulated MCEO recommend its large scale application as plant based preservative for the management of functional food deterioration caused by fungal and AFB1 contamination and free radical generation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Deepika is thankful to the University Grants Commission (UGC) [Grant No. F.16-6 (DEC. 2016)/2017] New Delhi, India for financial support. Authors are thankful to Head, CAS in Botany, DST-PURSE, ISLS, Banaras Hindu University, Varanasi for laboratory providing laboratory facility and Indian Institute of Technology, Banaras Hindu University for HR–SEM, FTIR and XRD analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream vol 18, pp 803–806
- Alcazar-Fuoli L, Mellado E. Ergosterol biosynthesis in Aspergillus fumigatus: its relevance as an antifungal target and role in antifungal drug resistance. Front Microbiol. 2013;3:439. doi: 10.3389/fmicb.2012.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Guha P. Use of predictive model to describe sporicidal and cell viability efficacy of betel leaf (Piper betle L.) essential oil on Aspergillus flavus and Penicillium expansum and its antifungal activity in raw apple juice. LWT-Food Sci Technol. 2017;80:510–516. doi: 10.1016/j.lwt.2017.03.024. [DOI] [Google Scholar]
- Basak S, Guha P. A review on antifungal activity and mode of action of essential oils and their delivery as nano-sized oil droplets in food system. J Food Sci Technol. 2018;55:4701–4710. doi: 10.1007/s13197-018-3394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chaudhari AK, Singh A, Singh VK, Dwivedy AK, Das S, Ramsdam MG, Dubey NK. Assessment of chitosan biopolymer encapsulated α-Terpineol against fungal, aflatoxin B1 (AFB1) and free radicals mediated deterioration of stored maize and possible mode of action. Food Chem. 2019 doi: 10.1016/j.foodchem.2019.126010. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Brown RL, Rajasekaran K, Damann KE, Cleveland TE. Identification of a maize kernel pathogenesis-related protein and evidence for its involvement in resistance to Aspergillus flavus infection and aflatoxin production. Phytopathology. 2006;96:87–95. doi: 10.1094/PHYTO-96-0087. [DOI] [PubMed] [Google Scholar]
- da Silva Gündel S, Velho MC, Diefenthaler MK, Favarin FR, Copetti PM, de Oliveira Fogaça A, Ourique AF. Basil oil-nanoemulsions: development, cytotoxicity and evaluation of antioxidant and antimicrobial potential. J Drug Deliv Sci Technol. 2018;46:378–383. doi: 10.1016/j.jddst.2018.05.038. [DOI] [Google Scholar]
- Dorman HD, Deans SG. Chemical composition, antimicrobial and in vitro antioxidant properties of Monarda citriodora var. citriodora, Myristica fragrans, Origanum vulgare ssp. Hirtum, Pelargonium sp. and Thymus zygis oils. J Essent Oil Res. 2004;16:145–150. doi: 10.1080/10412905.2004.9698679. [DOI] [Google Scholar]
- Dwivedy AK, Prakash B, Chanotiya CS, Bisht D, Dubey NK. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem Toxicol. 2017;106:175–184. doi: 10.1016/j.fct.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Dwivedy AK, Singh VK, Prakash B, Dubey NK. Nanoencapsulated Illicium verum Hook. f. essential oil as an effective novel plant-based preservative against aflatoxin B1 production and free radical generation. Food Chem Toxicol. 2018;111:102–113. doi: 10.1016/j.fct.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Ferreira FMD, Hirooka EY, Ferreira FD, Silva MV, Mossini SAG, Machinski M., Jr Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe in vitro. Food Addit Contam A. 2018;1:7. doi: 10.1080/19440049.2018.1520397. [DOI] [PubMed] [Google Scholar]
- Gago CML, Artiga-Artigas M, Antunes MDC, Faleiro ML, Miguel MG, Martín-Belloso O. Effectiveness of nanoemulsions of clove and lemongrass essential oils and their major components against Escherichia coli and Botrytis cinerea. J Food Sci Technol. 2019;56:2721–2736. doi: 10.1007/s13197-019-03762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholivand MB, Rahimi-Nasrabadi M, Batooli H, Ebrahimabadi AH. Chemical composition and antioxidant activities of the essential oil and methanol extracts of Psammogeton canescens. Food Chem Toxicol. 2010;48:24–28. doi: 10.1016/j.fct.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Gilman JC, Joseph C. A manual of soil fungi. 1. New Delhi: Daya Books; 1998. [Google Scholar]
- Haider J, Majeed H, Williams PA, Safdar W, Zhong F. Formation of chitosan nanoparticles to encapsulate krill oil (Euphausia superba) for application as a dietary supplement. Food Hydrocolloid. 2017;63:27–34. doi: 10.1016/j.foodhyd.2016.08.020. [DOI] [Google Scholar]
- Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym. 2013;95:50–56. doi: 10.1016/j.carbpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Jayashree T, Subramanyam C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Rad Biol Med. 2000;29:981–985. doi: 10.1016/S0891-5849(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Jingou J, Shilei H, Weiqi L, Danjun W, Tengfei W, Yi X. Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Colloid Surf B. 2011;83:103–107. doi: 10.1016/j.colsurfb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Kalapos MP. The tandem of free radicals and methylglyoxal. Chem-Biol Interact. 2008;171:251–271. doi: 10.1016/j.cbi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Kedia A, Prakash B, Mishra PK, Chanotiya CS, Dubey NK. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. Int Biodeter Biodegr. 2014;89:29–36. doi: 10.1016/j.ibiod.2013.10.027. [DOI] [Google Scholar]
- Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, Manzoor N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol. 2010;161:816–823. doi: 10.1016/j.resmic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Kohiyama CY, Ribeiro MMY, Mossini SAG, Bando E, da Silva Bomfim N, Nerilo SB, Machinski M., Jr Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015;173:1006–1010. doi: 10.1016/j.foodchem.2014.10.135. [DOI] [PubMed] [Google Scholar]
- Kong J, Zhang Y, Ju J, Xie Y, Guo Y, Cheng Y, Yao W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019;285:380–388. doi: 10.1016/j.foodchem.2019.01.099. [DOI] [PubMed] [Google Scholar]
- Kumar M, Sarma P, Dkhar MS, Kayang H, Raghuwanshi R, Dubey NK. Assessment of chemically characterised Gaultheria fragrantissima Wall. essential oil and its major component as safe plant based preservative for millets against fungal, aflatoxin contamination and lipid peroxidation during storage. J Food Sci Technol. 2018;55:111–119. doi: 10.1007/s13197-017-2842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacog Rev. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZG, Li XH, Li W. Chemical composition of antibacterial activity of essential oil from Monarda citriodora flowers. Adv Mat Res. 2011;183:920–923. [Google Scholar]
- Maryam I, Huzaifa U, Hindatu H, Zubaida S. Nanoencapsulation of essential oils with enhanced antimicrobial activity: A new way of combating antimicrobial Resistance. J Pharma Phytochem. 2015;4:165. [Google Scholar]
- Prakash B, Kedia A, Singh A, Yadav S, Singh A, Yadav A, Dubey NK. Antifungal, antiaflatoxin and antioxidant activity of plant essential oils and their in vivo efficacy in protection of chickpea seeds. J Food Quality. 2016;39:36–44. doi: 10.1111/jfq.12168. [DOI] [Google Scholar]
- Rawat S. Food Spoilage: microorganisms and their prevention. Asian J Plant Sci Res. 2015;5:47–56. [Google Scholar]
- Shojaee-Aliabadi S, Hosseini H, Mohammadifar MA, Mohammadi A, Ghasemlou M, Hosseini SM, Khaksar R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr Polym. 2014;101:582–591. doi: 10.1016/j.carbpol.2013.09.070. [DOI] [PubMed] [Google Scholar]
- Silva KP, de Carvalho Santos TA, Moutinho BL, da Silva RS, dos Santos Pinto V, Blank AF, Fernandes RPM. Using Varronia curassavica (Cordiaceae) essential oil for the biocontrol of Phytomonas serpens. Ind Crop Prod. 2019;139:111523. doi: 10.1016/j.indcrop.2019.111523. [DOI] [Google Scholar]
- Singh P, Srivastava B, Kumar A, Dubey NK. Fungal contamination of raw materials of some herbal drugs and recommendation of Cinnamomum camphora oil as herbal fungitoxicant. Microb Ecol. 2008;56:555–560. doi: 10.1007/s00248-008-9375-x. [DOI] [PubMed] [Google Scholar]
- Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE. 2012;7:e30147. doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012;130:520–527. doi: 10.1016/j.foodchem.2011.07.061. [DOI] [Google Scholar]
- Upadhyay N, Singh VK, Dwivedy AK, Das S, Chaudhari AK, Dubey NK. Cistus ladanifer L. essential oil as a plant based preservative against molds infesting oil seeds, aflatoxin B1 secretion, oxidative deterioration and methylglyoxal biosynthesis. LWT-Food Sci Technol. 2018;92:395–403. doi: 10.1016/j.lwt.2018.02.040. [DOI] [Google Scholar]
- USEPA (United States Environment Protection Agency) (1993) R.E.D. FACTS. Flower and Vegetable Oils. at http://www.epa.gov/oppsrrd1/REDs/factsheets/4097fact.pdf. Accessed 2 Feb 2008)
- USFDA (2013) GRAS notice inventory. GRN, No. 397, www.fda.gov
- Xu T, Gao C, Feng X, Huang M, Yang Y, Shen X, Tang X. Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum arabic polyelectrolyte complexed films. Carbohydr Polym. 2019;217:116–125. doi: 10.1016/j.carbpol.2019.03.084. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Bioph Res Co. 2005;337:61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- Yoksan R, Jirawutthiwongchai J, Arpo K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloid Surf B. 2010;76:292–297. doi: 10.1016/j.colsurfb.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang S, Fang J, Deng Y, Wang D, Zhao Y. Optimization of the fermentation conditions of Rhizopus japonicus M193 for the production of chitin deacetylase and chitosan. Carbohydr Polym. 2014;101:57–67. doi: 10.1016/j.carbpol.2013.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.