Abstract
Mead is an ancient alcoholic beverage produced through the fermentation of a diluted solution of honey. Due to the peculiar and varied composition of honey, mead production faces several problems, such as slow or stuck fermentations mainly due to the low nitrogen concentration, lack of uniformity of the final product and the production of unpleasant aromas. In this context, this work aimed to select low nitrogen-demand yeast strains and evaluate their potential for the production of mead. Therefore, among 21 commercial wine yeast strains, 5 were selected based on their fermentative behavior at low assimilable nitrogen concentrations. The selected strains were further evaluated for their contributions in meads produced with limited nitrogen availability, and the results showed significant differences on some physicochemical parameters like biomass production, residual sugars, glycerol concentration, and fermentative rate. Moreover, meads obtained with selected strains differed in the concentration of several volatile compounds. The volatile compounds concentration and the principal component analysis based on odor activity values allowed separating strains into three groups. In general, S. cerevisiae var bayanus strains (QA23, Spark, and AWRI-R2) were the largest producers of aromatic compounds, particularly those with floral and fruity descriptors. The selection of yeast strains with low nitrogen-demand and different volatile compounds production can be explored by mead makers to limit fermentation problems and obtain characteristic products.
Keywords: Mead, Honey, Yeast, Nitrogen demand, Volatile compounds
Introduction
Mead or honey-wine is produced by the fermentation of a diluted solution of honey, with a final ethanol concentration ranging from 8 to 18 (% v v−1) dependent on the proportion of honey/water (Ramalhosa et al. 2011). Mead has been produced since ancient times in several regions of the world, with particular relevance in Nordic countries and Eastern Europe. Although still small, mead has experienced an important growth in the alcoholic beverages market, as it represents an alternative for the use of surplus or residual honey, provides an alternative alcoholic beverage to consumers and is an economical option for beekeepers (Iglesias et al. 2014). Despite its long history and economic potential, mead is still produced in an empirical and artisanal manner with relative scarce scientific reports compared with other alcoholic beverages (Pereira et al. 2017).
The production of mead faces several problems such as the lack of product uniformity, slow fermentation, which may take months to complete, and stuck fermentations. These problems have been associated with several factors, including: the great variability of honey composition (Vidrih and Hribar 2016), the lack or limitation of essential nutrients for yeast development (Pereira et al. 2015), the low honey buffer capacity and pH, the use of inappropriate yeast strains (Pereira et al. 2009), or populations (Felipe et al. 2019), among others. Few studies related to mead making with immobilized cell have been reported. Although it may be a possibility to accelerate the fermentation of mead (Galanakis et al. 2012), the success of the method depends on the stability of the immobilization matrix (Iglesias et al. 2014).
Yeasts play an essential role in the production of alcoholic beverages and have a significant effect on their sensory characteristics. Yeast metabolism is responsible for the production of hundreds of compounds, which contributes to the characteristic aroma and flavor of fermented beverages, and the production and concentration of these compounds, desirable or not, formed during fermentation will depend on the yeast species or strain involved in the alcoholic fermentation (Lambrechts and Pretorius 2000). In this sense, it is important to know the potential differences in the biosynthesis of aroma compounds among yeast strains to select those more appropriate for the desired product (Pretorius 2000). Usually, yeasts used in mead production are strains selected for wine, beer and sparkling wine fermentations. These yeasts are selected for a set of particular characteristics of each product, such as vigorous fermentation, high tolerance to ethanol, tolerance to temperature variations, competition capacity, sedimentation capacity, aroma production, among others (Pretorius 2000). Commercial Saccharomyces cerevisiae cultures are currently used in fermentative processes to reduce risks and obtain more homogeneous products. Moreover, different S. cerevisiae strains exhibit particular metabolic properties which significantly influence on the quality and typicity of fermented beverages. However, the use of commercial yeasts that do not adapt to honey-must conditions may result in problems, like slow and incomplete fermentation and negative sensorial characteristics of the final product (Pereira et al. 2009).
One of the conditions that differentiate wine-musts from honey-musts is the amount of yeast assimilable nitrogen (Ramalhosa et al. 2011). The concentration of assimilable nitrogen in honey is commonly low, ranging from 0 to 0.13 (% w w−1), most of which is in the form of amino acids (Ball 2007). In honey-musts, depending on the water dilution, the assimilable nitrogen concentration can be four times lower than considered ideal for fermentation, approximately 150 mg L−1 yeast assimilable nitrogen (YAN) (Ribéreau-Gayon et al. 2006). The supplementation of nitrogen deficiencies with DAP addition is a widespread practice in mead production (Pereira et al. 2017), however, according to Ribéreau-Gayon et al. (2006), an ideal amount of YAN provides higher quality wines. Thus, the use of yeast strains with low nitrogen demand is essential to conduct proper honey-must fermentations with minimal addition of external nitrogen supplies to obtain high-quality meads (Ramalhosa et al. 2011).
In this context, this work aimed to select oenological S. cerevisiae strains with low nitrogen demand, to evaluate their behavior on honey-must fermentation, as well as their contribution to the concentration of volatile compounds of mead.
Material and methods
Must and fermentative conditions
The experiments were carried out with a multifloral commercial light honey from Rio Grande do Sul State, Brazil. The honey was characterized according to the Brazilian legislation (Normative Instruction No. 11/2000, October 20), using the official analytical methods for honey and showed: acidity (23.94 meq kg−1), ash (0.15% w w−1), solids (0.036% w w−1), moisture (17.9% v v−1), total sugar (79.8% w w−1), total soluble solids (82º Brix), and assimilable nitrogen (48.2 mg kg−1).
Honey-must was prepared by mixing the honey with distilled water to a final concentration of 20° Brix (soluble solids), heated at 70 °C for 10 min for pasteurization purposes, and the pH was adjusted to 3.6 with tartaric acid. Furthermore, honey-must was supplemented with ammoniacal nitrogen, by the addition of different concentrations of diammonium phosphate (DAP) in order to obtain 60, 75 and 90 mg L−1 YAN, and with a concentrated solution of minerals and vitamins in order to obtain a final concentration of 250 mg L−1 MgSO4·7H2O, 210 mg L−1 FeSO4, 150 mg L−1 NaCl, 150 mg L−1 CaCl2, 750 mg L−1 K2HPO4, 1 mg L−1 CuCl2, 15 mg L−1 ZnSO4, 2 mg L−1 KI, 1 mg L−1 H3BO3, 0.4 mg L−1 (NH4)6Mo7O24, 0.8 μg L−1 inositol, 2 mg L−1 nicotinic acid, 1.5 mg L−1 calcium pantothenate, 0.25 mg L−1 thiamine, 0.25 mg L−1 pyridoxine, 0.05 mg L−1 biotin.
Yeast strains were grown in Petri dishes containing complete medium-YEPD (1% yeast extract, 1% peptone, 2% dextrose, and 1.8% agar) for 48 h at 28 °C. Isolated colonies were transferred to liquid YEPD and grown for 24 h at 28 °C under constant shaking (150 rpm). The cells were centrifuged, washed twice with sterile saline solution (0.9% NaCl), and yeast suspension was inoculated into the honey-must in order to obtain a final population of 106 cells mL−1.
Yeast selection for low nitrogen demand
Twenty-one commercial oenological strains of S. cerevisiae were evaluated for their biochemical nitrogen demand: Cross Evolution, QA23, CY3079 and QD145 (Lallemand), PDM, BP725, AWRI-R2, AWRI 350, AWRI 796, Elegance, UCD 522 (Maurivin), Actiflore F33, Zymaflore F15, Zymaflore VL1, Zymaflore VL2, Zymaflore VL3, Zymaflore X5, Zymaflore Spark (Laffort), Red Fruit (Enartis), Rouge (Fermol), and Y-904 (Mauriferm).
The fermentations were conducted on a honey-must containing 15 mg L−1 YAN (yeast assimilable nitrogen) and adjusted to 60, 75, and 90 mg L−1 YAN by the addition of DAP. The nitrogen concentrations used in the experiments were based on the maximum DAP supplementation limit (300 mg L−1) established by the International Oenological Codex (OIV 2016), and the Brazilian legislation (Brasil 2016). Fermentations were monitored by CO2 evolution, and at the end of the experiment yeast strains were classified as low, medium, and high demand based on their fermentative behavior in the three musts. The fermentations were conducted in triplicates.
Mead fermentation with selected yeast strains
The yeast strains with low nitrogen demand, selected from the previous assay, were used to ferment 800 mL of honey-must with a final concentration of 75 mg L−1 YAN. Honey-must preparation and yeast inoculation were performed as described in the previous item. The fermentations were monitored by CO2 release and the fermentation rate estimated by linear regression of the exponential phase of the CO2 weight loss. The process was conducted in triplicates and, at the end of the experiment, the physical–chemical parameters were evaluated.
Physicochemical analysis
Total residual sugars (g L−1) and reducing sugars (g L−1) were evaluated by the colorimetric method using 3,5-dinitrosalicylic acid (DNS) according to Santos et al. (2017). The alcohol concentration (% v v−1) was determined by densitometry after steam distillation of mead samples, total acidity and volatile acidity (mEq L−1) were determined by titration with 0.1 N NaOH solution using phenolphthalein as indicator, and by steam distillation followed by titration, respectively following the method preconized by the International Organization of Vine and Wine. Color intensity was evaluated by absorbance at 420 nm using a spectrophotometer (Model 2800, Hitachi, Japan). Glycerol (g L−1) concentration was quantified by an enzymatic assay using the Megazyme® Glycerol kit following the manufacturer’s instructions. The residual yeast assimilable nitrogen, YAN (mg L−1) was determined by the formol titration technique. Yeast biomass (g dry weight L−1) was determined by the gravimetric method after centrifugation and drying at 50 °C. Fermentation rate was estimated by the linear regression of the exponential phase of the CO2 weight loss.
Volatile composition of meads
The volatile compounds were determined by headspace solid-phase micro-extraction (SPME) with DVB/CAR/PDMS (divinylbenzene/carboxenon polydimethylsiloxane) 50/30 μm fiber, according to the methodology proposed by Xiao et al. (2015) with some modifications. Briefly, 8 mL of sample, 2 g of NaCl, and 80 μL of 3-octanol (13.5 mg L−1) were added in a 20 mL vial with silicone septum. In a thermostatic water bath, the DVB/CAR/PDMS 50/30 μm fiber was exposed to the space above the liquid (headspace) and the sample was magnetically stirred at 50 °C for 45 min. A gas chromatograph—GC (model 6890, Agilent Technologies, USA), coupled to a mass selective detector (MS) 5973 (Agilent Technologies, USA), with HP-INNOWAX column (30 m × 0.25 mm × 0.25 μm) was used for determination of chemical composition. After the extraction, the fiber was put inside the GC/MS injector and the compounds were carried out at 230 °C.
The injection was performed in split mode at 1:20 maintaining the fiber in the injection port for 5 min. The temperature gradient of the oven was 40 °C for 2 min, increasing at a rate of 3 °C min−1 to 230 °C for 2 min. The MS parameters included electron impact ionization of 70 eV and a mass range of m/z 30–550, using the Selective Ion Monitoring mode (SIM). The software ChemStation (Agilent Technologies) was used for spectra processing. The compounds identification was performed by comparison of the Retention Indices (determined relatively to the retention times of n-alkanes homologous series) and fragmented mass standards with the authentic compounds or with mass spectra in the Wiley database (Hewlett-Packard, Palo Alto, CA) and NIST Database.
Quantification of the compounds was performed by comparing the area of the compound to the area of the internal standard (3-octanol). To estimate the contribution of each volatile compound to mead aroma, concentration data were transformed into odor activity values (OAV) by dividing the observed concentration by the odor thresholds values obtained from information available in the literature.
Statistical analyses
The results were analyzed statistically by analysis of variance (one-way ANOVA) and Tukey’s test and the results of the volatile compound above their olfactive thresholds also were submitted to Principal Components Analysis (PCA). The statistical analyses were performed using SPSS software and statistical significance was attributed to values of P ≤ 0.05.
Results
Selection of yeast strains with low nitrogen demand
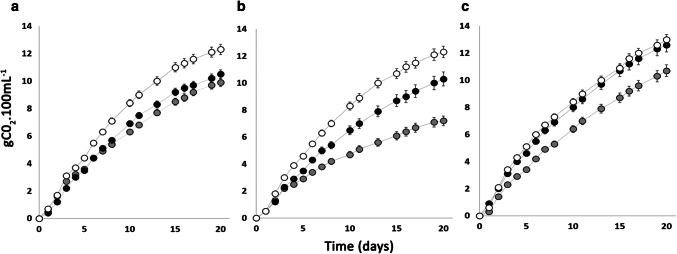
Considering that honey is poor in nitrogen and meads production without appropriate nutrient supplementation leads to slow or incomplete fermentation and products with organoleptic defects, the present study selected yeasts based on their nitrogen demand. The evaluation of the fermentative profile of the 21 oenological yeasts in honey-musts with different concentrations of nitrogen (60, 75 and 90 mg L−1 YAN), allowed to separate the strains in three groups. The typical behaviors of high, medium and low nitrogen-requiring strains are exemplified in Fig. 1.
Fig. 1.
Fermentative behavior in different nitrogen concentrations. a High nitrogen-requiring strain VL-1, b medium nitrogen-requiring strain X5, and c low nitrogen-requiring strain SPARK. In the graphs: (filled circle) 60 mg L−1 of assimilable nitrogen, (filled gray circle) 75 mg L−1 of assimilable nitrogen and (open circle) 90 mg L−1 of assimilable nitrogen. Values are the average of 3 fermentation repeats ± standard deviation
Based on the criteria proposed by Gardner et al. (2002) and Lemos Junior et al. (2017), yeast strains with low fermentation rates in the intermediate (75 mg L−1) and low (60 mg L−1) YAN concentrations, and a clear highest fermentation rate on the highest (95 mg L−1) YAN concentration were classified as “high nitrogen-requiring” strains (Fig. 1a). Yeasts classified within this group were: VL1, Red Fruit and QD-145. The largest group included yeasts classified as “medium nitrogen-requiring” strains that showed a nitrogen-dependent fermentation rate (Fig. 1b). This group was formed by twelve strains: Cross Evolution, PDM, BP725, F33, AWRI 350, AWRI 796, F15, VL2, UCD 522, X5, Elegance, and CY3079. The third group (Fig. 1c) showed a similar fermentation profile in high (90 mg L−1) and medium (75 mg L−1) YAN concentration and were classified as “low nitrogen-requiring” strains: AWRI-R2, QA23, Spark, Y-904, Rouge and VL3.
Five yeasts with low nitrogen demand were selected for the subsequent tests. Y904 was excluded because it did not complete the fermentation after 27 days at the intermediate nitrogen concentration. The selected strains included two S. cerevisiae var cerevisiae (Rouge and VL3), and three S. cerevisiae var bayanus (QA23, AWRI-R2, and Spark).
Mead fermentation with low nitrogen demand yeast strains
Selected strains were inoculated on honey-must with 75 mg L−1 YAN (intermediate nitrogen concentration) and the fermentations were monitored by CO2 release. As can be observed in Fig. 2, all the strains completed fermentations within 20 days, showing a short lag phase (1 day) and a constant exponential CO2 release up to the fifteenth day.
Fig. 2.
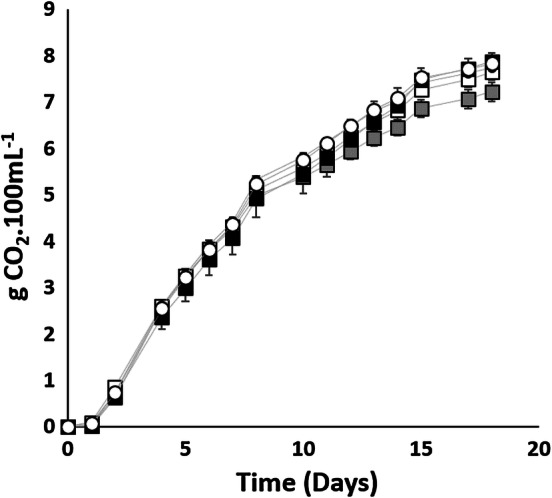
Fermentation kinetics of Saccharomyces cerevisiae var. bayanus QA23® (filled square), AWRI-R2® (open square), SPARK® (filled gray square) and Saccharomyces cerevisiae var. cerevisiae ROUGE® (open circle) and VL3® (filled circle). Values are the average of 3 fermentation repeats ± standard deviation
Spark showed the lowest total CO2 release and fermentation rate (0.46 gCO2 100 mL−1 day−1), followed by AWRI-R2 with 0.48 gCO2 100 mL−1 day−1. Rouge, QA23, and VL3 showed no significant differences in fermentation rates (0.51 gCO2 100 mL−1 day−1). Rouge produced the highest amount of biomass during fermentation (1.42 g L−1), followed by VL3 (1.33 g L−1) and AWRI-R2 (1.3 g L−1), which showed non-significative differences between them, while Spark (1.2 g L−1) and QA23 (1.07 g L−1) yielded the lowest biomass.
Non-significant differences were detected on the residual reducing sugars and total residual sugars concentration, except for meads produced with Spark that finished fermentation with 13.35 g L−1 and 21.66 g L−1, respectively (Table 1).
Table 1.
Physicochemical parameters of meads produced with selected yeast strains
| QA23 | AWRI-R2 | Rouge | Spark | VL 3 | |
|---|---|---|---|---|---|
| Total residual sugars (g L−1) | 16.8 ± 1.69b | 14.79 ± 1.49b | 14.35 ± 1.38b | 21.66 ± 3.12a | 16.84 ± 0.44ab |
| Reducing sugars (g L−1) | 10.34 ± 0.81b | 9.88 ± 0.95b | 9.75 ± 0.83b | 13.35 ± 1.57a | 10.47 ± 0.59b |
| Glycerol (g L−1) | 7.44 ± 0.95ab | 8.47 ± 0.54a | 7.72 ± 0.53ab | 6.47 ± 0.69b | 8.10 ± 0.66ab |
| Biomass (g L−1) | 1.07 ± 0.02d | 1.30 ± 0.02b | 1.42 ± 0.02a | 1.20 ± 0.03c | 1.33 ± 0.01b |
| Volatile acidity (mEq L−1) | 16.33 ± 4.73a | 17.00 ± 1.00a | 16.67 ± 0.58a | 11.00 ± 1.00a | 13.00 ± 3.00a |
| Total acidity (mEq L−1) | 51.33 ± 2.31a | 55.33 ± 3.06a | 53.33 ± 3.06a | 54.00 ± 5.29a | 52.67 ± 3.06a |
| YAN (mg L−1) | 8.60 ± 1.35a | 7.82 ± 1.35a | 7.04 ± 2.35a | 7.82 ± 1.35a | 7.04 ± 2.35a |
| Ethanol (% v v−1) | 10.97 ± 0.06a | 10.8 ± 0.00a | 11.23 ± 0.15a | 10.33 ± 0.35a | 10.43 ± 0.81a |
| Color intensity | 0.34 ± 0.05b | 0.19 ± 0.05c | 0.17 ± 0.01c | 0.52 ± 0.06a | 0.31 ± 0.03b |
| Fermentation rate* | 0.51 ± 0.02a | 0.48 ± 0.01ab | 0.51 ± 0.01a | 0.46 ± 0.01b | 0.51 ± 0.00a |
*Values identified by the same letter within a line are not significantly different at the 0.05 level (Tukey test)
Despite the differences in the residual sugars content of meads produced with the selected yeast strains, no significant differences were observed in the final ethanol concentration, which ranged from 10.33 (% v v−1) in meads produced with Spark to 11.23 (% v v−1) for those fermented with Rouge.
Glycerol concentration (g L−1) varied significantly between meads produced with different yeasts. Spark yielded meads with the lowest glycerol (6.47 g L−1), while AWRI-R2 led to the highest glycerol production (8.47 g L−1).
The volatile acidity in the fermentations varied between 11 and 17 mEq L−1 of acetic acid or 0.66–1.02 g L−1 of acetic acid (Table 1). As for total acidity, meads ranged from 51.33 to 54.00 mEq L−1, in fermentations performed with QA23 and Spark, respectively (Table 1). These values correspond to an increase of 39–42 mEq L−1 compared to honey-must (12 mEq L−1). The color intensity (absorbance at 420 nm) of meads produced with different yeast strains varied between 0.17 and 0.52 (Table 1). Considering that the color intensity of honey-must was 0.22, Rouge and AWRI-R2 caused a slight decrease in color (0.19), while the meads produced by Spark, QA23 (0.34) and VL3 (0.31) showed higher color intensity.
To determine the impact of the selected yeast strains on the aromatic characteristics of mead, volatile compounds were analyzed by GC/MS. The analysis of meads allowed identifying 52 compounds including higher alcohols, esters, fatty acids, terpenes, among other volatile molecules (Table 2).
Table 2.
The concentration of the aromatic compounds (mg L−1) identified in the meads produced with selected yeast strains and their respective olfactive threshold
| QA23 | AWRI-R2 | Rouge | Spark | VL3 | Olfative threshold (mg L−1) | Odour descriptor | |
|---|---|---|---|---|---|---|---|
| Higher alcohols | |||||||
| 1-Propanol | 1.97 ± 0.18a | 1.66 ± 0.15a | 0.28 ± 0.03c | 1.01 ± 0.09b | 0.56 ± 0.06c | 500 | Alcohol, ripe fruit |
| 2-Methyl-1-propanol | 2.72 ± 0.25a | n.d | 1.14 ± 0.10b | 1.81 ± 0.15ab | 2.04 ± 0.19ab | 75 | Alcohol |
| 3-Methyl-1-butanol | 53.72 ± 5.10c | 73.58 ± 2.73b | 26.61 ± 2.53d | 90.43 ± 6.49a | 67.66 ± 5.24b | 300 | Solvent |
| 3-Methyl-1-pentanol | 0.06 ± 0.00a | 0.08 ± 0.00a | 0.07 ± 0.00a | 0.10 ± 0.00a | 0.06 ± 0.00a | 1 | Herbaceous |
| 2,3-Butanodiol | 23.01 ± 2.13a | 16.23 ± 1.52b | 15.03 ± 0.98b | 7.50 ± 0.37c | 11.28 ± 0.89bc | 150 | Fruity |
| 2-Phenylethanol | 96.85 ± 7.18a | 84.56 ± 5.16a | 42.51 ± 0.18b | 88.00 ± 8.18a | 54.24 ± 4.32b | 7.5 | Rose, honey |
| Benzyl alcohol | 1.69 ± 0.12a | 0.78 ± 0.05b | 0.52 ± 0.02b | 0.45 ± 0.03b | 0.67 ± 0.01b | 200 | Sweet, fruity |
| 2-Ethyl hexanol | n.d | 0.13 | n.d | 0.22 | n.d | 8 | Floral |
| Total | 180.02 | 177.01 | 86.17 | 189.52 | 136.51 | ||
| Fatty alcohols | |||||||
| 1-Hexanol | n.d | n.d | n.d | 0.06 ± 0.00a | 0.03 ± 0.00a | 8 | Herbaceou, grass |
| 1-Heptanol | 0.14 ± 0.01a | 0.08 ± 0.00a | 0.07 ± 0.00a | n.d | n.d | 0.3 | Lemon, orange, copper |
| 1-Octanol | n.d | n.d | n.d | 0.29 | n.d | 0.9 | – |
| 1-Nonanol | 0.33 ± 0.02a | 0.43 ± 0.01a | 0.25 ± 0.01a | n.d | n.d | 0.6 | Fruity |
| 1-Dodecanol | 0.67 ± 0.06a | 0.36 ± 0.03ab | 0.10 ± 0.00b | 0.36 ± 0.01ab | 0.24 ± 0.00ab | 1 | Flowery |
| Total | 1.14 | 0.88 | 0.42 | 0.71 | 0.27 | ||
| Volatile fatty acids | |||||||
| Hexanoic acid | 6.53 ± 0.3a | 4.93 ± 0.23ab | 3.28 ± 0.31b | 5.82 ± 0.46a | 4.77 ± 0.12ab | 0.42 | Cheese, fatty |
| Heptanoic acid | 0.13 ± 0.00b | 0.28 ± 0.00a | 0.07 ± 0.00b | 0.29 ± 0.00a | 0.28 ± 0.01a | 3 | Sweaty, cheese |
| Octanoic acid | 46.31 ± 3.54a | 38.73 ± 2.19a | 18.71 ± 1.2b | 41.79 ± 2.32a | 33.99 ± 1.89a | 0.50 | Fatty, rancid |
| Nonanoic acid | 0.25 ± 0.01b | 0.66 ± 0.02a | 0.21 ± 0.00b | 0.67 ± 0.03a | 0.52 ± 0.01a | 3 | Fatty |
| Decanoic acid | 0.22 ± 0.00c | 13.16 ± 1.26a | 4.14 ± 0.33b | 11.64 ± 1.03a | 9.93 ± 0.89ab | 10 | Fatty, rancid |
| Decenoic acid | n.d | 1.87 ± 0.01a | 1.30 ± 0.05a | 1.19 ± 0.13a | 2.44 ± 0.15a | 0.04 | Waxy, fatty |
| Dodecanoic acid | n.d | 0.36 ± 0.02a | 0.10 ± 0.00a | 0.35 ± 0.00a | n.d | 1 | Dry, metallic |
| Tetradecanoic acid | 0.25 ± 0.00c | 0.44 ± 0.03b | 0.08 ± 0.00c | 0.70 ± 0.01a | 0.64 ± 0.05a | 10 | – |
| Total | 53.68 | 60.42 | 27.89 | 62.44 | 52.55 | ||
| Acetate esters | |||||||
| Isoamyl acetate | 3.49 ± 0.28a | 5.98 ± 0.51a | 1.65 ± 0.13b | 0.64 ± 0.03c | 0.93 ± 0.09bc | 0.26 | Banana |
| Hexyl acetate | n.d | n.d | n.d | n.d | 0.08 | 0.67 | Apple, pear, floral |
| 2-Phenylethyl acetate | 6.12 ± 0.27a | 7.24 ± 0.51a | 3.32 ± 0.15b | 6.38 ± 0.59a | 5.22 ± 0.42ab | 0.25 | Fruity |
| Total | 9.61 | 13.22 | 4.97 | 7.02 | 6.23 | ||
| Ethyl esters | |||||||
| Ethyl butyrate | n.d | 0.09 ± 0.00b | n.d | 0.46 ± 0.03a | 0.60 ± 0.01a | 0.40 | – |
| Ethyl hexanoate | 0.54 ± 0.00b | 0.67 ± 0.01b | 0.31 ± 0.00b | 1.79 ± 0.02a | n.d | 0.08 | Fruity, green, brandy |
| Ethyl heptanoate | n.d | 0.05 | n.d | 0.08 ± 0.00 | n.d | 0.0022 | Fruity |
| Ethyl octanoate | 3.51 ± 0.21b | 13.21 ± 0.9a | n.d | 14.60 ± 1.23a | 9.40 ± 0.85a | 0.58 | Sweet, fruity |
| Ethyl nonanoate | n.d | 0.42 ± 0.00a | 0.25 ± 0.01b | 0.57 ± 0.02a | 0.20 ± 0.00b | 0.34 | Floral, fruity |
| Ethyl decanoate | 3.14 ± 0.17c | 8.49 ± 0.56b | 2.42 ± 0.17c | 15.97 ± 0.13a | 3.93 ± 0.28c | 0.4 | Fruity, grape |
| Ethyl dodecanoate | 0.15 ± 0.02 | n.d | n.d | 0.41 ± 0.02 | n.d | 1.5 | Floral, fruity cream |
| Ethyl phenylacetate | 0.69 ± 0.05a | 0.41 ± 0.01a | 0.39 ± 0.02a | 0.33 ± 0.02a | 0.33 ± 0.03a | 0.65 | Floral |
| Ethyl decenoate | 1.54 ± 0.09c | 7.28 ± 0.65a | 2.53 ± 0.15bc | 4.91 ± 0.39ab | 3.28 ± 0.27b | 0.1 | Rose |
| Total | 9.23 | 30.42 | 5.71 | 38.97 | 17.57 | ||
| Terpenes | |||||||
| Linalool | 1.67 ± 0.16a | 1.86 ± 0.12a | 1.85 ± 0.15a | 1.61 ± 0.14a | 0.65 ± 0.02b | 0.05 | Citrus, floral |
| Nerol | 0.23 ± 0.02a | 0.25 ± 0.00a | n.d | 0.27 ± 0.06a | n.d | 0.4 | Rose, lime |
| Hotrienol | 2.89 ± 0.18a | 3.43 ± 0.27a | 1.68 ± 0.09a | 3.15 ± 0.28a | 3.05 ± 0.21a | n.d | – |
| Citronellol | 0.50 ± 0.02a | 0.54 ± 0.05a | 0.16 ± 0.02b | 0.31 ± 0.02a | 0.40 ± 0.03a | 0.4 | Citrus |
| Nerolidol | 1.68 ± 0.14b | 3.68 ± 0.28a | 1.45 ± 0.1b | 2.75 ± 0.19ab | 1.72 ± 0.16b | 0.7 | Rose, green, citrus |
| α-Terpineol | 0.43 ± 0.03a | n.d | 0.23 ± 0.01a | 0.23 ± 0.01a | 0.22 ± 0.00a | 0.4 | Floral, sweet |
| Limonene | n.d | n.d | n.d | n.d | 0.25 ± 0.01 | 0.02 | – |
| Cosmene | 0.30 ± 0.02a | 0.33 ± 0.03a | 0.12 ± 0.01a | 0.15 ± 0.01a | n.d | n.d | – |
| Total | 7.69 | 10.09 | 5.49 | 8.46 | 6.30 | ||
| Aldehydes | |||||||
| Acetaldehyde | 0.84 ± 0.03a | 1.22 ± 0.08a | 0.42 ± 0.00b | 1.70 ± 0.17a | 0.80 ± 0.06ab | 0.5 | Pungent |
| Benzaldehyde | 0.70 ± 0.01a | 0.35 ± 0.00b | 0.25 ± 0.04b | 0.27 ± 0.02b | 0.30 ± 0.01b | 2 | Almond |
| Total | 1.54 | 1.37 | 0.67 | 1.97 | 1.10 | ||
| Other compounds | |||||||
| Diethyl succinate | n.d | 0.65 ± 0.05a | 0.54 ± 0.03a | 0.81 ± 0.08a | 1.01 ± 0.89a | 200 | Fruity |
| Ethyl acetate | 14.71 ± 1.31a | 17.25 ± 1.57a | 8.28 ± 0.59b | 13.00 ± 0.96a | 14.39 ± 1,24a | 160 | Fruit, solvent |
| 3-Ethoxy-1-propanol | 2.48 ± 0.19a | n.d | 0.03 ± 0.00b | 0.79 ± 0.07b | n.d | 0.1 | Fruity |
| Monoethyl ester | n.d | n.d | 0.41 ± 0.04a | 0.28 ± 0.01a | 0.67 ± 0.02a | n.d | – |
| Propilene glycol | 1.20 ± 0.24a | 0.63 ± 0.06a | 0.56 ± 0.06a | n.d | 0.43 ± 0.04a | n.d | – |
| 3-(Methylthio)-1-propanol | 2.25 ± 0.17a | n.d | 1.06 ± 0.09b | 0.46 ± 0.02c | 0.60 ± 0.35c | 1.2 | Cooked vegetable |
| Isoamyl octanoate | n.d | n.d | n.d | 0.14 ± 0.00 | n.d | 0.15 | Sweet, fruity, cream |
| Volátil phenols | |||||||
| Eugenol | n.d | 0.20 ± 0.00 | 0.06 ± 0.00 | n.d | n.d | 0.005 | Spices, clove, honey |
| 2-Methoxy-4-vinylphenol | 0.63 ± 0.05 | n.d | n.d | n.d | n.d | 0.04 | – |
Meads obtained with QA23 exhibited the highest concentrations of 1-propanol, 2-methyl-1-propanol, 2,3-butanediol, 2-phenylethanol, benzyl alcohol, 1-heptanol, 1-dodecanol, hexanoic acid, octanoic acid, ethyl phenylacetate, terpineol, benzaldehyde, 1-propanol-3-ethoxy, 1-propanol-3-methylthiol, 1-dodecanol, and 2-methoxy-4-vinylphenol. Conversely, AWRI-R2 originated meads with a high concentration of total acetates and terpenes, and individual high concentrations of 1-nonanol, n-decanoic acid, isoamyl acetate, phenylethyl acetate, linalool, hotrienol, citronellol, nerolidol, cosmene, ethyl acetate, and eugenol.
Spark, a yeast strain recommended for second fermentation of sparkling wines, exhibited high production of higher alcohols, volatile fatty acids and their correspondent esters (ethyl esters), with emphasis on the concentrations of 3-methyl-1-butanol, 3-methyl-1-pentanol, 2-ethylhexanol, 1-hexanol, heptanoic acid, nonanoic acid, tetradecanoic acid, ethyl hexanoate, ethyl heptanoate, ethyl octanoate, ethyl nonanoate, ethyl decanoate, ethyl dodecanoate, nerol and acetaldehyde. Moreover, it was the only yeast to produce detectable concentrations of 1-octanol and, isoamyl octanoate. Meads obtained with VL3 originated meads with the highest concentration of hexyl acetate, ethyl butyrate, and diethyl succinate.
Rouge yielded more neutral meads with the lowest concentration of higher alcohols, fatty alcohols, volatile fatty acids, esters, terpenes and other compounds (Table 2).
To better understand the overall contribution of each yeast strain to the aromatic composition of meads, compounds with odor activity value (OAV) higher than 1, were used in principal component analysis. Twenty five out of 52 compounds (Table 2) were above their olfactive threshold (Guth 1997), and were considered in multivariate analysis (Fig. 3a, b).
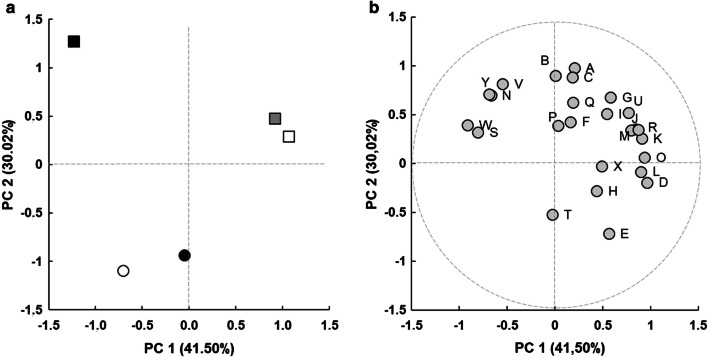
Fig. 3.
a Major component analysis (PC) based on the values of odoriferous activity of volatile compounds in meads fermented with S. cerevisiae var bayanus QA23® (filled square), AWRI-R2® (open square) and SPARK® (filled gray square), and S. cerevisiae var cerevisiae ROUGE® (open circle) and VL3® (filled circle). b Contribution of variables to principal components analysis: (A) 2-phenylethanol, (B) hexanoic acid, (C) octanoic acid, (D) n-decanoic acid, (E) 9-decenoic acid, (F) acetate isoamyl, (G) phenylethylacetate, (H) ethyl butyrate, (I) ethyl hexanoate, (J) ethyl heptanoate, (K) ethyl octanoate, (L) ethyl nonanoate, (M) ethyl decanoate, (N) ethyl phenylacetate, (O) ethyl 9-decenoate, (P) linalool, (Q) citronellol, (R) nerolidol, (S) terpineol, (T) limonene, (U) acetaldehyde, (V) 3-ethoxy-1-propanol, (W) 3-methylthiol-1-propanol, (X) eugenol and (Y) 2-methoxy-4-vinylphenol
The first two principal components accumulated 71.52% of variance, and allowed the separation of meads into three groups according to the yeast strain used: (a) QA23; (b) AWRI-R2 and Spark; (c) Rouge and VL3 (Fig. 3a). The first component (41.50% of the variance) separated the meads produced with AWRI-R2 and Spark from those obtained with QA23, Rouge, and VL3 (Fig. 3a). The compounds that highly contribute to this separation were decanoic acid, ethyl heptanoate, ethyl octanoate, ethyl nonanoate, ethyl decenoate, nerolidol, and acetaldehyde. Meads obtained with AWRI-R2 and Spark exhibited the highest concentrations (Fig. 3b) of these compounds. In contrast, meads obtained with QA23, Rouge and VL3 had higher concentrations for α-terpineol, and 3-(methylthio)-1-propanol.
The second component (30.02% of the variance) separated the meads obtained with QA23, AWRI-R2 and Spark (S. cerevisiae var bayanus) from those fermented with Rouge and VL3 (S. cerevisiae var cerevisiae). The yeasts S. cerevisiae var bayanus produced meads with higher concentrations of 2-phenylethanol, hexanoic acid, octanoic acid, phenylethyl acetate, ethyl phenylacetate, 1-propanol, 3-ethoxy, and 2-methoxy-4-vinylphenol, while meads produced with S. cerevisiae var cerevisiae exhibited higher concentration of decanoic acid.
Discussion
Selection of yeast strains with low nitrogen demand
Reports on yeast selection for mead production are scarce. Caridi et al. (1999) evaluated 122 autochthonous oenological yeasts for mead fermentation. A preliminary screening based on yeast growth and fermentation on honey-must excluded 38.5% of the strains, and after a second experiment they selected just four strains with good general performance. Pereira et al. (2009) evaluated the stress resistance (ethanol, sulphur dioxide, and osmotic shock) and fermentation behavior of five strains of S. cerevisiae isolated from honey showing that they are appropriate for mead production, but emphasize the importance of honey characteristics and must supplementation in order to achieve the best results in mead production.
In the present work, we evaluated the fermentation profile of 21 oenological S. cerevisiae strains in honey-must supplemented with different concentrations of YAN in order to select those with lower nitrogen demand. It is important to emphasize that the International Oenological Codex (OIV 2016), and the Brazilian legislation (Brasil 2016) for wines limits the maximum DAP supplementation to 300 mg L−1 that correspond to 63.6 mg L−1 YAN. Considering the low nitrogen content of honey (Ball 2007), the maximum YAN concentration that can be obtained by the addition of DAP to honey-musts (1:3 dilution) may range between 63 and 95 mg L−1. Moreover, the highest concentration of nitrogen used in the experiments (90 mg L−1) is much smaller than that considered minimal for a healthy wine fermentation (150 mg L−1 YAN) (Ribéreau-Gayon et al. 2006), and those previously reported on mead production (Pereira et al. 2015).
Among the evaluated yeast, only five strains showed a low nitrogen demand behavior and completed mead fermentation even with 75 mg L−1 YAN. The selected strains included three S. cerevisiae var cerevisiae, and three S. cerevisiae var bayanus, recommended for white, red and sparkling wine production. The difference in nitrogen demand from Saccharomyces strains is known by oenologists and companies, but few studies have been carried out aiming at unveiling the genetic and physiological mechanisms involved in these differences. Martínez-Moreno et al. (2012) showed that the production of yeast biomass depends on the nitrogen availability, yeast strain, and sugar concentration. However, they show that biomass production does not guarantee the total consumption of sugar, and that some amino acids, particularly leucine, isoleucine, valine, phenylalanine and threonine, increase the consumption of sugars and ensure greater cell viability. Moreover, Brice et al. (2014) comparing yeast strains with high and low nitrogen demand through transcriptomic analysis showed that the difference is not related to nitrogen accumulation, cellular protein content or protein synthesis, and that the variations in the glycolytic flux may be associated with nitrogen sensing and cell signaling under nitrogen stress.
Nitrogen supplementation through the addition of ammonium phosphate or sulfate is common in the production of wines (Ribéreau-Gayon et al. 2006) and meads (Pereira et al. 2013) but it can affect the absorption of amino acids, and consequently the synthesis of higher alcohols, esters, hydrogen sulfide, ethyl acetate, among other compounds involved in sensorial characteristics of the fermented products (Lambrechts and Pretorius 2000).
Mead fermentation with low nitrogen demand yeast strains
Mead fermentations were carried out with an intermediate nitrogen concentration (75 mg L−1 YAN) and the volatiles and physicochemical parameters of meads produced with selected yeast strains were evaluated. Rouge strain showed the highest fermentation rate and biomass production. As metabolic adaptation is associated with biomass production (Martínez-Moreno et al. 2012), Rouge can be considered as better adapted to a honey-must with 75 mg L−1 YAN, but it should be emphasized that according to Martínez-Moreno et al. (2012) biomass production does not guarantee total sugar consumption.
At the end of fermentations, between 14.35 and 21.66 g L−1 of residual sugar remained in meads. The relatively high residual sugars concentration in meads can be attributed to the presence of non-fermentable sugars, like rhamnose, trehalose, nigerobiose, maltotetraose, maltotriose, maltulose, melezitose, melibiose, nigerose, palatinose, raffinose, erlose among others, currently found in honeys (da Silva et al. 2016). Moreover, it is important to highlight that the presence of residual sugar is not necessarily a demerit, since according to Gomes et al. (2015), meads with a higher sugar content were better appreciated by consumers.
Glycerol concentration in meads was relatively low compared with wines (Ribéreau-Gayon et al. 2006), not exceeding 8.5 g L−1. In wine fermentation, the main role of glycerol synthesis is to provide the cell with a solute involved in osmotic and intracellular redox balance (Pretorius 2003). In addition, glycerol has an important sensory implication in wines, contributing mainly to the beverage body, texture and persistence (Nieuwoudt et al. 2002), and in high concentrations for the sweetness and softness (Cavalcante da Silva et al. 2018).
Regarding volatile acidity, there were no significant differences in volatile acidity concentrations between meads, however according to Ribéreau-Gayon et al. (2006), in high concentrations (> 1 g L−1) acetic acid can negatively affect alcoholic beverages attributing a vinegary character. Only meads fermented with AWRI-R2 exhibited more than 1 g L−1 of acetic acid. As for total acidity, as well as for volatile acidity, no significant differences were observed among meads produced with the selected strains. Since the acidity of the honey-must is very low, and it is important to add tartaric, malic or citric acid, it is advantageous to use strains that attribute to mead medium/high acidity (Caridi et al. 1999).
Color is one of the most important attributes in beverages since it is directly related to the appearance of the product, and is the first attribute perceived by a consumer. The color in honey is related to the contents of phenolics, flavonoids, and minerals (Pereira et al. 2017). Colored products formed from phenolics oxidation or condensation reactions, can be adsorbed by yeast during the fermentation (Mazauric and Salmon 2005). Rouge and AWRI-R2 originated meads with straw-yellow color and Spark, QA23 and VL3 meads with a higher yellow color.
The volatile compounds present in a particular fermented beverage may be derived from the raw material, the microbial metabolism, spontaneous oxide-reductive processes, and microbial biotransformation of precursors. The yeast ability to synthesize or biotransform compounds varies among different species and strains (Lambrechts and Pretorius 2000).
In general, meads produced with the S. cerevisiae var cerevisiae strains (Rouge and VL-3) showed lower concentrations of 2-phenylethanol, phenylethyl acetate, terpenes, higher alcohols, volatile fatty acids and acetate esters than S. cerevisiae var bayanus strains (QA23, AWRI and Spark). Higher production of 2-phenylethanol and phenylethyl acetate by S. cerevisiae var bayanus has been reported in wines (Antonelli et al. 1999). Higher alcohols, except 2-phanylethanol, are considered as negative aromatic products, with solvent and pungent smell. However, at low concentrations (< 300 mg L−1) they can contribute to the aroma complexity of alcoholic beverages (Swiegers et al. 2005). Meads, independent of the yeast strain, showed low higher alcohols concentration (< 200 mg L−1) when compared with wines and other fermented products. This is expected as higher alcohols are produced as by-products of the Ehrlich pathway during nitrogen recycling of aminoacids (Lambrechts and Pretorius 2000), which are scarce in honey. Conversely, esters derived from higher alcohol through acetyl transferase activity are considered as positive organoleptic molecules attributing floral or fruity characteristics to beverages (Saerens et al. 2010).
Volatile fatty acids usually have an unpleasant aroma, but their corresponding esters, which include ethyl hexanoate (fruity flavor), ethyl dodecanoate (fruity and floral aroma), among others, play a key role in the fruity notes of young white wines (Liu et al. 2016). In this sense, meads fermented with AWRI-R2 and Spark, showed the highest concentrations of ethyl esters, when compared with those produced with the other yeast strains. Terpenes are considered as positive factors in the quality of beverages, due to their floral olfactive character (Calleja and Falqué 2005). Monoterpenes can be produced by yeasts (Carrau et al. 2005), but most of them are present in raw material as free or glycosylated-terpenes. As reported by Felipe et al. (2019), the toxic compounds methanol and ethyl carbamate were not detected in meads, a fact that can be attributed to the absence of pectins and the low nitrogen concentration on honey-musts.
OAV values has being used to associated chemical data and sensory attributes, as they better represent the sensory response by human olfactory system than the absolute compound concentration (Zapata et al. 2012). The PCA based on OAV values clearly separates meads according to the yeast strain used on fermentation process. The first component separated the meads produced with AWRI-R2 and Spark from those obtained with the other strains, based on their higher concentration of acetaldehyde, nerolidol, decanoic acid, and several ethyl esters of volatile fatty acids. Although acetaldehyde and decanoic acid had a negative descriptor (pungent and rancid), the other compounds contribute with interesting fruity and floral aromas. The second component separated the meads obtained S. cerevisiae var bayanus (QA23, AWRI-R2 and Spark) and from those fermented with S. cerevisiae var cerevisiae (Rouge and VL3). Meads obtained with S. cerevisiae var bayanus strains showed high concentration (1 to 10 × their olfactive thresholds) of compounds with floral, herbaceous, and fruity descriptors, like 2-phenylethanol, phenylethyl acetate, ethyl phenylacetate, 3-ethoxy-1-propanol, and 2-methoxy-4-vinylphenol, while meads obtained with S. cerevisiae var cerevisiae were more neutral, with intermediary concentrations of the most important aromatic compounds. The highest production of 2-phenylethanol and esters by S. cerevisiae var. bayanus is well documented in wine production (Swiegers et al. 2005; Ribéreau-Gayon et al. 2006; Saerens et al. 2010), but this is the first report of this difference in meads. Although these results should be confirmed by sensory analysis, taking together, the analytical data indicate that S. cerevisiae var cerevisiae strains produce more balanced and neutral meads, while S. cerevisiae var bayanus strains produce more typical meads. Moreover, important differences were detected within each sub-species, indicating that the metabolic characteristics of yeast strains can be explored by producers in order to obtain particular products.
Our future works will be directed to: (1) confirm the effect of low nitrogen-requiring strains on the sensory characteristics of meads produce with different honeys, (2) compare the physicochemical, volatile compounds composition, and sensory attributes of meads produce with low and high nitrogen-requiring strains, and (3) the selection of low nitrogen-requiring native Saccharomyces and non-Saccharomyces yeasts for the production of mead.
Conclusion
The comparison of the fermentative profiles on honey-musts supplemented with different concentrations of YAN allowed the selection of five low nitrogen-demand strains: QA23, AWRI-R2, Spark, Rouge and VL3. Furthermore, the selected strains were evaluated for their contributions in meads produced with limited nitrogen availability (75 mg L−1). The results showed significant differences on some physicochemical parameters like biomass production, residual sugars, glycerol concentration, and fermentative rate. Moreover, meads obtained with selected strains differed on the concentration of several volatile compounds. The volatiles composition of meads and the principal component analysis based on OAV allowed separating yeasts strains in three groups. In general, S. cerevisiae var bayanus strains (QA23, Spark, and AWRI-R2) were the largest producers of aromatics compounds, particularly those with floral and fruity descriptors, while S. cerevisiae var cerevisiae strains (Rouge and VL3) produced more neutral meads. The selection of yeast strains based on their low nitrogen-demand and volatile compounds production can be explored by mead makers in order to avoid fermentation problems and to obtain characteristic products, and by yeast breeders to select yeast (commercial or native) strains to obtain high quality low input meads.
Acknowledgements
This study was funded by the Higher Education Personnel Improvement Coordination—Brazil (CAPES)—Financial Code 001 and University of Caxias do Sul.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Antonelli A, Castellari L, Zambonelli C, Carnacini A. Yeast influence on volatile composition of wines. J Agr Food Chem. 1999;47:1139–1144. doi: 10.1021/jf9807317. [DOI] [PubMed] [Google Scholar]
- Ball DW. The chemical composition of honey. J Chem Educ. 2007;84:1643–1646. doi: 10.1021/ed084p1643. [DOI] [Google Scholar]
- Brice C, Sanchez I, Tesnière C, Blondina B. Assessing the mechanisms responsible for differences between nitrogen requirements of Saccharomyces cerevisiae wine yeasts in alcoholic fermentation. Appl Environ Microbiol. 2014;80:1330–1339. doi: 10.1128/AEM.03856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja A, Falqué E. Volatile composition of Mencıa wines. Food Chem. 2005;90:357–363. doi: 10.1016/j.foodchem.2004.04.013. [DOI] [Google Scholar]
- Caridi A, Fuda S, Postorino S, Russo M, Sidari R. Selection of Saccharomyces sensu stricto for mead production. Food Technol Biotechnol. 1999;37:203–207. [Google Scholar]
- Carrau F, Medina K, Boido E, Farina L, Gaggero C, Dellacassa E, Versini G, Henschke PA. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett. 2005;243:107–115. doi: 10.1016/j.femsle.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Cavalcante da Silva SMP, de Carvalho CAL, Sodré GDS, Estevinho LM. Production and characterization of mead from the honey of Melipona scutellaris stingless bees. J Inst Brew. 2018;124:194–200. doi: 10.1002/jib.485. [DOI] [Google Scholar]
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Felipe ALD, Souza CO, Santos LF, Cestari A. Synthesis and characterization of mead: from the past to the future and development of a new fermentative route. J Food Sci Technol . 2019 doi: 10.1007/s13197-019-03968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis CM, Kordulis C, Kanellaki M, Koutinas AA, Bekatorou A, Lycourghiotis A. Effect of pressure and temperature on alcoholic fermentation by Saccharomyces cerevisiae immobilized on γ-alumina pellets. Bioresour Technol. 2012;114:492–498. doi: 10.1016/j.biortech.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Poole K, Jiranek V. Practical significance of relative assimilable nitrogen requirements of yeast: a preliminary study of fermentation performance and liberation of H2S. Aust J Grape Wine Res. 2002;8:175–179. doi: 10.1111/j.1755-0238.2002.tb00253.x. [DOI] [Google Scholar]
- Gomes T, Dias T, Cadavez V, Verdial J, Morais JS, Ramalhosa E, Estevinho LM. Influence of sweetness and ethanol content on mead acceptability. Pol J Food Nutr Sci. 2015;65:137–142. doi: 10.1515/pjfns-2015-0006. [DOI] [Google Scholar]
- Guth H. Identification of character impact odorants of different white varieties. J Agric Food Chem. 1997;45:3022–3026. doi: 10.1021/jf9608433. [DOI] [Google Scholar]
- Iglesias A, Pascoal A, Choupina AB, Carvalho CA, Feás X, Estevinho LM. Developments in the fermentation process and quality improvement strategies for mead production. Molecules. 2014;19:12577–12590. doi: 10.3390/molecules190812577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts MG, Pretorius IS. Yeast and its importance to wine aroma-a review. S Afr J Enol Vitic. 2000;21:97–129. [Google Scholar]
- Lemos Junior WJF, Viel A, Bovo B, Carlot M, Giacomini A, Corich V. Saccharomyces cerevisiae vineyard strains have different nitrogen requirements that affect their fermentation performances. Lett Appl Microbiol. 2017;65:381–387. doi: 10.1111/lam.12798. [DOI] [PubMed] [Google Scholar]
- Liu PT, Lu L, Duan CQ, Yan GL. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT-Food Sci Technol. 2016;71:356–363. doi: 10.1016/j.lwt.2016.04.031. [DOI] [Google Scholar]
- Martínez-Moreno R, Morales P, Gonzales R, Mas A, Beltran G. Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 2012;12:477–485. doi: 10.1111/j.1567-1364.2012.00802.x. [DOI] [PubMed] [Google Scholar]
- Mazauric JP, Salmon JM. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J Agric Food Chem. 2005;53:5647–5653. doi: 10.1021/jf050308f. [DOI] [PubMed] [Google Scholar]
- Nieuwoudt H, Prior B, Pretorius I, Bauer F. Glycerol and wine quality: fact and fiction. Wynboer. 2002;9:96–101. [Google Scholar]
- OIV (2016) International oenological codex. https://www.oiv.int/public/medias/5119/code-2017-en.pdf. Acessed 15 Oct 2019
- Pereira AP, Dias T, Andrade J, Ramalhosa E, Estevinho LM. Mead production: selection and characterization assays of Saccharomyces cerevisiae strains. Food Chem Toxicol. 2009;47:2057–2063. doi: 10.1016/j.fct.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Pereira AP, Mendes-Ferreira A, Oliveira JM, Estevinho LM, Mendes-Faia A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimization of mead production. Food Microbiol. 2013;33:114–123. doi: 10.1016/j.fm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Pereira AP, Mendes-Ferreira A, Oliveira JM, Estevinho LM, Mendes-Faia A. Mead production: effect of nitrogen supplementation on growth, fermentation profile and aroma formation by yeasts in mead fermentation. J Inst Brew. 2015;121:122–128. doi: 10.1002/jib.184. [DOI] [Google Scholar]
- Pereira AP, Oliveira JM, Mendes-Ferreira A, Estevinho LM, Mendes-Faia A (2017) Mead and other fermented beverages. In: Current developments in biotechnology and bioengineering. Elsevier, pp 407–434. 10.1016/B978-0-444-63666-9.00014-5
- Pretorius IS. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast. 2000;16:675–729. doi: 10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Pretorius IS. The genetic improvement of wine yeasts. In: Arora DK, editor. Handbook of fungal biotechnology. Boca Ratin: CRC Press; 2003. pp. 373–415. [Google Scholar]
- Ramalhosa E, Gomes T, Pereira AP, Dias T, Estevinho LM. Mead production: tradition versus modernity. Adv Food Nutr Res. 2011;63:101–118. doi: 10.1016/B978-0-12-384927-4.00004-X. [DOI] [PubMed] [Google Scholar]
- Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A. Handbook of enology, vol 1: the microbiology of wine and vinifications. Hoboken: Wiley; 2006. [Google Scholar]
- Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol. 2010;3:165–177. doi: 10.1111/j.1751-7915.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AAD, Deoti JR, Müller G, Dário MG, Stambuk BU, Alves Junior SL. Microwell plate-based method for the determination of reducing sugars with the DNS reagent. Braz J Food Technol. 2017;20:e2015113. doi: 10.1590/1981-6723.11315. [DOI] [Google Scholar]
- Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius I. Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res. 2005;11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]
- Vidrih R, Hribar J. Mead: the oldest alcoholic beverage. In: Kristbergsson K, Oliveira J, editors. Traditional foods. Integrating Food science and engineering knowledge into the food chain. Boston: Springer; 2016. pp. 325–338. [Google Scholar]
- Welke JE, Zanus M, Lazzarotto M, Zini CA. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res Int. 2014;59:85–99. doi: 10.1016/j.foodres.2014.02.002. [DOI] [Google Scholar]
- Xiao Z, Zhou X, Niu Y, Yu D, Zhu J, Zhu G. Optimization and application of headspace-solid-phase micro-extraction coupled with gas chromatography–mass spectrometry for the determination of volatile compounds in cherry wines. J Chromatogr B. 2015;978:122–130. doi: 10.1016/j.jchromb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Zapata J, Mateo-Vivaracho L, Lopez R, Ferreira V. Automated and quantitative headspace in-tube extraction for the accurate determination of highly volatile compounds from wines and beers. J Chromatogr A. 2012;1230:1–7. doi: 10.1016/j.chroma.2012.01.037. [DOI] [PubMed] [Google Scholar]